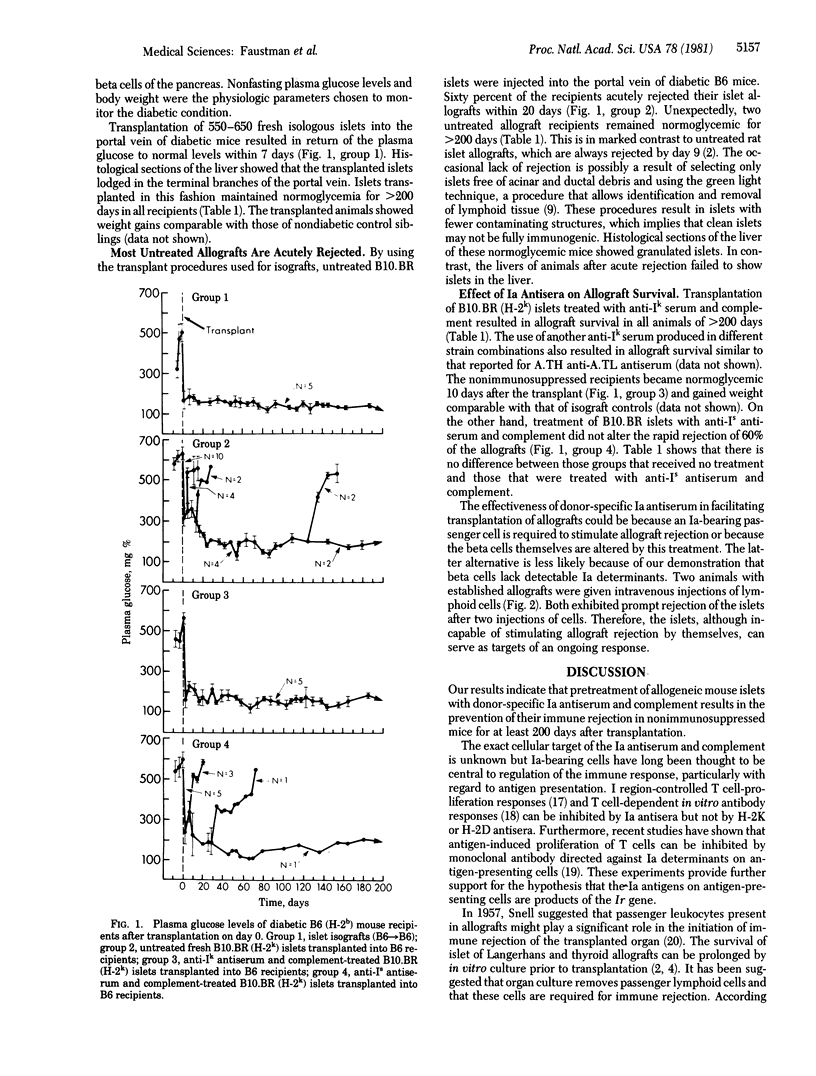
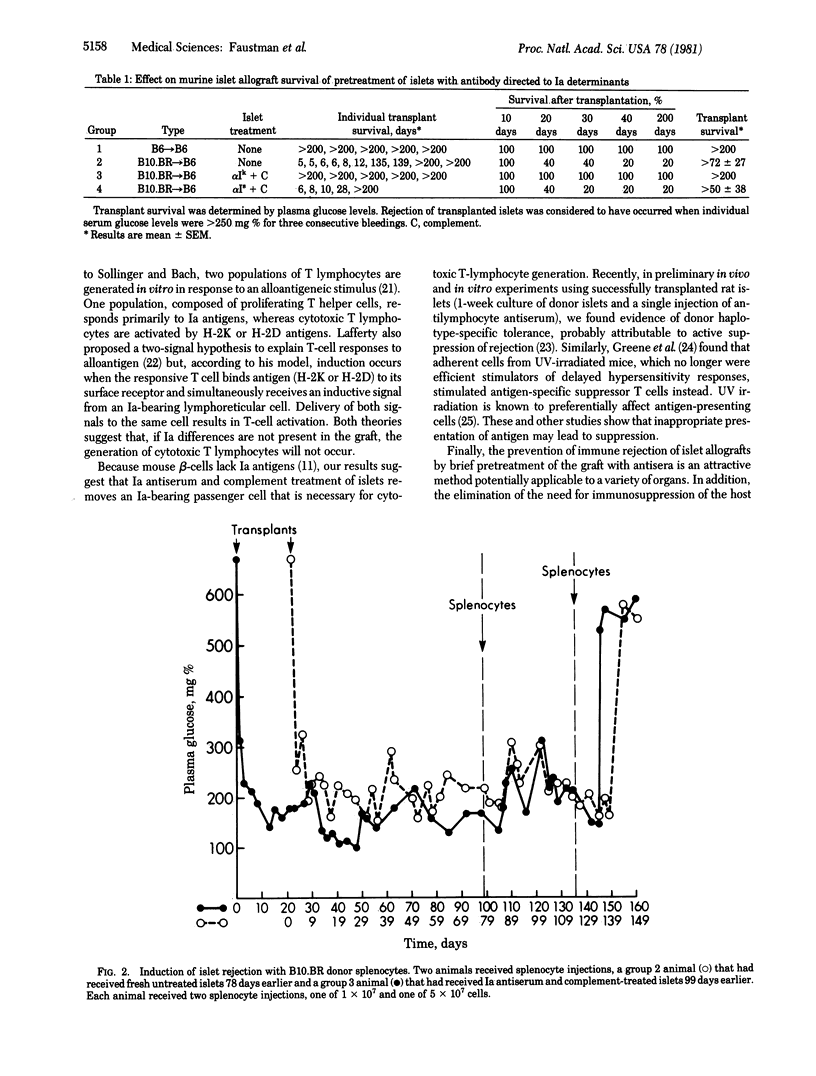
Abstract
Islets of Langerhans treated with donor-specific anti-Ia serum and complement were transplanted across a major histocompatibility barrier into nonimmunosuppressed diabetic mice. The allografts survived in all recipients for at least 200 days after transplantation. Rejection of an established allograft could be induced by intravenous injection of donor splenocytes. This demonstrates that allografts can serve as targets for immune rejection and supports the possible role of Ia-positive passenger lymphoid cells in initiation of immune rejection. The results show that immunosuppression of the recipient is not a prerequisite for successful transplantation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davies D. A., Staines N. A. A cardinal role for I-region antigens (Ia) in immunological enhancement, and the clinical implications. Transplant Rev. 1976;30:18–39. doi: 10.1111/j.1600-065x.1976.tb00213.x. [DOI] [PubMed] [Google Scholar]
- Faustman D., Hauptfeld V., Davie J. M., Lacy P. E., Shreffler D. C. Murine pancreatic beta-cells express H-2K and H-2D but not Ia antigens. J Exp Med. 1980 Jun 1;151(6):1563–1568. doi: 10.1084/jem.151.6.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finke E. H., Lacy P. E., Ono J. Use of reflected green light for specific identification of islets in vitro after collagenase isolation. Diabetes. 1979 Jun;28(6):612–613. doi: 10.2337/diab.28.6.612. [DOI] [PubMed] [Google Scholar]
- Frelinger J. A., Niederhuber J. E., Shreffler D. C. Inhibition of immune responses in vitro by specific antiserums to Ia antigens. Science. 1975 Apr 18;188(4185):268–270. doi: 10.1126/science.1118728. [DOI] [PubMed] [Google Scholar]
- Greene M. I., Sy M. S., Kripke M., Benacerraf B. Impairment of antigen-presenting cell function by ultraviolet radiation. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6591–6595. doi: 10.1073/pnas.76.12.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp C. B., Knight M. J., Scharp D. W., Ballinger W. F., Lacy P. E. Effect of transplantation site on the results of pancreatic islet isografts in diabetic rats. Diabetologia. 1973 Dec;9(6):486–491. doi: 10.1007/BF00461694. [DOI] [PubMed] [Google Scholar]
