Abstract
T30177 is a G-rich oligonucleotide with the sequence (GTGGTGGGTGGGTGGGT) which inhibits the HIV-1 integrase activity at nanomolar concentrations. Here we show that this DNA sequence forms in K+ solution a dimeric G-quadruplex structure comprising a total of six G-tetrad layers through the stacking of two propeller-type parallel-stranded G-quadruplex subunits at their 5′-end. All twelve guanines in the sequence participate in the G-tetrad formation, despite the interruption in the first G-tract by a thymine, which forms a bulge between two adjacent G-tetrads. In this work, we also propose a simple analytical approach to stoichiometry determination using concentration-dependent melting curves.
INTRODUTION
G-quadruplexes (1–5) are four-stranded structures formed by G-rich nucleic acid sequences. Formation of G-quadruplexes can affect a wide range of biological activities including telomere maintenance, gene regulation, transcription, DNA replication and repair (6). G-quadruplexes formed by synthetic oligonucleotides may possess anticancer (7–9) and anti-HIV (10–20) activity.
The basic unit of a G-quadruplex is a G-tetrad, a cyclic planar arrangement of four guanines linked by hydrogen bonds (21). The stability of a G-quadruplex depends on the stacking between different G-tetrads as well as electrostatic interaction mediated by cations, such as K+ or Na+, which are located at the center of the G-tetrad core. In most of the G-quadruplex structures reported so far, the G-tetrad core is formed by tracts of consecutive guanines (1–5). Formation of bulges has been observed in a G-quadruplex, where G-tracts are interrupted by non-guanine residues (22).
A series of G-rich DNA oligonucleotides (12–17) and their backbone-modified counterparts (11,12,23–25) have been reported to inhibit the activity of HIV-1 integrase (IN), an enzyme which is responsible for the integration of viral DNA into host genome (26). These include T30695 (or T30923) with the sequence (GGGTGGGTGGGTGGGT) (14,15) and T30177 (or I100-15) with the sequence (GTGGTGGGTGGGTGGGT) (11–13). These sequences were proposed to adopt a chair-type antiparallel-stranded G-quadruplex with two G-tetrads and three edgewise (T-G) loops (12,14,27). However, this proposed folding topology is at variance with the reported CD profile showing a positive peak at 260 nm (14,28), which is characteristic of a parallel-stranded G-quadruplex (29), and the mass spectrometry detection of a dimer for these sequences (28,30). Furthermore, a related G-rich oligonucleotide 93del was determined by NMR to form an interlocked dimer comprising two parallel-stranded G-quadruplex subunits (17); sequences with GGG tracts separated by single-residue linkers were shown to form stable parallel-stranded G-quadruplexes in both experimental (31–37) and computational (33,38) studies.
In the accompanying paper (39), the T30695 oligonucleotide with the sequence (GGGTGGGTGGGTGGGT) was determined to form a dimeric structure by the stacking of two propeller-type parallel-stranded G-quadruplex subunits, in which all G-tracts participate in the G-tetrad core formation. In this work, we investigate the structure of the G-rich oligonucleotide HIV-1 integrase inhibitor T30177, which differs from T30695 by the presence of a T residue that interrupts the first G-tract. Our results showed that T30177 forms a stacked dimeric G-quadruplex structure containing bulges. The knowledge of these structures might help us to uncover their inhibition mechanism against HIV-1 integrase. This work used a wide range of biophysical techniques including gel electrophoresis, CD, UV and NMR spectroscopy. We also propose a simple analytical approach to stoichiometry determination using concentration-dependent melting curves.
MATERIALS AND METHODS
Sample preparation
Unlabeled and site-specific labeled DNA oligonucleotides were chemically synthesized using products from Glen Research and Cambridge Isotope Laboratories. Samples were purified following Glen Research's protocol and then dialyzed successively against KCl solution and water. DNA oligonucleotides were dissolved in solution containing 70 mM potassium chloride and 20 mM potassium phosphate (pH 7.0). DNA concentration was expressed in strand molarity using a nearest-neighbor approximation for the absorption coefficients of the unfolded species (40).
NMR spectroscopy
NMR experiments were performed on 600 and 700 MHz NMR Bruker spectrometers. Guanine resonances were assigned by using site-specific 15N and 2H labeling (41,42) and through-bond correlations at natural abundance (43). Spectral assignments were completed by using NOESY, COSY, TOCSY and HSQC spectra (44). G-quadruplex folding topology was determined based on interproton distances obtained from NOESY experiments.
Gel electrophoresis
Electrophoresis experiment was performed at 120 V on native gels containing 20% polyacrylamide (Acrylamide: Bis-acrylamide = 37.5:1) in TBE buffer (89 mM Tris–borate, 2 mM EDTA, pH 8.3) supplemented with 3 mM KCl. Each sample contained 5 µg DNA. Gel was viewed by UV shadowing.
Circular dichroism
CD spectra were recorded on a JASCO-815 spectropolarimeter using 1 cm path-length quartz cuvette in a reaction volume of 600 µl at 20°C. Scans from 220 to 320 nm were performed with 200 nm/min, 1-nm pitch and 1-nm bandwidth. DNA concentration was 4–6 µM.
UV melting experiments
The stability of the G-quadruplex structure is measured in UV melting experiments conducted on a JASCO V-650 spectrophotometer. Absorbance at 295 nm was recorded as a function of temperature ranging from 30 to 90°C. The heating and cooling rates were 0.2°C/min. Experiments were performed with quartz cuvettes, with 1-cm path length. DNA concentration ranged from 0.5 to 200 µM. Solution contained 70 mM (or 30 mM) KCl and 20 mM potassium phosphate (pH 7.0).
