Abstract
Background. In glomerulonephritis (GN), an overload of free fatty acids (FFA) bound to albumin in urinary protein may induce oxidative stress in the proximal tubules. Human liver-type fatty acid-binding protein (hL-FABP) expressed in human proximal tubules, but not rodents, participates in intracellular FFA metabolism and exerts anti-oxidative effects on the progression of tubulointerstitial damage. We examined whether tubular enhancement of this anti-oxidative action modulates the progression of glomerular damage in immune-mediated GN in hL-FABP chromosomal gene transgenic (Tg) mice.
Methods. Anti-glomerular basement membrane antibody-induced glomerulonephritis (anti-GBM GN) was induced in Tg and wild-type mice (WT). Proteinuria, histopathology, polymorphonuclear (PMN) influx, expression of tubulointerstitial markers for oxidative stress 4-hydroxy-2-Nonenal (HNE) and fibrosis (α-smooth muscle actin), proximal tubular damage (Kim-1), Peroxisome Proliferator-Activated Receptor γ (PPAR γ) and inflammatory cytokines [Monocyte Chemotactic Protein-1, tumor necrosis factor-alpha (TNF-α) and Transforming growth factor beta (TGF-β)] were analyzed. The mice were also treated with an angiotensin type II receptor blocker (ARB).
Results. The urinary protein level in Tg mice decreased significantly during the acute phase (∼Day 5). Tg mice survived for a significantly longer time than WT mice, with an attenuation of tubulointerstitial damage score and expression of each tubulointerstitial damage marker observed at Day 7. Expression of inflammatory cytokines on Day 7 was higher in WT mice than Tg mice and correlated strongly with PPARγ expression in WT mice, but not in Tg mice. Interestingly, Tg mice showed insufficient PMN influx at 3 and 6 h, with simultaneous elevation of urinary L-FABP and reduction in HNE expression. The two strains of mice showed different types of glomerular damage, with mild mesangial proliferation in Tg mice and severe endothelial swelling with vascular thrombosis in WT mice. The glomerular damage in Tg mice was improved by administration of an ARB.
Conclusions. The present experimental model suggests that tubular enhancement of L-FABP may protect mice with anti-GBM GN from progression of both tubulointerstitial and glomerular injury.
Keywords: L-FABP, oxidative stress, tubulointerstitial damage
Introduction
Recent studies have shown that urinary protein is renotoxic and contribute to the progression of renal injury by causing tubulointerstitial abnormalities [1–5]. In massive proteinuria, there is an overload of free fatty acids (FFAs) bound to albumin in the proximal tubules. This induces production of certain inflammatory factors such as macrophage chemotactic factors, which in turn exacerbates urinary protein-related tubulointerstitial damage [6–9]. Oxidative stress in proximal tubules is induced by FFAs by the process of protein reabsorption. Oxidative stress is considered as one of the major causes of tubulointerstitial injury [10–13]. Following accumulation of FFAs in the proximal tubules, they become bound to cytoplasmic fatty acid-binding protein (FABP) and are then metabolized.
Human liver-type fatty acid-binding protein (hL-FABP, size 14 kDa) is expressed solely in the renal proximal tubules and acts by binding to overload FFAs in proteinuric renal diseases and is subsequently excreted in the urine. Thus, hL-FABP could potentially be useful in preventing FFA-induced tubulointerstitial cell damage [14–16]. In addition, L-FABP has a high affinity and capacity to bind long-chain fatty acid oxidation products, thereby reducing oxidative stress and ameliorating tubulointerstitial damage [17, 18]. In fact, recent studies have shown that the urinary levels of hL-FABP increases in proteinuric and/or tubular ischemic human kidney diseases [19, 20]. Therefore, in addition to its protective role against tubulointerstitial damage, L-FABP may also be a suitable biomarker of the progression of chronic kidney disease and tubular ischemia [21, 22].
Several recent reports have indicated that protection against tubular damage may improve renal prognosis independent of the type of glomerular disease [23]. This is mainly due to the fact that renal prognosis is closely linked to the expansion of tubulointerstitial fibrosis [24]. However, there is little information on whether protection against tubular damage influences the progression of glomerular injury and exacerbation of tubulointerstitial damage. We have recently reported that tubular L-FABP activated by mesangial cell-origin humoral factors may lessen progression of glomerular damage in IgA nephropathy by reducing oxidative stress and inflammatory mediators, suggesting that glomerulotubular/tubule glomerular cross-talk may underlie and affect renal prognosis, at least in the chronic renal disease [25].
Anti-glomerular basement membrane glomerulonephritis (anti-GBM GN) is a well-established model of severe and acute glomerular damage and is associated with severe proteinuria and tubulointerstitial damage [26, 27]. Because L-FABP is not expressed in the kidneys of rodents, we generated hL-FABP chromosomal transgenic (Tg) mice that expressed hL-FABP only in the renal proximal tubules. Using these hL-FABP Tg mice, we examined whether reinforcement of tubular protective capacity influenced the progression of the acute glomerular injury in anti-GBM GN.
Materials and methods
Animals
The Tg strains of mice bearing the hL-FABP gene were generated using a previously reported method (patent no. WO0073791) [23]. In brief, genomic DNA encoding the human L-FABP gene including its promoter region (13 kb) was microinjected into fertilized eggs obtained from BALB/c mice mated with CBA mice. Therefore, the distribution of hL-FABP was similar to that in humans, with the hL-FABP gene being selectively overexpressed in the renal proximal tubules of Tg mice. Imprinting control region mice were used as recipients of the transfected eggs. The resulting Tg mice were backcrossed for more than six generations with C57/BL6 mice to obtain homozygous mutant mice with an inbred background. Only female mice were used in the subsequent studies. The integration of the hL-FABP gene into the mouse genome was confirmed by the polymerase chain reaction (PCR) of genomic DNA, while limited expression of hL-FABP in the proximal tubules in the kidneys of the Tg mice was confirmed by northern blotting, western blotting and immunohistochemistry [7, 19, 23]. Tg mice did not show any obvious abnormalities in the appearance or behavior. The distribution of hL-FABP expression in kidneys, liver and intestine of Tg mice was confirmed by an enzyme-linked immunosorbent assay (ELISA).
