Abstract
The NF-κB family of transcription factors is a central regulator of chronic inflammation. The phosphorylation of IκB proteins by the IκB kinase (IKK) complex (IKKα, IKKβ, and NF-κB essential modulator or NEMO) is a key step in NF-κB activation. Peptides corresponding to the NEMO binding domain (NBD) of IKK blocks NF-κB activation without inhibiting basal NF-κB activity. In this report, we determined the effects of the IKK inhibitor peptide (NBD) in a model of spontaneously occurring chronic murine colitis, the IL-10-deficient (IL-10–/–) mouse. Using a novel cationic peptide transduction domain (PTD) consisting of eight lysine residues (8K), we were able to transduce the NBD peptide into cells and tissues. In a NF-κB reporter system, 8K-NBD dose-dependently inhibits TNF-induced NF-κB activation. Furthermore, 8K-NBD inhibited nuclear translocation of NF-κB family members. In NF-κBEGFP knock-in mice, 8K-NBD inhibited LPS-activated NF-κB (EGFP activity) in the ileum but did not inhibit basal NF-κB in Peyer's patches. IL-10–/– mice treated systemically with 8K-NBD demonstrate amelioration of established colitis, decreased NF-κB activation in the lamina propria, and a reduction in spontaneous intestinal IL-12 p40, TNF, IFN-γ, and IL-17 production. These results demonstrate that inhibitors of IKK, in particular a PTD-NBD peptide, may be therapeutic in the treatment of inflammatory bowel disease.
While the etiology of the human chronic inflammatory bowel diseases (IBD)4 Crohn's disease (CD) and ulcerative colitis remains unknown, research has identified contributing factors that include defects in the barrier mechanism of the lining of intestinal epithelial cells and a poorly regulated immune response against the normal enteric microbial flora.
NF-κB represents a group of structurally related proteins that includes five members in mammals (p65, c-Rel, Rel-B, p50, and p52). NF-κB is a central regulator of chronic inflammation in IBD (1–3). Many of the standard agents used to treat human IBD, including sulfasalazine, 5-aminosalicylates, and corticosteroids, have been postulated to exert some of their anti-inflammatory effects through NF-κB inhibition (4–6). In un-stimulated cells, NF-κB proteins are localized in the cytoplasm through their association with members of a family of inhibitory proteins known as IκB proteins. Proinflammatory cytokines such as TNF and IL-1 and bacterial products such as LPS induce phosphorylation of IκB proteins at specific N-terminal serine residues. Phosphorylation of IκB is mediated by the IκB kinase (IKK) complex. IκB phosphorylation leads to IκB degradation, the release of NF-κB subunits, and their subsequent translocation to the nucleus. Nuclear NF-κB regulates the transcription of proinflammatory genes including cytokines (IL-1β, TNF, IL-12/23), chemokines (IL-8, MIP-1α, MCP-1), and adhesion molecules (ICAM-1, VCAM, E-selectin). Cytokines that are stimulated by NF-κB, such as IL-1β and TNF, also directly activate NF-κB, thus establishing an autoregulatory loop that may be essential in the perpetuation of chronic inflammation (7).
IKK is made up of two catalytic subunits, IKKα and IKKβ, and a regulatory subunit named “NF-κB essential modulator” (NEMO; also named IKKγ) (8). An N-terminal region of NEMO associates with a hexapeptide sequence within the C terminus of both IKKα and IKKβ named the NEMO binding domain (NBD). A short peptide derived from the aa 735–745 NBD region of IKKβ disrupts the association of NEMO with IKKs in vitro and blocks TNF-induced NF-κB activation in vivo when delivered to cells using a cationic protein transduction domain (PTD). Because of the importance of the IKK complex in inflammation, the identification of such a selective IKK inhibitor as a potential therapeutic agent is of considerable interest.
PTDs have been shown to deliver a wide variety of therapeutic agents into cells, including peptides, proteins, nucleic acids, Abs, and small drugs. The first protein reported with transductional properties was the HIV transactivator protein TAT, in which the 11-aa PTD was identified by virtue of its cationic content (9). Recently, PTDs have been characterized that mediate efficient and rapid receptor-independent internalization of peptide-protein conjugates (10). These cationic transduction peptides transduce a wide variety of cells similar to the HIV TAT, mediating highly efficient transduction in vitro and in vivo (11, 12). The screening of a panel of cationic peptides has demonstrated that peptides of eight or 10 lysines are highly efficient transduction domains, working as or more effectively than other cationic PTDs for the delivery of peptides to numerous cell types, including mucosal cells and APCs (11).
PTD-mediated NBD delivery has been shown to be an efficacious therapeutic in models of autoimmunity and inflammation, including models of multiple sclerosis (13), islet transplantation (14), and rheumatoid arthritis (15). Accordingly, we postulated that a novel cell-permeable peptide IKK inhibitor, 8K-NBD (where 8K represents eight lysine residues), may efficiently target cells involved in the intestinal inflammatory response. In this report, we show that 8K-NBD dose-dependently inhibits TNF-induced NF-κB activation and translocation in cells. In vivo, 8KNBD inhibits activated but not basal NF-κB in the intestine as assessed by enhanced GFP (EGFP) activity in LPS-challenged NF-κBEGFP knock-in mice. Moreover, we demonstrate that treatment of IL-10-deficient (IL-10–/–) mice with 8K-NBD ameliorates established chronic colitis and inhibits intestinal Th1 and Th17 inflammatory responses.
Materials and Methods
Peptides
The peptides 8K-NBD (acetyl-KKKKKKKKGGTALDWSWLQTE-amide), inactive 8K-mutant NBD (mNBD) (acetyl-KKKKKKKKGGTALDASALQTE-amide, where the underlined amino acids represent tryptophan to alanine mutations), random-NBD (ARPLEHGSDKATGGTALDWSWLQTE), 8K-biotin (KKKKKKKK-biotin), and random peptide-biotin (ARPLEHGSDKAT-biotin) were synthesized by the peptide synthesis facility at the University of Pittsburgh, Pittsburgh, PA. The random-NBD and 8K-NBD peptides N-terminal ends were conjugated to 6-carboxyfluorescein (6CF, Molecular Probes) for localization experiments. Peptides were purified and characterized by reversed-phase high performance liquid chromatography and mass spectrometry.
Murine macrophages
The murine macrophage cell line, RAW264.7, was maintained in DMEM with 10% FBS and 1% penicillin/streptomycin. Bone marrow (BM)-derived murine macrophages were isolated from the femurs of C57BL/6 mice. BM was flushed with washing medium (RPMI 1640 with 1% penicillin/streptomycin), passed through a 70-μm nylon cell strainer into a 50-ml conical tube, and spun down at 1500 rpm for 5 min. RBCs were lysed using sterile filtered 0.8% ammonium chloride, washed twice with washing medium, and resuspended in complete medium (washing medium with 10% FBS). BM cells were seeded in complete medium in a 150-mm dish and differentiated using recombinant murine GM-CSF (20 ng/ml) (R&D Systems). On day 3 another 25 ml of fresh culture medium containing GM-CSF was added to the culture plates. On day 7 the cells, representing the BM-derived macrophage population, were harvested for experiments.