RESULTS AND DISCUSSION
T30177 and T30177-I11 form G-quadruplexes in K+ solution
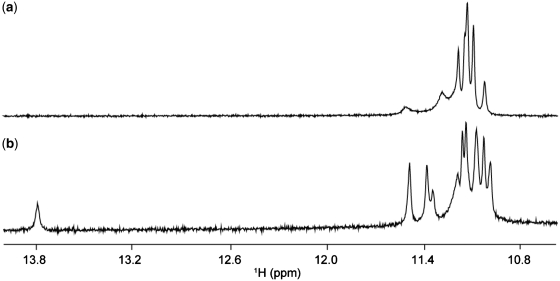
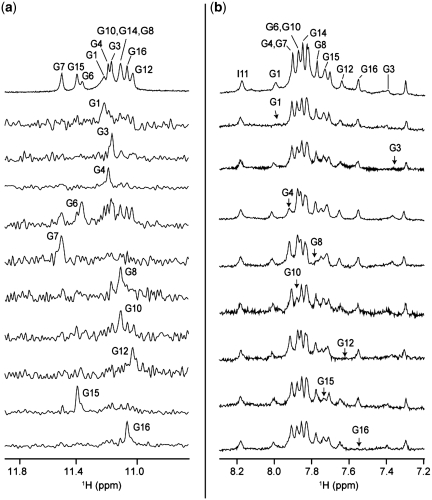
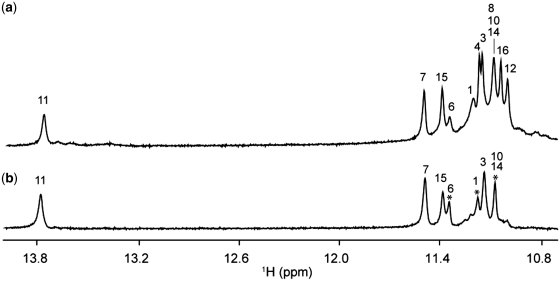
One-dimensional imino proton spectrum of T30177 in K+ solution (Figure 1a), similar to the one reported earlier (12), shows peaks in the range of 10.8–11.5 ppm, indicating the formation of a G-quadruplex. However, the heavy overlapping of these peaks makes further structural analysis difficult. This could be due to the structural symmetry, which arises from the repetitive nature of the sequence. We found that the DNA sequence with a single guanine-to-inosine substitution at position 11 showed greatly improved NMR spectra, and selected this sequence (henceforth designated as T30177-I11, Table 1) for further structural analysis. Similar spectral behavior was also observed (Supplementary Figure S1) for many different DNA sequences containing a single guanine-to-inosine substitution (Supplementary Table S1).
Figure 1.
Imino proton NMR spectra of (a) T30177 and (b) T30177-I11 in K+ solution at 25°C.
Table 1.
DNA sequences used in this work
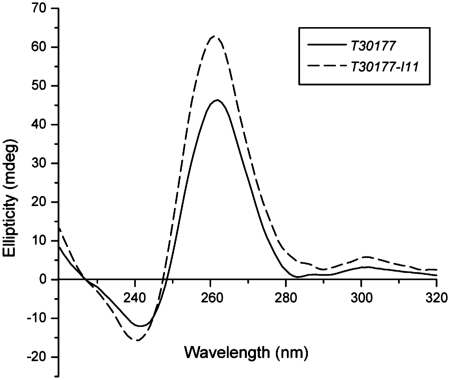
Imino proton spectrum of T30177-I11 in K+ solution (Figure 1b) shows peaks at 10.8–11.5 ppm corresponding to eleven guanine imino protons and an additional peak at 13.8 ppm corresponding to the imino proton of the inosine. This indicates the involvement of all 12 guanine and inosine bases in the G-tetrad formation, in contrast to only eight guanines for the previously proposed structure (12). Both T30177 and T30177-I11 give similar CD spectra (Figure 2) with a positive peak at 260 nm, which is characteristic of parallel-stranded G-quadruplexes (29).
Figure 2.