The mice were housed in the animal facilities of the Juntendo Faculty School of Medicine, with free access to food and water. Eight- to 12-week-old female Tg mice (n = 36; body weight 18–25 g) and wild-type (WT) littermates with a C57/BL6 background (n = 41; body weight 17–27 g) were used in this study. The presence of the transgene was ascertained by visualizing the mice under ultraviolet light. The transgene was fused with the green fluorescent protein gene, and mice expressing the transgene were identified by a green fluorescence signal. The experimental protocol was approved by the Ethics Committee for Animal Experimentation of Juntendo University Faculty of Medicine.
Preparation of nephrotoxic serum and experimental protocol for anti-GBM GN induction
The method used for the preparation of nephrotoxic serum (NTS) (Kyowa Hakko Kogyo Co., Tokyo, Japan) has been described previously [27]. Mouse GBM was purified from isolated glomeruli and anti-GBM antibodies raised in rabbits by repeated immunization with the purified GBM in complete Freund's adjuvant (Difco Laboratories Inc company, Detroit, MI). Anti-GBM GN was induced by intravenous injection of NTS (high dose, 200 μL/20 g body weight; low dose, 100 μL/20 g body weight) via the tail vein of mice who had been pre-immunized with rabbit IgG and complete Freund's adjuvant 4 days prior to administration of NTS. The selection of the injected dose was based on results of preliminary studies, which showed that the selected dose was sufficient to induce proteinuria and severe renal injury in WT mice. Long-term survival was evaluated using the low-dose model. No mice developed anaphylactic symptoms after injection of NTS.
Experimental design for investigating the therapeutic effects of angiotensin type II receptor blocker on anti-GBM GN
The angiotensin II (Ang II) type 1 receptor antagonist (ARB), olmesartan medoxomil (olmesartan), was synthesized and provided by Daiichi Sankyo Co., Ltd. (Tokyo, Japan). Since olmesartan is insoluble in water, it was suspended in 0.5% carboxymethyl cellulose sodium salt (CMC-Na). Olmesartan (6 mg/kg body weight/day) was administered orally daily to Tg and WT mice from 4 days before the high-dose NTS injection. After the NTS injection, oral administration was continued until the mice were sacrifice on Day 7 [28].
Evaluation of proteinuria
For detailed evaluation of proteinuria, urine samples were collected for 24 h using a metabolic cage (mouse metabolic cage; CLEA, Shizuoka, Japan). Urinary albumin and creatinine levels were measured by immunoassay (DCA 2000 system; Bayer Diagnostics, Elkhart, Ind., USA) and expressed as the urinary albumin/creatinine ratio (ACR). For simple evaluation of proteinuria, urine samples (10 μL) collected at each time point were also evaluated by Knight's method as described previously [27].
Measurement of urinary hL-FABP and urinary FFA
Urinary L-FABP was measured by a sandwich ELISA kit using a specific monoclonal antibody to human L-FABP (CMIC Co. Ltd, Tokyo, Japan) [21]. This assay system does not detect rodent L-FABP, particularly those derived from WT mice. Urinary hL-FABP (nanograms per milliliter) was expressed as the ratio of urinary hL-FABP to urinary creatinine.
Urinary FFAs were measured using a NEFA-SS kit (EIKEN, Tokyo, Japan) according to the manufacturer's protocol. This kit is based on the acyl enzymatic CoA synthetase-acyl CoA oxidase method, in which 3-octenoic acid is used to trap the remaining free CoA.
Histological analyses
The mice were sacrificed at 3 and 6 h of the acute phase and on Day 7 after the NTS injection. Their kidneys were perfused with ice-cold normal saline, following which one kidney was fixed in 20% formaldehyde overnight and then in 70% ethanol, and the other kidney was snap frozen in liquid nitrogen. Paraffin sections (3-μm thick) were stained by the periodic acid–Schiff's reagent and the histological changes were assessed by light microscopy. A tubulointerstitial injury score was evaluated based on the following morphological changes observed in the tubules that included dilatation, distortion of the tubular basement membrane and atrophy: 0, no morphological deformities; Grade 1, <10%; Grade 2, <25%; Grade 3, <50%; Grade 4, <75% and Grade 5, ≥75% involvement. More than 20 consecutive fields were examined in each slide under ×400 magnification and the values averaged. The number of infiltrating glomerular polymorphonuclear (PMN) cells was counted in 3-μm-thick sections stained with anti-Ly-6G/Ly-6C (Gr-1) antibody (Bio Legend, San Diego, CA). The number of PMN and Gr-1-positive cells was determined in at least 40 glomeruli of each mouse (three to four mice for each time point).
For the evaluation of mouse macrophages, frozen renal sections (3-μm thick) were stained with fluorescein-conjugated goat IgG fraction to rabbit IgG (Dako, Barcelona, Spain), fluorescein-conjugated goat IgG fraction to mouse IgG (BD PharMingen, San Diego, CA) and fluorescein-conjugated rat anti-mouse F4/80 antibody (Hycult Biotech, Uden, The Netherlands).