NF-κB luciferase assay
HEK293 cells stably transfected with a multimerized NF-κB DNA binding element-luciferase reporter (DMEM with 10% FBS and 1% penicillin/streptomycin) were pretreated for 1 h with 8K-NBD dissolved in Opti-MEM medium (Invitrogen Life Technologies) and activated for 2 h with 10 ng/ml TNF (R&D Systems). The cells were lysed in reporter lysis buffer and luciferase activity was measured with a luciferase assay system (Promega) using a Turner Designs TD20/20 luminometer.
Nuclear extracts and Western blotting
HEK293 cells were pretreated for 1 h with medium, 8K-NBD, or 8K-mNBD dissolved in Opti-MEM medium (Invitrogen Life Technologies) and activated for 15 min with 10 ng/ml TNF (R&D Systems). Nuclear extracts from treated HEK293 cells were isolated following the manufacturer's protocol (NE/PER reagents; Pierce). Protein concentration was determined using the Bradford assay (Pierce). Western blot analyses were performed on nuclear extracts as described previously (16). Anti-p65 and anti-c-Rel Abs were obtained from Santa Cruz Biotechnology, and anti-poly(ADP-ribose) and anti-phosphorylated (phospho) p65 Abs were obtained from Cell Signaling.
Mice
Male C57BL/6 (10–12 wk old) and female BALB/c (12–13 wk old) mice were obtained from The Jackson Laboratory. An IL-10–/– colony on a C57BL/6 background (breeder pairs from The Jackson Laboratory) was maintained in accordance with guidelines from the American Association for Laboratory Animal Care and Research Protocols and was approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh School of Medicine. The NF-κBEGFP knock-in mice (129/SvEv/C57BL6 background) were described previously (17, 18). Expression of EGFP was controlled by a chimeric promoter containing three HIV NF-κB cis elements in these mice. Mice were maintained in specific pathogen-free conditions. Research protocols were approved by the Institutional Animal Care and Use Committee at the University of North Carolina School of Medicine, Chapel Hill, NC.
In vivo PTD transduction
C57BL/6 or BALB/c mice were grouped randomly and treated i.p. with biotinylated peptides (random or 8K) linked to streptavidin-Cy3. After 30 min of treatment, organs were harvested, fixed in 2% paraformaldehyde, and then incubated in 30% sucrose in PBS at 4°C overnight. Samples were snap frozen in isopentane and cut into 6-μm-thick frozen sections and placed on microscope slides.
Immunohistochemistry
After transduced macrophages or cut tissue sections were placed on coverslips, slides were blocked in BSA and stained for nuclei with either Draq5 (Biostatus Limited) or propidium iodide (Molecular Probes) for 30 min. Phalloidin was used to visualize F-actin (Molecular Probes). After extensive washing, slides were mounted and viewed on an Olympus Fluo-View 1000 confocal microscope (Olympus America).
Colonic tissue was collected from control and treated animals, fixed overnight in paraformaldehyde, embedded in paraffin, and sectioned at 4 mm. Serial sections were stained for phospho-NF-κB p65. Following deparaffinization and rehydration, Ag unmasking was performed using Citra Plus Ag Retrieval (Biogenex Laboratories) per the manufacturer's protocol. Slides were cooled and washed and endogenous peroxidase was blocked using 0.3% hydrogen peroxide. Sections were next blocked with 1.5% goat serum (Vector Laboratories) in PBS for 1 h and incubated with rabbit anti-phospho-NF-κB p65 polyclonal Ab at a 1/50 dilution (Cell Signaling) in PBS at 4°C overnight in a humidified chamber. Slides were washed with PBS, incubated with biotinylated goat anti-rabbit secondary Ab (Vector Laboratories) for 45 min, washed with PBS, and Vectastain Elite ABC reagent was applied for 30 min. The slides were then washed with PBS, and diaminobenzene (Vector Laboratories) was used as a substrate. Sections were counterstained with hematoxylin, dehydrated, and mounted on coverslips. Stained sections were evaluated by an observer blinded to the treatment group for phospho-p65. Phospho-p65-positive cells were counted from 20 randomly selected high power fields in coded colonic sections to that the observers were blinded to the treatment group. Cells were enumerated from six mice per group (mNBD-, 2 mg/kg NBD-, and 10 mg/kg NBD-treated groups) and results are expressed as the number of positive cells per field.
In vivo NBD peptide treatment
Ten-week-old IL-10–/– mice were grouped randomly and treated with either mutant (10 mg/kg) or wild-type (2 or 10 mg/kg) NBD peptide linked to 8K. Treatment was administered in PBS in a total volume of 500 μl i.p. for 10 of 14 days. At the end of the study period, animals were euthanized using excess CO2 inhalation and intestinal tissue was harvested. NF-κBEGFP knock-in mice were pretreated with 8K-NBD or 8K-mNBD peptide (10 mg/kg) 1 h before LPS injection. LPS (25 mg/kg) or PBS was administered i.p. to the mice (two mice per treatment group). Sixteen hours later, the mice were sacrificed by excess CO2 inhalation and dissected.
Intestinal tissue explant cultures
Colons were isolated from individual mice, cut open longitudinally, and cleaned of fecal matter. The intestinal tissue was washed with PBS to remove residual fecal content. Intestinal sections were cut in half longitudinally and one-half was shaken at 250 rpm at room temperature for 30 min in RPMI 1640 supplemented with 1% antibiotic/antimycotic. Tissue fragments (0.05 g dry weight) were incubated in 1 ml of RPMI supplemented with 1% antibiotic/antimycotic and 10% FBS. Supernatants were collected after 24 h, assayed for spontaneous cytokine production via sandwich ELISAs, and normalized to dry gut weight.
Histology
Colons were isolated from individual mice, cut open longitudinally, and cleaned of fecal matter. Intestinal sections were cut in half longitudinally, and one-half was fixed in 10% buffered formalin and embedded in paraffin. Five-micrometer-thick sections were stained with H&E. Colitis scores (0–4) were determined by a staff pathologist using the criteria reported by Berg et al. (19). At least 20 separate microscopic fields (×10 magnification) were evaluated for each mouse by a pathologist (Dr. A. R. Sepulveda, University of Pittsburgh) blinded to the treatment groups.
EGFP imaging
Intestines removed from NF-κBEGFP knock-in mice were immediately imaged after dissection by using a charge-coupled device camera in a light-tight imaging box with a dual filtered light source and emission filters specific for EGFP (LT-99D2 Illumatools; Lightools Research). For confocal microscopy on intestinal tissue, terminal ilea were cut open longitudinally and placed on the stage of a Leica SP2 upright laser scanning confocal microscope (Leica), lumen side facing the lens, without further processing or fixation. EGFP was excited at 495-nm wavelength, and images were acquired using detection filters specific for the EGFP emission spectrum. Images were analyzed with the Leica SP2 laser scanning confocal imaging software (Leica).
