Abstract
In life, we must often learn new associations to people, places, or things we already know. The current functional magnetic resonance imaging study investigated the neural mechanisms underlying emotional memory updating. Nineteen participants first viewed negative and neutral pictures and learned associations between those pictures and other neutral stimuli, such as neutral objects and encoding tasks. This initial learning phase was followed by a memory updating phase, during which participants learned picture-location associations for old pictures (i.e., pictures previously associated with other neutral stimuli) and new pictures (i.e., pictures not seen in the first phase). There was greater frontopolar/ orbitofrontal (OFC) activity when people learned picture-location associations for old negative pictures than for new negative pictures, but frontopolar OFC activity did not significantly differ during learning locations of old versus new neutral pictures. In addition, frontopolar activity was more negatively correlated with the amygdala when participants learned picture-location associations for old negative pictures than for new negative or old neutral pictures. Past studies revealed that the frontopolar OFC allows for updating the affective values of stimuli in reversal learning or extinction of conditioning (e.g., Izquierdo & Murray, 2005); our findings suggest that it plays a more general role in updating associations to emotional stimuli.
Keywords: memory updating, emotion, frontal pole, orbitofrontal cortex, amygdala
The emotion elicited by stimuli or events affects later memory for that information (see Dolcos & Denkova, 2008; LaBar & Cabeza, 2006; Phelps, 2004 for reviews). Emotional items are remembered better than neutral items (e.g., Canli, Zhao, Brewer, Gabrieri, & Cahill, 2000; Dolcos, LaBar, & Cabeza, 2004; Hamann, Ely, Grafton, & Kilts, 1999). Intrinsic details (e.g., color, locations) of emotional items are also remembered better than those of neutral items (D’Argembeau & Van der Linden, 2004; Doerksen & Shimamura, 2001; Hadley & MacKay, 2006; Kensinger, Garoff-Eaton, & Schacter, 2006; MacKay & Ahmetzanov, 2005; Mather, 2007; Mather, Gorlick, & Nesmith, 2009; Mather & Nesmith, 2008; Nashiro & Mather, in press). These laboratory studies examined memory for the features of novel items. However, learning new information is only one aspect of human memory function. In life, we often re-encounter people, objects, or places in new contexts, and must learn new associations to things we already know. For instance, the first time one sees a rattlesnake might be very memorable, but how about the second time? Will it be harder to learn the new rattlesnake location? Updating existing knowledge is an important ability required in everyday life. The current study employed functional magnetic resonance imaging (fMRI) to investigate whether updating memory of emotional information depends on different neural mechanisms than learning new emotional information.
Most past studies on emotional memory updating used stimulus-reward or stimulus-punishment association learning paradigms. These studies suggest that updating emotional memory depends on the frontopolar/ orbitofrontal (OFC) regions. The first line of evidence comes from studies on reversal learning (e.g., Rolls, 2004; Rolls, McCabe, & Redoute, 2008; Schoenbaum, Saddoris, & Stalnaker, 2007). In a typical reversal-learning task, animals or humans are first presented with two cues and acquire associations between one of the cues and a reward. This learning phase is followed by reversal trials, in which participants have to respond to the previously unrewarded cue to obtain rewards. In fMRI studies, the frontopolar OFC activates during reversal learning (e.g., Budhani, Marsh, Pine, & Blair, 2007; Kringelbach & Rolls, 2003; Remijnse, Nielen, Uylings, & Veltman, 2005) and OFC lesions impair reversal learning in rats (e.g., Schoenbaum, Nugent, Saddoris, & Setlow, 2002), in monkeys (e.g., Dias, Robbins, & Roberts, 1996; Izquierdo, Suda, & Murray, 2004), and in humans (e.g., Fellows & Farah, 2003; Hornak, et al., 2004).
Another line of research, examining memory extinction, also suggests that the frontopolar OFC is necessary for updating emotional associations. For example, OFC lesions impair extinction of fear or reward conditioning in animals (e.g., Butter, 1969) and in humans (e.g., Rolls, Hornak, Wade, & McGrath, 1994). Neuroimaging studies also reveal enhanced frontopolar OFC activity during extinction of classical aversive conditioning (e.g., Gottfried & Dolan, 2004; Phelps, Delgado, Nearing, & LeDoux, 2004) and operant reinforcement conditioning (Finger, Mitchell, Jones, & Blair, 2008). Furthermore, in one study with humans, cortical thickness of the frontopolar OFC region predicted fear conditioning extinction (Milad, et al., 2005). Since extinction involves updating emotional memory (i.e., inhibiting the original conditioned responses and learning new associations with conditioned stimuli), these results are consistent with the view that updating emotional memories depends on the frontopolar OFC.
Although the role of the amygdala is still under investigation, recent research suggests that it protects memory for previous emotional events by preventing updating of these memories, while frontopolar OFC down-regulates the amygdala, allowing for flexible updating of emotional memories (Schoenbaum, et al., 2007). For example, lesion studies in monkeys revealed that while OFC lesions impaired reversal learning and extinction learning, amygdala lesions facilitate both extinction (Izquierdo & Murray, 2005) and reversal learning (Rudebeck & Murray, 2008). Stalnaker et al. (2007) also provided evidence that the amygdala mediates the effects of OFC on reversal learning in rats: Whereas lesioning OFC alone impaired reversal learning (as discussed above), lesioning OFC and amygdala together did not impair reversal learning. Thus, performance was actually better with an additional lesion—consistent with the notion that the amygdala works against updating of emotional memories, but is countered by the OFC. In line with these findings in animals, neuroimaging research with humans found that the frontopolar region had negative functional connectivity with the amygdala during extinction learning (Finger, et al., 2008). These results suggest that the frontopolar OFC facilitates flexible updating of emotional memories by inhibiting the amygdala’s protection of old emotional memories.
Since past studies on emotional memory updating predominantly relied on either reversal learning or extinction learning, however, several important questions remain. First, previous work has not examined how updating emotional information differs from updating non-emotional information. In typical reversal learning or extinction learning paradigms, memory updating always involves emotional components (e.g., learning associations between a previously rewarding cue and a non-rewarding event; learning associations between a previously neutral cue and a reward; inhibiting previous aversive or reinforcement conditioning). Thus, it is unclear whether frontopolar OFC is more involved when the information to be updated is emotional than when it is non-emotional. Second, while emotional memory updating does not necessarily mean updating affective values of stimuli (e.g., remembering the new location of a snake does not require updating the affective value of the snake), both reversal learning and extinction learning involve re-learning of affective values of stimuli (e.g., re-learning reward-stimulus association or re-learning punishment-stimulus associations). Thus, it is not clear whether or not the frontopolar OFC region is involved in learning new information about existing emotional memories even when affective values of stimuli do not need to be changed.
In fact, recent behavioral studies suggest that even when people do not have to re-learn affective values of stimuli, adding new associations to existing emotional memories involves different processing than learning similar associations to new emotional information (e.g., Mather & Knight, 2008; Novak & Mather, 2009). In one study (Mather & Knight, 2008), for example, participants first learned that some neutral faces predicted emotional pictures, and other neutral faces predicted neutral pictures. This initial phase was followed by an updating phase, where they learned intrinsic details (i.e., location) of those neutral faces that previously predicted either negative or neutral pictures. In addition to the old faces, the updating phase also involved new negative or new neutral faces that participants had not seen previously. Consistent with the emotional memory-enhancement shown in past studies (e.g., Mather & Nesmith, 2008), when faces were new, the locations of negative faces were remembered better than those of neutral faces. In contrast, the old faces showed the opposite pattern: Neutral faces previously associated with negative pictures produced worse memory for their locations than those previously associated with neutral pictures. Thus, it appears that even when the affective values of stimuli do not need to be updated, adding new associations to existing emotional memories requires different mechanisms than learning new emotional items.