The frozen kidney sections were stained with the following antibodies after acetone fixation: polyclonal goat anti-mouse 4-hydroxy-2-Nonenal (HNE) (1:500; Alpha Diagnostic International, San Antonio, TX), monoclonal mouse anti-human α-smooth muscle actin (α-SMA) antibody (1:1; Dako) and monoclonal rat anti-Kim-1 antibody (RMT-1-10, rat IgG2a), which was generated by immunizing rats with Kim-1-Fc fusion proteins [29]. Subsequently, the sections were incubated for 1 h in the secondary antibody, horseradish peroxidase-labeled rabbit anti-goat IgG (Simple Stain MAX-PO, goat; Nichirei, Tokyo, Japan). The bound antibodies were detected using an enhanced 3,3′-diaminobenzidine kit (Dako Cytomation). The percentage pixel density of brown staining for α-SMA in the tubulointerstitium, excluding the arteries, was measured using a KS version 3.0 image analysing system (KS400; Carl Zeiss Vision, Hallbergmoos, Germany). The staining intensity was evaluated in >10 different fields of each sample.
Quantitative real-time reverse transcriptase–PCR analysis
Total RNA was extracted from the whole kidney homogenates using TRIZOL (Invitrogen Corp., Carlsbad, CA), and the purity was checked by spectrophotometry. Isolated RNA was reverse transcribed using a random decamer primer (Ambion, Austin, TX) and M-MLV Reverse Transcriptase (Life Technologies). Quantitative PCR was performed using the SYBR Green PCR master mix (PerkinElmer Applied Biosystems, Foster City, CA) and analyzed using the ABI PRISM 7500 Sequence Detection System (PerkinElmer Applied Biosystems). The forward and reverse primers used for each molecule were as follows: 5'-CATTGTGGAAGGGCTCATGA-3' and 5'-TCTTCTGGGTGGCAGTGATG-3' for mouse Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5'-AAATCGTGCAGAATGGGAAG-3' and 5'-TCTCCCCTGTCATTGTCTCC-3' for human L-FABP, 5'-TCCCAATGAGTAGGCTGGAG-3' and 5'-CCTCTCTCTTGAGCTTGGTGA-3' for mouse MCP-1, 5'-TAGCCAGGAGGGAGAACAGA-3' and 5'-TTTTCTGGAGGGAGATGTGG-3' for mouse TNF-α, 5'-GCAACATGTGGAACTCTACCAGA-3' and 5'-GACGTCAAAAGACAGCCACTCA-3' for mouse TGF-β1 and 5'-ATGCCAAAAATATCCCTGGTTTC-3' and 5'-GGAGGCCAGCATGGTGTAGA-3' for PPARγ.
Western blotting
Proteins were separated on 7.5–12% sodium dodecyl sulfate-polyacrylamide gels and subsequently transferred to nitrocellulose membranes (Novex, San Diego, CA) by semi-dry blotting. Monoclonal rat anti-Kim-1 (RMT-1-10, rat IgG2a), polyclonal goat anti-mouse HNE (4-hydroxy-2-Nonenal) (1:500; Alpha Diagnostic International), rabbit anti-mouse GAPDH (1:5000; Abcam Inc., Cambridge, MA) antibodies and rabbit anti-β actin (1:5000; Sigma, Tokyo, Japan) antibodies were used as the primary antibodies. Horseradish peroxidase-conjugated secondary antibodies were diluted 1:20 000. The blots were visualized by the enhanced chemiluminescence reaction (Amersham Life Science, Piscataway, NJ).
Statistical analysis
All numerical data are expressed as the mean ± SD or mean ± SEM. Differences between the groups were examined for statistical significance by analysis of variance. A P-value <0.05 was considered as a statistically significant difference. Statistical analyses were performed using StatView 5.0 software (Abacus Concepts, Inc., Stanford, CA). Survival was determined by the Kaplan–Meier method using GraphPad Prism (GraphPad Software, La Jolla, CA).
Results
Attenuation of proteinuria associated with glomerular damage in the acute phase of anti-GBM GN
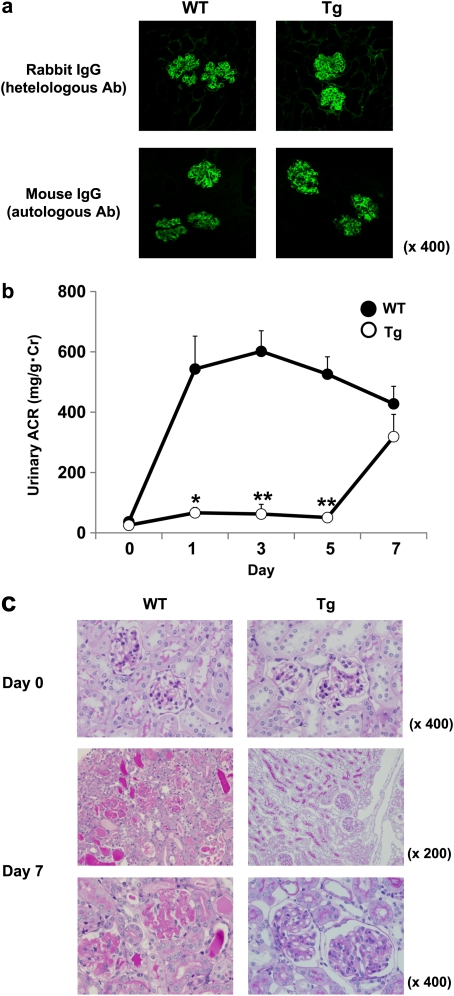
In this study, we adopted the augmented anti-GBM GN model in which each mouse was pre-immunized with rabbit IgG. We defined the phase before Day 7 as the acute phase. The amount of glomerular deposits of heterologous antibody (rabbit anti-mouse GBM immunoglobulin [Ig]) assessed by immunofluorescence 3 h after the NTS injection was similar in Tg and WT mice (Figure 1a, upper panels). The intensity of autologous IgG antibodies, which were reactive with heterologous Ig (rabbit IgG) fixed previously\ at the GBM, was also similar in glomeruli of Tg and WT mice (Figure 1a, lower panels).