Cytokine ELISAs
Murine IL-12 p40, TNF, IFN-γ (BD Pharmingen), and IL-17 (R&D Systems) immunoassay kits were used according to the manufacturer's instructions. Values were measured using a plate reader and the SoftMax Pro version 4.8 software (Molecular Devices).
Statistical analysis
Statistical significance in cell-based experiments (see Fig. 2) was assessed by the two-tailed Student's t test. Statistical significance from in vivo intervention experiments (see Figs. 6, 7, and 8) was assessed by the Mann-Whitney U Test (SPSS). p value ≤ 0.05 was considered to be statistically significant.
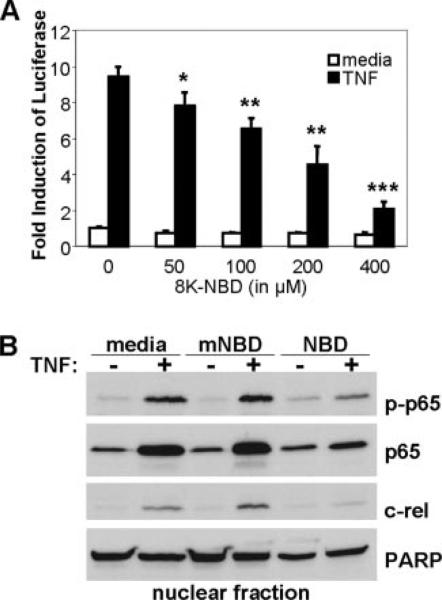
FIGURE 2.
TNF-induced NF-κB activation is inhibited by 8K-NBD transduction in cells. A, HEK293 cells stably transfected with a multimerized NF-κB DNA binding element-luciferase reporter were preincubated for 1 h with an increasing dose of 8K-NBD peptide in medium. Cells were subsequently stimulated for 2 h with 10 ng/ml recombinant human TNF. Cells were harvested and lysates were analyzed for luciferase activity. Results are expressed as fold induction of luciferase compared with unstimulated plus 0 μM peptide lysates (= 1). Experiments were performed in triplicate and repeated three times. A representative result is shown (mean ± SD). *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared with unstimulated sample. B, Nuclear extracts of HEK293 cells stimulated with or without 10 ng/ml recombinant human TNF in the presence of medium, 8K-mNBD, or 8K-NBD were isolated and run out for Western blotting. Blots were probed with phospho-p65 (p-p65), p65, and c-Rel. Blots were probed with poly(ADP-ribose) polymerase to assess equal loading. Experiments were repeated three times and a representative blot is shown.
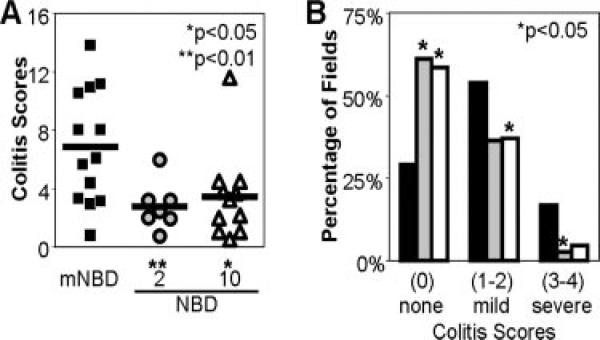
FIGURE 6.
8K-NBD treatment ameliorates histologic colitis. Colons were isolated from individual mice (black, 10 mg/kg mNBD; gray, 2 mg/kg NBD; white, 10 mg/kg NBD), cleaned, fixed in formalin, and embedded in paraffin. Sections were stained with H&E and colitis scores were determined using a modified scoring system (0–4) as described in Materials and Methods. At least 20 separate microscopic fields (magnification ×10) were evaluated for each mouse by a pathologist blinded to the treatment groups. Colitis scores were significantly lower in the 8K-NBD-treated mice. Histologic improvement in colitis is presented as a composite score (the average colitis score sum of five fields) (A) and as the percentage of histologic fields that demonstrate scores of 0 (no inflammation), 1 and 2 (mild inflammation), or 3 and 4 (severe inflammation) (B).
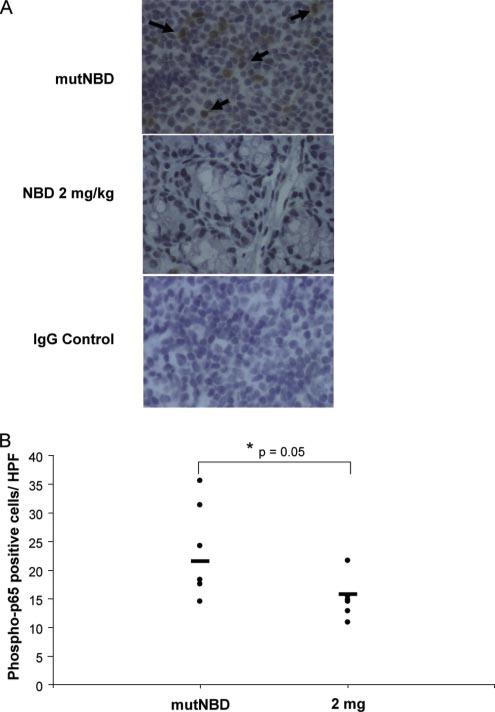
FIGURE 7.
8K-NBD inhibits the phosphorylation of intestinal NF-κB p65 in IL-10–/– mice. IL-10–/– mice were treated with 8K-NBD (2 mg/kg; n = 6) or 8K-mNBD (10 mg/kg; n = 6) for 14 days. Colonic sections were immunohistochemically stained for phospho-p65. A, Colonic sections from 8K-NBD and 8K-mNBD (mutNBD)-treated mice were stained with anti-phospho-p65 or an IgG control Ab. Representative tissue sections are shown. Black arrows identify phospho-p65-positive lamina propria cells in the upper panel. B, Phospho-p65-positive lamina propria cells were quantitated in stained colon sections of treated mice. Twenty high power fields were counted from each mouse and the results are expressed as the mean number of positive cells per high power field.
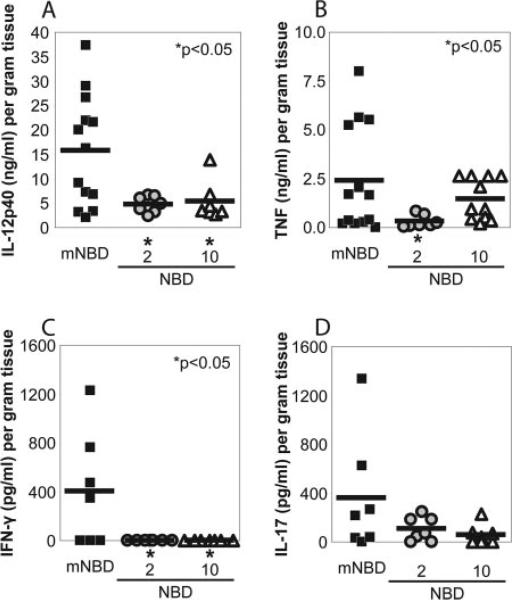
FIGURE 8.
