Abstract
A general procedure, based on a new activated alkyne linker, for the preparation of peptide–oligonucleotide conjugates (POCs) on solid support has been developed. With this linker, conjugation is effective at room temperature (RT) in millimolar concentration and submicromolar amounts. This is made possible since the use of a readily attachable activated triple bond linker enhances the Cu(I) catalyzed 1,3-dipolar cycloaddition (‘click’ reaction). The preferred scheme for conjugate preparation involves sequential conjugation to oligonucleotides on solid support of (i) an H-phosphonate-based aminolinker; (ii) the triple bond donor p-(N-propynoylamino)toluic acid (PATA); and (iii) azido-functionalized peptides. The method gives conversion of oligonucleotide to the POC on solid support, and only involves a single purification step after complete assembly. The synthesis is flexible and can be carried out without the need for specific automated synthesizers since it has been designed to utilize commercially available oligonucleotide and peptide derivatives on solid support or in solution. Methodology for the ready conversion of peptides into ‘clickable’ azidopeptides with the possibility of selecting either N-terminus or C-terminus connection also adds to the flexibility and usability of the method. Examples of synthesis of POCs include conjugates of oligonucleotides with peptides known to be membrane penetrating and nuclear localization signals.
INTRODUCTION
Delivery is a seriously limiting factor for antisense, siRNA and gene therapy in vivo (1–3). One approach to enhance nucleic acid uptake into cells is to utilize cell penetrating peptides (CPPs) either in the form of complexes or in the form of covalent conjugates, which also protects from digestion by intracellular enzymes (4). A recent successful example involves a CPP conjugate of a morpholino phosphorodiamidate oligomer that when given in low doses leads to highly efficient splice switching and production of therapeutically useful levels of dystrophin in skeletal and heart muscles, which makes this a promising approach for the treatment of Duchenne muscular dystrophy (DMD) (5).
Quite a few approaches have been reported for the attachment of peptides to oligonucleotides in solution. This includes a large variety of linkages between the peptide and oligonucleotide, with the most common being amide, disulfide, thioether and urea functionalities and also many other types that have different advantages or disadvantages (6,7). Two main strategies have been adopted for the synthesis of peptide–oligonucleotide conjugates (POCs): online solid-phase synthesis and fragment conjugation methods. In online methods, the peptide and oligonucleotide fragments are usually assembled sequentially on the same solid support. This strategy puts higher demands on compatibility of protecting groups or activating and deblocking agents, and conditions used during synthesis of the peptide are typically not compatible with the presence of the oligonucleotide part. Hence, protecting groups and other reaction conditions have to be chosen carefully to minimize subside reactions which in most cases requires expertise and a laboratory equipped with automated synthesizers. A solid-phase technique for the synthesis of 5′-POCs with a uniform protection strategy for the nucleic acid and the peptide fragments using acid-labile α-amino protections has been previously developed in our group (8). Another procedure, with similar limitations of the need for an in-house synthesizer and only allowing C-terminal conjugation, involves Fmoc-based solid phase synthesis (9).
Solution phase fragment conjugation involves separate isolation and purification of peptides and oligonucleotides specifically modified with the functional groups required for the conjugation reaction. One of the most efficient liquid-phase conjugation methods is ‘Native ligation’ (10, 11) where the peptides and oligonucleotides are modified so as to contain cysteine or S-protected thiols and thioesters at the site of ligation. This methodology appears to give high yields but requires specific synthesis of a number of building blocks and reagents, especially if the thiol function is to be transient. The disulfide linkage is frequently used in oligonucleotide peptide conjugations (6) giving high yields, but for some applications there are concerns regarding the stability of the linkage which can be cleaved in a reducing environment. Formation of a thioether linkage is also a common method for making POCs in solution, typically by one oligomer containing a thiol and the other a maleimide derivative, which react giving decent yields of conjugates (6,12). An attractive solution synthesis approach uses Diels–Alder cycloaddition for linking oligonucleotides with peptides and results in high yields with slight excess of peptide, while it will result in isomeric products due to the linkage formed (13). A reaction that would seem ideal for conjugation, due to the compatibility with many other functional groups, is the copper (I) catalyzed 1,3-dipolar cycloaddition between an azide and an alkyne, commonly referred to as click chemistry (14–17). Recently, synthesis of a Tat-oligonucleotide conjugate was reported using this approach in solution. The conjugate was formed in acceptable yields using a high excess of copper sulfate (×100) and peptide analog (×10), and only an oligonucleotide with a 3′-azide linker gave product and not oligonucleotides with 5′-alkyne or 5′-azide linkers (18). Another example of a DNA–peptide conjugate was also prepared by liquid-phase conjugation, which involves purification of both peptide and oligonucleotide precursor, but at a concentration that makes it difficult to perform in submicromole scale (19).
Solution phase conjugation of highly cationic peptides can be problematic due to the tendency to form complexes with the anionic oligonucleotide phosphate backbones. The reaction mixture can even become highly heterogeneous due to precipitation and reduce the conjugation yields considerably. One way to avoid this problem, and at the same time gain simplicity and reduce number of purification steps, is to perform the fragment conjugation of oligonucleotides and peptides with one of the oligomers still protected and attached to the solid phase. A method for solid-phase fragment conjugation (SPFC) in preparation of POCs by formation of urea linkages has been developed and results in yields of 3–50% (20,21). Gait and co-workers have synthesized different types of POCs by SPFC methods using a variety of functional groups to link the peptide and oligonucleotide fragments either directly or using a suitable spacer between the fragments (22,23). The oligonucleotide is assembled on solid phase and modified phosphoramidite building blocks are used in this strategy. The methods above can be used for successful synthesis of POCs. Each method has various limitations in linking position on the oligonucleotide and in whether the peptide can be linked at the N or C terminal or both. Other restrictions are that several of the methods give moderate degrees of conversion and that in most cases some of the work requires specialists and access to peptide and/or oligonucleotide synthesizers.
Despite the existence of the above methods, it seemed to us that there was still a need for versatile and high yielding method for the synthesis of POCs. It would be preferable to perform the conjugation with either peptide or oligonucleotide on solid support in order to minimize the number of purification steps and to make the handling user friendly. A specific aim was also that it should be possible to carry out without the need for specific synthesizers, through limited manipulation of commercially available oligonucleotide and peptide derivatives. This would be a particular advantage for laboratories that perform experiments with such conjugates but that do not have access to automated synthesizers. It would also be ideal if the general approach would be suitable for conjugation of other biomolecules under mild conditions as well. Oligonucleotides still attached to the solid support can be ordered from most companies selling oligonucleotides, and peptides still attached to the support can also be ordered. It would of course not be a disadvantage if the chemistry involved in the linking gives high versatility, thus allowing for conjugation to many different kinds of modified oligonucleotides. It is also advantageous to be able to choose conjugation to either the N or C terminal of the peptide as this could play a role in recognition/accessibility of the functional peptide. Furthermore, it would be desirable to be able to obtain near quantitative conjugation in a submicromole scale, especially for libraries.
Here, we present a versatile method for the synthesis of multiple types of POCs that can be performed on solid support in submicromole scale with commercially available oligonucleotide and peptide derivatives. Convenient handling and efficient reaction at low concentration under mild RT conditions is enabled by use of a new activated triple bond donor and by performing reaction on solid support. Convenient and efficient conversion of peptides into clickable derivatives also contributes to making the concept user friendly.
MATERIAL AND METHODS
Acetonitrile (MeCN, High Performance Liquid Chromatography (HPLC) grade, Merck), methanol (MeOH) and dichloromethane (DCM, Fisher Scientific, Analytical Grade) were of commercial grade and used as received. Dimethylformamide (DMF) and pyridine (Pyr) (both from Merck and analytical grade) were additionally dried over 4A molecular sieves. Silica gel column chromatography was performed on Merck G60, Thin Layer Chromatography (TLC) analysis was carried out on pre-coated Silica Gel 60 F254 (Merck), with detection by UV light. NMR spectra were recorded on a Bruker AVANCE DRX-400 instrument (400.13 MHz for 1H, 162.00 MHz for 31P, 100.62 MHz for 13C). Reverse phase HPLC was carried out on a Jasco HPLC system using the following columns: Hypersil 5 μm (250 × 4.6 mm) with 1 ml/min flow rate; Kromasil 100-5-C18, 5 μm (250 × 4.6 mm) with 1 ml/min flow rate; and Kromasil 100-5-C18, 5 μm (250 × 10 mm) at 4 ml/min flow rate. Buffers for reverse phase chromatography were as follows: (i) 50 mM triethylammonium acetate (TEAA), pH 6.5; (ii) 50 mM TEAA, pH 6.5, in 50% CH3CN. Mass spectra (TOF-MS, ES) were obtained by using a Micromass LCT electrospray time-of-flight (ES-TOF) instrument. Molecular weights of the conjugates were reconstructed from the m/z values of the multiply charged ions using the mass deconvolution program (MAXENT) of the instrument, which gave values with no decimal place. Oligonucleotide sequences were purchased from: Sigma-Proligo Sweden, a locked nucleic acid (LNA) sequence GLGLALMeCLTLTLTL-Am5 (LNA-Am5); Eurogentech of Seraing, Belgium, dAGAGCCATGAG-C6 (DNA-C6); Cybergene Sweden, dAGAGCCATGAG-Am5 (DNA-Am5), Rasayan Inc., Encinitas, CA, USA, 2′-OMe-CCUCUUACCUCAGUUACA-ODMT [18-merRNA(2′-OMe)]. Nucleobase protecting groups were benzoyl for cytosine and adenine and isobutyryl for guanine. Solid-supported peptide GAAKRVKLD (C-myc) was obtained from Eurogentech of Seraing, Belgium. The amino acid sequence was assembled by standard Fmoc synthesis on Wang resin and delivered on support with standard acid-labile protecting groups: t-butyloxycarbonyl (Boc) for lysine, t-butyl ester (O-tBu) for aspartic acid and 2,2,4,6,7-pentamethyl-dihydrobenzofuran-5-sulfonyl (Pbf) for arginine. AcPLG (AcMIF1) was purchased from Bachem. PKKKRKVG (SV40) was from AnaSpec Inc, and was obtained fully deprotected but not purified.