The present study examines the brain mechanisms underlying emotional memory updating, focusing on whether the frontopolar OFC is involved in learning new associations to old emotional stimuli. We also introduced a neutral memory updating condition, to see whether the frontopolar OFC plays a more important role in emotional memory updating than in non-emotional memory updating. The experiment involved two phases: an initial learning phase and an updating phase. During the initial learning phase, participants viewed emotional or neutral pictures and studied associations between those pictures and other information (such as neutral objects or encoding tasks). This initial learning phase was followed by the updating phase, in which participants learned the location of pictures. The pictures in the updating phase included those from the initial association phase (i.e., old condition) as well as new emotional and new neutral pictures (i.e., new condition). If the frontopolar OFC helps update emotional memory even when affective values of stimuli do not need to be changed, this brain region should show greater activity during learning locations of old compared with new emotional pictures. In contrast, the frontopolar OFC activity should not differ when learning locations of old versus new neutral pictures. In addition, if the frontopolar OFC counters amygdala’s protection of old emotional memories, the frontopolar OFC should have negative functional connectivity with the amygdala especially when participants learn new associations to old emotional pictures.
Methods
Participants
Twenty Japanese undergraduate and graduate students at University of Tsukuba took part in the experiment (13 males and 7 females; Mage = 21.2, SD = 1.72). They gave written informed consent in accordance with the MRI ethics committee of AIST and were paid for their participation. Prospective participants were screened and excluded for any medical, neurological, or psychiatric illness. There was an earthquake during one participant’s fMRI session. We did not include this participant’s data in the analyses.
Design and Materials
The updating phase involved learning 120 picture-location associations. Forty pictures were new pictures not seen in the initial learning phase (i.e., new condition), while the other 80 pictures were old pictures learned in the initial learning phase (i.e., old condition). To address the effects of the type of initial associations, half of the old pictures were associated with neutral objects in the initial learning phase (i.e., old-object), whereas the other half were associated with encoding tasks (i.e., old-task).
We employed 120 matched-picture pairs, in which each negative picture was yoked with a visually similar, but less arousing neutral picture (Mather & Nesmith, 2008). The 120 pairs were grouped into three sets of 40 matched-pair pictures. Each stimulus set was assigned to one of the three stimulus type conditions (i.e., old-object, old-task and new conditions). The assignment was counterbalanced across participants. Forty matched-picture pairs assigned to each stimulus type condition were further divided into two groups of 20 pairs (i.e., Set A and B). For half of the participants, we used only the negative versions from Set A and the neutral versions from Set B, while for the other half, we used the neutral versions from Set A and the negative versions from Set B. Thus, each participant was shown just one of the two pictures from a matched pair, resulting in 20 negative and 20 neutral pictures in each condition. In addition to the matched pictures, we employed 40 drawings of common neutral objects (e.g., apple, pen) obtained from a previous study (Snodgrass & Vanderwart, 1980), internet, and a commercialized DVD. Forty pictures assigned to the old-object condition were randomly paired with these neutral objects.
Behavioral Procedures
Participants completed a practice session of the experimental trials outside the scanner (with neutral stimuli not used in the main session). After participants entered the scanner, an initial T1-weighted sagittal localizer and a T1-weighted axial image were acquired, followed by study sessions. The study consisted of a) the initial learning phase and b) the updating phase, each of which was followed by a memory test (Figure 1). All tasks were conducted while participants were in the scanner, but they were not scanned during any of the memory tests.
Figure 1.
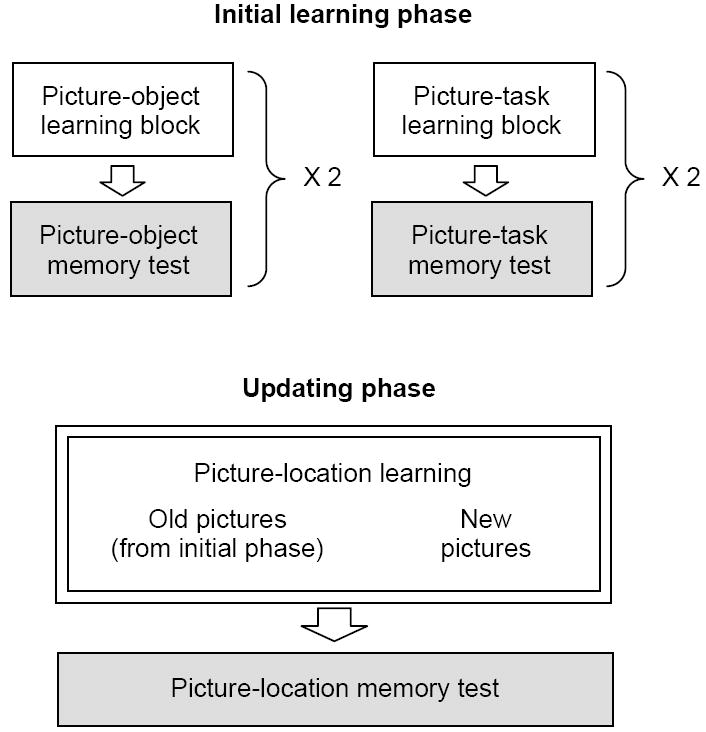
Overview of the study procedures. The experiment involved the initial learning phase and the updating phase. The initial learning phase included a block to learn 40 picture-object associations and another block to learn 40 pictures-task associations, each of which was immediately followed by a memory test. To enhance participants’ memory, the learning-test cycle was repeated once. This initial learning phase was followed by the updating phase, during which participants learned picture-location associations for 80 pictures from the initial learning phase (i.e., old pictures) and also for 40 new pictures (not shown during the initial learning phase). Immediately after they learned all of the 120 picture-location associations, their memory about the picture-location associations was tested. All sessions were conducted while participants were in a scanner, but they were not scanned during memory tests (shown in gray). FMRI data analyses reported in the paper were performed on the updating phase (highlighted by two lines).
Initial learning phase
The initial learning phase involved learning 40 picture-object pairs and 40 picture-task pairs. To enhance participants’ memory, each pair was repeated in a second block. The block order was counterbalanced across participants. Thus, half of the participants completed the initial learning phase in the following order: 1) picture-object, 2) picture-task, 3) picture-task, and 4) picture-object blocks. The other half received sessions in a different order: 1) picture-task, 2) picture-object, 3) picture-object, and 4) picture-task blocks.
In the picture-object block (Figure 2A), participants were first shown either a negative or neutral picture for 2 sec, which was followed by a neutral object for 3 sec. The picture remained on the screen during the object presentation. The object was shown either at the left bottom or the right bottom of the screen. Participants were asked to indicate the location of the object (i.e., left or right) by pressing keys, and to memorize an association between the picture and the object. After the object and the picture disappeared, a fixation cross was presented for 4-6 sec, which was followed by a next trial. The trial order was randomized regardless of the picture valence.
Figure 2.

Schematic representations of trials in each phase. (A) On each trial of the initial learning phase, participants viewed a picture, and 2 sec later, they saw a neutral object (in the picture-object block) or an encoding task (picture-task block) for 3 sec. The picture remained on a screen while the object and the task were shown. Participants were asked to learn each picture-object and picture-task pair as much as possible. (B) In the memory tests on the picture-object associations, participants were presented with one picture with two objects, and asked to indicate which object was paired with the picture in the preceding learning block. Similarly, in the memory tests on the picture-task associations, participants were told to indicate which task was paired with the picture. (B) During the updating phase, participants viewed 80 pictures studied in the initial learning phase (i.e., old condition) and 40 new pictures (i.e., new condition). Each picture was presented at one of the two locations (i.e., left-top or right-bottom), which was followed by a green or pink dot. Participants were told to the memorize picture-location associations and also to indicate the color of the dot by pressing keys. (D) During the memory test on the picture-location associations, participants saw each picture at the center of the screen and were told to indicate the location of the picture in the updating session.
A similar procedure was employed in the picture-task block (Figure 2A). On each trial, participants were presented with either a negative or neutral picture. Two seconds later, one of the following two questions was shown for 3 sec while the picture still remained on the screen: a) a question about themselves (self-task; “Have you seen similar scenes?”) or b) a question about their mothers (other-task; “Has your mother seen similar scenes?”). Half of the pictures were paired with the “self” task, while the other half were paired with the “other” task. The assignment of the tasks was counterbalanced across participants. Participants were asked to memorize an association between the picture and the task, and also to press keys to indicate their answers to the questions. On the self-task trials, they were told to answer whether they had seen a similar scene in other contexts (e.g., movies or TVs) or not. On the other-task trials, they were asked to answer whether they thought their mothers had seen similar pictures or not. The trial order was randomized regardless of the picture valence and the type of questions.