Fig. 1.
(a) Glomerular deposition of heterologous antibodies (rabbit IgG) at 3 h and autologous antibodies (mouse IgG) at Day 7 after NTS injection. There were no differences in heterologous and autologous antibodies in both Tg and WT mice. (b and c) Disease course in L-FABP Tg (n = 13) (open circles) and WT (n = 18) (closed circles) mice in high-dose anti-GBM GN model. The urinary albumin level (ACR) was attenuated significantly until Day 5 (versus WT; *P < 0.01, **P < 0.001) but finally became comparable with that of WT mice at Day 7. Data are expressed as mean ± SE. WT mice showed glomerular damage with severe endothelial swelling with thrombosis and fibrin deposition, while mesangial proliferative GN was found in Tg mice.
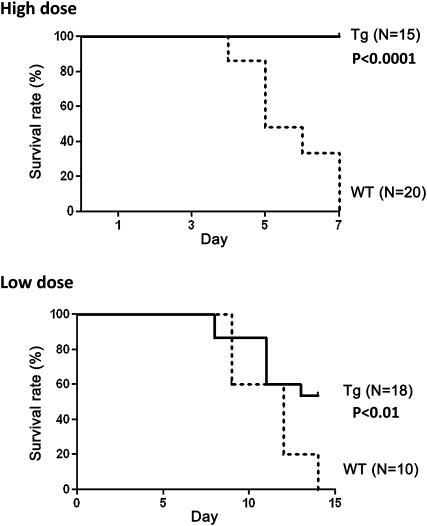
However, the urinary ACR in Tg mice decreased significantly from Day 1 after the injection of NTS (Figure 1b). The levels of urinary protein excretion in Tg mice increased gradually, reaching a level comparable to that observed in WT mice at Day 7 (Figure 1b). Figure 1c shows typical glomerular histology of each group in the high-dose model at Day 7. WT mice showed severe endothelial swelling and vascular thrombosis involving platelet accumulation and fibrin deposition with cellular necrosis. In contrast, fibrin deposition and cellular necrosis were not detected in Tg mice (Figure 1c). However, Tg mice showed proliferative GN, indicating that different morphological changes were induced in both strains of mice despite a comparable degree of ACR at Day 7 (Figure 1b and c). At the end of the study period in the high-dose model, all WT mice died with massive ascites, whereas Tg mice were significantly protected against such changes (P < 0.0001) (Figure 2, upper panel). In the low-dose model, Tg mice also showed significantly greater long-term survival (P < 0.01) (Figure 2, lower panel).
Fig. 2.
Survival rate of Tg and WT mice with anti-GBM GN. Tg mice showed significantly improved survival in both the high- (P < 0.0001) and low-dose (P < 0.01) models.
Attenuated progression of tubulointerstitial injury in Tg mice regardless of the level of proteinuria
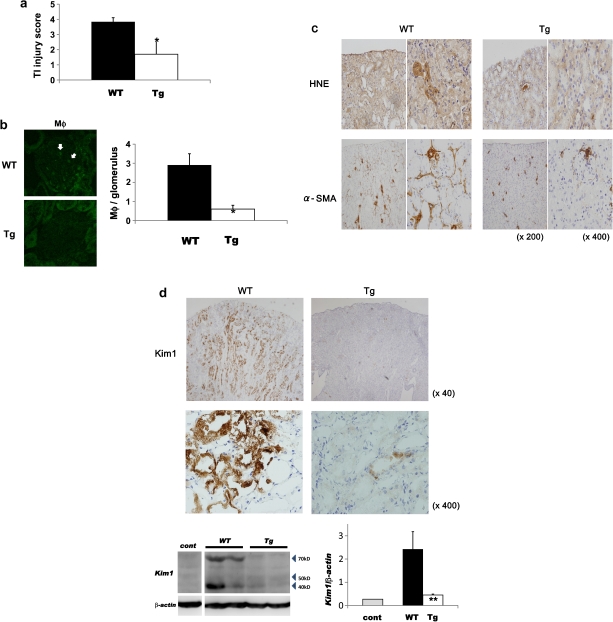
At Day 7, the degree of tubulointerstitial damage (P < 0.0001) (Figure 3a) and glomerular infiltration of macrophages (P < 0.001) (Figure 3b) was attenuated significantly in Tg mice, despite a similar level of ACR in the two strains of mice. Expression of HNE, an established peroxide lipid marker, and the number of α-SMA-positive cells (i.e. myofibroblasts) in the tubulointerstitial areas were reduced in Tg mice compared with WT mice (Figure 3c). Morphometric analysis by KS 400 provided further confirmation that the α-SMA-positive area in Tg mice was significantly and considerably smaller than in WT mice (WT versus Tg: 19.94 ± 6.69 versus 2.08 ± 0.93; P < 0.05). Moreover, renal Kim-1 expression, an established marker of proximal tubular damage by urinary protein, toxins and ischemia, was different in Tg and WT mice at Day 7. Marked expression of Kim-1 was detected in not only the cytoplasm but also the apical part of proximal tubules from the cortex to the outer medulla in WT mice, whereas very weak Kim-1 expression was observed in the cytoplasm of proximal tubules in Tg mice (Figure 3d). Western blotting further confirmed these findings (Figure 3d).
Fig. 3.