8K-NBD inhibits NF-κB-dependent cytokine production in intestinal explants. Colons were isolated from individual mice (black, 10 mg/kg mNBD; gray, 2 mg/kg NBD; white, 10 mg/kg NBD), cleaned, and processed for intestinal tissue explant cultures as described in Materials and Methods. Tissue fragments (0.05 g dry weight) were incubated in 1 ml of RPMI 1640 supplemented with 1% antibiotic/antimycotic and 10% FBS. Supernatants were collected after 24 h and spontaneous secretion of IL-12 p40 (A), TNF (B), IFN-γ (C), and IL-17 (D) were measured by ELISA. Values were normalized to dry weight of the intestinal explant. Asterisk (*) denotes comparisons where p < 0.05 compared with the mNBD control group.
Results
Transduction of 8K PTD peptide into macrophages
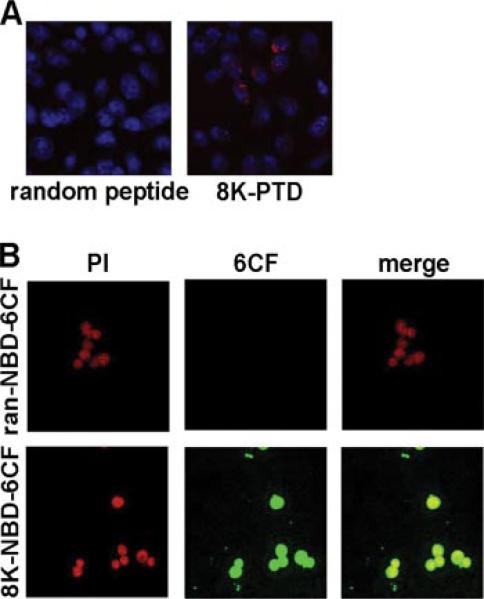
As previously reported (11), a panel of cationic protein transduction domains was screened for the ability to efficiently transduce a variety of cell types. Eight to 10 amino acid polylysine tracts have been shown to efficiently transduce a wide array of cell lines and primary cells, including islet β-cells, synovial cells, polarized airway epithelial cells, tumor cells, and dendritic cells (DCs) (11). To assess transduction of the 8K PTD into a relevant immunologic target cell, murine BM-derived macrophages were incubated with biotinylated 8K PTD linked to streptavidin-Cy3, and the murine macrophage cell line RAW264.7 was incubated with 8K PTD conjugated to the fluorescent label 6CF. Murine macrophages were efficiently transduced with the 8K PTD (>90% of cells demonstrate fluorescence) compared with a negative control peptide containing a random peptide sequence in place of the PTD (Fig. 1). This result demonstrates that in vitro macrophages are efficiently transduced with the 8K PTD.
FIGURE 1.
8K efficiently transduces murine macrophages. A, Biotinylated random peptide linked to streptavidin-Cy3 or 8K PTD linked to streptavidin-Cy3 was added to BM-derived macrophages. B, The fluorescently labeled random (ran) peptide-NBD-6CF or 8K-NBD-6CF was added to RAW264.7 macrophages. Following incubation for 1 h, cells were fixed, stained for nuclei (Draq5 in A, propidium iodide (PI) in B), and placed on a microscope slide. Localization of peptide was visualized by a confocal microscope system. Results were repeated three times and representative images are shown.
8K-NBD inhibits TNF-stimulated NF-κB activation and nuclear translocation in cells
To evaluate the functionality of the transduced peptide, the 8K PTD linked to the NBD peptide (8K-NBD) was preincubated with HEK293 cells expressing a stably transfected multimerized NF-κB DNA binding element-luciferase reporter gene. Cells were subsequently stimulated with TNF. Pretreatment with 8K-NBD demonstrated a dose-dependent inhibition in TNF-stimulated NF-κB activity (Fig. 2A). Moreover, 8K-NBD pretreatment without TNF stimulation did not alter basal levels of NF-κB activity, indicating that the peptide specifically targets activated NF-κB. To verify the mechanism by which 8K-NBD inhibited activity, nuclear translocation of NF-κB family members in activated cells was assessed. Western immunoblot analysis on nuclear extracts demonstrated decreased nuclear quantities of the NF-κB family members p65 (and its phosphorylated form, phospho-p65), and c-Rel (Fig. 2B) in 8K-NBD-pretreated, TNF-activated HEK293 cells.
Transduction of 8K-PTD in vivo
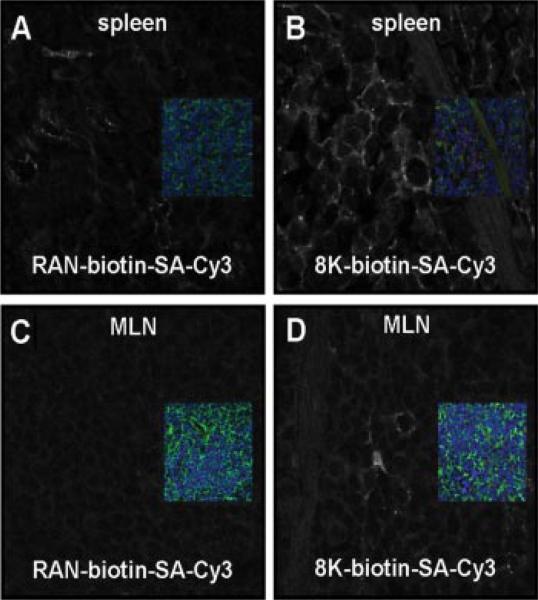
8K PTD efficiently transduces cells and inhibits NF-κB activity in cells. We next investigated whether 8K PTD transduces cells in vivo in mice. Mice were administered biotinylated peptides (random sequence or 8K PTD) linked to streptavidin-Cy3 i.p. In vivo uptake of peptide is observed as early as 30 min after administration. Intraperitoneal administration of 8K PTD revealed uptake in the spleen (Fig. 3, A and B) and mesenteric lymph nodes (Fig. 3, C and D). These results demonstrate that systemic and intestinal immune compartments are targeted by 8K peptide-mediated transduction in vivo.
FIGURE 3.