Memory test for the initial learning phase
Immediately after participants completed each learning block, their memory about the associations was tested by a forced-choice memory test. In the memory test on the picture-object associations (Figure 2B), participants were shown one of the 40 pictures presented in the preceding learning block with two neutral objects: a) the one associated with the picture, and b) another object associated with a different picture from the same valence category. Participants were told to indicate which object was associated with the picture. When they selected the wrong object, they saw “incorrect” on the screen for 1 sec. A similar procedure was used in the memory test on the picture-task association. Participants were shown each of the 40 pictures presented in the preceding learning block, and asked to indicate whether the picture was paired with the self question or with the other question. They saw “incorrect” on the screen for 1 sec when they selected the wrong question.
Updating phase
In the updating phase, participants were asked to learn 120 picture-location associations. During one-third of the trials, they saw new pictures that they had not seen in the initial learning sessions (i.e., new condition). On the remaining trials, they were shown pictures that had been associated with tasks or neutral objects (i.e., old condition). Unlike the initial learning phase, participants studied picture-location associations only once. They were told that they would have just one trial to learn each picture-location association, and to do their best to memorize each association during its presentation. On each trial (Figure 2C), participants were shown one picture at either the left top or the right bottom of the screen for 2 sec. They were asked to memorize an association between the picture and the location. The picture was followed by a 2-sec blank screen and then a pink or a green dot appeared at the center of the screen for 1 sec. Participants were asked to press keys to indicate the color of the dots. The dot was followed by a 4-6 sec interval and then the next trial started. The trial order was randomized regardless of picture valence and stimulus type. The 120 trials were divided into three blocks of 40 trials and participants took a 1-min rest between blocks.
Memory test for the updating phase
Immediately after the updating phase, participants were shown each of the 120 pictures presented in the updating phase in the center of the screen, and asked to remember whether the picture was shown at the left-top or the right-bottom of the screen (Figure 2D). We did not give participants “incorrect” feedback.
Functional MRI Data Acquisition and Preprocessing
All scanning was performed on a 3.0-T MRI Scanner (GE 3T Signa) equipped with EPI capability using the standard head coil for radiofrequency transmission and signal reception. 27 axial slices (4 mm thick and 0.2 mm gap, interleaved) were prescribed to cover the whole brain. A T2* weighted gradient echo EPI was employed. The imaging parameters were TR=2s, TE=30ms, FA=75, and FOV=20 cm×20 cm (64×64 mesh). To avoid head movement, participants wore a neck brace and were asked not to talk or move during scanning. Each participant’s data were individually pre-processed by SPM5 (Wellcome Trust Center for Neuroimaging). In the preprocessing analysis, images were corrected for slice timing and motion, then spatially normalized into the Montreal Neurological Institute (MNI) template and spatially smoothed using a Gaussian kernel of 8mm FWHM.
FMRI Data Analysis
The main purpose of the current study was to compare brain activity during learning locations of old versus new pictures. Therefore, we report functional MRI results from the updating phase and do not report those from the initial learning phase. Because we found similar results across two initial association types (i.e., old-object vs. old-task; see results from conjunction analyses), we report results from an analysis collapsing these two subcategory conditions as the main results. The comparisons across the initial association types (e.g., old-object vs. old-task; self vs. other) are beyond the scope of this paper and not reported here.
Whole brain analyses
For each participant, stimulus-dependent changes in BOLD signal were modeled with regressors for each event type (new negative, new neutral, old negative, and old neutral pictures). For the old picture conditions, pictures for which the participant could not recall correct associates in the second-round memory test of the initial learning phase were modeled separately. Because only a few trials fell in this category (see Table 1), we did not perform any statistical tests for these items. The model also involved additional regressors for dots and rests. The regressors were convolved with the canonical hemodynamic response function. A high-pass filter (cutoff period = 128 s) was applied to remove low-frequency artifacts from the data. The effects of each event type were estimated using a fixed-effects model. The data were then entered into a random effects analysis. The threshold was set at p < .05 –FDR combined with a cluster extent threshold of 10 contiguous voxels. Locations reported by SPM were converted into Talairach coordinates by the MNI-to-Talairach transformation algorithm (Lancaster, et al., 2007). These coordinates were used to determine the nearest gray matter using the Talairach Daemon version 2.4.2 (Lancaster, et al., 2000).
Table 1.
Memory performance in the initial learning and in the updating phases.
| Initial learning | Updating | ||||
|---|---|---|---|---|---|
| 1st round | 2nd round | ||||
| Old | Object | Negative | .91 (0.09) | .99 (0.02) | .76 (0.17) |
| Neutral | .87 (0.15) | .98 (0.04) | .74 (0.18) | ||
| Task | Negative | .79 (0.09) | .93 (0.07) | .78 (0.14) | |
| Neutral | .79 (0.13) | .96 (0.06) | .74 (0.17) | ||
| New | Negative | - | - | .76 (0.13) | |
| Neutral | - | - | .75 (0.19) | ||
Note: Standard deviations in parentheses.
Conjunction analyses
To examine whether the frontopolar OFC activated in response to old negative pictures regardless of the type of initial associations (i.e., old-object vs. old-task), we conducted a conjunction analysis. The analysis method was similar to the analysis described above, except that the GLM design file had separate regressors for pictures in old-object condition and those in old-task condition for each valence category. After the effects of each event type were estimated using a random effects analysis, conjunction analyses were performed, using a masking function of SPM. Each contrast entered into the conjunction analyses was thresholded at p < .001 -uncorrected, resulting in conjunction probability of p < .00001. Clusters of activations that involved less than 10 contiguous voxels were discarded.
Regions-of-interest (ROI) analyses
Given past findings that the amygdala plays an important role in emotion and memory (LaBar & Cabeza, 2006; Phelps, 2006), we structurally defined bilateral amygdala based on the AAL atlas (Tzourio-Mazoyer, et al., 2002) and performed ROI analyses, using the MarsBar toolbox (http://marsbar.sourceforge.net/).
Functional connectivity analyses
To examine functional connectivity, we applied a beta series correlation analysis (Gazzaley, Cooney, Rissman, & D’Esposito, 2005; Rissman, Gazzaley, & D’Esposito, 2004). This allowed us to use trial-to-trial variability to characterize dynamic inter-regional interactions. First, a new GLM design file was constructed where each individual trial for each condition was coded with a unique covariate, resulting in 120 independent variables. To reduce the confounding effects of the global signal change, the global mean signal level over all brain voxels was calculated for each time point and was used as a covariate. The model also involved additional regressors for dots and rests. Second, the least squares solution of the GLM yielded a beta value for each trial for each individual participant. These beta values were then sorted by stimulus type. As a third step, mean activity (i.e., mean parameter estimates) was extracted for each individual trial from a seed region. As a fourth step, for each stimulus type, we computed correlations between the seed’s beta series and the beta series of all other voxels in the brain, thus generating condition-specific seed correlation maps. Correlation magnitudes were converted into z-scores using the Fisher’s r-to-z transformation. To assess the map-wise significance of the correlation at the group level, random-effects t tests were conducted on the z-transformed correlation maps of the individual participants. Cluster-based FWE corrections (p < .05) were employed with t = 3.61 for height threshold, resulting in thresholded correlational maps for each condition. Condition-dependent changes in functional connectivity were also assessed using random-effects analyses, which were thresholded at p < .005-uncorrected at voxel-level combined with a cluster extent threshold of 20 contiguous voxels. To further examine interactions among brain regions involved in learning locations of old negative pictures, multi-level mediation analyses were performed (Zhang, Zyphur, & Preacher, 2009). For each trial of the old negative conditions in each participant, we extracted the percent signal change from our regions of interests, such as bilateral hippocampus, bilateral amygdala, and the frontal pole. The frontal pole was defined based on an activation cluster obtained in the whole brain analysis (see results section). The hippocampus and amygdala were defined anatomically based on the AAL atlas. Then, we tested whether the interactions between two regions were mediated by another region with treating each trial as a level-1 unit and each participant as a level-2 unit.
