Abstract
Early Eocene land bridges allowed numerous plant and animal species to cross between Europe and North America via the Arctic. While many species suited to prevailing cool Arctic climates would have been able to cross throughout much of this period, others would have found dispersal opportunities only during limited intervals when their requirements for higher temperatures were met. Here, we present Titanomyrma lubei gen. et sp. nov. from Wyoming, USA, a new giant (greater than 5 cm long) formiciine ant from the early Eocene (approx. 49.5 Ma) Green River Formation. We show that the extinct ant subfamily Formiciinae is only known from localities with an estimated mean annual temperature of about 20°C or greater, consistent with the tropical ranges of almost all of the largest living ant species. This is, to our knowledge, the first known formiciine of gigantic size in the Western Hemisphere and the first reported cross-Arctic dispersal by a thermophilic insect group. This implies intercontinental migration during one or more brief high-temperature episodes (hyperthermals) sometime between the latest Palaeocene establishment of intercontinental land connections and the presence of giant formiciines in Europe and North America by the early middle Eocene.
Keywords: Formicidae, Formiciinae, Titanomyrma, Holarctic dispersal, hyperthermals
1. Introduction
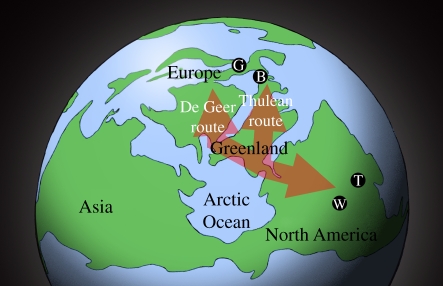
Holarctic interchanges of plants and animals during the Eocene have long been known [1–13]; however, details of their nature and timing remain little understood. Eocene North America and Europe were only a short distance apart (figure 1), which, combined with eustatic sea-level drop, resulted in land connections between them. Dispersal was possible across a forested, unglaciated Arctic via Greenland by a northern ‘De Geer’ route through Fennoscandia and a southern ‘Thulean’ route through Iceland, the Faroe Islands and Great Britain [2–7,10,12]. An epicontinental seaway (figure 1) probably restricted dispersal between Europe and Asia at least some of this time.
Figure 1.
Reconstructed early Eocene northern continental positions and shorelines in polar view with Formiciinae fossil localities (G, Germany; B, Britain; W, Wyoming; T, Tennessee), and dispersal routes across the Arctic indicated by red arrows.
The mammal fossil record shows discrete waves of intercontinental migrations during this interval, most notably at the Palaeocene/Eocene boundary ‘mammalian dispersal event’, when major groups simultaneously appeared across the Holarctic [4,10]. Early Eocene Europe and North America shared the highest number of mammal genera in the Cenozoic [2]; they also had pronounced floral similarities, indicating numerous intercontinental range extensions of plant taxa [3,5,7]. While it has long been recognized that the distributions of modern insect taxa implies Eocene cross-North Atlantic migrations [1], direct fossil evidence has only been reported recently [8,9,11,13].
Increasingly, fine-scale variations in early Eocene Arctic climate are becoming known, with mostly cool, but seasonally equable temperatures prevailing, but with episodic hyperthermal intervals of increased temperatures, some brief and intense [14–18]. Insect taxa previously shown to have co-occurred in Europe and North America in the early Eocene all had ranges including localities where mean annual temperature (MAT) is estimated as cool (far-western North America) or is not known (Fur Formation, Denmark). The new, giant formiciine ant from the Eocene of Wyoming described here, however, was a thermophilic insect, providing evidence of the role played by early Eocene hyperthermal events in facilitating selective trans-Arctic intercontinental dispersals [3–7,12]. Variations in climate would have then acted as a filter gate across high latitudes, mediating differential access between continents for species populations according to their individual physiological requirements.
2. Material and methods
We follow the morphological terminology used by Lutz [19,20] and Wappler [21], except in referring to abdominal, not gaster, segment identifying numbers (abdominal segments A3–7 = gaster segments I–V). Palaeoclimatic analysis of MAT of the fossil ant localities (table 1) used either published proxies for MAT [18,22–24], or new analyses based on previously published data on the proportion of dicot leaf morphotypes with non-toothed margins using the leaf margin analysis equation of Miller et al. [25], and the leaf area analysis equation for estimating mean annual precipitation (MAP) from Wilf et al. [26]. Occurrence data for modern large ants were gathered from published records and personal communications (electronic supplementary material). The latitude and longitudes of these records were entered into the WorldClim software [27], which provided estimates of MAT, coldest yearly quarter mean temperature and MAP to a square kilometre level of spatial resolution.
Table 1.
Mean annual temperature (MAT) estimates for formiciine-bearing fossil localities (Messel, Puryear, Bournemouth) and localities regional to the Farson Fish beds, Wyoming (Little Mountain, Niland Tongue). MAT estimates for Messel were previously published as points on a figure [56], and these values are now stated here, as well as newly published estimates based on both leaf margin analysis (LMA) and the coexistence interval of nearest living relatives of plant fossils [66] (Wilde et al. [67] inferred a similar climate for Eckfeld and Messel). A MAT estimate for the Farson Fish Beds has not been reported; values are given for the slightly older Green River Formation Laney Member Little Mountain locality, the regional Wasatch Formation Niland Tongue locality and an unspecified Green River Basin [68]. Values for all sites include both leaf margin analysis and either taxon analogue [66] or isotopic methods [68], except for Bournemouth, which is from isotopic analysis of fossil gastropod shells [24]. An isotopic estimate from the San Juan Basin (SJB) [68] is supplied alongside the LMA-based estimate for Puryear, being of the same palaeolatitude and age [68]. The standard error for LMA values is set at 4.0°C unless the calculated error is greater than 4.0°C, following the recommendation of Peppe et al. [69]; for SJB isotope value, it is 5.5°C [68]; error not provided for Bournemouth isotope value [24].