A simple method for stoichiometry determination: T30177-I11 forms a dimeric G-quadruplex
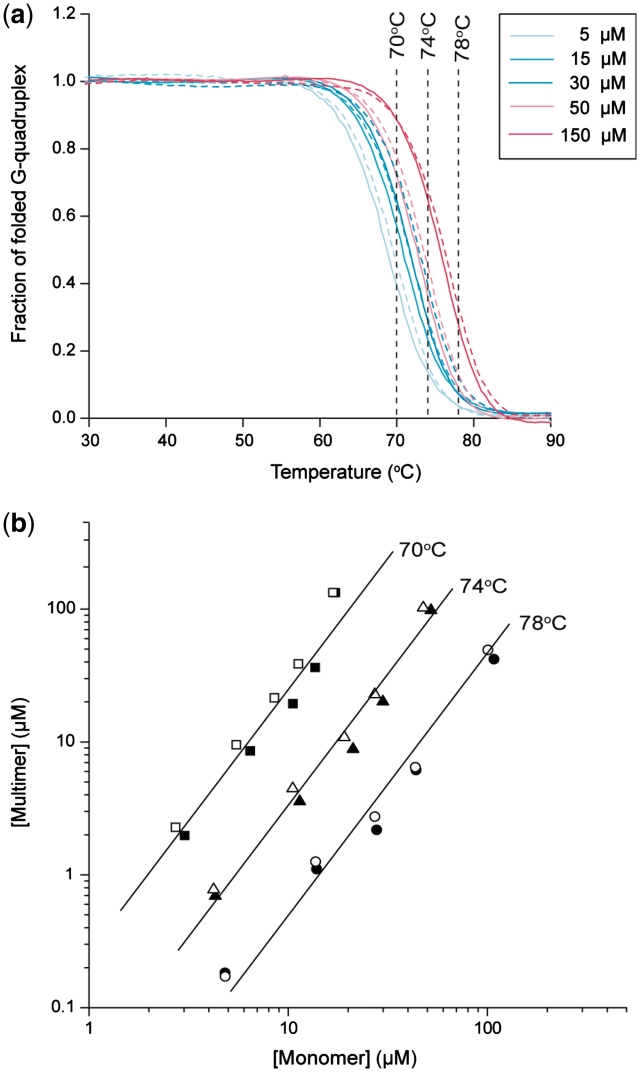
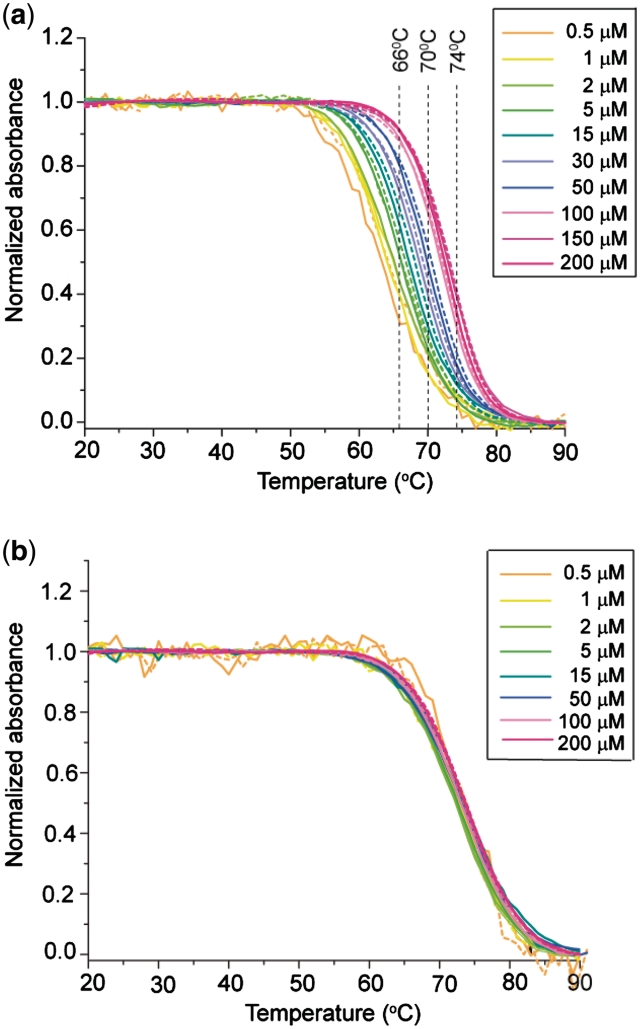
The temperature-driven folding/unfolding of T30177-I11 was monitored by the UV absorbance at 295 nm (45) (Figures 3a and 4a). The 295-nm absorbance decreased with an increase of temperature, as typically observed for G-quadruplexes. The heating and cooling curves were almost superimposed indicating near equilibrium processes. The melting temperature (Tm) was dependent on DNA concentration: in ∼100 mM K+ solution, Tm increased from 68 to 76°C when the DNA concentration increased from 5 to 150 µM (Figure 3a); in ∼60 mM K+ solution, Tm increased from 63 to 73°C when the DNA concentration increased from 0.5 to 200 µM (Figure 4a). These results indicated the formation of a multimeric G-quadruplex.
Figure 3.
UV-melting data of T30177-I11. (a) Cooling and heating curves for a particular DNA concentration are shown in one color, with continuous and dashed lines respectively. Color codes are shown at the top right corner. (b) Plots of multimer concentrations against monomer concentrations at three different temperatures. Data were obtained from the melting curves in (a) with filled and open points corresponding to cooling and heating processes, respectively. Lines of slope 2 are drawn through the points. Samples contained 70 mM KCl and 20 mM potassium phosphate (pH 7.0).
Figure 4.
UV-melting data of (a) T30177-I11 and (b) T30177-I11-TT. Cooling and heating curves for a particular DNA concentration are shown in one color, with continuous and dashed lines respectively. Color codes are shown at the top right corner. Samples contained 30 mM KCl and 20 mM potassium phosphate (pH 7.0).
Consider the equilibrium between a monomer and a multimer (n-mer):
where [C] and [Cn] are the concentrations of the monomer and the n-mer respectively. The equilibrium concentrations of the two species are related via the dissociation constant (Kd) by:
The plot of the concentration of the n-mer against the concentration of the monomer in log scales should be a straight line with the slope n (44).
Here we propose a simple method to quantitatively analyze the concentration-dependent melting curves for stoichiometry determination. For a given temperature where the structural transition was observed (e.g. 70, 74 and 78°C, Figure 3a), concentrations of the folded and unfolded species were determined for each melting curve. The concentration of the folded species (n-mer) was plotted against the concentration of the unfolded species (monomer) in log scales (Figure 3b, Supplementary Figures S2, S3). The slope 2 would fit well the data points in ∼100 mM K+ solution at each temperature (Figure 3b) [the slope of the best fits ranged from 1.74 to 2.16 (Supplementary Figure S2)], indicating the formation of a dimer by T30177-I11. At 70°C, the dissociation constant Kd of this dimer is ∼10 µM in this condition.