Tubulointerstitial damage in Tg and WT mice with anti-GBM GN at Day 7. (a) The tubulointerstitial injury score was attenuated significantly in Tg mice (versus WT; *P < 0.001) (n = 5 in each group). (b) The number of macrophages was also significantly lower in Tg mice (versus WT; *P < 0.001). (c) Interstitial expression of markers of oxidative stress (HNE) and fibrosis (α-SMA) were attenuated in Tg mice. (d) Immunohistochemical analysis showed reduced tubular expression of the marker of proximal tubular damage, Kim-1, in Tg mice. Western blotting further confirmed this significant reduction (versus WT; **P < 0.01). Arrows indicate the position of Kim-1-specific bands at ∼40 and 70–80 kDa. Data are expressed as mean ± SD.
Significant correlation between renal expression of PPARγ and inflammatory cytokines at Day 7 in WT mice, but not in Tg mice
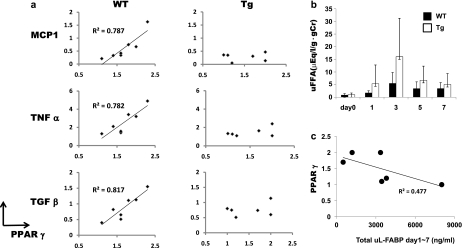
At Day 7, renal PPARγ expression showed significant correlations with renal expression of inflammatory cytokines including MCP-1, TNF-α and TGF-β (Figure 4a, left panels). However, these associations were not found in Tg mice (Figure 4a, right panels). In addition, the basal expression of PPARγ in Tg mice was lower than that observed in WT mice. Since FFAs can activate PPARγ, we subsequently measured the urinary excretion of FFAs in both strains of mice. Although we did not find statistically significant differences, urinary FFA excretion measurement tended to be higher in Tg mice than in WT mice during the course of the disease (Figure 4b). Furthermore, from Days 1 to 7, Tg mice showed a trend toward an inverse association between the total amount of urinary hL-FABP and expression of PPARγ (Figure 4c).
Fig. 4.
(a) Although PPARγ expression at Day 7 well correlated with expression of inflammatory cytokines (Monocyte Chemotactic Protein-1, TNF-α and TGF-β) in WT mice, such correlation was not found in Tg mice. (b) Time course of urinary FFA in Tg and WT mice (n ≥ 6 at each time point). (c) Correlation between PPARγ expression at Day 7 and total urinary hL-FABP from Days 1 to 7 in Tg mice. T, which is an addition to urinary hL-FABP measured at Days 1, 3, 5 and 7.
Significant attenuation of PMN influx in Tg mice with anti-GBM GN
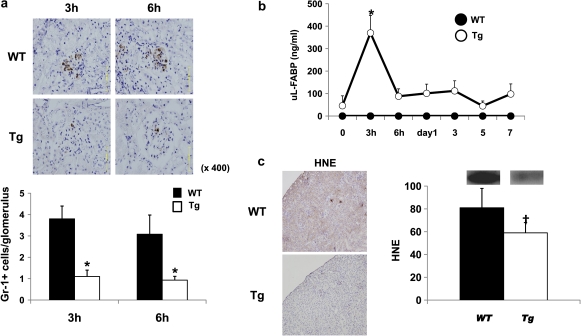
To elucidate the mechanism responsible for different glomerular morphological changes induced in WT and Tg mice, we counted the number of glomerular PMN cells in both strains of mice 3 and 6 h after the NTS injection [27, 30, 31]. The influx of Gr-1-positive PMN in Tg mice was significantly attenuated at both 3 and 6 h after the NTS injection (Figure 5). Interestingly, a marked elevation in urinary L-FABP excretion and strong expression of HNE were observed at 3 h (Figure 5b and c).
Fig. 5.
Differences in the initial damage observed between Tg and WT mice with anti-GBM GN. (a) The numbers of infiltrating Gr-1-positive PMN in the glomeruli at 3 and 6 h in Tg mice (n = 5) were significantly lower than those in WT mice (n = 5) (versus WT; *P < 0.001). Data are expressed as mean ± SD. (b) In Tg mice, urinary excretion of L-FABP at 3 h (open circles) was significantly higher than that at baseline (**P < 0.01). WT mice (closed circles) showed no urinary excretion of L-FABP. Data are expressed as mean ± SD. (c) HNE expression was detected at 3 h in WT mice but was attenuated significantly in Tg mice (versus WT; †P < 0.005). Data are expressed as mean ± SD.
Inhibitory effects of ARB on glomerular lesions in Tg mice with anti-GBM GN
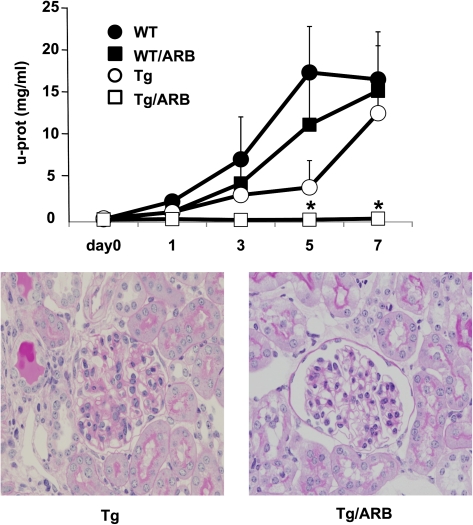
Finally, to examine the pathological mechanisms responsible for the development of mesangial proliferative lesions in Tg mice with anti-GBM GN [27, 29, 31–33], we blocked Ang II type 1 receptor (AT1) signaling using the ARB olmesartan. Daily oral administration of olmesartan commenced 4 days before the NTS injection. Administration of olmesartan in WT mice did not prevent the development of a lethal state, with massive proteinuria and severe glomerular damage at Day 7 being similar to that observed in untreated WT mice (Figure 6). Although olmesartan had no effect on the amount of linear deposition of the injected antibodies and autologous antibodies along GBM, the Tg mice demonstrated a marked improvement in the level of proteinuria and glomerular pathological changes (Figure 6).