| locality | no. of leaf morphotypes | pmargin(%) | MAT |
|---|---|---|---|
| with Formiciinae | |||
| Messel | 49 | 65.0 | 20.3°Ca, 21.1°Cb, 22°C (up to ∼24°C) [66] |
| Puryear | 47 | 91.5 | 23.9°C [23], 27.7°Ca, 29.0°Cb, (27.8°C SJB [68]) |
| Bournemouth | n.a. | n.a. | 22°C [24] |
| Green River Formation | |||
| Little Mountain | 49 | 60.2 | 19.6°C [22], 18.8°Cc, 18.3°C [68] |
| Niland Tongue | 14 | 71.4 | 23.0°C [22], 22.0°Cc |
3. Systematic palaeontology
Formicidae Latreille 1802;
Formiciinae Lutz [20];
Titanomyrma lubei gen. et sp. nov.
(a). Etymology
Genus name derived from the Greek Titan, often used to indicate large size, and myrmex (Greek: ant), gender feminine. The specific epithet is formed from the surname of the collector of the holotype, Louis Lube.
(b). Holotype
Denver Museum of Nature and Science no. 9041 (labelled DMNH 9041); part only, body in dorsal aspect, anterior portions of right forewing, very little of left.
(c). Locality and age
DMNS locality 784, Farson Fish Beds, Laney Member of the Green River Formation; Sweetwater County, Wyoming; latest early Eocene, approximately 49.5 Ma [28].
(d). Type and included species of Titanomyrma
Titanomyrma giganteum comb. nov. is here designated as the type species of the genus, which also includes Titanomyrma simillimum comb. nov and T. lubei sp. nov.
(e). Diagnosis: Titanomyrma
As provided for Formicium by Lutz [20], amended here by: gaster shape variable: ovate or more slender/cylindrical; A5 width relative to other gaster segments variable; relative lengths of A3–7 variable.
(f). Diagnosis: Titanomyrma lubei
Queens separated from other species of Titanomyrma most easily by gaster characters: more slender, length/width: 2.14 (T. giganteum, 1.40; T. simillimum, 1.50); middle half roughly cylindrical (others ovate, A5 widest); A3 length about a quarter width (others about a third); A4 about three times A3 length (others less than twice); A5–6 about half as long as wide (others about a third); A3 not curved around the petiole at joining.
(g). Description: Titanomyrma
As provided for Formicium by Lutz [20], amended here by change in description of gaster (cf. diagnosis, above).
(h). Description: Titanomyrma lubei queen
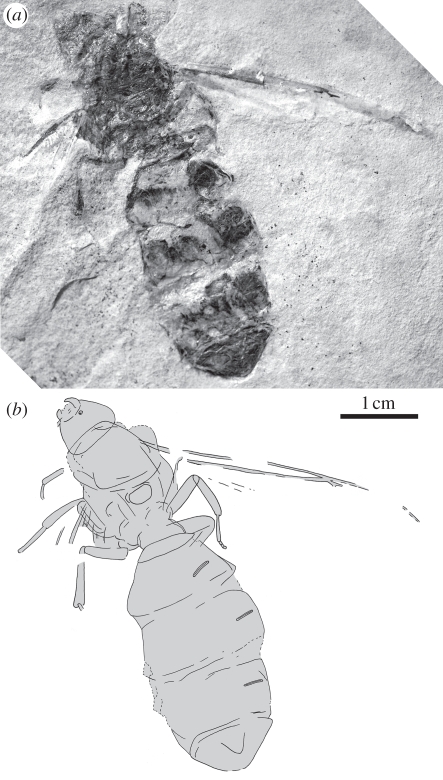
As in diagnosis; figures 2 and 3a,d; immense size, body approximately 51 mm long; head rounded-triangular, approximately 5.5 mm long; approximately 7 mm wide, about a third alitrunk length; antennae, eyes not known; mandibles about a third head length; alitrunk approximately 15 mm long, wing bearing; legs short, tibia III 6 mm; forewing probably in size range of T. giganteum queens; waist single segmented, petiole approximately 5.5 mm wide, lacking anterior peduncle; gaster 31 mm long, 14.5 mm wide, without constriction between A3 and A4; spiracles (known on A4–6) long, narrow.
Figure 2.
Holotype of T. lubei gen. et sp. nov.: (a) photograph and (b) drawing.
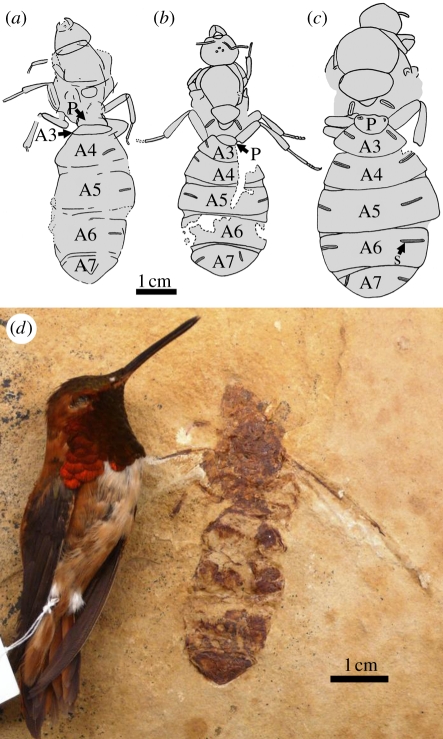
Figure 3.