Preparation of azide-peptides for C-terminal conjugation, e.g. AcMIF1-L1-azide (4)
The acetylated tripeptide AcPLG-COOH (100 mg, 0.3 mmol) was dissolved in 3 ml DMF containing 2 eq. of N-methylmorpholine (NMM) (67 μl). HBTU was added (1 eq., 116 mg) and the reaction was stirred at RT for 30 min. After this time, 2-azidoethylamine was added (0.95 eq., 25 mg) and the reaction was stirred overnight at RT. The solvent was then evaporated and the residue was dried by evaporation of added toluene and dichloromethane. The purification was done by silica gel column chromatography using a linear gradient of methanol in DCM from 0% to 6%. AcMIF1-L1-azide (4): Yield 45 mg, 38%. 1H-NMR (400 MHz, CDCl3), δ (ppm): 0.85 (3H, d, CH3 Leu, J = 6.2 Hz), 0.90 (3H, d, CH3 Leu), 1.59 (2H, m, CH Leu + 1H from CH2 Leu), 1.71 (1H, m, 1H from CH2 Leu), 2.00 (3H, m, CH2 Pro), 2.07 (3H, s, CH3, Ac from Ac-Pro), 2.20 (1H, m, from CH2 Pro), 3.39 (4H, bs, 2 × CH2, ethylene from azidoamine), 3.48 (1H, m, from CH2 Pro), 3.59 (1H, m, from CH2 Pro), 3.88 (2H, AB-q, CH2 Gly, J = 5.8 Hz, J = 11.0 Hz), 4.17 (1H, m, αCH Leu), 4.42 (1H, m, αCH Pro), 7.21 (2H, bs, 2 × NH, Leu + Gly) 13C-NMR (100.62 MHz, CDCl3), δ (ppm): 21.7 (CH3, Ac from Ac-Pro), 22.7 (CH3 Leu), 23.1 (CH3 Leu), 25.2 (CH Leu + CH2 Pro), 28.5 (CH2 Pro), 39.1 (CH2, ethylene from azidoamine), 40.6 (CH2 Leu), 43.4 (CH Gly), 48.7 (CH2 Pro), 50.5 (CH2, ethylene from azidoamine), 53.3 (CH Leu), 60.7 (CH Pro), 169,9 (C=O), 171.1 (C=O), 172.7 (C=O), 172.9 (C=O). ES–MS, calcd m/z (M-H)−1 394.2281, found 394.2279 (a similar procedure used for TFA-SV40-L1-azide and SV40-L1-azide is presented in Supplementary Data).
Preparation of azide-peptides for N-terminal conjugation on solid support: C-myc-L2-azide (7) and TFA-C-myc-L2-azide (8)
The peptide GAAKRVKLD 5 (0.035 mmol) still attached to solid support and with free N-terminus was placed in a sealed Eppendorf tube. In another sealed Eppendorf tube 2-(2-azidoethoxy)ethoxyacetic acid (L2, 3.3 eq.), 2-(1H-Benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU, 3.3 eq.) and NMM (6 eq.) in 350 μl of DMF were shaken for 0.5 h at RT. This solution was then added to the peptide on solid support. The tube was sealed and the reaction was agitated on a vortex for 2 h at RT. The tube was centrifuged, the solution was carefully removed with a syringe and 200 μl of DMF was added to the support. The Eppendorf tube was then agitated on a vortex, centrifuged and the solution above the solid support was removed. This washing was repeated 5 times. After this 500 μl of a solution containing 90% trifluoroacetic acid (TFA), 4% triisopropylsilane (TIS), 4% H2O and 2% 3,5-dioxo-1,8-octanedithiol was added and the suspension was agitated on a vortex for 4 h. The tube was centrifuged and the solution was carefully collected with a syringe. Another portion of the deblocking/support cleaving solution was added and the suspension was agitated and centrifuged whereupon the solution was collected with a syringe (this washing was repeated an additional two times). The combined solutions were collected in round-bottomed flask and TFA was removed by flushing air into the flask, whereupon water was added and the solution was washed with cold diethyl ether (repeated two times). The water layer was collected and concentrated in vacuo. Methanol was added and evaporated under reduced pressure whereupon the residue was dried under vacuum for 0.5 h (peptide 7). Peptide 7 was purified as detailed in (i). If protection of basic amino acids (e.g. lysines) is desired, the procedure indicated in (ii) was followed.
The peptide was dissolved in 50 mM triethylammonium acetate buffer, pH 6.5, containing 15% acetonitrile and then purified by RP HPLC using a linear gradient of buffer B in A from 15% to 27% in 30 min, detection at 220 nm. C-myc-L2-azide (7): tR = 15.7 min, yield = 60% (HPLC); ES–MS, calcd m/z (M-H)+1 1128.6, found 1128.8.
Methanol (5 ml) was added to form a suspension and the reaction was put on an ice bath. Ethyl trifluoroacetate (0.3 ml) was added, followed by 0.1 ml of triethylamine. The reaction was left stirring for 3 days slowly warming up to RT (melting ice bath). After the reaction was complete, the solvents are evaporated under reduced pressure and the peptide was dissolved in 50 mM TEAA buffer, pH 6.5, containing 15% acetonitrile and purified by RP HPLC using a linear gradient of buffer B in A from 30% to 100% in 30 min, detection at 220 nm. TFA-C-myc-L2-azide (8): tR = 16.8 min, yield = 44% (HPLC); ES–MS, calcd m/z (M-H)−11318.6, found 1318.6.
(N-Propynoylamino)-p-toluic acid (PATA, 11)
Propynoic acid (1 mmol, 61 μl) was added to stirred cold (ice bath) solution of dicyclohexylcarbodiimide (DCC, 1.2 eq., 247 mg) in dry acetonitrile (7 ml) under argon. After 10 min, 4-(aminomethyl)benzoic acid (1 eq., 151 mg) dissolved in 1.5 ml of acetonitrile was added dropwise and the resulting reaction mixture was stirred for 1 h at 0°C, allowed to warm up to RT and then reacted for an additional 1 h at this temperature. The solution was evaporated and the compound purified by column chromatography using a linear gradient of methanol in dichloromethane (0–10%). After collecting fractions containing the product, the solvent was evaporated under reduced pressure and the compound precipitated by the addition of dichloromethane. Pure 11 was collected by filtration, dried under vacuum and can be kept at −20°C for several weeks. Yield of 102 mg, 50%. 1H-NMR (400 MHz, DMSO d6), δ (ppm): 4.20 (1H, s, CH), 4.34 (2H, d, CH2, J = 6.1 Hz), 7.32 (2H, d, 2 × CH Ph, J = 8.1 Hz), 7.90 (2H, d, 2 × CH Ph, J = 8.1 Hz), 9.32 (1H, t, NH, J = 6.1 Hz), 13C-NMR (100.62 MHz, DMSO d6), δ (ppm): 42.1 (CH2), 76.3 (CH), 78.1 (CH), 127.1 (2 × CH Ph), 129.4 (2 × CH Ph), 142.9 (C=O), 151.8 (C=O) ES–MS, calcd m/z (M-H)−1 202.0582, found 202.0570.
Synthesis of POCs by conjugation of peptide-azides to solid-supported, aminolinker-carrying commercial oligonucleotides
Commercially available solid-supported 5′-aminolinker oligonucleotides were placed in sealed 500 μl Eppendorf tubes (1.5–3 mg support, 0.1 μmol in each tube). In another Eppendorf tube, HBTU (479 eq., 18 mg) and active linker PATA (500 eq., 10 mg) were dissolved in 560 μl of dry DMF containing 55 μl (4950 eq.) of NMM. The tube was sealed and placed horizontally on a vortex and shaken for 0.5 h, after which time a one-quarter of the solution was taken by syringe and added to the solid-supported oligonucleotide. The Eppendorf tubes were sealed, connected together by means of Parafilm and placed horizontally on a vortex, and then shaken for 2 h. After this time, the mixture was centrifuged, the solution was carefully removed from the solid support with a syringe and 200 μl of DMF was added to each tube. The tube was closed, centrifuged and washed with an additional portion of DMF; this process is repeated to have, in total, 10 washings with DMF and finally 2 with tBuOH/H2O (1:1). The protected peptide-azide TFA-C-myc-L2-azide (1.6 μmol, 2.1 mg) or Ac-MIF1-L2-azide (1.6 μmol, 0.63 mg) dissolved in 140 μl of tBuOH/H2O (1:1) and 35 μl of this peptide solution (4 eq., 0.4 μmol) was added to the supported oligonucleotide. To each reaction was added 5 μl of tBuOH/H2O (1:1) followed by sodium ascorbate solution [6 eq., 0.6 μmol, 5 μl from a stock solution of 24 mg/ml in tBuOH/H2O (1:1)], whereupon the tube was gently shaken and CuSO4 was added (2.4 eq., 0.24 μmol, 5 μl from a stock solution of 12 mg/ml in H2O). The Eppendorf tubes were sealed, connected together with Parafilm and horizontally shaken on the vortex over night at RT. After this time, the tube was centrifuged, the solution was carefully removed from the solid support with a syringe and the residue was washed two times with 200 µl of tBuOH/H2O (1:1) and two times with DMF. To each mixture was 500 μl of 28% NH3 (aq) added and the Eppendorf tube was sealed, agitated on a vortex for 1 min and left at 55°C over night. The tube was centrifuged and the solution was carefully collected with a syringe. Another portion of NH3 solution was added and the suspension agitated, centrifuged and the solution was collected. To the combined solutions, water was added (5 ml) and the solution with the POC was concentrated in vacuo. The reaction was analyzed and purified by RP-HPLC using a linear gradient of buffer B in A from 0% to 50% in 20 min (UV detection at 260 nm) at 50°C.