Quantitative analysis of concentration-dependent melting curves for stoichiometry determination has been previously reported (46,47). However, the proposed analysis was relatively complicated: the authors theoretically derived the analytical expression for the variation of Tm as a function of DNA concentration, i.e. using the interception points of a horizontal line at 0.5 with the melting curves; furthermore, measurements of the enthalpy (ΔH) associated with the transition were required for the stoichiometry determination. In contrast, our approach used interception points of vertical lines with the melting curves, and the analysis was quite simple. To our knowledge, this approach is being used here for the first time. We should note that the application of both approaches for quantitative analysis of concentration-dependent melting curves requires the assumption that these melting curves are equilibrium processes for a transition between two states: one folded and one unfolded. In practice, the equilibrium transitions can be achieved when the interconversion between these states is fast compared to the heating/cooling rate of the spectrometer.
The described analysis is based on the assumption that the observed changes in the UV absorbance reflect the multimer–monomer transition. However, in the present example (see structure below) the changes in the 295-nm UV absorbance only directly reflect the G-quadruplex unfolding, which in turn may depend on the dimer–monomer transition. In the given example, at very low DNA concentrations, monomeric intramolecular G-quadruplex can exist at a higher abundance. This can explain why the observed data points for low DNA concentrations (∼1 µM) deviated from the slope 2 towards a shallower slope (Supplementary Figure S3).
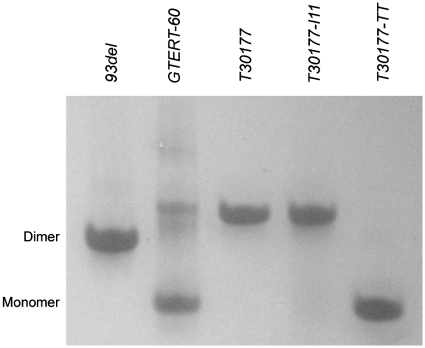
Gel electrophoresis (Figure 5) showed that the migration of T30177 and T30177-I11 was similar to that of the interlocked dimeric G-quadruplex 93del comprising six G-tetrad layers (17) and slower than that of a monomeric parallel-stranded propeller-type G-quadruplex GTERT-60 with three tetrad layers (48), consistent with the formation of a dimeric G-quadruplex by T30177 and T30177-I11. This result was corroborated by NMR data (discussion below).
Figure 5.
Gel electrophoresis analysis of T30177 and its derivatives. Reference markers are 93del (d[GGGTGGGAGGAGGGT]), an interlocked dimeric G-quadruplex (17) and GTERT-60 (d[AGGGIAGGGGCTGGGAGGGC]), a monomeric propeller-type G-quadruplex (48). T30177-TT has two additional thymines at the 5′-end as compared to T30177.
NMR spectral assignments of T30177-I11
The downfield-shifted peak at 13.8 ppm was assigned to the imino proton of I11 (49). Guanine imino protons were unambiguously assigned to their respective positions in the sequence (Figure 6a) using the site-specific low-enrichment approach (41), in which one guanine at a time was 15N-labeled at 2% (Supplementary Table S2). These assignments further confirmed that all guanines and inosine in the sequence participated in G-tetrad formation. Guanine H8 protons were assigned independently by site-specific 2H substitutions at the H8 position of guanines one at a time (42,50) (Supplementary Table S2), which led to the disappearance of a single peak corresponding to the substituted guanine (Figure 6b).
Figure 6.
Resonance assignments of T30177-I11 (a) Imino proton reference spectrum (top) with assignments indicated over the spectrum. Below are the 15N-filtered spectra of samples, 2% 15N labeled at the indicated position. (b) Aromatic reference spectrum (top) with assignments indicated over the spectrum. Below are the individual spectra, where the H8 protons are replaced by deuterium (2H) at the indicated positions.
Determination of folding topology: T30177-I11 forms a stacked dimeric G-quadruplex
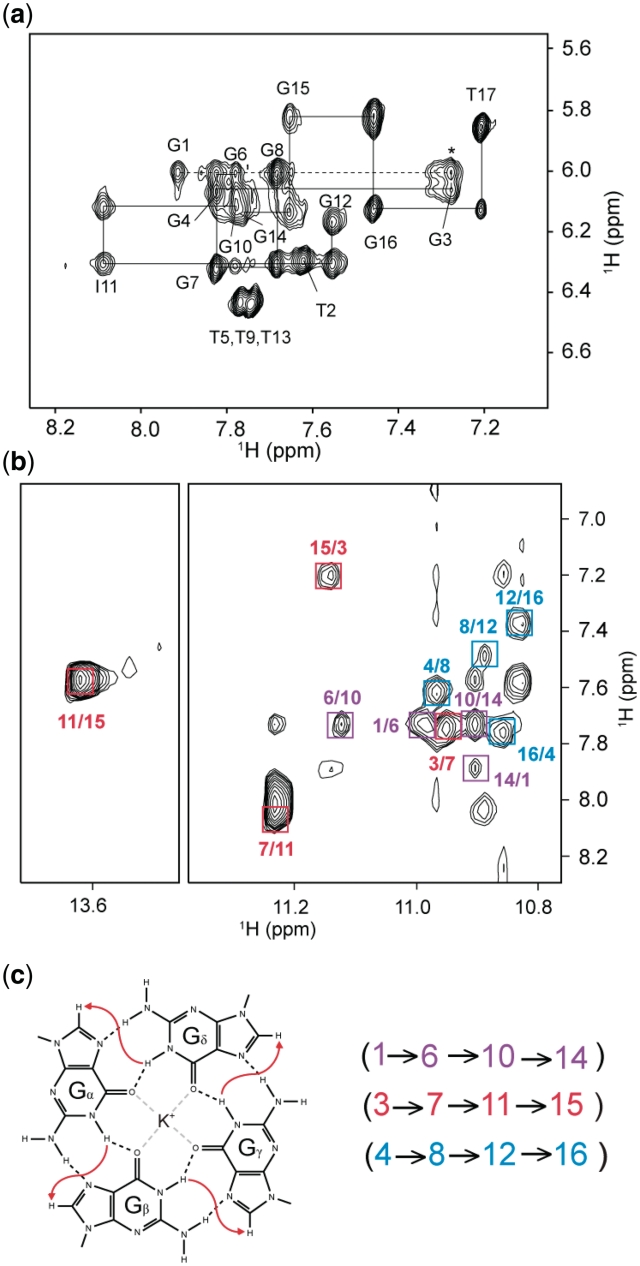
Using the complete assignments of imino (H1) and H8 protons, the G-tetrad alignments were determined from NOESY spectra based on the specific imino-H8 connectivities within a G-tetrad (Figure 7). For example, we observed NOE cross-peaks between G4(H1) and G8(H8), G8(H1) and G12(H8), G12(H1) and G16(H8), and G16(H1) and G4(H8), which established the formation of the (G4•G8•G12•G16) tetrad. In the same manner, we determined the arrangements of (G3•G7•I11•G15) and (G1•G6•G10•G14) tetrads (Figure 7c).