Fig. 6.
Effect of an ARB in Tg (open circles) and WT (closed circles) mice with anti-GBM GN (n = 5 in each group). ARB treatment provided significant protection against glomerular damage and proteinuria in Tg/ARB (open squares) mice (versus Tg mice; *P < 0.001). However, ARB treatment did not modulate the disease course in WT/ARB (closed circles). Data are expressed as mean ± SE.
Discussion
The present study demonstrated that tubular over-expression of L-FABP provided a renoprotective action not only to the progression of tubulointerstitial injury but also against acute glomerular damage in Tg mice with anti-GBM GN. This latter protective effect occurred despite no difference between Tg and WT mice in the various immunopathological factors, such as the amount of NTS injected and basal immune responses. These findings suggest that oxidative stress in proximal tubules appears to influence the progression and/or severity of glomerular lesions in acute immune-mediated GN.
FFA are bound to albumin, filtered through the glomeruli, and then reabsorbed by the proximal tubules. In massive proteinuria, FFA overload occurs in the proximal tubules and induces oxidative stress and production of inflammatory cytokines by increasing mitochondrial reactive oxygen species, subsequently leading to tubulointerstitial damage [34, 35]. The proximal tubules of the human kidney express L-FABP [36], which participates in intracellular fatty acid metabolism. FFA in the hepatocyte are bound to cytoplasmic L-FABP and transported to the mitochondria or peroxisomes [37, 38], where they are metabolized by β-oxidation. Although the function of tubular L-FABP is not fully understood, it is presumed to be the same as in the liver [34]. In the present study, protection against tubulointerstitial damage was observed in L-FABP Tg mice on Day 7, despite the level of proteinuria at that time being similar to that measured in WT mice. This may be partly due to the anti-oxidative action of L-FABP against fatty acid overloading by reabsorbed urinary protein as described previously [39–42]. In fact, not only improvement of tubulointerstitial damage score but also oxidative and fibrotic stress markers, HNE and α-SMA, expression were attenuated in Tg mice. Furthermore, we observed a conspicuous reduction in tubular Kim-1 expression, a marker of ischemic proximal tubular damage, and urinary protein levels in L-FABP Tg mice, indicating that tubular enhancement of L-FABP may improve relative tubular ischemia even during severe proteinuria. In addition, conserved peritubular flow due to a lack of severe glomerular endothelial damage may contribute to the anti-ischemic action in Tg mice. This finding is compatible with our previous report that peritubular flow has a greater impact on tubulointerstitial injury than proteinuria [29].
The level of urinary FFA excretion in Tg mice was higher than that in WT mice during the course of our study, despite the total urinary protein level in WT mice being considerably higher than that measured in Tg mice. This finding suggests that over-expression of tubular L-FABP may play a role in the effective excretion of FFA in the urine, presumably including metabolized FFA, thus protecting against the harmful effects of FFAs. In this regard, it is clear that FFAs can activate PPARγ, resulting in potent anti-inflammatory effects. In fact, WT mice showed a significant correlation between the expression of PPARγ and inflammatory cytokines, such as MCP-1, TNF-α and TGF-β. These findings suggest that reabsorbed FFAs may induce not only a strong inflammatory action but also endogenous anti-inflammatory PPARγ expression. From Days 1 to 7, the total amount of urinary L-FABP inversely correlated with PPARγ expression, suggesting that the anti-inflammatory action of PPARγ may be also involved in the renoprotection that occurs in Tg mice. However, Tg mice showed no clear correlation between PPARγ and inflammatory cytokines, suggesting that the renoprotective actions against tubulointerstitial and glomerular damage in Tg mice were not primarily related to PPARγ expression.
We have previously examined the pathological mechanisms during the acute stage of anti-GBM GN [27, 29, 31–33]. Using several FcR-related knockout mice and their bone marrow chimeras, we demonstrated that the Fc receptor (FcR) is a critical molecule for PMN influx in the initial phase of anti-GBM GN [27, 31]. These studies demonstrated that PMN cells directly recognize antibodies deposited on the GBM via their FcRs and then induce tethering of the anti-GBM antibody. The respiratory bursts of PMN cells that result from cross-linking of FcRs at the tethering point may activate glomerular endothelial cells to induce TNF-α production, with subsequent expression of various adhesion molecules [31]. This suggests that overload of oxidative stress by FcR-dependent respiratory bursts is a crucial step in full PMN influx. In fact, pretreatment with catalase has been reported to inhibit PMN influx and attenuate initial glomerular damage [31]. Other reports also indicated that PMN influx during the initial phase of this disease is a key process in the development of acute glomerular lesions, primarily severe endothelial damage [27, 30, 43–46]. Therefore, the absence of PMN influx and subsequent initial severe endothelial damage in L-FABP Tg mice with anti-GBM GN may be associated with a reduction in oxidative stress and lipid peroxidation. In fact, in the present study, we observed that HNE expression was also reduced in the tubulointerstitial areas of Tg mice. There is evidence that the activation of L-FABP in proximal tubules can be triggered not only by proteinuria but also by oxidative stress due to tubular ischemia [19, 23, 34]. Therefore, mediators of the initial respiratory burst in PMN cells, such as H2O2 may also induce oxidative stress in the proximal tubules as a result of peritubular flow after the glomerular efferent artery, thereby further influencing the glomerular status.