(a–c) Comparative bodies (wings not drawn) of (a) T. lubei, (b) T. simillimum, (c) T. giganteum and (d) T. lubei holotype with rufous hummingbird (Selasphorus rufus) for size comparison; (b,c) redrawn from Lutz [20]. P, petiole; s, spiracle; A3–7, abdominal segments three through to seven (=gaster segments I–V).
4. Discussion
(a). The status of formiciine species
Titanomyrma is confidently assigned to the Formiciinae by character states unique within Formicidae: extremely large size combined with large, slit-like spiracles [19,20,29], which associate it with T. giganteum and T. simillimum (early middle Eocene Germany: Messel, and cf. these species from Ekfeld Maar [19–21]), both transferred here to Titanomyrma from Formicium. Formicium brodiei Westwood, Formicium mirabile Cockerell [30–32] (both Bournemouth, UK; middle Eocene) and Formicium berryi Carpenter [33] (middle Eocene Claiborne Formation, Puryear, TN, USA; incorrectly called Wilcox Formation by Carpenter [33,34]) are only known from forewings, and were grouped with the German species by the similarity of their venations.
The distinctive gaster shape of T. lubei is confidently not an artefact of preservation; the tergites are clearly shaped differently than are those of the German species, where A5 is wider than A4 (gaster segments III and II) [20] (figure 3b,c). In T. lubei, however, these are of similar lengths and maximum widths (figures 2 and 3a). Its legs are notably more gracile than those of T. giganteum and somewhat more so than those of T. simillimum. The mandibles are poorly preserved, but appear smaller than those of the German species (figures 2 and 3a–c). Wing length basad the pterostigma in T. lubei is about 24 mm, similar to that of the T. giganteum holotype queen [20], indicating a forewing length within the range of T. giganteum, larger than in T. simillimum [20]. However, the body of T. lubei is closer in length to the T. simillimum holotype, 53 mm [20].
We propose the genus Titanomyrma for the new species and transfer the two German species to it, as grouping them within Formicium would compound an existing problem that can no longer be avoided.
Lutz [20] recognized the difficulty of defining the genus based on the limited information provided by a single forewing specimen of the type species, F. brodiei. He, therefore, used the characters of the new species ‘F.’ giganteum and ‘F.’ simillimum to form his genus diagnosis and description, effectively employing them as sort of ‘functional type species’ (‘Since Westwood only illustrated the wing, but did not describe it formally, the following new finds from Messel will provide a more detailed diagnosis and description’. p. 182, our translation from the German).
Further, in ant taxonomy generally, because of the variability of wing characters and the small amount of their study compared with those of other insect groups, and by the traditional availability of more commonly collected wingless worker caste specimens, wings are usually ignored or de-emphasized in defining modern species; they are invariably defined by workers.
There appears to be considerable plasticity of wing characters in Formiciinae in particular. Venational morphology of the holotype wings of F. brodiei ([20], fig. 1c) and F. berryi ([20], fig. 2c) falls within variability between the left and right wings of a specimen of ‘F.’ aff. giganteum ([21], fig. 95). Further, note the differences between the forewings of this specimen of ‘F.’ aff. giganteum and the ‘F.’ giganteum holotype ([19], fig. 3b) and paratype 6 ([19], fig. 5). Also, size differences between males and females of the German species indicate the possibility that smaller and larger wing species could be conspecific [19]. Such examples indicate deep problems in the usefulness of formiciine wings for defining species.
By these reasons, we propose to clarify formiciine taxonomy by considering Formicium as a collective genus containing the wing species, which we consider tentative, and erecting the nominal genus Titanomyrma to include the new species and the two German species. As a collective group, Formicium would have neither a diagnosis nor a description, but would be defined as including all species of Formiciinae that cannot be placed in a nominal genus owing to lack of sufficient preserved characters. Formicium brodiei ceases to be Formicium's type species (a collective genus has none: ICZN Article 67.14 [35]). Formicium, however, remains the type genus of Formiciinae, and the subfamily name is not affected.
A team of palaeoentomologists is currently undertaking a major examination of the Formiciinae (T. Wappler 2011, personal communication), with large numbers of new specimens available with which to confidently establish intra- and interspecific variability. We expect that this work will greatly clarify the positions of the wing species. Here, we provide what we recognize is a brief, interim diagnosis and description of Titanomyrma, with the understanding that these will soon be established with greater detail by the Wappler team.