Results
Behavioral Results
Memory test for the initial learning phase
A 2 (type: object vs. task) X 2 (valence: negative vs. neutral) X 2 (repetition: 1st round vs. 2nd round) analysis of variance (ANOVA) was performed on correct recall rates in the memory tests during the initial learning phase. The ANOVA revealed significant effects of repetition, F (1, 18) = 85.56, p < .01, R2 = .35, and of type, F (1, 18) = 19.08, p < .01, R2 = .12, and a significant interaction between type and repetition, F (1, 18) = 5.01, p < .05, R2 = .59. Participants remembered picture-object associations better than picture-task associations in the first round, F (1, 18) = 28.22, p < .01, but not in the second round (p > .05). Indeed, their performance was at ceiling levels in the second round (see Table 1). Thus, although participants had more difficulty learning picture-task associations than picture-object associations, they could acquire most of the picture-task and picture-object associations within two rounds.
Memory test for the updating phase
A 3 (type: old-object, old-task, vs. new) X 2 (valence: negative vs. neutral) ANOVA on the correct location recall rates in the updating phase did not yield any significant effects (ps > .20; Table 1). Thus, contrary to previous findings (e.g., Mather & Nesmith, 2008), we did not observe emotion enhancement of picture-location associations for new items. Similarly, although past studies revealed that people are worse at remembering picture-location associations for old emotional items than for old neutral items (Mather & Knight, 2008; Novak & Mather, 2009), we did not find the same pattern in the current study. While the past studies employed eight locations with no overlaps, the current study employed only two locations (i.e., left-top or right-bottom) and they were spatially overlapping with each other. This might have resulted in less salient picture-location associations that were more difficult to learn in the current study, which might have obscured the effects of emotion.
FMRI Results
Brain regions showing different activity between old and new pictures
The whole-brain analysis during the updating phase revealed increased activity in the anterior prefrontal region, including the frontal pole and the inferior OFC (BA 11; 47), when people learned picture-location associations for old negative pictures than for new negative pictures (Figure 3A; Table 2). Old negative pictures also induced greater activity in the angular gyrus, posterior cingulate, superior frontal gyrus, superior temporal gyrus and occipital gyrus than did new negative pictures. In contrast, the old - new contrast for neutral pictures did not reveal any significant differences at the same threshold level. When we employed a lower threshold (p = .001-uncorrected), old neutral pictures produced greater activity in posterior cingulate (0 -30 26 in MNI; BA 23), occipital lobe (-4 -86 32; BA 18), angular gyrus (-38 -80 42, BA 39) than did new neutral pictures, but we still did not find a significant difference in frontopolar OFC activity (Figure 3B). Reverse contrasts (i.e., new negative – old negative; new neutral – old neutral) did not reveal any significant differences. Thus, it appears that frontopolar OFC is involved more in making associations to old items than to new items when items are emotional but not when items are neutral. Supporting this idea, an interaction contrast, [old negative – new negative] > [old neutral – new neutral], revealed significant activation in right frontal pole (Figure 3C, 3D; Table 2) with p < .001 –uncorrected with a cluster threshold of 10 voxels. There were no other regions showing significant results in this interaction analysis.
Figure 3.
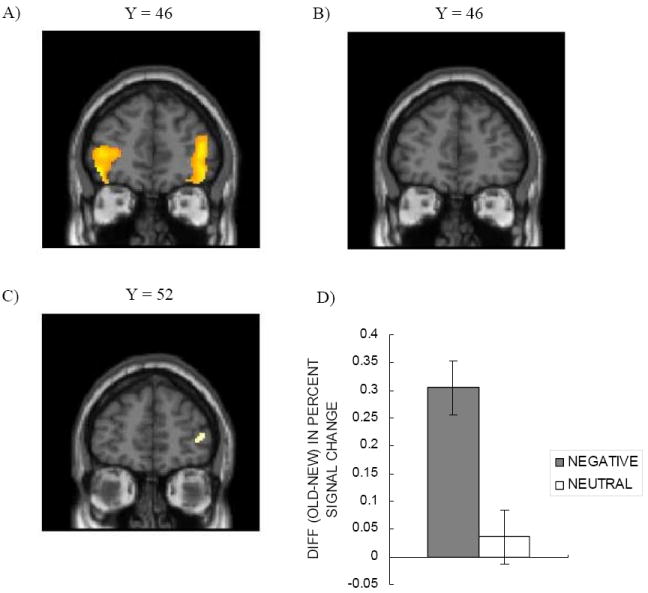
Differential frontopolar/OFC activity when learning associations for old pictures and for new pictures during the updating phase. (A) Frontopolar/ OFC regions showed greater activity for old emotional items than for new emotional items. (B) The old vs. new contrast for neutral pictures did not show differences. (C, D) The greater frontopolar activity for old negative but not old neutral pictures led to a significant interaction between valence and novelty in right BA 10.
Table 2.
Brain activity showing significant differences between old versus new pictures in the updating phase.
| Area | H | BA | MNI | Talairach | ||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | |||
| Old negative > New negative | ||||||||
| Angular Gyrus | L | 39/19 | -54 | -62 | 44 | -52 | -63 | 37 |
| R | 39/19 | 48 | -74 | 38 | 43 | -74 | 32 | |
| Frontal Pole | R | 10 | 44 | 50 | 6 | 40 | 44 | 15 |
| L | 10 | -44 | 44 | -12 | -41 | 40 | -3 | |
| Posterior Cingulate | L/R | 23/30 | -4 | -30 | 26 | -5 | -32 | 25 |
| Superior/Middle Frontal Gyrus | R | 6 | 22 | 28 | 56 | 19 | 19 | 57 |
| R | 8 | 48 | 30 | 44 | 43 | 22 | 47 | |
| L | 9/8 | -42 | 28 | 36 | -40 | 21 | 38 | |
| L | 8 | -20 | 34 | 50 | -20 | 26 | 51 | |
| Superior Temporal Gyrus | L | 22 | -52 | -10 | -8 | -49 | -10 | -5 |
| 22 | -66 | -32 | 4 | -62 | -32 | 4 | ||
| Occipital Lobe, Cuneus | L/R | 18 | -4 | -74 | 38 | -5 | -74 | 31 |
| Cingulate Gyrus | L | 32 | -6 | 32 | 40 | -7 | 25 | 43 |
| Paracentral Lobule | R | 5 | 2 | -30 | 52 | 0 | -34 | 48 |
| Pons | R | 2 | -26 | -32 | 1 | -23 | -27 | |
|
| ||||||||
| Old neutral > New neutral | ||||||||
| No significant results | ||||||||
|
| ||||||||
| New negative > Old negative | ||||||||
| No significant results | ||||||||
|
| ||||||||
| New neutral > Old neutral | ||||||||
| No significant results | ||||||||
|
| ||||||||
| [Old negative> New negative]>[Old neutral > New neutral]* | ||||||||
| Frontal Pole | R | 10 | 36 | 56 | 2 | 32 | 50 | 11 |
| R | 10 | 42 | 52 | 6 | 38 | 46 | 15 | |
Note:
p < .001 –uncorrected.
Conjunction analyses
Next, we examined brain activity shared by the two contrasts: a) [old-object negative > new negative], and b) [old-task negative > new negative]. This conjunction analysis confirmed greater activity in the frontal pole for old negative pictures than for new negative pictures (see Table 3). The activation extended into inferior OFC (BA 11). A similar conjunction analysis on neutral pictures did not reveal any significant activity. These results suggest that the frontopolar OFC was involved in learning locations of old emotional pictures, regardless of the type of initial associations.
Table 3.