LNA-AcMIF1 (entry 1, Table 1): tR = 15.2 min, ES–MS, calcd (M) 3067, found 3066; DNA-Am5-AcMIF1 (entry 6, Table 1): tR = 16.4 min ES–MS, calcd (M) 4138, found 4138; LNA-Am5-C-myc (entries 2 and 3, Table 1): tR = 16.2 min; ES–MS, calcd (M) 3802, found 3803; DNA-Am5-C-myc (entries 7 and 8, Table 1): tR = 15.1 min; ES–MS, calcd (M) 4870, found 4870; DNA-C6-C-myc (entry 9, Table 1): tR = 16.3 min; ES–MS, calcd (M) 4882, found 4882; LNA-Am5-L2-azide (oligonucleotide with azide linker used in conjugate synthesis for entry 5, Table 1): tR = 15.0 min; ES–MS, calcd (M) 2659, found 2659; LNA-TFA-SV40 (non-isolated, entry 5, Table 1): ES–MS, calcd (M) 3680, found 3680.
Table 1.
Oligonucleotides, peptide derivatives and linkers used in synthesis of peptide-oligonucleotide conjugates (POCs)
| Entry | Oligonucleotide sequence 3′ → 5′ | Peptide derivative | Peptide terminus | Linker reagent (MMT protected) |
|---|---|---|---|---|
| 1 | GLGLALMeCLTL TLTL-Am5 (LNACPG-Am5) | AcMIF1-L1-azide (4) | C | Am5-amidite |
| 2 | GLGLALMeCLTL TLTL-Am5 (LNACPG-Am5) | C-myc-L2-azide (7) | N | Am5-amidite |
| 3 | GLGLALMeCLTL TLTL-Am5 (LNACPG-Am5) | TFA-C-myc-L2-azide (8) | N | Am5-amidite |
| 4 | GLGLALMeCLTL TLTL-Am5 (LNACPG-Am5) | TFA-SV40-L1-azide (3) | C | Am5-amidite |
| 5a | GLGLALMeCLTL TLTL-Am5- L2-azide, (LNACPG-Am5) | TFA-SV40-PATA (18)a | C | Am5-amidite |
| 6 | dAGAGCCATGAG-Am5 (DNAPS-Am5) | AcMIF1-L1-azide (4) | C | Am5-amidite |
| 7 | dAGAGCCATGAG-Am5 (DNAPS-Am5) | C-myc-L2-azide (7) | N | Am5-amidite |
| 8 | dAGAGCCATGAG-Am5 (DNAPS-Am5) | TFA-C-myc-L2-azide (8) | N | Am5-amidite |
| 9 | dAGAGCCATGAG-C6 (DNACPG-C6) | TFA-C-myc-L2-azide (8) | N | C6-amidite |
| 10 | (2′OMe)CCUCUUACCUCAGUUACA-Am5 (RNACPG) | AcMIF1-L1-azide (4) | C | Am5-H-phosphonate |
| 11 | (2′OMe)CCUCUUACCUCAGUUACA-Am5 (RNACPG) | TFA-C-myc-L2-azide (8) | N | Am5-H-phosphonate |
aSynthesis in solution (see Supplementary Data for details).
XL, locked nucleic acid monomer; dX, deoxynucleotides; (OMe)X, 2′-metoxy oligonucleotide. Commercial names of aminolinkers: Am5, amino modifier 5; C6, aminolinker C6; Ac, acetyl; MeC, 5-methyl cytosine; L1/L2, azide linkers (Scheme 1).
2-(N-(4-monomethoxytrityl)aminoethoxy)ethyl H-phosphonate triethylammonium salt (13)
To 2-(2-aminoethoxy)ethanol (2 mmol, 200 μl) dissolved in 20 ml of dry pyridine, triethylamine (2 mmol, 288 μl) was added, followed by monomethoxytrityl chloride (2.2 mmol, 679 mg). The reaction was stirred under argon atmosphere for 2 h (RT). After confirmation (by TLC with ninhydrine staining) of consumption of the starting material, diphenyl H-phosphonate was added to the reaction mixture (4 mmol, 780 μl). The reaction was left stirring for an additional 1 h. After confirmation that all monomethoxytritylated linker was consumed (TLC and MS), 5 ml of water was added to the reaction that was then allowed to stir overnight at RT. The reaction was then evaporated to dryness under reduced pressure, dissolved in dichloromethane and washed with a saturated aqueous solution of NaHCO3 (two times). The organic phase was collected, dried over MgSO4, concentrated and purified by column chromatography on silica using a gradient of methanol in dichloromethane (0–5%, with 0.3% triethylamine). Fractions containing 13 were collected and concentrated whereupon added dichloromethane was evaporated (three times) giving a pale orange oil. Yield 1 g, 92%. 1H-NMR (400 MHz, CDCl3), δ (ppm): 1.18 (9H, t, J = 7.3 Hz, CH3), 2.24 (2H, t, CH2, J = 5.4 Hz), 2.89 (6H, q, CH2, J = 7.3 Hz), 3.47 (2H, t, CH2, J = 5.0 Hz), 3.51 (2H, t, CH2, J = 5.2 Hz), 3.67 (3H, s, OCH3), 3.87 (2H, m), 6.01 (0.5H, s, 1/2PH, J = 614 Hz), 6.72 (4H, m, PhH), 7.07-7.38 (10H, m, PhH), 7.55 (0.5H, s, 1/2PH, J = 614 Hz), 31P-NMR (161.9 MHz, CDCl3) δ (ppm): 4.01, 7.86 (1P, dt, J = 614 Hz, J = 7.7 Hz), 13C-NMR (100.62 MHz, CDCl3), δ (ppm): 7.6 (CH3), 38.3 (CH2), 44.1 (CH2), 54.1 (OCH3), 61.6 (CH2), 69.1 (CH2), 69.5 (C), 70.1 (CH2), 112.1 (CH, Ph), 125.1 (CH, Ph), 126.8 (CH, Ph), 127.1 (CH, Ph), 127.5 (CH, Ph), 137.1 (CH, Ph), 145.2 (CH, Ph), 157.1 (CH, Ph), ES–MS, calcd m/z (M-H)−1 440.1785, found 439.9181.
2-(N-(2-(4-Biphenylyl)-prop-2-yl-oxycarbonyl)aminoethoxy)ethyl H-phosphonate triethylammonium salt (16)
2-(Biphenyl)prop-2-yl 4-methoxycarbonylphenyl carbonate (Bpoc reagent) (2 mmol, 780 mg) was dissolved in 10 ml of DMF containing 10% of triethylamine and placed in at 55°C in a silicon oil bath. A 1.1 equivalent of 2-(2-aminoethoxy)-ethanol (2.2 mmol, 220 μl) was added to the solution and the mixture was stirred for 3.5 h, after which the solvent was removed under reduced pressure. The resulting oil was diluted with H2O (20 ml), 5% NaHCO3 (2 ml) and extracted three times with DCM (3 × 20 ml). The aqueous phase was cooled in an ice bath and extracted once more with DCM (20 ml). The combined organic layers were dried over Na2SO4, filtered and concentrated in vacuo, to a pale yellow oil. The crude product was purified by column chromatography on silica using a mixture of DCM/MeOH/NEt3 (98:2:10). Fractions containing pure compound were collected and the solvent was evaporated under reduced pressure to give the desired product (15) in 87% yield. 1H-NMR (400 MHz, CDCl3), δ (ppm): 1.80 (6H, s, CH3), 3.32 (2H, m, CH2), 3.53 (2H, t, CH2, J = 4.5 Hz), 3.57 (2H, t, CH2, J = 4.5 Hz), 3.74 (2H, t, CH2, J = 4.6 Hz), 7.32 (1H, m, PhH), 7.40-7.45 (4H, m, PhH), 7.54-7.59 (4H, m, PhH); ES–MS, calcd m/z (M-H +Na+)−1 366.4074, found 366.4141.