(b). Thermophily, size and the Formiciinae
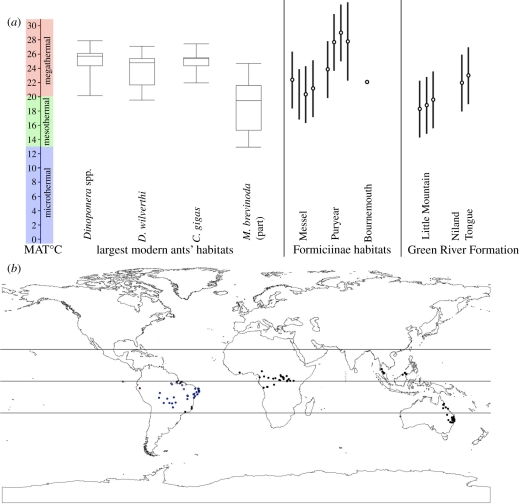
To assess the climatic constraints of Formiciinae, we reconstructed the MAT, coldest quarter mean temperature (CQMT) and the MAP of the sites where they have been recovered. Further, we calculated the climate space occupied by the largest living ants to ascertain whether there are common climatic trends associated with great size that are general to ants. Based on analysis of their habitats, the Formiciinae were clearly thermophilic; they are only known from hot climates, with MAT > 20°C (table 1 and figure 4a). This is also supported by their great size, as almost all of the largest modern ant species are restricted to the megathermal climates of the tropics.
Figure 4.
Climates and distributions of formiciine ants and modern ants with any caste over 3 cm in length. (a) Mean annual temperature (MAT) values for the largest living ants (box plots: minimum, 25%, median, 75% and maximum values), the Eocene localities where formiciine ants have been recovered and Green River Formation localities comparable with the Farson Fish Beds where T. lubei was found (references for fossil site MAT values as in table 1); (b) ranges of modern ant species with any caste over 3 cm in length (locality references in the electronic supplementary material), equator, tropics of Capricorn and Cancer indicated: South America, Dinoponera spp.; Africa, D. wilverthi; Southeast Asia, C. gigas; eastern Australia, M. brevinoda (in part, see text).
The great size of T. lubei was only exceeded by that of the largest known ants, T. giganteum and T. simillimum, the reproductives of which are estimated to have had the mass of a small bird [19]; indeed, the body of T. lubei is larger than that of a rufous hummingbird (figure 3d).
Although the average size of workers within modern ant communities tends to increase with latitude [36,37], almost all of the largest individual living ant species (any caste ≥3 cm long) inhabit tropical regions with MAT over 20°C (figure 4a,b). They are scattered among subfamilies, have differing castes as the largest achieve great size by different morphologies and are widely separated on different continents.
— Dinoponera spp. (subfamily Ponerinae): tropical Brazil, Bolivia and Peru. Dinoponera species (Dinoponera gigantea, Dinoponera lucida, Dinoponera longipes, Dinoponera mutica and Dinoponera quadriceps) reach and may exceed 30 mm in length [38], except Dinoponera australis (25 mm or less), whose range extends outside the tropics. They lack morphologically distinct worker and queen castes [39,40].
— Dorylus wilverthi (subfamily Dorylinae): equatorial Africa [41–45]. The largest living ant, with queens reaching 52 mm long [46], similar in size to T. lubei. Great size is achieved here, however, in a much different manner: the gaster is approximately 80 per cent of body length (cf. fig. 16–13a of Hölldobler & Wilson [46]), compared with approximately 60 per cent in T. lubei. Queens show a suite of characteristics restricted to legionary ants, including physogastry, where the gaster becomes swollen, distended with sclerites widely separated by a membrane, containing expanded ovaries that allow rapid production of large numbers of eggs and so extremely large colonies [42,47,48].
— Camponotus gigas (subfamily Formicinae): southeast Asian region [45,48,49]. Queens average slightly over 31 mm in length, and major workers approximately 28 mm, but reach 30 mm [48,50].
— Myrmecia brevinoda (subfamily Myrmeciinae): eastern Australia [51]. Major workers reach 36 mm and queens 31 mm in length [52]. Only a subset of those ant groups synonymized as M. brevinoda reach lengths over 3 cm [52,53].
Seven of these eight species inhabit distinctly megathermal environments (figure 4a,b): the five large Dinoponera species have a distribution with a median MAT of 25.7°C, with a CQMT of 23.9°C; D. wilverthi median MAT is 24.7°C, CQMT 23.4°C; and C. gigas median MAT is 25.3°C, CQMT 24.2°C. Only M. brevinoda breaks this pattern, with a range extending through eastern Australia from the hot tropics of northern Queensland (approx. 16° S) south to warm-temperate Victoria (approx. 39° S). This is, then, the only modern ant species of this great size that includes part of its range outside of the tropics. The species, as it is currently defined, however, includes those that Clark [52] discussed to be over 3 cm in length as a subset, as well as various groups whose largest individuals (any caste) are recorded only reaching about 24 mm in length. The records of great size appear to be only from Queensland and adjoining northern New South Wales, i.e. the northern part of this range [52]. The range of M. brevinoda as a whole has a median MAT of 17.0°C and a CQMT of 11.6°C. The range of Clark's [52] larger form, however, has a nearly megathermal MAT of 19.4°C and a CQMT of 14.3°C.
All of these modern ants inhabit mesic to wet climates, in agreement with MAP estimates for Messel, Puryear (new analyses here) and Green River [22] (see the electronic supplementary material, table S1). The Eocene Arctic was probably a rainforest [15,16], so moisture availability would not have been a barrier.
High temperatures extended well out of the modern tropical belt in the Eocene [15–18,22,23,54–56]. Formiciines are exclusively known from climates with an estimated MAT of about 20°C or greater; MAT of Messel, Puryear and Bournemouth were all megathermal. The climate of the Farson Fish Beds within which T. lubei was recovered has not been characterized, but estimates for comparable Green River Formation localities indicate similarly hot conditions (table 1 and figure 4a).