Figure 7.
(a) NOESY spectrum (mixing time, 200 ms) showing the H8/H6-H1′ connectivity of T30177-I11 in K+ solution. The assignments and H8/6-H1′ NOE sequential connectivities are shown. Intra-residue cross-peaks are labeled with the corresponding residue numbers. NOE connectivity between G1 and G3 across the bulge T2 is indicated with a dashed line; The cross-peak between G1(H1′) and G3(H8) is labeled with an asterisk. (b) NOESY spectrum (mixing time, 200 ms) showing the imino-H8 connectivity around different G-tetrads. The characteristic guanine imino-H8 cross-peaks for G-tetrads are framed and labeled with the imino proton assignment in the first position and that of the H8 proton in the second position. The residues in different G-tetrads are indicated by different colors. (c) Specific imino-H8 connectivity pattern around a Gα•Gβ•Gγ•Gδ tetrad indicated with arrows. Characteristic guanine imino-H8 NOE connectivities observed for G1•G6•G10•G14 (purple), G3•G7•I11•G15 (red), and G4•G8•G12•G16 (blue) tetrads.
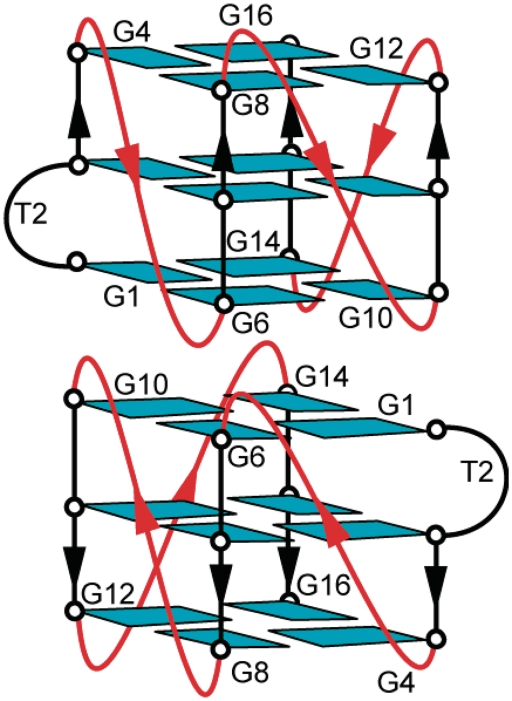
Figure 8 shows a dimeric folding topology for T30177-I11 that satisfies the established alignments of the three G-tetrads. This is a dimeric G-quadruplex comprising two identical subunits of propeller-type parallel-stranded G-quadruplexes each containing three G-tetrad layers, three double-chain-reversal loops (T5, T9 and T13) and a bulge (T2). The two subunits are stacked at their 5′-end; there could be various isomers, where the two subunits are rotated with respect to each other about the common central helical axis. However, the broadening of peaks at the interface (see below) and the symmetric nature of the structure prevented us from definite determination of the orientation and the detailed structure of the stacking interface. The stacking mode shown in Figure 8 was proposed on the basis of the stacked dimeric structure of the homologue sequence T30695 (39).
Figure 8.
Schematic structure of the G-quadruplex adopted by T30177-I11 in K+ solution.
This folding topology is consistent with the results of a solvent-exchange experiment showing that imino protons belonging to the central (G3•G7•I11•G15) and the 5′-end (G1•G6•G10•G14) tetrads are the most protected (Figure 9). The glycosidic conformations of all residues are anti, as shown by the moderate intensities of H1′-H8/6 NOE cross-peaks (Figure 7a), consistent with the formation of a parallel-stranded G-quadruplex (29). NOE cross-peaks between G1 and G3 (Figure 7a) indicated continuous stacking between these bases across the bulge (T2). Note that there might be a motion at the bulge as indicated by the broadening of the H8 proton of G3. The structure of a parallel-stranded G-quadruplex of T30177 with T2 being looped out from the G-tetrad core was recently reported to be stable in a molecular dynamics simulation (38).
Figure 9.
Imino proton spectra of T30177-I11 (a) in H2O and (b) after 13.5 h in 2H2O. Imino proton peaks belonging to the central G-tetrad and the G-tetrad at the stacking dimeric interface are protected from the exchange with solvent. Peaks at the interface are labeled with asterisks.
Dimeric interface: motion and control of stacking between the monomers
In this section, we describe the nature and stability of the dimeric interface where the stacking between two monomers occurs. Different NMR spectra provided evidence for considerable motion in this region. We observed the broadening of imino protons corresponding to guanines 1, 6, 10 and 14 (Figure 9 and data not shown). Broadening was also observed for some non-exchangeable protons of these residues (Figure 7a). Since all these guanines are located at the dimeric interface, this clearly establishes motion in this region. Possible types of motion include inter-conversion between dimer and monomer or rotation of two subunits about the central axis.