The 8K PTD transduces lymphoid tissue in vivo. Biotinylated (A and C) random (RAN) peptide linked to streptavidin (SA)-Cy3 or (B and D) the 8K PTD linked to streptavidin-Cy3 was injected i.p. for 30 min. Paraformaldehyde-fixed (A and B) spleens and (C and D) mesenteric lymph nodes (MLN) were stained for actin (green) and nuclei (blue; Draq5) and viewed by confocal microscopy. Insets represent the three-color image for each sample. To specifically visualize the location of the peptide (Cy3; red), the signal from the red channel was given a false white color while the other channels were turned off. This is represented in the larger black and white image. Background threshold levels were adjusted to random peptide samples for each organ.
8K-NBD inhibits LPS-stimulated intestinal NF-κB but does not inhibit basal NF-κB in vivo
To determine whether 8K-NBD inhibits activated but not basal NF-κB in the intestine in vivo, we used NF-κBEGFP knock-in mice (17, 18) where the expression of EGFP is controlled by a chimeric promoter containing three HIV NF-κB cis elements. Markedly increased EGFP expression (NF-κB activity) has been described in jejunal and ileal lamina propria T cells and monocytes following LPS injection (18). Furthermore, gross analysis of whole organs from cis-NF-κBEGFP mice demonstrated basal levels of EGFP expression in Peyer's patches, which are known to exhibit high basal levels of NF-κB activation (18).
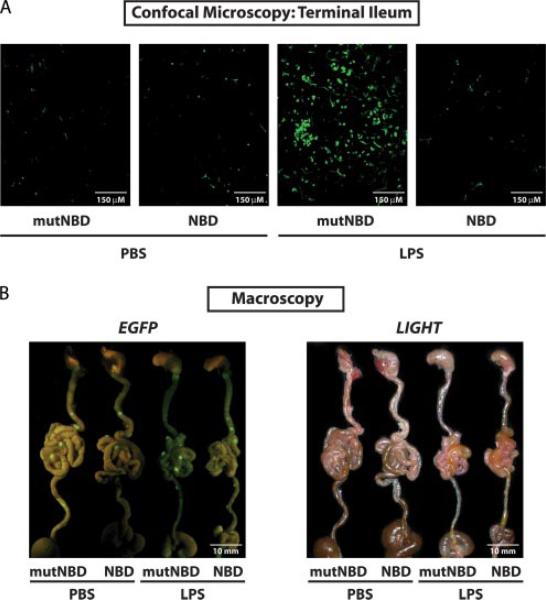
NF-κBEGFP knock-in mice were pretreated with 8K-NBD (10 mg/kg) or 8K-mNBD peptide (10 mg/kg) 1 h before i.p. LPS (25 mg/kg) or PBS administration. After 16 h, mice were sacrificed and intestines were immediately analyzed for EGFP expression, which is indicative of NF-κB activity. Terminal ilea were cut open longitudinally and the lamina propria was directly imaged by confocal microscopy without further processing or fixation (Fig. 4A). Mice injected with PBS demonstrate few EGFP-positive cells in the lamina propria whether pretreated with 8K-NBD or 8K-mNBD (Fig. 4A, left). In contrast, mice injected with LPS pretreated with 8K-mNBD demonstrate strong NF-κB activation in the ileal lamina propria. Mice pretreated with 8K-NBD before LPS challenge show markedly fewer EGFP-positive cells in the lamina propria, similar to the number observed in PBS-treated mice (Fig. 4A, right). To study NF-κBEGFP transgene expression in whole organs, a specific EGFP imaging system was used to visualize EGFP expression in the intestine following challenge with LPS. Strong basal EGFP expression (NF-κB activity) is observed in Peyer's patches (visualized macroscopically as nodules on the serosal surface of the small intestine) of PBS- and LPS-treated mice. This activity is not inhibited by administration of 8K-NBD (Fig. 4B, left). Taken together, these experiments demonstrate that i.p. administration of 8K-NBD inhibits activated but not basal NF-κB in the intestinal immune compartment in vivo.
FIGURE 4.
8K-NBD inhibits LPS-stimulated NF-κB but not basal NF-κB in vivo. NF-κBEGFP knock-in mice were pretreated with 8K-NBD (10 mg/kg) or 8K-mNBD (mutNBD) (10 mg/kg) 1 h before LPS injection. LPS (25 mg/kg) or PBS was administered i.p to the mice (two mice per treatment group). After 16 h, mice were sacrificed and the guts were collected. A, EGFP expression in the ileal lamina propria was visualized by confocal microscopy. B, EGFP fluorescence of whole intestines was macroscopically assessed using the Lightools Research macroimaging system. Fluorescent (right) and white light imaging (left) are depicted.
8K-NBD treatment ameliorates colitis in IL-10–/– mice
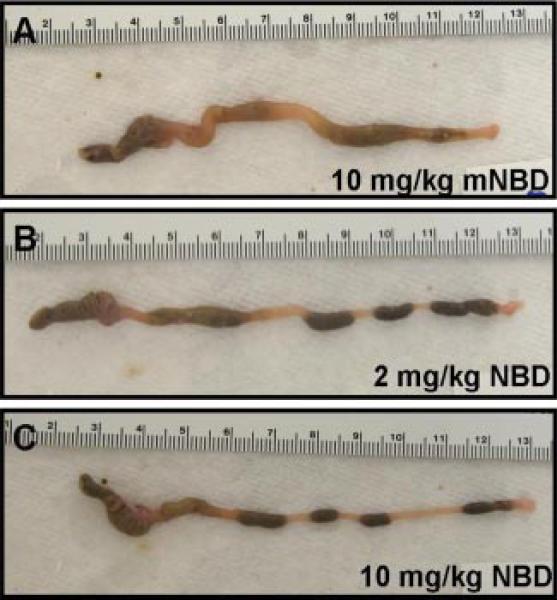
Next, we tested the hypothesis that 8K-NBD may ameliorate active chronic colitis in vivo in IL-10–/– mice. As previously reported (20), in our colony 80% of 10-wk-old IL-10–/– mice have active intestinal inflammation; thus, therapeutic intervention was initiated in IL-10–/– mice with established colitis. IL-10–/– mice were treated from 10 to 12 wk of age with either 8K-NBD at 2 or 10 mg/kg or 8K-mNBD at 10 mg/kg by i.p. injection for 10 of 14 days. Gross inspection of the intestines revealed increased colonic lengths, decreased colonic wall thickening, and formed fecal pellets in the 8K-NBD treatment groups (Fig. 5, B and C) compared with the 8K-mNBD controls (Fig. 5A). Histologic severity of colitis was graded over the entire length of the colon for each mouse by a single pathologist blinded to treatment groups. Due to the incomplete penetrance and the segmental, patchy pattern of colitis in IL-10–/– mice, colitis scores are depicted in two different ways. First, composite scores are represented using a modified standard scoring system described by Berg et al. (19) (Fig. 6A). Mice treated with 8K-NBD at 2 mg/kg and 10 mg/kg demonstrated a 50% reduction in colitis scores compared with the control-treated group (mNBD). To illustrate the spectrum of disease encountered over the entire length of the colon, scores are presented as the percentage of fields that demonstrate no histological inflammation (colitis score of 0), mild to moderate inflammatory changes (colitis scores of 1 and 2), and severe inflammation (colitis scores of 3 and 4). Compared with the 8K-mNBD group (Fig. 6B, filled bars), 2 mg/kg and 10 mg/kg 8K-NBD-treated mice (Fig. 6B, gray and open bars, respectively) displayed more fields demonstrating no evidence of histologic inflammation and consequently fewer fields with significant inflammatory changes.