The cooler, microthermal to lower mesothermal [54,55] early Eocene Okanagan Highlands localities of far-western North America bear a number of ant species over 1.5 cm in length—in one case greater than 2.5 cm—but none are known to reach 3 cm [9,57]. A species from the early Eocene Danish Fur Formation is also about 2.5 cm long [9,58]. Ants from the late Eocene cool upland at Florissant, Colorado and from Palaeocene and Eocene ambers (Sakhalin, Baltic, Ukranian, etc.; indeed, any amber) are not known to reach such sizes (e.g. [59–63]).
(c). Eocene Arctic climate, hyperthermal events and intercontinental dispersal
Thermophilic organisms face physiological barriers to dispersal outside of the high MAT climate spaces to which they are adapted [12,23,64]. Eocene Arctic fossil floras indicate that temperate (i.e. upper microthermal to lower mesothermal) conditions predominated across intercontinental connections, with early to middle Eocene floras from Greenland and Axel Heiberg Island (50–40 Myr ago) giving MAT estimates of 12–16°C, probably too cool to support dispersal of Formiciinae throughout much of the time when this must have occurred [12–16]. Brief, cyclic warming events of approximately 2–4°C possibly driven by ventilation of oceanic carbonates were unlikely to have been of sufficient intensity to facilitate formiciine dispersal [65]. However, during larger global hyperthermal events linked to injection of greenhouse gasses into the atmosphere from sedimentary reservoirs, Arctic MAT increased by 5–10°C to perhaps approximately 23°C, with the coldest month mean temperature greater than 8°C at approximately 85° N palaeolatitude [18], which would have been suitable for formiciine dispersal across high latitudes. These include the brief (approx. 170 kyr) Palaeocene–Eocene Thermal Maximum at the Palaeocene–Eocene boundary (about 55.5 Ma); the Eocene Thermal Maximum 2 (about 53.5 Ma); and the longer Early Eocene Climatic Optimum, about 2 Myr of the latest early Eocene [14,17,18]. That is, the early Eocene physical bridge between Europe and North America that was normally climatically impassable for Formiciinae must have had episodic openings of a physiological gate allowing the dispersal of these giant ants and other thermophilic organisms across the Arctic.
Acknowledgements
We thank L. Lube, collector and donor of the specimen; J. Baldwin for software consultation; G. Alpert, B. Bolton, E. Jarzembowski, H. Lutz, V. Makarkin, M. Moffett, D. Notton, M. Pfeiffer, Z. Qian, S. Robson, A. Ross, B. Taylor, R. Taylor, T. Wappler and S. Watanasit for discussion and information. We thank C. Labandeira and an anonymous reviewer for helpful comments. This research was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC) through a postdoctoral fellowship to S.B.A., and Discovery Grants to R.W.M. and D.R.G.
References
- 1.Matthews J. V., Jr 1979. Tertiary and Quaternary environments: historical background for an analysis of the Canadian insect fauna. In Canada and its insect fauna, Memoirs of the Entomological Society of Canada, no. 108, (ed. Danks H. V.), pp. 31–86 Ottawa, Canada: Entomological Society of Canada. [Google Scholar]
- 2.Woodburne M. O., Swisher C. C., III 1995. Land mammal high-resolution geochronology, intercontinental overland dispersals, sea level, climate, and vicariance. In Geochronology, time scales, and global stratigraphic correlations, vol. 54 (eds Berggren W. A., Kent D. V., Aubry M.-P., Hardenbol J.), pp. 335–364 Tulsa, OK: Society for Sedimentary Geology [Google Scholar]
- 3.Manchester S. R. 1999. Biogeographical relationships of North American Tertiary floras. Ann. Miss. Bot. Gard. 86, 472–522 10.2307/2666183 (doi:10.2307/2666183) [DOI] [Google Scholar]
- 4.Hooker J. J. 2000. Paleogene mammals: crisis and ecological change. In Biotic responses to global change: the last 145 million years (eds Culver S. J., Rawson P. F.), pp. 333–349 Cambridge, UK: Cambridge University Press [Google Scholar]
- 5.Tiffney B. H. 2000. Geographic and climatic influences on the Cretaceous and Tertiary history of Euramerican floristic similarity. Acta. Univ. Carol. Geol. 44, 5–16 [Google Scholar]
- 6.Sanmartín I., Enghoff H., Ronquist F. 2001. Patterns of animal dispersal, vicariance and diversification in the Holarctic. Biol. J. Linn. Soc. Lond. 73, 343–390 10.1006/bijl.2001.0542 (doi:10.1006/bijl.2001.0542) [DOI] [Google Scholar]