Injection of NTS into the renal artery of an isolated kidney induces elevation of renin activity in perfusates collected from the renal vein [45, 47]. Moreover, NTS administration also changes venous pressure in glomerular capillaries and the single nephron glomerular filtration rate in isolated kidneys [47]. Therefore, antibody deposition on GBM may induce local activation of the renin–angiotensin system (RAS), thereby modulating glomerular filtrate rate mainly by contraction of the efferent glomerular arterioles [47]. Stagnation of glomerular capillary flow by contraction may aid in complete PMN influx. Relative ischemia due to a reduction in peritubular flow mediated by contraction of the efferent arterioles may further enhance oxidative stress in tubular cells [41]. In addition, increased levels of renin or Ang II in urine may also further enhance oxidative stress generated by NADH/nicotinamide adenine dinucleotide phosphate-oxidase in the tubular lumens [40]. In fact, the rapid rise in urinary L-FABP immediately after the NTS injection may suggest an acute loading of oxidative stress in the proximal tubules.
Additionally, we have demonstrated using the bone marrow chimeras of FcR, AT1 receptor knockout mice and ARB treatment that renal RAS activation associated with anti-GBM antibody deposition may determine the susceptibility of glomerular injury, which is characterized by mesangial proliferation resulting from Ang II-induced chemokine expression of renal resident cells [27, 32]. Several studies have reported that AT1-dependent mesangial proliferative GN is observed in FcR-deficient mice with anti-GBM GN, due to the absence of severe endothelial cell damage caused by FcR-dependent PMN influx [27, 31, 32]. The disease course in Tg mice with anti-GBM GN was characterized by mesangial proliferative GN and massive proteinuria, changes that mimicked those observed in FcR-deficient mice with this disease [27, 29, 31, 32]. In fact, ARB treatment completely abrogated this glomerular injury in the L-FABP Tg mice, similar to that seen in the FcR-deficient mice [27]. This suggested that renal AT1-dependent cellular immunity was involved in the pathogenesis of glomerular lesions in Tg mice [32]. On the other hand, ARB treatment did not alter the fulminant outcome in WT mice resulting from FcR-dependent PMN influx and subsequent severe endothelial damage. Therefore, local RAS activation following antibody deposition may be responsible for induction of the acute hemodynamic changes associated with tubular oxidative stress, as well as other acute immune-mediated mechanisms that occur in this disease [27, 32, 47].
Careful analyses are required to determine the mechanisms by which the tubular anti-oxidative action of L-FABP abrogated PMN influx. However, as local activation of RAS has a major role in the acute phase of this disease [27, 32, 47], the underlying mechanisms related to tubuloglomerular feedback should be considered in this disease in L-FABP Tg mice. Local RAS activation by anti-GBM antibody deposition and subsequent oxidative stress in the tubules may jointly induce pathophysiological signals to the juxta-glomerular apparatus. These signals may induce acute hemodynamic changes in glomeruli and thus contribute to the initial pathological setting in the acute immune-mediated GN. The present study indicated that enhancement of the anti-oxidative environment in the tubules may be linked to the progression of not only tubulointerstitial damage but also to the amplitude of leukocyte migration and severity of acute glomerular injury. We recently reported that the anti-oxidative action by tubular L-FABP also protected the progression of glomerular damage in chronic GN [25]. Therefore, increasing anti-oxidative activity is potentially important for future development of new therapies to prevent glomerular damage.
Acknowledgments
We thank CMIC Co. Ltd. for measuring urinary L-FABP levels, Daiichi Sankyo Co. Ltd. for providing olmesartan medoxomil (Olmetec®) and Kyowa Hakko Kogyo Co. for providing anti-GBM nephrotoxin. We also thank Ms Terumi Shibata, Mr Kazutaka Yoshida and Ms Mari Yamada for their excellent technical and secretarial assistance.
Conflict of interest statement. None declared.
References
- 1.Remuzzi G, Bertani T. Pathophysiology of progressive nephropathies. N Engl J Med. 1998;339:1448–1456. doi: 10.1056/NEJM199811123392007. [DOI] [PubMed] [Google Scholar]
- 2.Remuzzi G, Ruggenenti P, Benigni A. Understanding the nature of renal disease progression. Kidney Int. 1997;51:2–15. doi: 10.1038/ki.1997.2. [DOI] [PubMed] [Google Scholar]
- 3.Peterson JC, Adler S, Humsicker LG, et al. Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease Study. Ann Intern Med. 1995;123:754–762. doi: 10.7326/0003-4819-123-10-199511150-00003. [DOI] [PubMed] [Google Scholar]
- 4.Eddy AA. Experimental insights into the tubulointerstitial disease accompanying primary glomerular lesions. J Am Soc Nephrol. 1994;5:1273–1287. doi: 10.1681/ASN.V561273. [DOI] [PubMed] [Google Scholar]
- 5.Eddy AA, McCulloch L, Adams J, et al. A relationship between proteinuria and acute tubulointerstitial disease in rats with experimental nephritic syndrome. Am J Pathol. 1991;138:1111–1123. [PMC free article] [PubMed] [Google Scholar]