Results from conjunction analyses between old-object and old-task conditions in the updating phase.
| Area | H | BA | MNI | Talairach | ||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | |||
| [Old-object negative > New negative] and [Old-task negative > New negative] | ||||||||
| Angular Gyrus | L | 39/19 | -54 | -62 | 44 | -52 | -63 | 37 |
| R | 39/19 | 48 | -70 | 40 | 43 | -71 | 34 | |
| R | 39 | 52 | -58 | 34 | 47 | -59 | 30 | |
| Frontal Pole | L | 10 | -44 | 44 | -10 | -42 | 40 | -2 |
| L | 10 | -42 | 46 | 6 | -40 | 41 | 13 | |
| R | 10 | 36 | 56 | 4 | 32 | 50 | 13 | |
| Superior Temporal Gyrus | L | 22 | -54 | -8 | -10 | -51 | -8 | -6 |
| Superior Frontal Gyrus | R | 6 | 22 | 24 | 56 | 19 | 16 | 57 |
| L | 9 | -44 | 30 | 34 | -42 | 23 | 36 | |
| Posterior Cingulate | L/R | 30/23 | -4 | -34 | 26 | -5 | -36 | 24 |
| Occipital Lobe, Cuneus | L | 18 | -14 | -74 | 26 | -14 | -73 | 21 |
| L | 7 | -6 | -74 | 38 | -7 | -74 | 31 | |
| Occipital Lobe, Precuneus | R | 31 | 16 | -68 | 24 | 13 | -67 | 20 |
|
| ||||||||
| [Old-object neutral > New neutral] and [Old-task neutral > New neutral] | ||||||||
| No significant results | ||||||||
Brain regions showing different activity for old negative vs. old neutral pictures
A comparison between old negative and old neutral pictures did not reveal any significant results at p < .05 -FDR. When we employed a lower threshold (p < .001 –uncorrected with a cluster threshold of 10 voxels), however, we found that old negative pictures produced greater activity in bilateral amygdala and parahippocampal gyrus (Figure 4). In addition, the frontal pole and the ventrolateral prefrontal cortex (VLPFC) showed greater activity to old negative pictures than to old neutral pictures (Table 4). Although these clusters are not overlapping with the regions where we found significant differences when contrasting old negative versus new negative pictures in the previous whole brain analysis, past studies reported activity in similar regions for reversal learning (e.g., Cools, Clark, Owen, & Robbins, 2002; Remijnse, et al., 2005). Thus, the brain regions implicated in reversal learning may help not only to update affective values of stimuli as shown in previous reversal learning studies, but also to learn new information about old emotional memories in general. A reverse contrast did not reveal any significant results even at p < .001 –uncorrected.
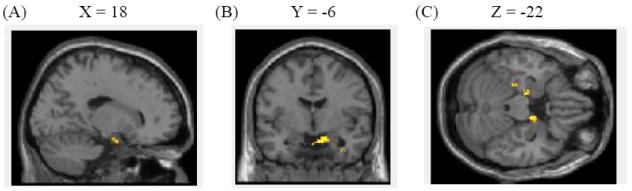
Figure 4.
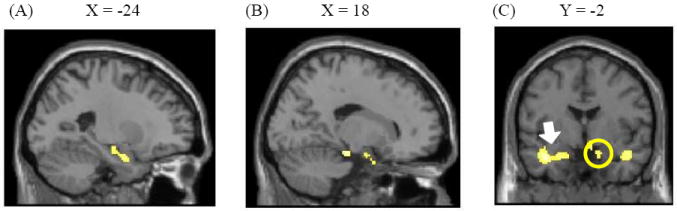
(A) Left and (B) right amygdala and adjacent parahippocampal gyri showed greater activity to old negative pictures than to old neutral pictures. (C) A coronal view of the activations (the left amygdala/ parahippocampal gyrus cluster is pointed by an arrow and the right amygdala/ parahippocampal gyrus cluster is circled).
Table 4.
Brain activity showing significant differences between old negative versus old neutral pictures in the updating phase.
| Area | H | BA | MNI | Talairach | ||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | |||
| Old negative > Old neutral | ||||||||
| Parahippocampal Gyrus | R | 34 | 14 | 4 | -26 | 12 | 4 | -19 |
| R | 34 | 14 | -2 | -20 | 12 | -2 | -14 | |
| R | 35 | 18 | -24 | -18 | 16 | -23 | -14 | |
| L | 35 | -12 | -24 | -18 | -12 | -22 | -14 | |
| Amygdala | L | -24 | -2 | -24 | -23 | -1 | -18 | |
| L | -24 | -14 | -14 | -23 | -13 | -10 | ||
| Hypothalamus | 4 | -4 | -10 | 3 | -5 | -5 | ||
| Frontal Pole | L | 10 | -18 | 64 | 18 | -18 | 56 | 26 |
| Ventrolateral PFC | R | 45 | 54 | 28 | 4 | 49 | 24 | 11 |
| Superior Frontal Gyrus | R | 8 | 8 | 56 | 42 | 6 | 47 | 47 |
| R | 8 | 10 | 50 | 50 | 8 | 40 | 53 | |
| L | 8 | -6 | 58 | 38 | -7 | 49 | 43 | |
| L | 6 | -4 | 40 | 56 | -5 | 31 | 58 | |
| Superior Temporal Gyrus | R | 38 | 42 | 0 | -22 | 38 | 0 | -15 |
| L | 38 | -40 | 0 | -24 | -38 | 1 | -18 | |
| Middle Occipital Gyrus | L | 37 | -52 | -70 | -8 | -49 | -66 | -10 |
| Cerebellum | R | 38 | -62 | -26 | 34 | -57 | -24 | |
| R | 36 | -46 | -32 | 33 | -42 | -28 | ||
| R | 44 | -50 | -32 | 40 | -46 | -28 | ||
|
| ||||||||
| Old neutral > Old negative | ||||||||
| No significant results. | ||||||||
ROI analyses
Separate 2 (valence) X 2 (novelty) ANOVAs were performed on the percent signal changes from left and right amygdala. These ANOVAs found significant effects of valence, F (1, 18) = 13.53, 8.13, ps < .05, with no other significant effects. This indicates that negative pictures induced greater activity in the bilateral amygdala than neutral pictures, regardless of the novelty (left: Mneg = 0.15; Mneut = 0.05; right: Mneg = 0.15; Mneut = 0.04).
Functional connectivity analysis
To address the role of the frontal pole, a beta series correlation analysis was employed, using the right frontal pole cluster previously identified as showing a significant interaction between valence and novelty as a seed region. The analysis revealed that right frontal pole was negatively correlated with bilateral medial temporal lobe (MTL), including hippocampus, amygdala and parahippocampal gyrus, during learning associations to old negative pictures (Figure 5A). Although this frontopolar cluster had similar negative connectivity with MTL for new negative (Figure 5B) and old neutral pictures (Figure 5C), direct comparison revealed that old negative pictures produced greater negative correlations between frontal pole and MTL than did old neutral or new negative pictures (Tables 5-6; Figure 6). Thus, the frontal pole had negative connectivity with MTL especially when learning associations to old emotional pictures.
Figure 5.
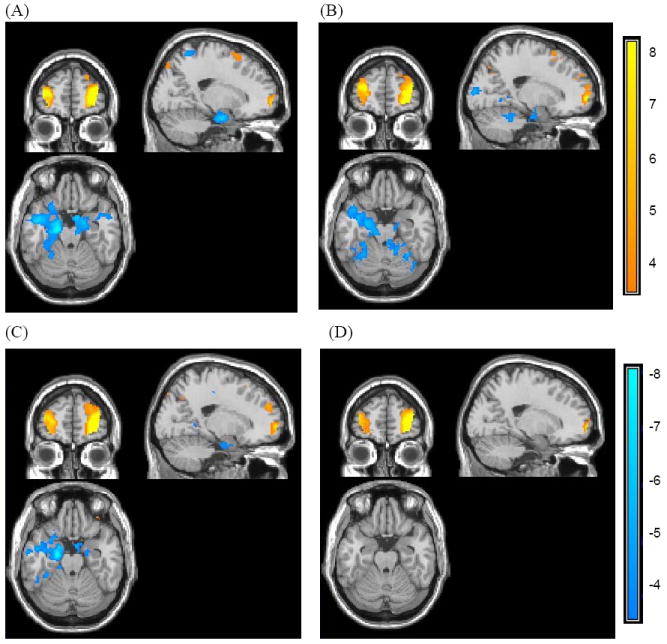
Functional connectivity with right frontal pole cluster for each condition (x = 20, y = 56, z = -22). The right frontal pole had significant negative connectivity with MTL for (A) old negative, (B) new negative, and (C) old neutral pictures, but not for (D) new neutral pictures.
Table 5.