We could remove the stacking between the two G-quadruplex monomers by the addition of two extra thymine bases at the 5′-end (sequence T30177-TT, Table 1). Gel electrophoresis experiments (Figure 5) clearly showed the difference between two structures: the monomer (T30177-TT) migrated much faster than the dimer (T30177 or T30177-I11). The monomeric nature of T30177-TT and T30177-I11-TT were supported by the independence of their melting temperature on the DNA concentration (ranging from 0.5 to 200 µM) (Figure 4b and data not shown). Our unpublished NMR data confirmed that T30177-TT forms a monomeric propeller-type parallel-stranded G-quadruplex in this condition.
CONCLUSION
G-rich oligonucleotide T30177 (or T30177-I11) forms a dimeric structure involving two subunits of propeller-type parallel-stranded G-quadruplexes, which are stacked at their 5′-end. All guanines in the sequence participate in G-tetrad formation and there is a bulge of a T residue in each subunit. This work along with other structural studies (39). pointed to the formation of dimeric parallel-stranded G-quadruplexes comprising a total of six G-tetrad layers by various G-rich oligonucleotides that possess HIV-1 integrase inhibition activity.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Singapore Ministry of Education grants (ARC30/07 and RG62/07); Singapore Biomedical Research Council (grant 07/1/22/19/542 to A.T.P.). Funding for open access charge: Waived by Oxford University Press.
Conflict of interest statement. None declared.
Supplementary Material
REFERENCES
- 1.Davis JT. G-quartets 40 years later: from 5'-GMP to molecular biology and supramolecular chemistry. Angew. Chem. Int. Ed. Engl. 2004;43:668–698. doi: 10.1002/anie.200300589. [DOI] [PubMed] [Google Scholar]
- 2.Phan AT, Kuryavyi V, Patel DJ. DNA architecture: from G to Z. Curr. Opin. Struct. Biol. 2006;16:288–298. doi: 10.1016/j.sbi.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burge S, Parkinson GN, Hazel P, Todd AK, Neidle S. Quadruplex DNA: sequence, topology and structure. Nucleic Acids Res. 2006;34:5402–5415. doi: 10.1093/nar/gkl655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel DJ, Phan AT, Kuryavyi V. Human telomere, oncogenic promoter and 5'-UTR G-quadruplexes: diverse higher order DNA and RNA targets for cancer therapeutics. Nucleic Acids Res. 2007;35:7429–7455. doi: 10.1093/nar/gkm711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neidle S. The structures of quadruplex nucleic acids and their drug complexes. Curr. Opin. Struct. Biol. 2009;19:239–250. doi: 10.1016/j.sbi.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Maizels N. Dynamic roles for G4 DNA in the biology of eukaryotic cells. Nat. Struct. Mol. Biol. 2006;13:1055–1059. doi: 10.1038/nsmb1171. [DOI] [PubMed] [Google Scholar]
- 7.Bates PJ, Kahlon JB, Thomas SD, Trent JO, Miller DM. Antiproliferative activity of G-rich oligonucleotides correlates with protein binding. J. Biol. Chem. 1999;274:26369–26377. doi: 10.1074/jbc.274.37.26369. [DOI] [PubMed] [Google Scholar]
- 8.Jing N, Li Y, Xiong W, Sha W, Jing L, Tweardy DJ. G-quartet oligonucleotides: a new class of signal transducer and activator of transcription 3 inhibitors that suppresses growth of prostate and breast tumors through induction of apoptosis. Cancer Res. 2004;64:6603–6609. doi: 10.1158/0008-5472.CAN-03-4041. [DOI] [PubMed] [Google Scholar]
- 9.Qi H, Lin C-P, Fu X, Wood LM, Liu AA, Tsai Y-C, Chen Y, Barbieri CM, Pilch DS, Liu LF. G-quadruplexes induce apoptosis in tumor cells. Cancer Res. 2006;66:11808–11816. doi: 10.1158/0008-5472.CAN-06-1225. [DOI] [PubMed] [Google Scholar]
- 10.Wyatt JR, Vickers TA, Roberson JL, Buckheit RW, Klimkait T, DeBaets E, Davis PW, Rayner B, Imbach JL, Ecker DJ. Combinatorially selected guanosine-quartet structure is a potent inhibitor of human immunodeficiency virus envelope-mediated cell fusion. Proc. Natl Acad. Sci. USA. 1994;91:1356–1360. doi: 10.1073/pnas.91.4.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ojwang JO, Buckheit RW, Pommier Y, Mazumder A, De Vreese K, Este JA, Reymen D, Pallansch LA, Lackman-Smith C, Wallace TL, et al. T30177, an oligonucleotide stabilized by an intramolecular guanosine octet, is a potent inhibitor of laboratory strains and clinical isolates of human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 1995;39:2426–2435. doi: 10.1128/aac.39.11.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rando RF, Ojwang J, Elbaggari A, Reyes GR, Tinder R, McGrath MS, Hogan ME. Suppression of human immunodeficiency virus type 1 activity in vitro by oligonucleotides which form intramolecular tetrads. J. Biol. Chem. 1995;270:1754–1760. doi: 10.1074/jbc.270.4.1754. [DOI] [PubMed] [Google Scholar]