- 7.Tiffney B. H., Manchester S. R. 2001. The use of geological and paleontological evidence in evaluating plant phylogenetic hypotheses in the Northern Hemisphere Tertiary. Int. J. Plant Sci. 162(Suppl. 6), S3–S17 10.1086/323880 (doi:10.1086/323880) [DOI] [Google Scholar]
- 8.Archibald S. B., Makarkin V. N. 2006. Tertiary giant lacewings (Neuroptera: Polystoechotidae) revision and description of new taxa from western North America and Denmark. J. Syst. Palaeontol. 4, 119–155 10.1017/S1477201906001817 (doi:10.1017/S1477201906001817) [DOI] [Google Scholar]
- 9.Archibald S. B., Cover S. D., Moreau C. S. 2006. Bulldog ants of the Eocene Okanagan Highlands, and the history of the subfamily (Hymenoptera: Formicidae: Myrmeciinae). Ann. Entomol. Soc. Am. 99, 487–523 10.1603/0013-8746(2006)99[487:BAOTEO]2.0.CO;2 (doi:10.1603/0013-8746(2006)99[487:BAOTEO]2.0.CO;2) [DOI] [Google Scholar]
- 10.Smith T., Rose K. D., Gingerich P. D. 2006. Rapid Asia–Europe–North America geographic dispersal of earliest Eocene primate Teilhardina during the Paleocene–Eocene Thermal Maximum. Proc. Natl Acad. Sci. USA 103, 11 223–11 227 10.1073/pnas.0511296103 (doi:10.1073/pnas.0511296103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrulevičius J. F., Nel A., Rust J., Bechly G., Kohls D. 2007. New Paleogene Epallagidae (Insecta: Odonata) recorded in North America and Europe. Biogeographic implications. Alavesia 1, 15–25 [Google Scholar]
- 12.Tiffney B. H. 2008. Phylogeography, fossils, and Northern Hemisphere biogeography: the role of physiological uniformitarianism. Ann. Miss. Bot. Gard. 95, 135–143 10.3417/2006199 (doi:10.3417/2006199) [DOI] [Google Scholar]
- 13.Archibald S. B. 2009. New Cimbrophlebiidae (Insecta: Mecoptera) from the Early Eocene at McAbee, British Columbia, Canada and Republic, Washington, USA. Zootaxa 2249, 51–62 [Google Scholar]
- 14.Westerhold T., Röhl U., Laskar J., Raffi I., Bowles J., Lourens L. J., Zachos J. C. 2007. On the duration of magnetochrons C24r and C25n and the timing of early Eocene global warming events: implications from the Ocean Drilling Program Leg 208 Walvis Ridge depth transect. Paleoceanography 22, 1–19 10.1029/2006PA001322 (doi:10.1029/2006PA001322) [DOI] [Google Scholar]
- 15.Eldrett J. S., Greenwood D. R., Harding I. C., Huber M. 2009. Increased seasonality through the Eocene to Oligocene transition in northern high latitudes. Nature 459, 969–974 10.1038/nature08069 (doi:10.1038/nature08069) [DOI] [PubMed] [Google Scholar]
- 16.Greenwood D. R., Basinger J. F., Smith R. Y. 2010. How wet was the Arctic Eocene rainforest? Estimates of precipitation from Paleogene Arctic macrofloras. Geology 38, 15–18 10.1130/G30218.1 (doi:10.1130/G30218.1) [DOI] [Google Scholar]
- 17.Zachos J. C., Dickens G. R., Zeebe R. E. 2008. An early Cenozoic perspective on greenhouse warming and carbon-cycle dynamics. Nature 451, 279–283 10.1038/nature06588 (doi:10.1038/nature06588) [DOI] [PubMed] [Google Scholar]
- 18.Sluijs A., et al. 2009. Warm and wet conditions in the Arctic region during Eocene thermal maximum 2. Nat. Geosci. 2, 777–780 10.1038/NGEO668 (doi:10.1038/NGEO668) [DOI] [Google Scholar]
- 19.Lutz H. 1990. Systematische und palökologische Untersuchungen an Insekten aus dem Mittel-Eozän der Grube Messel bei Darmstadt. Cour. Forschungstinst. Senckenb. 124, 1–165 [Google Scholar]
- 20.Lutz H. 1986. Eine neue Unterfamilie der Formicidae (Insecta: Hymenoptera) aus dem mittel-eozänen Ölschiefer der ‘Grube Messel’ bei Darmstadt (Deutschland, S-Hessen). Senckenb. lethaea 67, 177–218 [Google Scholar]
- 21.Wappler T. 2003. Die Insekten aus dem Mittel-Eozän des Eckfelder Maars, Vulkaneifel. Mainzer naturwiss. Arch. Beiheft. 27, 1–234 [Google Scholar]
- 22.Wilf P. 2000. Late Paleocene–early Eocene climate changes in southwestern Wyoming: paleobotanical analysis. Geol. Soc. Am. Bull. 112, 292–307 (doi:10.1130/0016-7606(2000)112<292:LPECCI>2.0.CO;2) [DOI] [Google Scholar]
- 23.Greenwood D. R., Wing S. L. 1995. Eocene continental climates and latitudinal gradients. Geology 23, 1040–1048 (doi:10.1130/0091-7613(1995)023<1044:ECCALT>2.3.CO;2) [DOI] [Google Scholar]
- 24.Andreasson F. P., Schmitz B. 2000. Seasonality in the early Middle Eocene North Atlantic region. Geol. Soc. Am. Bull. 112, 628–640 (doi:10.1130/0016-7606(2000)112<628:TSITEM>2.0.CO;2) [DOI] [Google Scholar]
- 25.Miller I. M., Brandon M. T., Hickey L. J. 2006. Using leaf margin analysis to estimate the Mid-Cretaceous (Albian) paleolatitude of the Baja BC block. Earth Planet. Sci. Lett. 245, 95–114 10.1016/j.epsl.2006.02.022 (doi:10.1016/j.epsl.2006.02.022) [DOI] [Google Scholar]