One millimole of 15 (343 mg) was dissolved in 5 ml of 2 M H3PO3 solution in dry pyridine (10 mmol). The reaction mixture was stirred and pivaloyl chloride (PvCl; 675 μl, 5.5 mmol) was added slowly. The reaction was monitored, for disappearance of the starting alcohol and the formation of a baseline material, by TLC on silica gel plates using 10:1 DCM/MeOH as solvent. When the reaction was complete (after overnight at RT), 1 ml of 1 M triethylammonium bicarbonate (TEAB, pH 7.8) was added and the mixture was partitioned between 40 ml of DCM and 25 ml of 2 M TEAB (pH 7.8). The organic layer was dried over Na2SO4, filtered and concentrated in vacuo. The crude product was purified by column chromatography on silica using a mixture of DCM/MeOH/NEt3 (9:1:0.1). Fractions containing the desired product were combined and concentrated under reduced pressure to yield pure 16 as the triethylammonium salt in 88% yield. 1H-NMR (400 MHz, CDCl3), δ (ppm):1.27 (9H, t, CH3 (TEAH+), J = 7.3 Hz,), 1.80 (6H, s, CH3), 2.99 (6H, q, CH2 (TEAH+), J = 7.3 Hz), 3.30 (2H, q, CH2, J = 5.0 Hz), 3.54 (2H, t, CH2, J = 4.9 Hz), 3.66 (2H, t, CH2, J = 4.8 Hz), 4.04 (2H, m, CH2), 6.14 (0.5H, s, 1/2PH, J = 624 Hz), 7.33 (1H, m, PhH), 7.41-7.47 (4H, m, PhH), 7.55-7.60 (4H, m, PhH), 7.70 (0.5H, s, 1/2PH, J = 624 Hz); 31P-NMR (161.9 MHz, CDCl3) δ (ppm): 4.30, 8.13 (1P, dt, J = 620 Hz, J = 8.3 Hz); 13C-NMR (100.62 MHz, CDCl3), δ (ppm): 27.9 (CH3), 29.1 (CH3), 40.6 (CH2), 62.8 (CH2), 70.0 (CH2), 70.9 (CH2), 80.4 (C), 124.8, 127.0, 127.1, 128.7, 139.5, 140.9, 145.7, (C, CH, Ph), 155.4 (C=O). ES–MS, calcd m/z (M-H)−1 405.3870, found 405.9834.
General Method for synthesis of POCs on solid support by subsequent coupling of aminolinker H-phosphonate and PATA followed by Cu(I) catalyzed cycloaddition with peptide-azides
The oligonucleotide [52 mg (2 μmol) of controlled pore glass (CPG) supported 18-mer RNA(2′-OMe)] was placed in a syringe with a frit and then detritylated with 2% dichloroacetic acid (DCA) in DCM (two times 1 min) and subsequently washed with DCM (five times), MeCN (2 times) and pyridine. The protected (Bpoc or MMT)-aminolinker H-phosphonate was dissolved in anhydrous pyridine (62 mM, coupling solution) and added to the supported oligonucleotide. 1.1 eq. of pivaloyl chloride (PvCl) in acetonitrile was then added so that the final proportion of pyr/MeCN is 10:1 and the reaction mixture was slowly agitated at RT for 20 min. After this time, the coupling solution was removed and the support washed with pyridine. Two percent of I2 in pyridine/water (98:2) was added to the support which was left shaking for 15 min. The support was then washed extensively with pyridine (five times), MeCN (five times) and DCM (five times). The 5′-amino protecting group (MMT or Bpoc) was then removed by treatment with 2% DCA in DCM (two times for 2 min) and the support was washed with DCM (five times) and MeCN (two times). In a separate tube, compound 11 (PATA linker, 60 mg, 150 eq.) was dissolved in 2 ml of anhydrous DMF and mixed with HBTU (150 eq., 112 mg). NMM (400 μl, 1800 eq.) was added and the tube was agitated slowly on a vortex for 30 min at RT. This preactivation solution was then transferred to the support in the syringe which was then gently agitated on a vortex for 2 h at RT. The resulting solid supported alkyne-modified oligonucleotide was then washed with DMF (25 ml), MeCN (20 ml) and DCM (3 ml), dried and split in 0.2 μmol fractions to perform click reactions. Thus, 0.2 μmol of the supported 5′-alkyneoligonucleotide (5 mg resin) was placed in a small glass vial with a screw cap. The 4 eq. of peptide-azide (0.316 mg of Ac-MIF1-L2-azide or 1.1 mg of TFA-C-myc-L2-azide) was dissolved in 80 μl of tBuOH/H2O (1:1) and added into the vial. The 1 eq. of CuI in 10 μl of DMSO (from a stock solution of 4.3 mg/ml in DMSO) was added to the mixture followed by diisopropyl ethyl amine (DIPEA) solution [8 eq., 1.6 μmol, 10 μl from a stock solution of 28 μl in 1 ml of tBuOH/H2O (1:1)] and sodium ascorbate [3 eq., 0.6 μmol, 10 μl from a stock solution of 12 mg/ml in tBuOH/H2O (1:1)] whereupon the solution was gently agitated on a vortex at RT over night. The solution was then removed from the support and the resin was transferred to a syringe with a frit and rinsed with H2O (20 ml), 0.05 M ethylenediamine tetraacetate (EDTA) (aq, 20 ml), acetonitrile (10 ml) and DCM (3 ml). The support was transferred to an Eppendorf tube with a screw cap and 0.5 ml of 28% NH3 (aq) was added. The tube was then heated to and kept at 55°C over night. Purification was done by RP-HPLC using a linear gradient of buffer B in A from 0% to 64% for 20 min, detection at 260 nm and oven temperature set at 50°C.
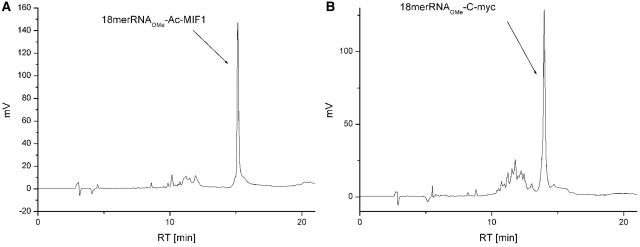
The 18-merRNAOMe-AcMIF1 (entry 10, Table 1): tR = 15.1 min, ES–MS, calcd (M) 6570, found 6570; 18-merRNAOMe-C-myc (entry 11, Table 1): tR = 14.0 min, ES–MS, calcd (M) 7306, found 7304.
RESULTS AND DISCUSSION
Clickable peptides
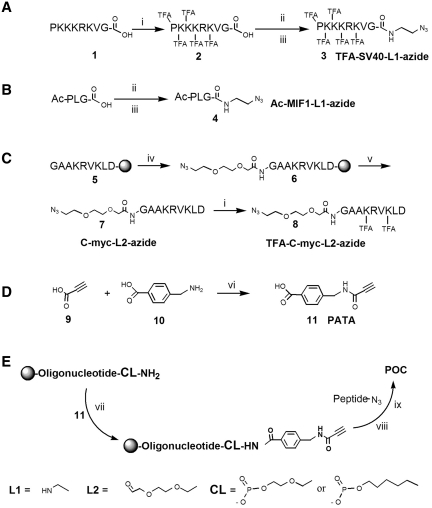
The first phase of working out the method was development of an efficient way, involving minimum manipulation, for conversion of peptides into clickable derivatives. Non-supported fully deprotected peptides can be purchased and easily converted into azido derivatives by intermediate TFA protection and subsequent attachment of an azide containing linker, as exemplified for the highly basic SV-40-derived NLS peptide (24) (Scheme 1A). Amino groups of peptide 1 were converted into trifluoroacetamides (2) and subsequent coupling to 2-azidoethylamine (linker L1-azide) was performed to obtain the desired peptide 3 (TFA-SV40-L1-azide), from which a deprotected derivative could also readily be made (25). In addition, a short non-charged tripeptide, Ac-MIF1 [N-acetylated PLG which is a blood–brain barrier penetrating peptide (26)], was similarly converted to the azide derivative 4 (Ac-MIF-L1-azide, Scheme 1B). An N-terminal azido derivatized [C-myc-L2-azide (7, Scheme 1C) less basic peptide (from the C-myc cell penetrating peptide (27)] could be readily made from a solid support bound peptide (5) by condensation with 2-(2-azidoethyl)ethoxyacetic acid (linker L2-azide) to give (6) followed by cleavage from support. Cold ether washing of the peptide removes most impurities and it can then be reacted with ethyl trifluoroacetate to form TFA-C-myc-L2-azide (8, Scheme 1C). If a more hydrophilic derivative is desired, the crude unprotected azidopeptide 7 can be directly HPLC purified and used for cycloaddition.
Scheme 1.
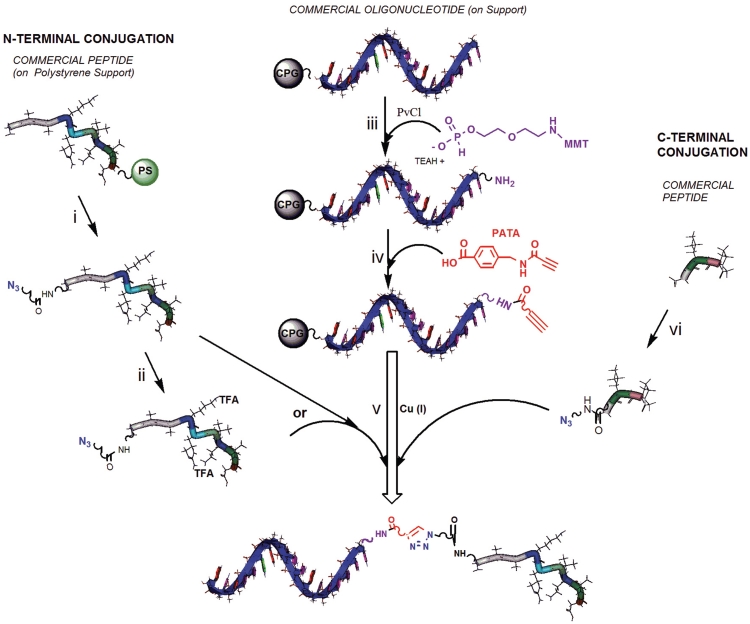
(A–C) Synthesis of azido-functionalized peptides (D) Synthesis of active alkyne linker, PATA. (E) Scheme for POC synthesis on solid support using commercial oligonucleotides with aminolinkers. Reagents and conditions: (i) ethyl trifluoroacetate, methanol, 3 days, RT; (ii) preactivation with HBTU and NMM in DMF 0.5 h, RT; (iii) azidoethylamine, 2 h, RT; (iv) 2-(2-azidoethoxy)ethoxyacetic acid (preactivated with HBTU and NMM in DMF, 0.5 h, RT), DMF, 2 h, RT; (v) 90% TFA, 4% TIS, 4% H2O, 2% 3,5-dioxo-1,8-octanedithiol, 4 h, RT; (vi) preactivation of 9 with DCC, in acetonitrile, 0°C, then addition of 10; (vii) preactivation of 11 with HBTU, and NMM, DMF, 0.5 h, RT, then addition to solid supported oligonucleotide 2 h; (viii) 1.2 eq. CuSO4, 3 eq. sodium ascorbate, 2 eq. of peptide-azide; overnight; (ix) NH3, (aq) sat. 55°C overnight (HBTU, O-benzotriazole-N,N,N′,N′-tetramethyl-uronium-hexafluoro-phosphate; azide, N3; Ac, acetyl; TIS, triisopropyl silane; TFA, trifluoroacetic acid).