- 13.Mazumder A, Neamati N, Ojwang JO, Sunder S, Rando RF, Pommier Y. Inhibition of the human immunodeficiency virus type 1 integrase by guanosine quartet structures. Biochemistry. 1996;35:13762–13771. doi: 10.1021/bi960541u. [DOI] [PubMed] [Google Scholar]
- 14.Jing N, Rando RF, Pommier Y, Hogan ME. Ion selective folding of loop domains in a potent anti-HIV oligonucleotide. Biochemistry. 1997;36:12498–12505. doi: 10.1021/bi962798y. [DOI] [PubMed] [Google Scholar]
- 15.Jing NJ, Marchand C, Liu J, Mitra R, Hogan ME, Pommier Y. Mechanism of inhibition of HIV-1 integrase by G-tetrad-forming oligonucleotides in vitro. J. Biol. Chem. 2000;275:21460–21467. doi: 10.1074/jbc.M001436200. [DOI] [PubMed] [Google Scholar]
- 16.de Soultrait VR, Lozach PY, Altmeyer R, Tarrago-Litvak L, Litvak S, Andréola ML. DNA aptamers derived from HIV-1 RNase H inhibitors are strong anti-integrase agents. J. Mol. Biol. 2002;324:195–203. doi: 10.1016/s0022-2836(02)01064-1. [DOI] [PubMed] [Google Scholar]
- 17.Phan AT, Kuryavyi V, Ma JB, Faure A, Andreola ML, Patel DJ. An interlocked dimeric parallel-stranded DNA quadruplex: a potent inhibitor of HIV-1 integrase. Proc. Natl Acad. Sci. USA. 2005;102:634–639. doi: 10.1073/pnas.0406278102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michalowski D, Chitima-Matsiga R, Held DM, Burke DH. Novel bimodular DNA aptamers with guanosine quadruplexes inhibit phylogenetically diverse HIV-1 reverse transcriptases. Nucleic Acids Res. 2008;36:7124–7135. doi: 10.1093/nar/gkn891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oliviero G, Amato J, Borbone N, D'Errico S, Galeone A, Mayol L, Haider S, Olubiyi O, Hoorelbeke B, Balzarini J, et al. Tetra-end-linked oligonucleotides forming DNA G-quadruplexes: a new class of aptamers showing anti-HIV activity. Chem. Commun. 2010;46:8971–8973. doi: 10.1039/c0cc02866e. [DOI] [PubMed] [Google Scholar]
- 20.Marchand C, Maddali K, Metifiot M, Pommier Y. HIV-1 IN inhibitors: 2010 update and perspectives. Curr. Top. Med. Chem. 2009;9:1016–1037. doi: 10.2174/156802609789630910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gelleert M, Lipsett MN, Davies DR. Helix formation by guanylic acid. Proc. Natl Acad. Sci. USA. 1962;48:2013–2018. doi: 10.1073/pnas.48.12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan BC, Xiong Y, Shi K, Sundaralingam M. Crystal structure of a bulged RNA tetraplex at 1.1 angstrom resolution: Implications for a novel binding site in RNA tetraplex. Structure. 2003;11:1423–1430. doi: 10.1016/j.str.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 23.Koizumi M, Koga R, Hotoda H, Momota K, Ohmine T, Furukawa H, Agatsuma T, Nishigaki T, Abe K, Kosaka T, et al. Biologically active oligodeoxyribonucleotides–IX. Synthesis and anti-HIV-1 activity of hexadeoxyribonucleotides, TGGGAG, bearing 3'- and 5'-end-modification. Bioorg. Med. Chem. 1997;5:2235–2243. doi: 10.1016/s0968-0896(97)00161-2. [DOI] [PubMed] [Google Scholar]
- 24.Urata H, Kumashiro T, Kawahata T, Otake T, Akagi M. Anti-HIV-1 activity and mode of action of mirror image oligodeoxynucleotide analogue of Zintevir. Biochem. Biophys. Res. Commun. 2004;313:55–61. doi: 10.1016/j.bbrc.2003.11.094. [DOI] [PubMed] [Google Scholar]
- 25.Pedersen EB, Nielsen JT, Nielsen C, Filichev VV. Enhanced anti-HIV-1 activity of G-quadruplexes comprising locked nucleic acids and intercalating nucleic acids. Nucleic Acids Res. 2011;39:2470–2481. doi: 10.1093/nar/gkq1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Craigie R. HIV integrase, a brief overview from chemistry to therapeutics. J. Biol. Chem. 2001;276:23213–23216. doi: 10.1074/jbc.R100027200. [DOI] [PubMed] [Google Scholar]
- 27.Jing N, Gao X, Rando RF, Hogan ME. Potassium-induced loop conformational transition of a potent anti-HIV oligonucleotide. J. Biomol. Struct. Dyn. 1997;15:573–585. doi: 10.1080/07391102.1997.10508967. [DOI] [PubMed] [Google Scholar]
- 28.Li H, Yuan G, Du D. Investigation of formation, recognition, stabilization, and conversion of dimeric G-quadruplexes of HIV-1 integrase inhibitors by electrospray ionization mass spectrometry. J. Am. Soc. Mass. Spectrom. 2008;19:550–559. doi: 10.1016/j.jasms.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Balagurumoorthy P, Brahmachari SK, Mohanty D, Bansal M, Sasisekharan V. Hairpin and parallel quartet structures for telomeric sequences. Nucleic Acids Res. 1992;20:4061–4067. doi: 10.1093/nar/20.15.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smargiasso N, Rosu F, Hsia W, Colson P, Baker ES, Bowers MT, De Pauw E, Gabelica V. G-quadruplex DNA assemblies: Loop length, cation identity, and multimer formation. J. Am. Chem. Soc. 2008;130:10208–10216. doi: 10.1021/ja801535e. [DOI] [PubMed] [Google Scholar]