FIGURE 5.
8K-NBD-treated mice demonstrate improvement in gross colonic appearance. Representative photographs of colons from the control group 10 mg/kg 8K-mNBD (A) and the treatment groups 2 mg/kg 8K-NBD (B) and 10 mg/kg 8K-NBD (C). Colons from mice treated with 8K-NBD peptide demonstrated increased length, decreased tissue thickening, and formed stool pellets compared with the control group.
Activated NF-κB was determined by immunohistochemistry for phosphorylated NF-κB p65 in colonic sections from 8K-mNBD-and 8K-NBD-treated (2 mg/kg) IL-10–/– mice (n = 6 per group). Significantly fewer phospho-p65 positive cells were detected in the lamina propria from 8K-NBD compared with 8K-mNBD treated mice (Fig. 7), suggesting that 8K-NBD inhibits activated NF-κB in the colon of IL-10–/– mice, correlating with histological improvement.
Finally, the effect of 8K-NBD treatment on mucosal inflammatory cytokine production in IL-10–/– mice was investigated. Spontaneous release of the NF-κB-regulated proinflammatory cytokines IL-12 p40 (Fig. 8A) and TNF (Fig. 8B) were determined in cell-free supernatants from colonic mucosal tissue explants. Explants from 8K-NBD-treated mice secreted significantly less IL-12 p40 and TNF compared with 8K-mNBD-treated control mice. The IL-12 p40 subunit is a component of the bioactive cytokines IL-12 and IL-23. To assess the downstream consequences of attenuated mucosal IL-12 p40 production, the prominent T cell targets of IL-12 and IL-23 signaling, IFN-γ and IL-17, respectively, were measured. Intestinal explants from 8K-NBD-treated mice secreted less spontaneous IFN-γ (Fig. 8C) and IL-17 (Fig. 8D) compared with 8K-mNBD-treated control mice. Therefore, decreased mucosal innate and T cell inflammatory cytokine expression correlates with histological findings, suggesting that specific targeting of the IKK complex with cell-permeable NBD peptides may be effective in treating chronic IBD.
Discussion
In this study, delivery of an IKK inhibitor peptide using a novel PTD (8K) was investigated in cells and in vivo. This peptide efficiently transduces cells in culture and transduces intestinal lymphoid tissue following i.p. administration in vivo. In cells, 8KNBD inhibits TNF-induced NF-κB transcriptional activity and nuclear translocation. In vivo, 8K-NBD inhibited LPS-activated NF-κB (EGFP expression) in the lamina propria but did not affect basal NF-κB in Peyer's patches in NF-κBEGFP knock-in mice. Moreover, we demonstrate that 8K-NBD ameliorates chronic colitis in IL-10–/– mice, and histological improvement correlated with a reduction in the mucosal levels of inflammatory cytokines.
The critical role of NF-κB in chronic intestinal inflammation is best illustrated by a nonspecific as well as a selective blockade of NF-κB activation in animal models of IBD (21–23). In spontaneously occurring colitis in IL-10–/– mice, increased NF-κB DNA binding activity and increased p65 protein expression were found in lamina propria macrophages. The essential role of p65 in maintaining chronic intestinal inflammation was demonstrated by successful treatment of established colitis in these mice with p65 antisense oligonucleotides (23).
Furthermore, activated NF-κB is found in human IBD. A significant increase of p65 protein in lamina propria macrophages and intestinal epithelial cells from CD patients was correlated with increased production of the inflammatory cytokines IL-1β, IL-6, and TNF (1). In vitro treatment of lamina propria macrophages from CD patients with p65 antisense oligonucleotides was effective in down-regulating inflammatory cytokine production, suggesting a key role for NF-κB p65 in inflammatory cytokine expression in CD (1). Activated NF-κB has been reported in macrophages and intestinal epithelial cells from inflamed mucosa of patients with IBD in situ by using a specific p65 Ab that exclusively detects the activated form of NF-κB (3).
Accordingly, selective IKK inhibition represents a potential therapeutic strategy in IBD. A concern about the use of inhibitors that completely suppress IKK activity is that they may also inhibit the ability of basally active NF-κB to act as a physiologic survival factor, thereby raising the possibility of toxicity. Activation of the IKK complex in response to inflammatory mediators depends critically on the presence of the NEMO subunit of the IKK complex. For example, NEMO-deficient cells lack detectable NF-κB binding activity in response to TNF, IL-1β, and LPS (24). Furthermore, recent studies have shown that continuous administration of the NBD peptide effectively ameliorates inflammatory responses in animal models of inflammation without overt side effects or liver or kidney toxicity (25, 26). Additionally, the NBD peptide preserves the alternative pathway of NF-κB activation that is necessary for B cell development and lymphoid organogenesis, again minimizing potential toxicity concerns. Our data showed that while there was an inhibition of activated NF-κB, the levels of basal NF-κB translocation stayed constant (Fig. 2B) in cells. Moreover, in vivo 8K-NBD inhibited activated NF-κB (EGFP expression) in the ileal lamina propria, but basal NF-κB appeared to be preserved in the Peyer's patches in LPS challenged NF-κBEGFP knock-in mice (Fig. 4). This feature theoretically allows for dampened inflammatory responses without altering other roles of NF-κB within the cell.
Specific inhibition of IKK activity by NBD peptides may have pleiotropic mechanistic and durable immunologic effects in inflammatory diseases. In mouse models of chronic inflammation including collagen-induced arthritis (15, 25) and experimental allergic encephalomyelitis (13), in vivo treatment with NBD peptides blocked disease activity, proinflammatory cytokine expression, and homing of cells to inflammatory sites due to the inhibition of expression of cellular adhesion molecules. In experimental allergic encephalomyelitis, clinical recovery was correlated with a durable alteration of the T cell phenotype, as NBD-treated mice demonstrated Th2 cytokine production rather than disease-associated Th1 cytokine production (13). Furthermore, mice treated systemically with an NBD peptide for 5 days after the induction of collagen-induced arthritis maintained clinical and histological improvement for nearly 3 wk following the termination of peptide administration (25). In our study, the 8K-PTD was detected in important immune inductive sites (spleen and mesenteric lymph nodes) following i.p. administration; however we could not identify the peptide in the intestinal lamina propria or epithelia (data not shown). This finding suggests that the mechanism of action of 8K-NBD in IBD is likely to be more complicated than can be explained by the direct inhibition of activated NF-κB in the inflamed intestine. Future mechanistic studies in IBD models will be necessary to characterize the functional and phenotypic alterations in immune cell populations and the durability of clinical responses with 8K-NBD.