POCs from commercial aminolinker oligonucleotides
Thus having ready access to peptide-azides, we attempted to synthesize POCs by Cu(I) catalyzed 1,3-dipolar cycloaddition of an oligonucleotide 4-pentynoic amide derivative, but this failed totally as no reaction was observed (Supplementary Data, Scheme 1). Non-catalyzed Huisgen 1,3-dipolar cycloaddition with an azide is accelerated by electron-withdrawing substituents on the dipolarophile (28,29) and it seems to be the case also for the Cu (I) catalyzed case (16,17). In view of the most recent mechanistic suggestions (30,31) and in particular the experimentally supported claim that the rate-limiting step involves bond formation between the copper acetylide and coordinated azide (31), this could also be expected. We found that activation of propynoic acid gave side reactions and colored byproducts. To increase the reaction rate of the click reaction but at the same time have a cleaner reaction when attaching it to amino groups, we developed a linker with an activated triple bond donor, α-(N-propynoylamino)-p-toluic acid (11, PATA, Scheme 1D). The electron withdrawing carbonyl of the amide group vicinal to the triple bond makes it considerably more reactive in the Cu(I) catalyzed cycloaddition. At the same time, this reagent has a handle to readily attach it to oligonucleotides carrying aminolinkers and is UV visible, which is convenient upon preparation and purification. This PATA-linker is straightforward to make from (Scheme 1D) propynoic acid (9) and aminomethylbenzoic acid (10). Some side products are formed but the yield after purification is quite acceptable and since the starting materials are inexpensive, the cost for the reagent is quite low. The PATA linker can be made in a relatively large scale (grams, RT and column chromatography) and is stable upon storage.
For the first studies on synthesis of POCs, we used DNA and LNA oligonucleotides, purchased still attached to CPG or polystyrene support (PS) and carrying either 2-(aminoethoxy)ethyl phosphate (aminomodifier Am5) or aminohexyl phosphate (aminomodifier C6) as aminolinkers (initially MMT protected). The PATA reagent was then coupled to the solid supported aminolinker oligonucleotide, and after washing and subsequent Cu(I) catalyzed cycloaddition under mild conditions with a peptide-azide the corresponding oligonucleotide peptide conjugate is formed (Scheme 1E).
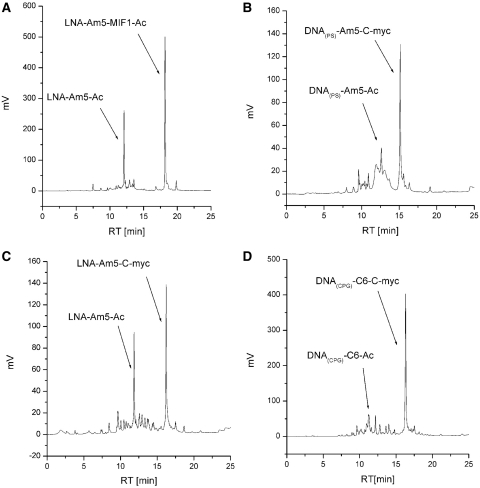
Conjugation reactions were initially performed with an azido derivative of the tripeptide MIF1 (AcMIF1-L1-azide, 4, Scheme 1B). Attachment of this peptide to solid-supported oligonucleotide LNA-Am5-PATA in 0.1 μmol scale (of oligonucleotide on support), using 2 equivalents of peptide at different dilutions (down to 2 mM of peptide which would correspond to 1 mM of oligonucleotide if this was in solution, Supplementary Data, Figure 1) in the presence of CuSO4/ascorbate gave conversion to the corresponding conjugate (Figure 1A). After conjugation of the PLG-tripeptide (Ac-MIF1) to CPG supported LNA, a series of additional POCs were produced (Table 1, entries 2–4, 6–9) using the same approach and conditions. With the MIF peptide, similar results were obtained upon conjugation to polystyrene-supported DNA (entry 6, Table 1). This indicates that although Cu(II)/ascorbate has been reported to cause oxidative damage and cleavage to DNA (32,33), this is not significant enough to pose any major problem in the synthesis, which could perhaps be expected since the active alkyne linker allows for relatively mild conditions at RT. Reaction of the same supported oligonucleotide sequences with an N-terminal linked peptide-azide from C-myc (C-myc-L2-azide, 7) was then investigated. With the LNACPG-Am5, conversion was good using the unprotected C-myc-L2-azide (entry 2, Table 1), but conjugation to a DNA fragment on PS gave, although still respectable, somewhat lower conversion (entry 7, Table 1). The free unprotected side chains of lysines could potentially interfere to some degree, by Michael conjugate addition to the active linker and perhaps the lower lipophilicity of the peptide (compared with AcMIF1-azide) could give less than ideal permeation of the solid support. Conjugation of the peptide with trifluoroacetyl-protected lysines (TFA-C-myc-L2-azide, 8) to the polystyrene-bound DNA also gave better conversion (Figure 1B, entry 8, Table 1). With this protected peptide good conversions to LNA and DNA conjugates on CPG was also obtained (Figure 1C and D, entries 3 and 9, Table 1). In the above conjugations, CuSO4 and sodium ascorbate were used. It has been reported that use of a Cu–TBTA complex (Cu-([tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine]) as catalyst can improve click reactions (34). However, little improvement was seen when the Cu(I)-stabilizing ligand TBTA was used in the reaction with DNA on polystyrene (Supplementary Data, Figure 2). It thus seems like there is not much benefit to use TBTA here, but it is not excluded that it could be beneficial for some conjugations not tested here. After deprotection and simultaneous cleavage from support [with conc. NH3 (aq) at 55°C overnight], the POCs were readily purified by RP-HPLC.
Figure 1.
HPLC chromatograms of crude POCs formed after addition of PATA and CuSO4/ascorbate catalyzed peptide attachment. (A) LNA-Am5-MIF1-Ac conjugate (entry 1, Table 1); (B) DNA(PS)-Am5-C-myc conjugate [entry 7, Table 1, DNA(PS) indicates that this POC was synthesized on PS]; (C) LNA-Am5-C-myc conjugate (entry 3, Table 1); (D) DNA(CPG)-C6-C-myc conjugate [entry 9, Table 1, DNA(CPG) indicates that this POC was synthesized on CPG support]. Reaction conditions: support with 0.1 μmol oligonucleotide, 4 eq. peptide sodium ascorbate solution 6 eq., 2.4 eq. CuSO4 in 50 μl tBuOH–water (45:55), overnight at RT {RP-HPLC: 0–50% linear gradient of B [0.05 M triethylammonium acetate (aqueous, pH = 6.5) in 50% acetonitrile (aq)] in A [0.05 M triethylammonium acetate (aqueous, pH = 6.5)] over 20 min, detection at 260 nm, at 50°C}.
Figure 2.
HPLC chromatograms of crude peptide oligonucleotide products synthesized according to Scheme 3 (coupling of H-phophonate linker, addition of PATA and CuI catalyzed peptide conjugation): (A) after performing click reaction of solid-supported oligonucleotide-PATA with azido-modified peptide AcMIF1-L1-azide in the presence of CuI/ascorbate/DIPEA. (B) After performing click reaction of solid-supported oligonucleotide-PATA with azido-modified peptide TFA-C-myc-L2-azide in the presence of CuI/ascorbate/DIPEA. Reaction conditions: support with 0.2 μmol oligonucleotide, 4 eq. peptide sodium ascorbate solution 3 eq., 1 eq. CuI in 100 μl tBuOH–Water–DMSO–DIPEA (43.5:43.5:10:3), overnight at RT {RP-HPLC: 0–80% linear gradient of B [0.05 M triethylammonium acetate (aqueous, pH = 6.5) in 50% acetonitrile (aq)] in A [0.05 M triethylammonium acetate (aqueous, pH = 6.5)] over 20 min, detection at 260 nm, at 50°C}.