- 31.Phan AT, Modi YS, Patel DJ. Propeller-type parallel-stranded G-quadruplexes in the human c-myc promoter. J. Am. Chem. Soc. 2004;126:8710–8716. doi: 10.1021/ja048805k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seenisamy J, Rezler EM, Powell TJ, Tye D, Gokhale V, Joshi CS, Siddiqui-Jain A, Hurley LH. The dynamic character of the G-quadruplex element in the c-MYC promoter and modification by TMPyP4. J. Am. Chem. Soc. 2004;126:8702–8709. doi: 10.1021/ja040022b. [DOI] [PubMed] [Google Scholar]
- 33.Hazel P, Huppert J, Balasubramanian S, Neidle S. Loop-length-dependent folding of G-quadruplexes. J. Am. Chem. Soc. 2004;126:16405–16415. doi: 10.1021/ja045154j. [DOI] [PubMed] [Google Scholar]
- 34.Rachwal PA, Findlow IS, Werner JM, Brown T, Fox KR. Intramolecular DNA quadruplexes with different arrangements of short and long loops. Nucleic Acids Res. 2007;35:4214–4222. doi: 10.1093/nar/gkm316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bugaut A, Balasubramanian S. A sequence-independent study of the influence of short loop lengths on the stability and topology of intramolecular DNA G-quadruplexes. Biochemistry. 2008;47:689–697. doi: 10.1021/bi701873c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guédin A, De Cian A, Gros J, Lacroix L, Mergny J-L. Sequence effects in single-base loops for quadruplexes. Biochimie. 2008;90:686–696. doi: 10.1016/j.biochi.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 37.Guedin A, Gros J, Alberti P, Mergny JL. How long is too long? Effects of loop size on G-quadruplex stability. Nucleic Acids Res. 2010;38:7858–7868. doi: 10.1093/nar/gkq639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li MH, Zhou YH, Luo Q, Li ZS. The 3D structures of G-Quadruplexes of HIV-1 integrase inhibitors: molecular dynamics simulations in aqueous solution and in the gas phase. J. Mol. Model. 2010;16:645–657. doi: 10.1007/s00894-009-0592-0. [DOI] [PubMed] [Google Scholar]
- 39.Do NQ, Lim KW, Teo MH, Heddi B, Phan AT. Stacking of G-quadruplexes: NMR structure of a G-rich oligonucleotide with potential anti-HIV and anticancer activity. Nucleic Acids Res. 2011 doi: 10.1093/nar/gkr539. (doi:10.1093/nar/gkr539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cantor CR, Warshaw MM, Shapiro H. Oligonucleotide interactions. 3. Circular dichroism studies of the conformation of deoxyoligonucleotides. Biopolymers. 1970;9:1059–1077. doi: 10.1002/bip.1970.360090909. [DOI] [PubMed] [Google Scholar]
- 41.Phan AT, Patel DJ. A site-specific low-enrichment (15)N,(13)C isotope-labeling approach to unambiguous NMR spectral assignments in nucleic acids. J. Am. Chem. Soc. 2002;124:1160–1161. doi: 10.1021/ja011977m. [DOI] [PubMed] [Google Scholar]
- 42.Huang XN, Yu PL, LeProust E, Gao XL. An efficient and economic site-specific deuteration strategy for NMR studies of homologous oligonucleotide repeat sequences. Nucleic Acids Res. 1997;25:4758–4763. doi: 10.1093/nar/25.23.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phan AT. Long-range imino proton-13C J-couplings and the through-bond correlation of imino and non-exchangeable protons in unlabeled DNA. J. Biomol. NMR. 2000;16:175–178. doi: 10.1023/a:1008355231085. [DOI] [PubMed] [Google Scholar]
- 44.Phan AT, Gueron M, Leroy JL. Investigation of unusual DNA motifs. Methods Enzymol. 2001;338:341–371. doi: 10.1016/s0076-6879(02)38228-4. [DOI] [PubMed] [Google Scholar]
- 45.Mergny JL, Phan AT, Lacroix L. Following G-quartet formation by UV-spectroscopy. FEBS Lett. 1998;435:74–78. doi: 10.1016/s0014-5793(98)01043-6. [DOI] [PubMed] [Google Scholar]
- 46.Marky LA, Breslauer KJ. Calculating thermodynamic data for transitions of any molecularity from equilibrium melting curves. Biopolymers. 1987;26:1601–1620. doi: 10.1002/bip.360260911. [DOI] [PubMed] [Google Scholar]
- 47.Jin RZ, Breslauer KJ, Jones RA, Gaffney BL. Tetraplex formation of a guanine-containing nonameric DNA fragment. Science. 1990;250:543–546. doi: 10.1126/science.2237404. [DOI] [PubMed] [Google Scholar]
- 48.Lim KW, Lacroix L, Yue DJ, Lim JK, Lim JM, Phan AT. Coexistence of two distinct G-quadruplex conformations in the hTERT promoter. J. Am. Chem. Soc. 2010;132:12331–12342. doi: 10.1021/ja101252n. [DOI] [PubMed] [Google Scholar]
- 49.Smith FW, Feigon J. Strand orientation in the DNA quadruplex formed from the Oxytricha telomere repeat oligonucleotide d(G4T4G4) in solution. Biochemistry. 1993;32:8682–8692. doi: 10.1021/bi00084a040. [DOI] [PubMed] [Google Scholar]
- 50.Lim KW, Alberti P, Guedin A, Lacroix L, Riou JF, Royle NJ, Mergny JL, Phan AT. Sequence variant (CTAGGG)n in the human telomere favors a G-quadruplex structure containing a G.C.G.C tetrad. Nucleic Acids Res. 2009;37:6239–6248. doi: 10.1093/nar/gkp630. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.