Although PTD-NBD has demonstrated efficacy in other inflammatory models, the important and distinguishing features of colitis in IL-10–/– mice compared with these other experimental systems are that it is a chronic, spontaneously occurring model of inflammation and that 8K-NBD was an effective therapeutic intervention when administered after the onset of disease (27). Recently, the NBD peptide linked to the Antennapedia homeodomain was shown to be effective in blocking inflammatory injury in two murine models of acute colonic inflammation, dextran sulfate sodium (DSS)- and trinitrobenzene sulfonic acid (TNBS)-induced colitis (28). In this study, the NBD peptide was administered prior to the induction of acute intestinal injury with DSS or TNBS. Therefore, there are important biologic distinctions in the model and methodology in the current study that likely provide a more relevant proof of concept for human IBD. Importantly, we demonstrate that 8K-NBD ameliorates established inflammation in spontaneously occurring chronic colitis. Moreover, in contrast to the prior study, we show in vivo localization of the peptide to mucosal and systemic immune inductive sites and present evidence that 8K-NBD blocks NF-κB activation in the intestine but does not inhibit basal NF-κB.
To further study the inhibition of NF-κB in IBD, it will be critical to dissect the protective from the detrimental properties of NF-κB activation in mucosal inflammation. Although increased activation of NF-κB is implicated in the pathogenesis of numerous chronic disorders, NF-κB activation pathways may be protective and serve to maintain homeostasis in the intestine (29, 30). For example, the TLR family recognizes extracellular microbial constituents resulting in the downstream activation of NF-κB. TLR-deficient mice or deletions in signaling intermediates such as MyD88 demonstrate a decrease in survival compared with wild-type mice when colitis is induced with DSS (30). This study suggested that TLRs, expressed on intestinal epithelial cells, may recognize luminal microbial constituents and mediate a protective response through NF-κB activation. Most relevant to this study, mice with a targeted deletion of NEMO in intestinal epithelial cells develop severe spontaneous intestinal inflammation through TNF-and MyD88-dependent pathways, further suggesting that IKK activation in the intestinal epithelium mediates homeostatic pathways (31). In summary, NF-κB inhibition, particularly in the intestinal epithelium, may lead to abrogation of mucosal protective effects.
Conversely, the preponderance of evidence suggests that inhibiting NF-κB in lamina propria macrophages and DCs may be of therapeutic benefit in IBD. A recent study demonstrated that the development of colitis in IL-10–/– mice is completely dependent on TLR signaling pathways (32). In IL-10–/– × MyD88–/– mice, colitis is abrogated and intestinal IL-12 p40 levels are markedly decreased. Furthermore, BM chimera experiments reveal that BM-derived cells are responsible for the recognition of commensal microbial signals and mucosal innate immune activation.
Taken as a whole, the spectrum of NF-κB biology in the gut is complex. Our results and those of others suggest that the inhibition of activated NF-κB in mucosal macrophages and DCs may ameliorate innate immune responses that underlie chronic IBD; however, NF-κB may play a protective role in the epithelium. Thus, in contemplating therapeutic strategies that target NF-κB, many interrelated factors may be important to determine the ultimate clinical applicability, including inhibition of activated vs basal NF-κB, targeting of specific cell types (macrophages vs gut epithelium), and route of delivery (systemic vs local).
This study supports the proof of concept that selective inhibition of IKK by 8K-NBD is an effective strategy for suppressing intestinal inflammatory responses. Compared with other NF-κB inhibitors tested in chronic inflammatory diseases, 8K-NBD has the theoretic advantages of inhibiting the activation of NF-κB, a hallmark of chronic inflammation, while not inhibiting basal NF-κB activity that may be involved in fundamental cellular processes.
Footnotes
This work was supported by National Institutes of Health Grants AI35098 (to A.S.B.), R01 DK54452, R41 DK074193 (to S.E.P.), and P30 DK34987 (to S.E.P. and C.J.), National Research Service Award F30 ES013617 (to S.H.D.), and funding from the Broad Medical Research Program (to S.E.P.).
Abbreviations used in this paper: IBD, inflammatory bowel disease; BM, bone marrow; CD, Crohn's disease; 6CF, 6-carboxyfluorescein; DC, dendritic cell; DSS, dextran sulfate sodium; EGFP, enhanced GFP; IKK, IκB kinase; mNBD, mutant NBD; NBD, NEMO binding domain; NEMO, NF-κB essential modulator; phospho, phosphorylated; PTD, protein transduction domain; TNBS, trinitrobenzene sulfonic acid.
Disclosures
Albert S. Baldwin declares an equity position in Theralogics, Inc.
References
- 1.Neurath MF, Fuss I, Schurmann G, Pettersson S, Arnold K, Muller-Lobeck H, Strober W, Herfarth C, Buschenfelde KH. Cytokine gene transcription by NF-κB family members in patients with inflammatory bowel disease. Ann. NY Acad. Sci. 1998;859:149–159. doi: 10.1111/j.1749-6632.1998.tb11119.x. [DOI] [PubMed] [Google Scholar]
- 2.Schreiber S, Nikolaus S, Hampe J. Activation of nuclear factor κB inflammatory bowel disease. Gut. 1998;42:477–484. doi: 10.1136/gut.42.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rogler G, Brand K, Vogl D, Page S, Hofmeister R, Andus T, Knuechel R, Baeuerle PA, Scholmerich J, Gross V. Nuclear factor κB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology. 1998;115:357–369. doi: 10.1016/s0016-5085(98)70202-1. [DOI] [PubMed] [Google Scholar]
- 4.Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-κB activity through induction of IκB synthesis. Science. 1995;270:286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 5.Bantel H, Berg C, Vieth M, Stolte M, Kruis W, Schulze-Osthoff K. Mesalazine inhibits activation of transcription factor NF-κB in inflamed mucosa of patients with ulcerative colitis. Am. J. Gastroenterol. 2000;95:3452–3457. doi: 10.1111/j.1572-0241.2000.03360.x. [DOI] [PubMed] [Google Scholar]