When conjugates between polyanionic oligonucleotides and polycationic peptides are fully deprotected, problems with aggregation can occur but a solution has been suggested where a conjugate with a protected peptide is mixed with a complementary target strand and only then deprotected (35). Direct block coupling of TFA-protected TFA-SV40-L1-azide (3) with the solid-supported oligonucleotide LNA-Am5 (entry 4, Table 1) gave the desired conjugate (this was clearly the major product as evidenced by MS of the crude mixture after NH3 deprotection). Unfortunately, any handling that included evaporation, redissolving, freeze drying and filtering of the product resulted in severe aggregation, which made it difficult to isolate the deprotected conjugate by HPLC. In order to synthesize a POC with unprotected oligonucleotide but protected peptide, we developed a solution phase conjugation method between a protected peptide PATA derivative and 5′-azido-functionalized oligonucleotide (Supplementary Data). This is more cumbersome and gives more loss of material than the solid phase procedure, but it shows that PATA derivatives can be useful in solution conjugation as well.
Especially, in the solid-supported syntheses conjugates from the commercial amino-linked oligonucleotides gave the oligonucleotide with N-acetylated aminolinker as a side product, in varying amounts depending on the manufacturer. This side product has previously been reported, and avoided, either by removing one capping step and include morpholine washing when synthesizing POCs from 5′-aminonucleoside-containing oligonucleotides (8) or use of active esters when conjugating acridine derivatives (36). This is a separate problem, occurring before and not connected to the conjugation method per se, and the acetylated fraction will not react further. The presence of the side product did not affect purification since it did not overlap with starting material or products in the chromatograms. Although the above method is quick, convenient and suitable for use in many research laboratories, it may not always be acceptable with this loss of oligonucleotide, especially if considered for larger scale synthesis. Another aspect is that, when purchasing amino-linked oligonucleotides, a substantial part of the cost is due to the linker, while for laboratories that do not produce large volumes, it is more expensive with in-house synthesis of standard oligonucleotides than to purchase them from a commercial source. Taking this into account, we opted for an alternative approach for solid-phase conjugation of peptides to oligonucleotides which would not only lower the cost but also hoping that this would reduce the amount of acetylated side product. This is based on purchasing oligonucleotides on support and then attach an aminolinker manually followed by conjugation to the peptide, thus still enabling POC synthesis without the need to employ a DNA/RNA synthesizer.
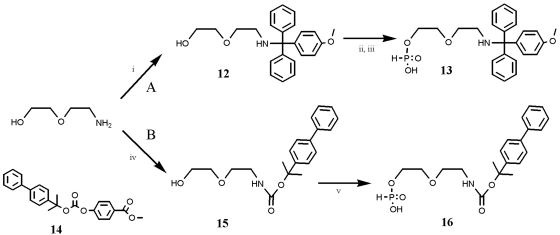
Aminolinker H-phosphonates
Since we have extensive practical experience in H-phosphonate handling and preparation, and because of which the monoesters are stable and can be stored for months without changes in quality of coupling reactions, it seemed natural to make linkers that would be attached with this chemistry (37–41). We synthesized protected 2-(aminoethoxy)ethyl linkers (commonly known as amino modifier 5, Am5) as H-phosphonate monoesters (Scheme 2). Two different N-protecting groups were chosen, the most commonly used monomethoxytrityl group and the 2-(biphenyl)-2-propyloxycarbonyl (Bpoc) previously used in online step wise POC synthesis (8). Both linkers are readily made, especially the MMT protected H-phosphonate 13, because it can be synthesized in a one-pot, three-step reaction [monomethoxytritylation in pyridine to 12 followed by addition of diphenyl-H-phosphonate and subsequent hydrolysis (42,43)]. The Bpoc derivative involves protection with 14 to give 15 and then phosphonylation with H-pyrophosphonate (42,43) to give 16. The two new linkers were used in an overall approach with subsequent attachment of H-phosphonate linker, PATA and peptide-azide (Scheme 3) whereby POCs were made from an 18-mer 2′-O-methyl RNA [a splice-correcting sequence we used in a luciferase assay to estimate nuclear delivery (44)].
Scheme 2.
Synthesis of 2-(2-aminoethoxy)-ethanol based 5′-amino modifiers for solid-supported oligonucleotides. (A) Synthesis of H-phosphonate of monomethoxytrityl protected amino modifier (15): (i) pyridine, monomethoxytrityl chloride, RT, triethylamine, 2 h; (ii) diphenyl-H-phosphonate, 1 h, RT; (iii) water, RT, overnight; (B) synthesis of H-phosphonate of 2-(p-biphenyl)-2-propyloxycarbonyl (Bpoc) protected amino modifier (18); (iv) DMF, Bpoc reagent, RT, triethylamine, 3.5 h; (v) 2 M H3PO3 and PvCl, pyridine, overnight, RT.
Scheme 3.
Schematic presentation of the synthesis of different POCs by ‘click’ reaction on solid support. (i) (a). N-terminal functionalization of peptide with linker L2-azide: pre-activation of L2-azide with HBTU, and NMM, DMF, 0.5 h, RT, then addition to peptide 2 h; (b). deprotection from solid support with trifluoroacetic acid: 90% TFA, 4% TIS, 4% H2O, 2% 3,5-dioxo-1,8-octanedithiol, 4 h, RT; (ii) trifluoroacetyl (TFA) protection of amino groups: ethyl trifluoroacetate, methanol, 3 days, RT; (iii) functionalization of solid-supported oligonucleotide with Am5: 1, compound 15, pyridine, PvCl, 20 min, RT, 2, I2/H2O/pyridine; (iv) functionalization of amino-linked oligonucleotide with the active alkyne linker: preactivation of PATA (11) with HBTU, and NMM, DMF, 0.5 h, RT, then addition to solid-supported oligonucleotide 2 h; (v) CuI [3 + 2] dipolar cycloaddition of oligonucleotide PATA with a peptide-azide derivative 8 or 4; (vi) preactivation of peptide with HBTU and NMM in DMF 0.5 h, RT, and then addition of azidoethylamine, 2 h, RT (HBTU = O-benzotriazole-N,N,N′,N′-tetramethyl-uronium-hexafluoro-phosphate; azide, N3; Ac, acetyl; TIS, triisopropyl silane; PvCl, pivaloyl chloride; TFA, trifluoroacetic acid).
POC synthesis from supported oligonucleotides, with ‘in house’ attachment of aminolinker
The oligonucleotide was purchased with 5′-monomethoxytrityl group on and attached to the solid support (CPG). The MMT was removed just before coupling with H-phosphonate linkers 13 or 16 in the presence of pivaloyl chloride whereupon the H-phosphonate was oxidized with iodine in pyridine water. The PATA linker was then attached as before whereupon the peptide-azide was conjugated by Cu (I) catalyzed cycloaddition. Although results with both linkers are comparable, a slightly better HPLC profile was obtained with the MMT protected linker, which then was chosen for synthesis of the POCs. For the solid-supported conjugation of the 2′-OMeRNA to the Ac-MIF-L1-azide peptide (entry 10, Table 1), we also compared a few conditions for the ‘click reaction’ using different copper (I) and (II) salts (CuI, CuI/ascorbate, CuCl and CuSO4/ascorbate as above). One equivalent of copper catalyst was used in all cases and the results were nearly comparable (Supplementary Figure S5) but with somewhat better results for CuI/ascorbate (Figure 2A). We then also prepared the C-myc 2′-OMeRNA conjugate (Figure 2B, entry 11, Table 1) using the MMT protected H-phosphonate 13 and the TFA-C-myc-L2-azide (7) with CuI/ascorbate according to same overall scheme (Scheme 3). When preparing conjugates with the ‘in house’ attachment of the different H-phosphonate linkers, we can see no clear presence of N-acetylated product. We cannot completely exclude that N-acetylated side product could be present in minor amounts as well as it is possible that the presence of this side product when using purchased aminolinker oligonucleotides is dependent on other factors than the linker used. Nevertheless, there is clearly not much N-acylation occurring when following the procedure in Scheme 3.
The overall procedure, as performed here, involves transfers of the solid support between different containers. If this is done with milligram amounts of support, it will result in some material loss. Then in addition, there is a typical loss occurring upon HPLC purification (especially if this is performed by multiple injections). The loss upon HPLC purification is exemplified by comparing the UV absorbance of the crude non-conjugated 2′-OMeRNA after removal from support with NH3 with that of the HPLC-purified isolated non-conjugated 2′-OMeRNA. Doing this (with 5 mg support) gave 53% isolated non-conjugated oligonucleotide, which is not untypical after HPLC purification. To investigate what the isolated yield would be in the worst possible scenario of material transfer and conversion, synthesis of the C-myc 2′-OMeRNA POC was performed in milligram scale (as described above and depicted in Scheme 3, after which 77% of the mass of support remained). This gave an overall isolated yield of 27% of HPLC-purified POC after all reaction steps, transfers and HPLC purification. Judging from the HPLC profile, we could expect a higher percentage for the MIF conjugates. This is then to be compared with the 53% obtained of HPLC-isolated non-conjugated oligonucleotide.