- 26.Wilf P., Wing S. L., Greenwood D. R., Greenwood C. L. 1998. Using fossil leaves as paleoprecipitation indicators: an Eocene example. Geology 26, 203–206 (doi:10.1130/0091-7613(1998)026<0203:UFLAPI>2.3.CO;2) [DOI] [Google Scholar]
- 27.Hijmans R. J., Cameron S. E., Parra J. L., Jones P. G., Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. Int. Climatol. 25, 1965–1978 10.1002/joc.1276 (doi:10.1002/joc.1276) [DOI] [Google Scholar]
- 28.Smith M. E., Singer B. S., Carroll A. R. 2008. Synoptic reconstruction of a major ancient lake system: Eocene Green River Formation, western United States. Geol. Soc. Am. Bull. 120, 54–84 10.1130/1326073.1 (doi:10.1130/1326073.1) [DOI] [Google Scholar]
- 29.Bolton B. 2003. Synopsis and classification of the Formicidae. Mem. Am. Entomol. Inst. 71, 1–370 [Google Scholar]
- 30.Westwood J. O. 1854. Contributions to fossil entomology. Quart. J. Geol. Soc. Lond. 10, 378–396 10.1144/GSL.JGS.1854.010.01-02.43 (doi:10.1144/GSL.JGS.1854.010.01-02.43) [DOI] [Google Scholar]
- 31.Cockerell T. D. A. 1920. Fossil arthropods in the British Museum 1. Ann. Mag. Nat. Hist. 5, 273–279 [Google Scholar]
- 32.Jarzembowski E. A. 1996. Fossil insects of the Bournemouth Group (Eocene: Late Ypresian–Lutetian) of southern England. Tert. Res. 16, 203–211 [Google Scholar]
- 33.Carpenter F. M. 1929. A fossil ant from the Lower Eocene (Wilcox) of Tennessee. J. Wash. Acad. Sci. 19, 300–301 [Google Scholar]
- 34.Dilcher D. L. 1973. Vegetation and vegetational history of Northern Latin America (ed. Graham A.), pp. 39–59 Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 35.ICZN 1999. International code of zoological nomenclature, 4th edn. London, UK: International Trust for Zoological Nomenclature [Google Scholar]
- 36.Cushman J. H., Lawton J. H., Manly B. F. J. 1993. Latitudinal patterns in European ant assemblages: variation in species richness and body size. Oecologia 95, 30–37 [DOI] [PubMed] [Google Scholar]
- 37.Kaspari M. 2005. Global energy gradients and size in colonial organisms: worker mass and worker number in ant colonies. Proc. Natl Acad. Sci. USA 102, 5079–5083 10.1073/pnas.0407827102 (doi:10.1073/pnas.0407827102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kempf W. W. 1971. A preliminary review of the ponerine ant genus Dinoponera Roger (Hym. Formicidae). Stud. Entomol. 14, 369–394 [Google Scholar]
- 39.Haskins C. P., Whelden R. M. 1965. ‘Queenlessness’, Worker sibship, and colony versus population structure in the formicid genus Rhytidoponera. Psyche (MA) 72, 87–112 10.1155/1965/40465 (doi:10.1155/1965/40465) [DOI] [Google Scholar]
- 40.Peeters C. 1991. The occurrence of sexual reproduction among ant workers. Biol. J. Linn. Soc. Lond. 44, 141–152 10.1111/j.1095-8312.1991.tb00612.x (doi:10.1111/j.1095-8312.1991.tb00612.x) [DOI] [Google Scholar]
- 41.Gotwald W. H., Jr 1982. Army ants. In Social insects 4 (ed. Hermann H. R.), pp. 157–254 New York, NY: Academic Press [Google Scholar]
- 42.Schöning C., Humle T., Möbius Y., McGrew W. C. 2008. The nature of culture: technological variation in chimpanzee predation on army ants revisited. J. Hum. Evol. 55, 48–59 10.1016/j.jhevol.2007.12.002 (doi:10.1016/j.jhevol.2007.12.002) [DOI] [PubMed] [Google Scholar]
- 43.California Academy of Sciences 2009. AntWeb. See http://www.antweb.org/
- 44.Peters M. K., Fischer G., Schaab G., Kraemer M. 2009. Species compensation maintains abundance and raid rates of African swarm-raiding army ants in rainforest fragments. Biol. Conserv. 142, 668–675 10.1016/j.biocon.2008.11.021 (doi:10.1016/j.biocon.2008.11.021) [DOI] [Google Scholar]
- 45.Taylor B. The ants of (sub-Saharan) Africa (Hymenoptera: Formicidae), 10th edn. 2009. See http://antbase.org/ants/africa/ [Google Scholar]
- 46.Hölldobler B., Wilson E. O. 1990. The ants. Cambridge, MA: Belknap Press [Google Scholar]
- 47.Berghoff S. M. 2002. Sociobiology of the hypogaeic army ant Dorylus (Dichthadia) laevigatus Fr. Smith. PhD thesis, University of Würzburg, Faculty of Biology, Theodor-Boveri-Institut für Biowissenschaften, Würzburg, Germany [DOI] [PubMed]
- 48.Pfeiffer M., Linsenmair K. 2000. Contributions to the life history of the Malaysian giant ant Camponotus gigas (Hymenoptera, Formicidae). Insect. Soc. 47, 123–132 10.1007/PL00001690 (doi:10.1007/PL00001690) [DOI] [Google Scholar]
- 49.Watanasit S., Saewai J., Phlapplueng A. 2007. Ants of the Klong U-Tapao Basin, Southern Thailand. Asian Myrmecol. 1, 69–79 [Google Scholar]