- 6.Scheinman RI, Gualberto A, Jewell CM, Cidlowski JA, Baldwin AS., Jr. Characterization of mechanisms involved in transrepression of NF-κB by activated glucocorticoid receptors. Mol. Cell. Biol. 1995;15:943–953. doi: 10.1128/mcb.15.2.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tak PP, Firestein GS. NF-κB: a key role in inflammatory diseases. J. Clin. Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.May MJ, Marienfeld RB, Ghosh S. Characterization of the IκB-kinase NEMO binding domain. J. Biol. Chem. 2002;277:45992–46000. doi: 10.1074/jbc.M206494200. [DOI] [PubMed] [Google Scholar]
- 9.Wadia JS, Dowdy SF. Protein transduction technology. Curr. Opin. Biotechnol. 2002;13:52–56. doi: 10.1016/s0958-1669(02)00284-7. [DOI] [PubMed] [Google Scholar]
- 10.Mi Z, Lu X, Mai JC, Ng BG, Wang G, Lechman ER, Watkins SC, Rabinowich H, Robbins PD. Identification of a synovial fibroblast-specific protein transduction domain for delivery of apoptotic agents to hyperplastic synovium. Mol. Ther. 2003;8:295–305. doi: 10.1016/s1525-0016(03)00181-3. [DOI] [PubMed] [Google Scholar]
- 11.Mi Z, Mai J, Lu X, Robbins PD. Characterization of a class of cationic peptides able to facilitate efficient protein transduction in vitro and in vivo. Mol. Ther. 2000;2:339–347. doi: 10.1006/mthe.2000.0137. [DOI] [PubMed] [Google Scholar]
- 12.Mai JC, Mi Z, Kim SH, Ng B, Robbins PD. A proapoptotic peptide for the treatment of solid tumors. Cancer Res. 2001;61:7709–7712. [PubMed] [Google Scholar]
- 13.Dasgupta S, Jana M, Zhou Y, Fung YK, Ghosh S, Pahan K. Antineuroinflammatory effect of NF-κB essential modifier-binding domain peptides in the adoptive transfer model of experimental allergic encephalomyelitis. J. Immunol. 2004;173:1344–1354. doi: 10.4049/jimmunol.173.2.1344. [DOI] [PubMed] [Google Scholar]
- 14.Rehman KK, Bertera S, Bottino R, Balamurugan AN, Mai JC, Mi Z, Trucco M, Robbins PD. Protection of islets by in situ peptide-mediated transduction of the IκB kinase inhibitor Nemo-binding domain peptide. J. Biol. Chem. 2003;278:9862–9868. doi: 10.1074/jbc.M207700200. [DOI] [PubMed] [Google Scholar]
- 15.Dai S, Hirayama T, Abbas S, Abu-Amer Y. The IκB kinase (IKK) inhibitor, NEMO-binding domain peptide, blocks osteoclastogenesis and bone erosion in inflammatory arthritis. J. Biol. Chem. 2004;279:37219–37222. doi: 10.1074/jbc.C400258200. [DOI] [PubMed] [Google Scholar]
- 16.Xiong H, Zhu C, Li F, Hegazi R, He K, Babyatsky M, Bauer AJ, Plevy SE. Inhibition of interleukin-12 p40 transcription and NF-κB activation by nitric oxide in murine macrophages and dendritic cells. J. Biol. Chem. 2004;279:10776–10783. doi: 10.1074/jbc.M313416200. [DOI] [PubMed] [Google Scholar]
- 17.Karrasch T, Kim JS, Muhlbauer M, Magness ST, Jobin C. Gnotobiotic IL-10–/–;NF-κB(EGFP) mice reveal the critical role of TLR/NF-κB signaling in commensal bacteria-induced colitis. J. Immunol. 2007;178:6522–6532. doi: 10.4049/jimmunol.178.10.6522. [DOI] [PubMed] [Google Scholar]
- 18.Magness ST, Jijon H, Van Houten Fisher N, Sharpless NE, Brenner DA, Jobin C. In vivo pattern of lipopolysaccharide and anti-CD3-induced NF-κB activation using a novel gene-targeted enhanced GFP reporter gene mouse. J. Immunol. 2004;173:1561–1570. doi: 10.4049/jimmunol.173.3.1561. [DOI] [PubMed] [Google Scholar]
- 19.Berg DJ, Davidson N, Kuhn R, Muller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4+ TH1-like responses. J. Clin. Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hegazi RA, Rao KN, Mayle A, Sepulveda AR, Otterbein LE, Plevy SE. Carbon monoxide ameliorates chronic murine colitis through a heme oxygenase 1-dependent pathway. J. Exp. Med. 2005;202:1703–1713. doi: 10.1084/jem.20051047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neurath MF, Pettersson S. Predominant role of NF-κB p65 in the pathogenesis of chronic intestinal inflammation. Immunobiology. 1997;198:91–98. doi: 10.1016/s0171-2985(97)80030-7. [DOI] [PubMed] [Google Scholar]
- 22.Lawrance IC, Wu F, Leite AZ, Willis J, West GA, Fiocchi C, Chakravarti S. A murine model of chronic inflammation-induced intestinal fibrosis down-regulated by antisense NF-κB. Gastroenterology. 2003;125:1750–1761. doi: 10.1053/j.gastro.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 23.Neurath MF, Pettersson S, Meyer zum Buschenfelde KH, Strober W. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-κB abrogates established experimental colitis in mice. Nat. Med. 1996;2:998–1004. doi: 10.1038/nm0996-998. [DOI] [PubMed] [Google Scholar]
- 24.Rudolph D, Yeh WC, Wakeham A, Rudolph B, Nallainathan D, Potter J, Elia AJ, Mak TW. Severe liver degeneration and lack of NF-κB activation in NEMO/IKKγ-deficient mice. Genes Dev. 2000;14:854–862. [PMC free article] [PubMed] [Google Scholar]
- 25.Jimi E, Aoki K, Saito H, D'Acquisto F, May MJ, Nakamura I, Sudo T, Kojima T, Okamoto F, Fukushima H, et al. Selective inhibition of NF-kappa B blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nat. Med. 2004;10:617–624. doi: 10.1038/nm1054. [DOI] [PubMed] [Google Scholar]
- 26.di Meglio P, Ianaro A, Ghosh S. Amelioration of acute inflammation by systemic administration of a cell-permeable peptide inhibitor of NF-κB activation. Arthritis Rheum. 2005;52:951–958. doi: 10.1002/art.20960. [DOI] [PubMed] [Google Scholar]
- 27.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 28.Shibata W, Maeda S, Hikiba Y, Yanai A, Ohmae T, Sakamoto K, Nakagawa H, Ogura K, Omata M. Cutting edge: the IκB kinase (IKK) inhibitor, NEMO-binding domain peptide, blocks inflammatory injury in murine colitis. J. Immunol. 2007;179:2681–2685. doi: 10.4049/jimmunol.179.5.2681. [DOI] [PubMed] [Google Scholar]
- 29.Araki A, Kanai T, Ishikura T, Makita S, Uraushihara K, Iiyama R, Totsuka T, Takeda K, Akira S, Watanabe M. MyD88-deficient mice develop severe intestinal inflammation in dextran sodium sulfate colitis. J. Gastroenterol. 2005;40:16–23. doi: 10.1007/s00535-004-1492-9. [DOI] [PubMed] [Google Scholar]
- 30.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, Danese S, Huth M, Nikolaev A, Neufert C, Madison B, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 32.Rakoff-Nahoum S, Hao L, Medzhitov R. Role of toll-like receptors in spontaneous commensal-dependent colitis. Immunity. 2006;25:319–329. doi: 10.1016/j.immuni.2006.06.010. [DOI] [PubMed] [Google Scholar]