Residual amounts of copper ions
The use of an activated alkyne in combination with solid-phase conjugation enables the use of just one equivalent of copper salt, which makes the possible contamination of the product with this toxic metal ion less of an issue. A simple calculation reveals that even if all copper (equimolar amounts) used in the reaction remains with the POC, this would be at the maximum limit for drinking water at ∼30 µM concentration of POC. For most applications, the POC would be used in considerably lower concentration and would be non-toxic for human intake. However, there may be situations where even low amounts of copper ions could have an influence, e.g. when conjugates to chelating agents are prepared such as those used in oligonucleotide-based artificial nucleases (45–47) and PNAzymes (48,49). An estimation of copper ions in the crude product (entry 17 in Supplementary Data) gives that after cleavage from support, the POC has largely retained the copper [about 85–90% of the concentration expected if all copper added during reaction remained with the POC (the theoretical max concentration)]. However, an additional advantage of performing the ‘click’ reaction on solid support is that the support can be conveniently washed with EDTA solution after conjugation but before cleavage of POC from support, which gave a substantial reduction in copper ion concentration [to ∼20% of the theoretical max concentration and at the same level as the drinking water (at a POC concentration of 30 µM) that we have in the Stockholm area]. This level (∼1 μM Cu if diluting the POC to 4 μM concentaration) should be sufficiently low, even for most applications involving oligonucleotide chelates. It is certainly low enough for human intake and should probably not, in general, cause acute toxicity for cell experiments where 3 μM hardly affected astrocytes (50) and concentrations up to 100 μM did not affect the growth of HeLa cells (51). However, effects on the metabolism of cells can be found for low micromolar concentrations (50,51) which suggest that caution should be exerted, especially if a higher concentration of the POC is to be used. Thus, if an ultra low concentration is needed for some particular application, it may be advisable to include more extensive EDTA washing and perhaps evaluate the use of lower amounts of Cu (I) than used here.
CONCLUSIONS
Alternative methods for the synthesis of POCs by use of ‘click chemistry’ enhanced by the PATA moiety have been developed. LNA conjugates with the N-terminal of a C-myc-derived peptide as well as with the C-terminal of the MIF peptide were prepared on solid support (CPG). DNA–peptide conjugates with the C-myc peptide were made on both polystyrene and CPG supports. Apart from the oligoether aminolinker (aminomodifier Am5), an aminohexylphosphate linker (aminolinker C6) was also used.
Peptides made by standard Fmoc synthesis can be purchased on solid support [e.g. the acid-labile Wang resin which gives release of carboxylic acid at the C-terminus or Rink amide resin to obtain a C-terminal amide (for N-terminal conjugation only)]. This makes N-terminal attachment of an azide-containing linker a convenient procedure which just involves coupling of the linker to the support-bound peptide, washing and then removal from the support. There is also no apparent limitation with respect to amino acid sequence for this N-terminal attachment approach, and lysines can be subsequently protected for increased lipophilicity. Peptides containing hydroxyl functions, e.g. serine, threonine and tyrosine have not been included in this study, but it is unlikely that these would pose any major problems. Hydroxyls would not be expected to be protected using ethyl trifluoroacetate in methanol and there should also be no need for protection of these in the click reaction. For C-terminal attachment, non-supported fully deprotected peptides can be readily converted into both azide and activated alkyne derivatives by intermediate TFA protection (one would expect that hydroxyl containing amino acids can be incorporated also in this case since the activated carboxyl should react preferentially with the aminolinker and if necessary the coupling could be performed in water). The peptide should preferably have a C-terminal glycine or other non-chiral linker to avoid racemization upon coupling of the peptide to the linker.
Although the approach with use of a commercial aminolinker oligonucleotide is a convenient and viable approach, that in some cases gave acceptably low levels of concomitant N-acetylation, we would consider the overall approach in Scheme 3 to be the preferred method. A variety of oligonucleotides, including modified ones, can be purchased on solid support (with or without a number of different aminolinkers) but are in general more available and at a lower cost without the aminolinker. Both the H-phosphonate aminolinker and the PATA reagent are readily prepared, made from inexpensive materials and stable upon storage. Subsequent coupling of these two reagents to a solid-supported oligonucleotide occur in high yields and when used together in the overall approach, the N-acetylated side product is not observed. This may be due to these aminolinkers, but we cannot exclude that the approach of attaching the linker and PATA consecutively at the same occasion with minimal time in between is a major reason as well. We consider the overall conversion from oligonucleotide to POC to be excellent, especially since there is no need to isolate and purify any oligonucleotide intermediate (neither aminolinked nor the alkyne containing one) but only the final POC.
The use of an activated alkyne enables submicromolar scale synthesis and cycloaddition with a peptide-azide leads to the oligonucleotide conjugate using mild conditions and only one equivalent of copper. This, in combination with solid-phase conjugation, gives very low levels of copper contamination, which makes this method particularly suitable for the synthesis of molecules aimed at biological applications. The approach makes it possible to readily make both N- and C-terminal conjugation of peptides to oligonucleotides. In this study, we have prepared oligonucleotide 5′-conjugates, which is the most common type of construct. Although use of the PATA linker and peptide-azides is in principle not limited to this, it would require ‘in house’ synthesis or special order of oligonucleotides with additional functionalities if conjugation to other positions on solid support is desired. Nevertheless, the developed methodology enables preparation of POCs without access to machine-assisted synthesis and this should be possible without much experience in oligonucleotide or peptide chemistry. In addition, the methodology should be highly suitable for the parallel synthesis of libraries of POCs in which either or both components are varied. Use of the 1,3-dipolar cycloaddition with an activated alkyne should also make the approach suitable for conjugation with other biomolecules or labels, thus enabling the use of a single type of conjugation method in the synthesis of different conjugates.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Swedish Research Council, VINNOVA and European Council (FP7:ITN-2008-238679). Funding for open access charge: VINNOVA and Swedish Research Council.
Conflict of interest statement. None declared.
Supplementary Material
REFERENCES
- 1.Akhtar S, Hughes MD, Khan A, Bibby M, Hussain M, Nawaz Q, Double J, Sayyed P. The delivery of antisense therapeutics. Adv. Drug Deliv. Rev. 2000;44:3–21. doi: 10.1016/s0169-409x(00)00080-6. [DOI] [PubMed] [Google Scholar]
- 2.Lundin P, Johansson H, Guterstam P, Holm T, Hansen M, Langel Ã, El Andaloussi S. Distinct uptake routes of cell-penetrating peptide conjugates. Bioconjug. Chem. 2008;19:2535–2542. doi: 10.1021/bc800212j. [DOI] [PubMed] [Google Scholar]
- 3.Lebleu B, Moulton HM, Abes R, Ivanova GD, Abes S, Stein DA, Iversen PL, Arzumanov AA, Gait MJ. Cell penetrating peptide conjugates of steric block oligonucleotides. Adv. Drug Deliv. Rev. 2008;60:517–529. doi: 10.1016/j.addr.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frederic H, May Catherine M, Gilles D. Twenty years of cell-penetrating peptides: from molecular mechanisms to therapeutics. Br. J. Pharmacol. 2009;157:195–206. doi: 10.1111/j.1476-5381.2009.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jearawiriyapaisarn N, Moulton HM, Buckley B, Roberts J, Sazani P, Fucharoen S, Iversen PL, Kole R. Sustained dystrophin expression induced by peptide-conjugated morpholino oligomers in the muscles of mdx mice. Mol. Ther. 2008;16:1624–1629. doi: 10.1038/mt.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Venkatesan N, Kim BH. Peptide conjugates of oligonucleotides: synthesis and applications. Chem. Rev. 2006;106:3712–3761. doi: 10.1021/cr0502448. [DOI] [PubMed] [Google Scholar]
- 7.Lu K, Duan Q-P, Ma L, Zhao D-X. Chemical strategies for the synthesis of peptide-oligonucleotide conjugates. Bioconjug. Chem. 2010;21:187–202. doi: 10.1021/bc900158s. [DOI] [PubMed] [Google Scholar]
- 8.Zaramella S, Yeheskiely E, Strömberg R. A method for solid-phase synthesis of oligonucleotide 5′-peptide-conjugates using acid-labile alpha-amino protections. J. Am. Chem. Soc. 2004;126:14029–14035. doi: 10.1021/ja046945o. [DOI] [PubMed] [Google Scholar]