- 50.Pfeiffer M. 1997. Sozialstruktur und Verhaltensökologie von Riesenameisen Camponotus gigas Latreille 1802 im Regenwald Malaysias auf Borneo. Berlin, Germany: Wissenschaft und Technik [Google Scholar]
- 51.Ogata K., Taylor R. W. 1991. Ants of the genus Myrmecia fabricius: a preliminary review and key to the named species (Hymenoptera: Formicidae: Myrmeciinae). J. Nat. Hist. 25, 1623–1673 10.1080/00222939100771021 (doi:10.1080/00222939100771021) [DOI] [Google Scholar]
- 52.Clark J. 1951. The Formicidae of Australia 1. Subfamily Myrmeciinae. Melbourne, Australia: CSIRO [Google Scholar]
- 53.Brown W. L., Jr 1953. Revisionary notes on the ant genus Myrmecia of Australia. Bull. Mus. Comp. Zool. 111, 1–35 [Google Scholar]
- 54.Greenwood D. R., Archibald S. B., Mathewes R. W., Moss P. T. 2005. Fossil biotas from the Okanagan Highlands, southern British Columbia and northeastern Washington State: climates and ecosystems across an Eocene landscape. Can. J. Earth Sci. 42, 167–185 10.1139/E04-100 (doi:10.1139/E04-100) [DOI] [Google Scholar]
- 55.Smith R. Y., Basinger J. F., Greenwood D. R. 2009. Depositional setting, fossil flora, and paleoenvironment of the Early Eocene Falkland site, Okanagan Highlands, British Columbia. Can. J. Earth Sci. 46, 811–822 10.1139/E09-053 (doi:10.1139/E09-053) [DOI] [Google Scholar]
- 56.Wing S. L., Greenwood D. R. 1993. Fossils and fossil climates: the case for equable Eocene continental interiors. Phil. Trans. R. Soc. Lond. B 341, 243–252 10.1098/rstb.1993.0109 (doi:10.1098/rstb.1993.0109) [DOI] [Google Scholar]
- 57.Archibald S. B., Mathewes R. W. 2000. Early Eocene insects from Quilchena, British Columbia, and their paleoclimatic implications. Can. J. Zool. 78, 1441–1462 10.1139/cjz-78-8-1441 (doi:10.1139/cjz-78-8-1441) [DOI] [Google Scholar]
- 58.Rust J., Andersen N. M. 1999. Giant ants from the Paleogene of Denmark with a discussion of the fossil history and early evolution of ants (Hymenoptera: Formicidae). Zool. J. Linn. Soc. 125, 331–348 10.1111/j.1096-3642.1999.tb00596.x (doi:10.1111/j.1096-3642.1999.tb00596.x) [DOI] [Google Scholar]
- 59.Carpenter F. M. 1930. The fossil ants of North America. Mus. Comp. Zool. Harv. 70, 1–66 [Google Scholar]
- 60.Dlussky G. M. 1988. Ants from (Paleocene?) Sakhalin amber. Paleont. J. 22, 50–61 [English transl. of Paleont. Zh. 1988 1, 50–61] [Google Scholar]
- 61.Dlussky G. M. 1996. Genera of ants (Hymenoptera: Formicidae) from Baltic amber. Paleont. J. 31, 616–627 [English transl. of Paleont. Zh. 1997 6, 50–62] [Google Scholar]
- 62.Dlussky G. M., Perkovsky E. E. 2002. Ants (Hymenoptera, Formicidae) from the Rovno Amber. Vestnik. Zool. 36, 3–20 [Google Scholar]
- 63.Dlussky G. M., Rasnitsyn A. P. 2003. [2002] Ants (Hymenoptera: Formicidae) of Formation Green River and some other Middle Eocene deposits of North America. Russ. Entomol. J. 11, 411–436 [Google Scholar]
- 64.Eberle J., Fricke H., Humphrey J. 2009. Lower-latitude mammals as year-round residents in Eocene Arctic forests. Geology 37, 499–502 10.1130/G25633A.1 (doi:10.1130/G25633A.1) [DOI] [Google Scholar]
- 65.Sexton P. F., Norris R. D., Wilson P. A., Pälike H., Westerhold T., Röhl U., Bolton C. T., Gibbs S. 2011. Eocene global warming events driven by ventilation of oceanic dissolved organic carbon. Nature 471, 349–353 10.1038/nature09826 (doi:10.1038/nature09826) [DOI] [PubMed] [Google Scholar]
- 66.Grein M., Utescher T., Wilde V., Roth-Nebelsick A. In press Reconstruction of the middle Eocene climate of Messel using palaeobotanical data. In Neues Jahrbuch für Geologie und Paläontologie. Stuttgart, Germany: 10.1127/0077-7749/2011/0139 (doi:10.1127/0077-7749/2011/0139) [DOI] [Google Scholar]
- 67.Wilde V., Kvaček Z., Bogner J. 2005. Fossil leaves of the Araceae from the European Eocene and notes on other aroid fossils. Int. J. Plant Sci. 166, 157–183 10.1086/425673 (doi:10.1086/425673) [DOI] [Google Scholar]
- 68.Fricke H. C., Wing S. L. 2004. Oxygen isotope and paleobotanical estimates of temperature and δ18O-latitude gradients over North America during the early Eocene. Am. J. Sci. 304, 612–635 10.2475/ajs.304.7.612 (doi:10.2475/ajs.304.7.612) [DOI] [Google Scholar]
- 69.Peppe D. J., et al. 2011. Sensitivity of leaf size and shape to climate: global patterns and paleoclimatic applications. New Phytol. 190, 724–739 10.1111/j.1469-8137.2010.03615.x (doi:10.1111/j.1469-8137.2010.03615.x) [DOI] [PubMed] [Google Scholar]