Brain regions showing differential negative connectivity with right frontal pole across conditions.
| Area | H | BA | MNI | Talairach | T | ||||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | ||||
| Old negative > New negative | |||||||||
| Hippocampus | R | 30 | -14 | -28 | 27 | -13 | -22 | 3.54 | |
| Amygdala | R | 34 | -6 | -32 | 31 | -5 | -24 | 3.36 | |
| Parahippocampal gyrus | R | 34 | 12 | 0 | -22 | 10 | 0 | -15 | 5.49 |
| Uncus | R | 28 | 36 | 4 | -30 | 33 | 4 | -22 | 4.50 |
| Caudate | L | -6 | 16 | -12 | -6 | 14 | -5 | 4.97 | |
| R | 8 | 18 | 4 | 6 | 15 | 9 | 3.21 | ||
| Putamen | L | -18 | 10 | -18 | -17 | 9 | -11 | 3.54 | |
| Inferior Frontal Gyrus | L | 47 | -24 | 14 | -28 | -23 | 14 | -20 | 4.42 |
| L | 47 | -28 | 12 | -20 | -27 | 11 | -13 | 3.21 | |
| Anterior Cingulate | R | 24 | 4 | 30 | -2 | 3 | 26 | 5 | 3.77 |
| L | 24 | -2 | 30 | 4 | -3 | 26 | 10 | 3.47 | |
| R | 24 | 6 | 24 | -8 | 5 | 21 | -1 | 2.98 | |
| Cingulate Gyrus | L | 24 | -8 | 8 | 26 | -9 | 3 | 28 | 4.85 |
|
| |||||||||
| Old negative > Old neutral | |||||||||
| Hippocampus/Parahippocampal Gyrus | L | 28 | -16 | -18 | -24 | -16 | -16 | -19 | 4.39 |
| L | 35 | -26 | -28 | -26 | -25 | -25 | -22 | 3.88 | |
| R | 34 | 14 | -4 | -20 | 12 | -4 | -14 | 3.96 | |
| R | 35 | 28 | -12 | -30 | 25 | -10 | -23 | 5.49 | |
| Pons | L | -6 | -20 | -32 | -6 | -18 | -26 | 3.68 | |
| Superior Frontal Gyrus | L | 6 | -12 | 36 | 58 | -13 | 27 | 59 | 4.30 |
| Superior Temporal Gyrus | R | 34 | 8 | -36 | 31 | 9 | -27 | 4.27 | |
| Inferior Parietal Lobule | L | 40 | -42 | -38 | 58 | -41 | -42 | 52 | 3.84 |
| L | 40 | -46 | -38 | 50 | -44 | -41 | 45 | 3.40 | |
|
| |||||||||
| New negative > Old negative | |||||||||
| Inferior Occipital Gyrus | L | 18 | -40 | -90 | -8 | -38 | -85 | -12 | 3.67 |
| L | 19 | -44 | -74 | -6 | -42 | -70 | -8 | 4.53 | |
| L | 19 | -38 | -84 | -2 | -36 | -80 | -6 | 3.85 | |
| R | 17 | 18 | -94 | -4 | 15 | -89 | -8 | 3.24 | |
| Middle Occipital Gyrus | L | 17 | -20 | -92 | 2 | -20 | -88 | -3 | 3.13 |
| R | 19 | 38 | -80 | 20 | 34 | -78 | 15 | 4.08 | |
| R | 19 | 40 | -84 | 12 | 36 | -81 | 8 | 3.69 | |
|
| |||||||||
| Old neutral > Old negative | |||||||||
| Middle Temporal Gyrus | L | 36 | -46 | -22 | -16 | -43 | -21 | -13 | 4.21 |
| R | 19 | 38 | -60 | 14 | 34 | -59 | 12 | 3.23 | |
| Precentral Gyrus | L | 6 | -60 | 6 | 12 | -57 | 3 | 14 | 4.09 |
| Inferior Occipital Gyrus | L | 18 | -34 | -84 | -2 | -33 | -80 | -6 | 3.61 |
Table 6.
Brain regions showing differential positive connectivity with right frontal pole across conditions.
| Area | H | BA | MNI | Talairach | T | ||||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | ||||
| Old negative > New negative | |||||||||
| Frontal pole* | R | 10 | 40 | 52 | 4 | 36 | 46 | 13 | 7.91 |
| R | 10 | 34 | 58 | 10 | 30 | 51 | 19 | 4.15 | |
| R | 10 | 26 | 56 | 4 | 23 | 50 | 13 | 4.01 | |
| Superior Occipital Gyrus | L | 17 | -12 | -98 | 4 | -12 | -93 | -1 | 4.04 |
| L | 18 | -22 | -94 | 22 | -22 | -91 | 15 | 4.43 | |
| R | 18 | 18 | -96 | 16 | 15 | -93 | 10 | 4.85 | |
| Superior Occipital Gyrus | L | 18 | -16 | -98 | 16 | -16 | -94 | 9 | 3.47 |
| Middle Occipital Gyrus | L | 18 | -22 | -100 | 8 | -22 | -96 | 2 | 3.09 |
| L | 18 | -18 | -100 | 10 | -18 | -96 | 4 | 3.20 | |
| R | 18 | 28 | -94 | 16 | 24 | -91 | 10 | 3.86 | |
| Supramarginal Gyrus | R | 40 | 60 | -54 | 32 | 54 | -55 | 29 | 4.02 |
|
| |||||||||
| Old negative > Old neutral | |||||||||
| Middle Occipital Gyrus | L | 18 | -12 | -96 | 20 | -13 | -93 | 13 | 4.27 |
| Superior Temporal Gyrus | R | 22 | 46 | -56 | 22 | 41 | -56 | 19 | 4.08 |
| Occipital Lobe, Cuneus | L | 18 | -10 | -82 | 24 | -11 | -80 | 18 | 3.61 |
|
| |||||||||
| New negative > Old negative | |||||||||
| Superior Frontal Gyrus | L | 8 | -36 | 24 | 50 | -35 | 16 | 50 | 4.39 |
|
| |||||||||
| Old neutral > Old negative | |||||||||
| No significant results | |||||||||
Note:
represents the seed region.
Figure 6.
Differential correlation maps with right frontal pole. Frontal pole had more negative correlations with (A) right amygdala and (B) right parahippocampal gyrus for old negative pictures than for new negative pictures. Frontal pole also had greater negative correlations with (C) bilateral parahippocampal gyrus for old negative pictures than for old neutral pictures.
The inverse correlation between the right frontal pole and the amygdala suggests that the frontal pole might inhibit the amygdala’s protection of old emotional memories (Izquierdo & Murray, 2005). If the frontal pole counters the amygdala to allow for emotional memory updating, however, it does not seem reasonable that the frontal pole would inhibit hippocampus which is known to help emotional memory updating (Corcoran, Desmond, Frey, & Maren, 2005). Given that the amygdala has strong reciprocal connections with the hippocampus (Pitkanen, Pikkarainen, Nurminen, & Ylinen, 2000), one possible explanation is that the amygdala mediates the negative relationships between the frontal pole and the hippocampus. Multilevel mediation analyses supported this possibility; The left amygdala significantly mediated the negative relationships between the left hippocampus and the frontal pole cluster (Sobel test: z = 2.88, p < .01), and that the right amygdala mediated the negative relationships between the right hippocampus and the frontal pole cluster (z = 2.98, p < .01). In addition, the effects of the frontal pole on the hippocampus were no longer significant after controlling the effects of the amygdala (ps > .10). To confirm the meditational role of the amygdala, a Multilevel Structural Equation Modeling (MSEM) analysis was also performed (Muthén & Asparouhov, 2008). The Akaike information criterion (AIC) and Bayesian Information Criterion (BCI) were used to compare the fit of the following two models: The first model assumed that the left amygdala mediates the left hippocampus-frontal pole interactions and the right amygdala mediates the right hippocampus-the frontal pole interactions, and the second model posited that the left hippocampus mediates the left amygdala-frontal pole interactions and the right hippocampus mediates the right amygdala-frontal pole interactions. The MSEM analysis revealed a better fit for the first model (AIC = 4266.77; BIC = 4443.77) than the second model (AIC = 4295.26; BIC = 4455.13). These results suggest that the negative relationships between the hippocampus and the frontal pole for old negative pictures were mediated by the amygdala.