- 9.Ocampo SM, Albericio F, Fernandez I, Vilaseca M, Eritja R. A straightforward synthesis of 5′-peptide oligonucleotide conjugates using N-alpha-Fmoc-protected amino acids. Org. Lett. 2005;7:4349–4352. doi: 10.1021/ol0514698. [DOI] [PubMed] [Google Scholar]
- 10.Stetsenko DA, Gait MJ. New phosphoramidite reagents for the synthesis of oligonucleotides containing a cysteine residue useful in peptide conjugation. Nucleosides Nucleotides Nucleic Acids. 2000;19:1751–1764. doi: 10.1080/15257770008045457. [DOI] [PubMed] [Google Scholar]
- 11.Stetsenko DA, Gait MJ. Efficient conjugation of peptides to oligonucleotides by “native ligation”. J. Org. Chem. 2000;65:4900–4908. doi: 10.1021/jo000214z. [DOI] [PubMed] [Google Scholar]
- 12.Tung CH, Rudolph MJ, Stein S. Preparation of oligonucleotide-peptide conjugates. Bioconjug. Chem. 1991;2:464–465. doi: 10.1021/bc00012a015. [DOI] [PubMed] [Google Scholar]
- 13.Marchan V, Ortega S, Pulido D, Pedroso E, Grandas A. Diels-Alder cycloadditions in water for the straightforward preparation of peptide-oligonucleotide conjugates. Nucleic Acids Res. 2006;34:e24/1–e24/9. doi: 10.1093/nar/gnj020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolb HC, Finn MG, Sharpless KB. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 15.Kolb HC, Sharpless KB. The growing impact of click chemistry on drug discovery. Drug Discov. Today. 2003;8:1128–1137. doi: 10.1016/s1359-6446(03)02933-7. [DOI] [PubMed] [Google Scholar]
- 16.Meldal M, Tornoe CW. Cu-catalyzed azide-alkyne cycloaddition. Chem. Rev. 2008;108:2952–3015. doi: 10.1021/cr0783479. [DOI] [PubMed] [Google Scholar]
- 17.Tornoe CW, Christensen C, Meldal M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 18.Brown SD, Graham D. Conjugation of an oligonucleotide to Tat, a cell-penetrating peptide, via click chemistry. Tet. Lett. 2010;51:5032–5034. [Google Scholar]
- 19.Gogoi K, Mane MV, Kunte SS, Kumar VA. A versatile method for the preparation of conjugates of peptides with DNA/PNA/analog by employing chemo-selective click reaction in water. Nucleic Acids Res. 2007;35:e139. doi: 10.1093/nar/gkm935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kubo T, Zhelev Z, Rumiana B, Ohba H, Doi K, Fujii M. Controlled intracellular localization and enhanced antisense effect of oligonucleotides by chemical conjugation. Org. Biomol. Chem. 2005;3:3257–3259. doi: 10.1039/b507691a. [DOI] [PubMed] [Google Scholar]
- 21.Diala I, Osada A, Maruoka S, Imanisi T, Murao S, Ato T, Ohba H, Fujii M. Synthesis of phosphorothioate oligonucleotide-peptide conjugates by solid phase fragment condensation. Bioorg. Med. Chem. Lett. 2007;17:6576–6578. doi: 10.1016/j.bmcl.2007.09.101. [DOI] [PubMed] [Google Scholar]
- 22.Stetsenko DA, Gait MJA. Convenient solid-phase method for synthesis of 3′-conjugates of oligonucleotides. Bioconjug. Chem. 2001;12:576–586. doi: 10.1021/bc000157g. [DOI] [PubMed] [Google Scholar]
- 23.Kachalova A, Zubin E, Stetsenko D, Gait M, Oretskaya T. Oligonucleotides with 2′-O-carboxymethyl group: synthesis and 2'-conjugation via amide bond formation on solid phase. Org. Biomol. Chem. 2004;2:2793–2797. doi: 10.1039/B409496D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adam SA, Lobl TJ, Mitchell MA, Gerace L. Identification of specific binding proteins for a nuclear location sequence. Nature. 1989;337:276–279. doi: 10.1038/337276a0. [DOI] [PubMed] [Google Scholar]
- 25.Steunenberg P, Wenska M, Strömberg R. Conversion of commercial peptides into clickable derivatives for labeling and conjugate synthesis. Nat. Protocols. 2010 doi: 10.1038/nprot.2010.94. [Google Scholar]
- 26.Kastin AJ, Pan W, Maness LM, Banks WA. Peptides crossing the blood-brain barrier: some unusual observations. Brain Res. 1999;848:96–100. doi: 10.1016/s0006-8993(99)01961-7. [DOI] [PubMed] [Google Scholar]
- 27.Saphire ACS, Bark SJ, Gerace L. All four homochiral enantiomers of a nuclear localization sequence derived from c-Myc serve as functional import signals. J. Biol. Chem. 1998;273:29764–29769. doi: 10.1074/jbc.273.45.29764. [DOI] [PubMed] [Google Scholar]
- 28.Huisgen R, Szeimies G, Möbius L. 1.3-Dipolare Cycloadditionen, XXXII. Kinetik der Additionen organischer Azide an CC-Mehrfachbindungen. Chem. Ber. 1967;100:2494–2507. [Google Scholar]
- 29.Sustmann R. Orbital energy control of cycloaddition reactivity. Pure Appl. Chem. 1974;40:569–593. [Google Scholar]
- 30.Hein JE, Fokin VV. Copper-catalyzed azide–alkyne cycloaddition (CuAAC) and beyond: new reactivity of copper(I) acetylides. Chem. Soc. Rev. 2010;39:1302–1315. doi: 10.1039/b904091a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun S, Wu P. Mechanistic insights into Cu(I)-catalyzed azide-alkyne “click” cycloaddition monitored by real time infrared spectroscopy. J. Phys. Chem. A. 2010;114:8331–8336. doi: 10.1021/jp105034m. [DOI] [PubMed] [Google Scholar]
- 32.Chiou S-H. DNA- and protein-scission activities of ascorbate in the presence of copper ion and a copper-peptide complex. J. Biochem. 1983;94:1259–1267. doi: 10.1093/oxfordjournals.jbchem.a134471. [DOI] [PubMed] [Google Scholar]
- 33.Zareie MH, Erdem G, Öner C, Öner R, Ögus A, Piskin E. Investigation of ascorbate-Cu(II) induced cleavage of DNA by scanning tunneling microscopy. Int. J. Biol. Macromol. 1996;19:69–73. doi: 10.1016/0141-8130(96)01103-8. [DOI] [PubMed] [Google Scholar]
- 34.Ustinov AV, Dubnyakova VV, Korshun VA. A convenient “click chemistry” approach to perylene diimide-oligonucleotide conjugates. Tetrahedron. 2008;64:1467–1473. doi: 10.1080/15257770701490936. [DOI] [PubMed] [Google Scholar]
- 35.Fraley AW, Pons B, Dalkara D, Nullans G, Behr J-P, Zuber G. Cationic oligonucleotide peptide conjugates with aggregating properties enter efficiently into cells while maintaining hybridization properties and enzymatic recognition. J. Am. Chem. Soc. 2006;128:10763–10771. doi: 10.1021/ja060873e. [DOI] [PubMed] [Google Scholar]
- 36.Casals J, Debethune L, Alvarez K, Risitano A, Fox KR, Grandas A, Pedroso E. Directing quadruplex-stabilizing drugs to the telomere: synthesis and properties of acridine - Oligonucleotide conjugates. Bioconjug. Chem. 2006;17:1351–1359. doi: 10.1021/bc060194t. [DOI] [PubMed] [Google Scholar]
- 37.Garegg PJ, Regberg T, Stawinski J, Strömberg R. Formation of inter-nucleotidic bonds via phosphonate intermediates. Chem. Scr. 1985;25:280–282. [Google Scholar]
- 38.Garegg PJ, Regberg T, Stawinski J, Strömberg R. Nucleoside hydrogen-phosphonates in oligonucleotide synthesis. Chem. Scr. 1986;26:59–62. [Google Scholar]
- 39.Garegg PJ, Stawinski J, Strömberg R. Nucleoside 3′-H-phosphonates. 8. Activation of hydrogen phosphonate monoesters by chlorophosphates and arenesulfonyl derivatives. J. Org. Chem. 1987;52:284–287. [Google Scholar]
- 40.Regberg T, Stawinski J, Strömberg R. Nucleoside H-phosphonates. IX. Possible side-reactions during hydrogen phosphonate diester formation. Nucleosides Nucleotides. 1988;7:23–35. [Google Scholar]
- 41.Sigurdsson S, Strömberg R. The H-phosphonate approach to oligonucleotide synthesis. An investigation on the mechanism of the coupling step. J. Chem. Soc., Perkin Trans. 2002;2:1682–1688. [Google Scholar]
- 42.Stawinski J, Strömberg R. Deoxyribo- and ribonucleoside H-phosphonates. Curr. Protocols Nucleic Acid Chem. 2001;1:2.6.1–2.6.15. doi: 10.1002/0471142700.nc0206s04. [DOI] [PubMed] [Google Scholar]
- 43.Stawinski J, Strömberg R. Di- and oligonucleotide synthesis using H-phosphonate chemistry. Methods Mol. Biol. 2005;288:81–100. [PubMed] [Google Scholar]
- 44.Moreno P, Wenska M, Lundin K, Wrange Ö, Strömberg R, Smith E. A synthetic snRNA m3G-CAP enhances nuclear delivery of exogenous proteins and nucleic acids. Nucleic Acids Res. 2009;37:1925–1935. doi: 10.1093/nar/gkp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Åström H, Williams NH, Strömberg R. Oligonucleotide based artificial nuclease (OBAN) systems. Bulge size dependence and positioning of catalytic group in cleavage of RNA-bulges. Org. Biomol. Chem. 2003;1:1461–1465. doi: 10.1039/b212216b. [DOI] [PubMed] [Google Scholar]
- 46.Åström H, Strömberg R. Synthesis of new OBAN's and further studies on positioning of the catalytic group. Org. Biomol. Chem. 2004;2:1901–1907. doi: 10.1039/b403652b. [DOI] [PubMed] [Google Scholar]
- 47.Murtola M, Strömberg R. 2'-O-methyloligoribonucleotide based artificial nucleases (2′-O-MeOBANs) cleaving a model of the leukemia related M-BCR/ABL m-RNA. ARKIVOC. 2008;2009:84–94. [Google Scholar]
- 48.Murtola M, Strömberg R. PNA based artificial nucleases displaying catalysis with turnover in the cleavage of a leukemia related RNA model. Org. Biomol. Chem. 2008;6:3837–3842. doi: 10.1039/b810106j. [DOI] [PubMed] [Google Scholar]
- 49.Murtola M, Wenska M, Strömberg R. PNAzymes that are artificial RNA restriction enzymes. J. Am. Chem. Soc. 2010;132:8984–8990. doi: 10.1021/ja1008739. [DOI] [PubMed] [Google Scholar]
- 50.Scheiber IF, Schmidt MM, Dringen R. Zinc prevents the copper-induced damage of cultured astrocytes. Neurochem. Int. 2010;57:314–322. doi: 10.1016/j.neuint.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 51.Hultberg B, Andersson A, Isaksson A. Copper ions differ from other thiol reactive metal ions in their effects on the concentration and redox status of thiols in HeLa cell cultures. Toxicology. 1997;117:89–97. doi: 10.1016/s0300-483x(96)03554-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.