- Kripke M. L., Lofgreen J. S., Beard J., Jessup J. M., Fisher M. S. In vivo immune responses of mice during carcinogenesis by ultraviolet irradiation. J Natl Cancer Inst. 1977 Oct;59(4):1227–1230. doi: 10.1093/jnci/59.4.1227. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Davie J. M., Finke E. H. Induction of rejection of successful allografts of rat islets by donor peritoneal exudate cells. Transplantation. 1979 Nov;28(5):415–420. doi: 10.1097/00007890-197911000-00014. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Davie J. M., Finke E. H. Prolongation of islet allograft survival following in vitro culture (24 degrees C) and a single injection of ALS. Science. 1979 Apr 20;204(4390):312–313. doi: 10.1126/science.107588. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Davie J. M., Finke E. H. Prolongation of islet xenograft survival without continuous immunosuppression. Science. 1980 Jul 11;209(4453):283–285. doi: 10.1126/science.6770465. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Lafferty K. J., Cooley M. A., Woolnough J., Walker K. Z. Thyroid allograft immunogenicity is reduced after a period in organ culture. Science. 1975 Apr 18;188(4185):259–261. doi: 10.1126/science.1118726. [DOI] [PubMed] [Google Scholar]
- Lafferty K. J. Immunogenicity of foreign tissues. Transplantation. 1980 Mar;29(3):179–182. [PubMed] [Google Scholar]
- Lindall A., Steffes M., Sorenson R. Immunoassayable insulin content of subcellular fractions of rat islets. Endocrinology. 1969 Aug;85(2):218–223. doi: 10.1210/endo-85-2-218. [DOI] [PubMed] [Google Scholar]