Discussion
The current fMRI study addressed the neural mechanisms of learning new associations to old emotional memories. Our results indicated that the frontopolar OFC showed greater activity when participants learned the locations of old emotional items than of new emotional items. In contrast, this region did not show increased activity to old neutral items compared to new neutral items. In fact, we found an interaction of valence and novelty in the right frontal pole. In addition, the frontal pole had negative correlations with MTL when people learned locations of old emotional pictures during the updating phase. Importantly, this negative connectivity was modulated by the type of stimuli; the frontal pole had less negative connectivity with MTL for new emotional and old neutral pictures, and the negative connectivity between the frontal pole and MTL did not emerge for new neutral pictures. Although the frontal pole showed negative correlations not only with the amygdala but also with the hippocampus which is known to help emotional memory updating (Corcoran, et al., 2005), subsequent mediation analyses suggested that the negative hippocampus-frontopolar relation was mediated by the amygdala. The meditational role of the amygdala was also confirmed by the MSEM analysis. Thus, it appears that while the frontal pole had negative connectivity with the amygdala when learning new associations to old emotional items, the amygdala had positive relations with the hippocampus, which resulted in the negative correlations between the frontal pole and the hippocampus.
These findings of the current study are consistent with recent evidence that the frontopolar OFC helps re-learn affective values of stimuli, by countering amygdala’s protection of previous emotional memories (e.g., Stalnaker, et al., 2007). Finger et al. (2008), for example, found that during extinction learning, the frontal pole showed enhanced activity and had negative connectivity with the amygdala. While those past studies relied on the stimulus-reward/ stimulus-punishment association paradigm and examined re-learning of affective values of stimuli, the present study observed similar patterns when learning new associations to old emotional items without changing the affective values of the emotional items. Thus, it appears that the frontopolar OFC plays an important role in emotional memory updating, even when the affective values of stimuli do not need to be updated. However, the frontal pole has been also implicated in memory retrieval (e.g., Prince, Daselaar, & Cabeza, 2005). This suggests an alternative possibility that people tend to have stronger memories about emotional items than neutral items during the initial learning phase, and that the enhanced frontopolar activity to old negative stimuli reflects retrieval of the initial exposures to those items. Furthermore, the negative correlations between the amygdala and the frontal pole have been reported in other situations where emotional memory updating is not necessarily required, such as emotion regulation (Urry, et al., 2006), and resting (Roy, et al., 2009). Future research is needed to elucidate the precise role of the frontopolar OFC in learning associations to old emotional memories.
In addition to the frontopolar OFC, old negative pictures produced greater activity in the angular gyrus and posterior cingulate than did new negative pictures. Although a similar contrast on neutral items [old neutral > new neutral] did not find any significant effects at the same threshold level, an additional analysis with a lower threshold revealed greater activity in these regions for old neutral than for new neutral pictures. In addition, we did not find a significant valence and novelty interaction in these two regions. Thus, it seems likely that the angular gyrus and posterior cingulate are involved in memory updating situations in general, regardless of the affective natures of memories. Past studies suggest that the angular gyrus implements stimulus-driven/ bottom-up attention (e.g., Chambers, Payne, Stokes, & Mattingley, 2004; Corbetta, Patel, & Shulman, 2008). Transcranial magnetic stimulation administered on this region disrupted shifts of attention from one visual cue to another visual stimulus (Chambers, et al., 2004). The angular gyrus also activates when shifting attention to mentally generated stimuli, such as other person’s mind or viewpoint (Saxe & Kanwisher, 2003) and retrieved episodic memories (Cabeza, Ciaramelli, Olson, & Moscovitch, 2008). These findings suggest that when people viewed old items, they remembered memories about initial exposures to those stimuli, and that the greater angular gyrus activity to old items than new items reflects bottom-up attention shifts to those retrieved memories.
The posterior cingulate has also been implicated in memory retrieval (Nielsen, Balslev, & Hansen, 2005). Increased activity in this region is observed when people remember their autobiographical memories in detail (Addis, Wong, & Schacter, 2007), and recognize familiar people (Maddock, Garrett, & Buonocore, 2001), objects and places (Sugiura, Shah, Zilles, & Fink, 2005). The posterior cingulate also activates during successful memory retrieval, especially when items were encoded with a deep encoding task than with a shallow encoding task (Shannon & Buckner, 2004). In addition, the posterior cingulate has strong reciprocal connections with MTL memory structures (e.g., Vogt & Pandya, 1987). These findings suggest that the posterior cingulate activity we observed with old items reflects retrieved memories acquired in the initial association phase. However, the posterior cingulate has been also implicated in self-referential processing (e.g., Johnson, et al., 2006) and consciousness (e.g., Vogt, Vogt, & Laureys, 2006). Further research should address the precise role of this region in memory updating.
Another question for future research concerns participants’ behavioral memory performance. Contrary to previous behavioral studies (Mather & Knight, 2008; Mather & Nesmith, 2008; Novak & Mather, 2009), our participants showed neither emotional memory enhancement for new pictures, nor emotional memory impairment for old pictures. Since we employed only two overlapping locations, the location manipulation was less salient in the current study than in past studies that employed eight locations. Fewer locations in the current study also resulted in higher chance level in the memory test (50% rather than around 12%). Thus, our behavioral memory measures were less sensitive than previous ones. Even though the memory performance was not significantly different, however, the brain activity patterns were substantially modulated by the stimulus novelty and valence. While our memory measure was a discrete variable, brain activity signals were continuous variables and should have greater statistical power (e.g., Cohen, 1983), which could explain significant effects in the neural measures but not in the behavioral measures. Related to this issue, we did not have enough trials in which participants failed to remember the locations in the updating phase, and could not separately analyze results from remembered items and those from failed items. Future research should employ more sensitive behavioral measures and address the brain mechanisms of emotional memory updating while considering differential memory performance.
In summary, the current study addressed the brain mechanisms underlying emotional memory updating. The results indicated that the frontopolar OFC is activated when learning new associations to old emotional items, and that this region has negative connectivity with the amygdala during updating old emotional memories. This pattern is consistent with the idea that the frontopolar OFC counters the amygdala’s protection of old emotional memories, allowing for more flexible updating of old emotional memories. This region has been implicated in re-learning reward-stimulus or punishment-stimulus associations (e.g., Finger, et al., 2008; Kringelbach & Rolls, 2003). Our results suggest that the frontopolar OFC plays a more general role in learning new associations to old emotional stimuli.
Acknowledgments
This work was supported by Grant-in-Aid for JSPS Fellows (19-8978) and by the National Institute on Aging [grants K02AG032309 and RO1AG025340].
References
- Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45(7):1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budhani S, Marsh AA, Pine DS, Blair KS. Neural correlates of response reversal: Considering acquisition. Neuroimage. 2007;34:1754–1765. doi: 10.1016/j.neuroimage.2006.08.060. [DOI] [PubMed] [Google Scholar]
- Butter CM. Perseveration in extinction and in discrimination reversal tasks following selective frontal ablations in Macaca mulatta. Physiology & Behavior. 1969;4(2):163–171. doi: 10.1016/0031-9384(69)90075-4. [DOI] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nature Reviews Neuroscience. 2008;9(8):613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Brewer J, Gabrieri JDE, Cahill L. Event-related activity in the human amygdala associates with later memory for individual emotional experience. Journal of Neuroscience. 2000;20:RC99. doi: 10.1523/JNEUROSCI.20-19-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers CD, Payne JM, Stokes MG, Mattingley JB. Fast and slow parietal pathways mediate spatial attention. Nature Neuroscience. 2004;7(3):217–218. doi: 10.1038/nn1203. [DOI] [PubMed] [Google Scholar]
- Cohen J. The cost of dichotomization. Applied Psychological Measurement. 1983;7(3):249–253. doi: 10.1177/014662168300700301. [DOI] [Google Scholar]
- Cools R, Clark L, Owen AM, Robbins TW. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. Journal of Neuroscience. 2002;22:4563–4567. doi: 10.1523/JNEUROSCI.22-11-04563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: From environment to theory of mind. Neuron. 2008;58(3):306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. Journal of Neuroscience. 2005;25(39):8978–8987. doi: 10.1523/jneurosci.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Argembeau A, Van der Linden M. Influence of affective meaning on memory for contextual information. Emotion. 2004;4:173–188. doi: 10.1037/1528-3542.4.2.173. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380(6569):69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Doerksen S, Shimamura AP. Source memory enhancement for emotional words. Emotion. 2001;1:5–11. doi: 10.1037/1528-3542.1.1.5. [DOI] [PubMed] [Google Scholar]
- Dolcos F, Denkova E. Neural correlates of encoding emotional memories: a review of functional neuroimaging evidence. Cell Science Reviews. 2008;5:78–122. [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004;42(5):855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003;126(8):1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- Finger EC, Mitchell DGV, Jones M, Blair RJR. Dissociable roles of medial orbitofrontal cortex in human operant extinction learning. Neuroimage. 2008;43(4):748–755. doi: 10.1016/j.neuroimage.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D’Esposito M. Top-down suppression deficits underlies working memory impairment in normal aging. Nature Neuroscience. 2005;8(10):1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, Dolan RJ. Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nature Neuroscience. 2004;7(10):1144–1152. doi: 10.1038/nn1314. [DOI] [PubMed] [Google Scholar]
- Hadley CB, MacKay DG. Does emotion help or hinder immediate memory?: Arousal versus priority-binding mechanisms. Journal of Experimental Psychology: Learning, Memory and Cognition. 2006;32:79–88. doi: 10.1037/0278-7393.32.1.79. [DOI] [PubMed] [Google Scholar]
- Hamann S, Ely TD, Grafton ST, Kilts CD. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nature Neuroscience. 1999;2(3):289–293. doi: 10.1038/6404. [DOI] [PubMed] [Google Scholar]
- Hornak J, O’Doherty J, Bramham J, Rolls ET, Morris RG, Bullock PR, et al. Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. Journal of Cognitive Neuroscience. 2004;16:463–478. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Murray EA. Opposing effects of amygdala and orbital prefrontal cortex lesions on the extinction of instrumental responding in macaque monkeys. European Journal of Neuroscience. 2005;22:2341–2346. doi: 10.1111/j.1460-9568.2005.04434.x. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. Journal of Neuroscience. 2004;24(34):7540–7548. doi: 10.1523/jneurosci.1921-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK, Raye CL, Mitchell KJ, Touryan SR, Greene EJ, Nolen-Hoeksema S. Dissociating medial frontal and posterior cingulate activity during self-reflection. Social Cognitive and Affective Neuroscience. 2006;1(1):56–64. doi: 10.1093/scan/nsl004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. Memory for specific visual details can be enhanced by negative arousing content. Journal of Memory and Language. 2006;54(1):99–112. doi: 10.1016/j.jml.2005.05.005. [DOI] [Google Scholar]
- Kringelbach ML, Rolls ET. Neural correlates of rapid reversal learning in a simple model of human social interaction. Neuroimage. 2003;20:1371–1383. doi: 10.1016/S1053-8119(03)00393-8. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nature Review Neuroscience. 2006;7(1):54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, et al. Bias between MNI and Talairach coordinates analyzed using the ICBN-152 brain template. Human Brain Mapping. 2007;28(11):1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach Atlas labels for functional brain mapping. Human Brain Mapping. 2000;10(3):120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay DG, Ahmetzanov MV. Emotion, memory, and attention in the taboo stroop paradigm. Psychological Science. 2005;16:25–32. doi: 10.1111/j.0956-7976.2005.00776.x. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience. 2001;104(3):667–676. doi: 10.1016/S0306-4522(01)00108-7. [DOI] [PubMed] [Google Scholar]
- Mather M. Emotional arousal and memory binding: An object-based framework. Perspectives on Psychological Science. 2007;2(1):33–52. doi: 10.1111/j.1745-6916.2007.00028.x. [DOI] [PubMed] [Google Scholar]
- Mather M, Gorlick M, Nesmith K. The limits of arousal’s memory impairing effects on nearby information. The American journal of psychology. 2009;122(3):349–369. [PMC free article] [PubMed] [Google Scholar]
- Mather M, Knight M. The emotional harbinger effect: Poor context memory for cues that previously predicted something arousing. Emotion. 2008;8(6):850–860. doi: 10.1037/a0014087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Nesmith K. Arousal-enhanced location memory for pictures. Journal of Memory and Language. 2008;58:449–464. doi: 10.1016/j.jml.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proceedings of the National Academy of Sciences. 2005;102(30):10706–10711. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén BO, Asparouhov T. Growth mixture modeling: Analysis with non-Gaussian random effects. In: Fitzmaurice G, Davidian M, Verbeke G, Molenberghs G, editors. Longitudinal data analysis. Boca Raton, FL: Chapman & Hall/CRC Press; 2008. pp. 143–165. [Google Scholar]
- Nashiro K, Mather M. How arousal affects younger and older adults’ memory binding. Experimental Aging Research. doi: 10.1080/0361073X.2011.536746. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen F, Balslev D, Hansen LK. Mining the posterior cingulate: Segregation between memory and pain components. Neuroimage. 2005;27:520–532. doi: 10.1016/j.neuroimage.2005.04.034. [DOI] [PubMed] [Google Scholar]
- Novak DL, Mather M. The tenacious nature of memory binding for arousing negative items. Memory & Cognition. 2009;37(7):945–952. doi: 10.3758/mc.37.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Current Opinion in Neurobiology. 2004;14(2):198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: Insights from studies of the human amygdala. Annual Review of Psychology. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat: A review. Annals of the New York Academy of Sciences. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Prince SE, Daselaar SM, Cabeza R. Neural correlates of relational memory: Successful encoding and retrieval of semantic and perceptual associations. Journal of Neuroscience. 2005;25(5):1203–1210. doi: 10.1523/jneurosci.2540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remijnse PL, Nielen MMA, Uylings HBM, Veltman DJ. Neural correlates of a reversal learning task with an affectively neutral baseline: An event-related fMRI study. Neuroimage. 2005;26:609–618. doi: 10.1016/j.neuroimage.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D’Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23(2):752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The functions of the orbitofrontal cortex. Brain & Cognition. 2004;55:11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Hornak J, Wade D, McGrath J. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. Journal of Neurology, Neurosurgery & Psychiatry. 1994;57(12):1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, McCabe C, Redoute J. Expected value, reward outcome, and temporal difference error representations in a probabilistic decision task. Cerebral Cortex. 2008;18(3):652–663. doi: 10.1093/cercor/bhm097. [DOI] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AMC, Uddin LQ, Gotimer K, et al. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45(2):614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Murray EA. Amygdala and orbitofrontal cortex lesions differentially influence choices during object reversal learning. Journal of Neuroscience. 2008;28(33):8338–8343. doi: 10.1523/jneurosci.2272-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind”. Neuroimage. 2003;19(4):1835–1842. doi: 10.1016/S1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Nugent SL, Saddoris MP, Setlow B. Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. Neuroreport. 2002;13(6):885. doi: 10.1097/00001756-200205070-00030. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Saddoris MP, Stalnaker TA. Reconciling the roles of orbitofrontal cortex in reversal learning and the encoding of outcome expectancies. Annals of the New York Academy of Sciences. 2007;1121:320–335. doi: 10.1196/annals.1401.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon BJ, Buckner RL. Functional-anatomic correlates of memory retrieval that suggest nontraditional processing roles for multiple distinct regions within posterior parietal cortex. Journal of Neuroscience. 2004;24(45):10084–10092. doi: 10.1523/jneurosci.2625-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. Journal of Experimental Psychology: Human Learning and Memory. 1980;6(2):174–215. doi: 10.1037/0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Franz TM, Singh T, Schoenbaum G. Basolateral amygdala lesions abolish orbitofrontal-dependent reversal impairments. Neuron. 2007;54(1):51–58. doi: 10.1016/j.neuron.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Shah NJ, Zilles K, Fink GR. Cortical representations of personally familiar objects and places: Functional organization of the human posterior cingulated cortex. Journal of Cognitive Neuroscience. 2005;17:183–198. doi: 10.1162/0898929053124956. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM: Using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN. Cingulate cortex of the rhesus monkey II: Cortical afferents. Journal of Comparative Neurology. 1987;262:271–289. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Vogt L, Laureys S. Cytology and functionally correlated circuits of human posterior cingulate areas. NeuroImage. 2006;29(2):452. doi: 10.1016/j.neuroimage.2005.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zyphur MJ, Preacher KJ. Testing multilevel mediation using hierarchical linear models. Organizational Research Methods. 2009;12(4):695–719. doi: 10.1177/1094428108327450. [DOI] [Google Scholar]