- Longo D. L., Schwartz R. H. Inhibition of antigen-induced proliferation of T cells from radiation-induced bone marrow chimeras by a monoclonal antibody directed against an Ia determinant on the antigen-presenting cell. Proc Natl Acad Sci U S A. 1981 Jan;78(1):514–518. doi: 10.1073/pnas.78.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie I. F., Henning M. M. The I region transplantation antigens: immunogenicity and enhancement. J Immunogenet. 1977 Aug;4(4):259–269. doi: 10.1111/j.1744-313x.1977.tb00908.x. [DOI] [PubMed] [Google Scholar]
- SNELL G. D. The homograft reaction. Annu Rev Microbiol. 1957;11:439–458. doi: 10.1146/annurev.mi.11.100157.002255. [DOI] [PubMed] [Google Scholar]
- Shreffler D. C., Amos D. B., Mark R. Serological analysis of a recombination in the H-2 region of the mouse. Transplantation. 1966 May;4(3):300–322. doi: 10.1097/00007890-196605000-00008. [DOI] [PubMed] [Google Scholar]
- Silberberg-Sinakin I., Gigli I., Baer R. L., Thorbecke G. J. Langerhans cells: role in contact hypersensitivity and relationship to lymphoid dendritic cells and to macrophages. Immunol Rev. 1980;53:203–232. doi: 10.1111/j.1600-065x.1980.tb01045.x. [DOI] [PubMed] [Google Scholar]
- Sollinger H. W., Bach F. H. Collaboration between in vivo responses to LD and SD antigens of major histocompatibility complex. Nature. 1976 Feb 12;259(5543):487–488. doi: 10.1038/259487a0. [DOI] [PubMed] [Google Scholar]
- Sollinger H. W., Burkholder P. M., Rasmus W. R., Bach F. H. Prolonged survival of xenografts after organ culture. Surgery. 1977 Jan;81(1):74–79. [PubMed] [Google Scholar]
- Streilein J. W., Toews G. B., Bergstresser P. R. Corneal allografts fail to express Ia antigens. Nature. 1979 Nov 15;282(5736):326–327. doi: 10.1038/282326a0. [DOI] [PubMed] [Google Scholar]
- Yamashita U., Shevach E. M. The expression of Ia antigens on immunocompetent cells in the guinea pig. II. Ia antigens on macrophages. J Immunol. 1977 Nov;119(5):1584–1588. [PubMed] [Google Scholar]
- Yano A., Schwartz R. H., Paul W. E. Antigen presentation in the murine T lymphocyte proliferative response. II. Ir-GAT-controlled T lymphocyte responses require antigen-presenting cells from a high responder donor. Eur J Immunol. 1978 May;8(5):344–347. doi: 10.1002/eji.1830080510. [DOI] [PubMed] [Google Scholar]