- 6.Kamijo-Ikemori A, Sugaya T, Kimura K, et al. Liver-type fatty acid-binding protein attenuates renal injury induced by unilateral ureteral obstruction. Am J Pathol. 2006;169:1107–1117. doi: 10.2353/ajpath.2006.060131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Negishi K, Noiri E, Fujita T, et al. Renal L-type fatty acid-binding protein mediates the bezafibrate reduction of cisplatin-induced acute kidney injury. Kidney Int. 2008;73:1374–1384. doi: 10.1038/ki.2008.106. [DOI] [PubMed] [Google Scholar]
- 8.Negishi K, Noiri E, Sugaya T, et al. A role of liver fatty acid-binding protein in cisplatin-induced acute renal failure. Kidney Int. 2007;72:348–358. doi: 10.1038/sj.ki.5002304. [DOI] [PubMed] [Google Scholar]
- 9.Wang G, Gong Y, Burczynski FJ, et al. Antioxidative function of L-FABP in L-FABP stably transfected Chang liver cells. Hepatology. 2006;42:871–879. doi: 10.1002/hep.20857. [DOI] [PubMed] [Google Scholar]
- 10.Arici M, Chana R, Brunskill NJ, et al. Stimulation of proximal tubular cell apoptosis by albumin-bound fatty acids mediated by peroxisome proliferator activated receptor-gamma. J Am Soc Nephrol. 2003;14:17–27. doi: 10.1097/01.asn.0000042167.66685.ea. [DOI] [PubMed] [Google Scholar]
- 11.Thomas ME, Morrison AR, Schreiner GF. Metabolic effects of fatty acid-bearing albumin on a proximal tubule cell line. Am J Physiol. 1995;268:F1177–1184. doi: 10.1152/ajprenal.1995.268.6.F1177. [DOI] [PubMed] [Google Scholar]
- 12.Thomas ME, Schreiner GF. Contribution of proteinuria to progressive renal injury: consequences of tubular uptake of fatty acid bearing albumin. Am J Nephrol. 1993;13:385–398. doi: 10.1159/000168653. [DOI] [PubMed] [Google Scholar]
- 13.Kees-Folts D, Sadow JL, Schreiner GF. Tubular catabolism of albumin is associated with the release of an inflammatory lipid. Kidney Int. 1994;45:1697–1709. doi: 10.1038/ki.1994.222. [DOI] [PubMed] [Google Scholar]
- 14.Veerkamp JH, van Kuppevelt TH, Prinsen CF, et al. Structural and functional aspects of cytosolic fatty acid-binding proteins. Prostaglandins Leukot Essent Fatty Acids. 1993;49:887–906. doi: 10.1016/0952-3278(93)90174-u. [DOI] [PubMed] [Google Scholar]
- 15.Veerkamp JH, Peeters RA, Maatman RG. Structural and functional features of different types of cytoplasmic fatty acid-binding proteins. Biochim Biophys Acta. 1991;1081:1–24. doi: 10.1016/0005-2760(91)90244-c. [DOI] [PubMed] [Google Scholar]
- 16.Sweetser DA, Heuckeroth RO, Gordon JI. The metabolic significance of mammalian fatty-acid-binding proteins: abundant proteins in search of a function. Annu Rev Nutr. 1987;7:337–359. doi: 10.1146/annurev.nu.07.070187.002005. [DOI] [PubMed] [Google Scholar]
- 17.Ek-Von Mentzer BA, Zhang F, Hamilton JA. Binding of 13-HODE and 15-HETE to phospholipid bilayers, albumin, and intracellular fatty acid binding proteins. Implications for transmembrane and intracellular transport and for protection from lipid peroxidation. J Biol Chem. 2001;276:15575–15580. doi: 10.1074/jbc.M011623200. [DOI] [PubMed] [Google Scholar]
- 18.Raza H, Pongubala JR, Sorof S. Specific high affinity binding of lipoxygenase metabolites of arachidonic acid by liver fatty acid binding protein. Biochem Biophys Res Commun. 1989;161:448–455. doi: 10.1016/0006-291x(89)92619-3. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto T, Noiri E, Sugaya T, et al. Renal L-type fatty acid–binding protein in acute ischemic injury. J Am Soc Nephrol. 2007;18:2894–2902. doi: 10.1681/ASN.2007010097. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura T, Sugaya T, Koide H. Urinary liver-type fatty acid-binding protein levels for differential diagnosis of idiopathic focal glomerulosclerosis and minor glomerular abnormalities and effect of low-density lipoprotein apheresis. Clin Nephrol. 2006;65:1–6. doi: 10.5414/cnp65001. [DOI] [PubMed] [Google Scholar]
- 21.Kamijo A, Kimura K, Omata M, et al. Urinary fatty acid-binding protein as a new clinical marker of the progression of chronic renal disease. J Lab Clin Med. 2004;143:23–30. doi: 10.1016/j.lab.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Ferguson MA, Vaidya VS, Waikar SS, et al. Urinary liver-type fatty acid-binding protein predicts adverse outcomes in acute kidney injury. Kidney Int. 2010;77:708–714. doi: 10.1038/ki.2009.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamijo A, Sugaya T, Kimura K, et al. Urinary excretion of fatty acid-binding protein reflects stress overload on the proximal tubules. Am J Pathol. 2004;165:1243–1255. doi: 10.1016/S0002-9440(10)63384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nangaku M. Mechanisms of tubulointerstitial injury in the kidney: final common pathways to end-stage renal failure. Intern Med. 2004;43:9–17. doi: 10.2169/internalmedicine.43.9. [DOI] [PubMed] [Google Scholar]
- 25.Zuo N, Suzuki Y, Sugaya T, et al. Nephrol Dial Transplant. Protective effects of tubular liver-type fatty acid-binding protein against glomerular damage in murine IgA nephropathy. (in press) [DOI] [PubMed] [Google Scholar]
- 26.Assman KJ, Tangelder MM, Lange WP, et al. Anti-GBM nephritis in the mouse: severe proteinuria in the heterologous phase. Virchows Arch A Pathol Anat Histopathol. 1985;406:285–299. doi: 10.1007/BF00704298. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki Y, Shirato I, Tomino Y, et al. Distinct contribution of Fc receptors and angiotensin II-dependent pathways in anti-GBM glomerulonephritis. Kidney Int. 1998;54:1166–1174. doi: 10.1046/j.1523-1755.1998.00108.x. [DOI] [PubMed] [Google Scholar]
- 28.Tanabe Y, Morikawa Y, Nakayama K. Effects of olmesartan, an AT1 receptor antagonist, on hypoxia-induced activation of ERK1/2 and pro-inflammatory signals in the mouse lung. Naunyn-Schmiedebergs Arch Pharmacol. 2006;374:235–248. doi: 10.1007/s00210-006-0110-1. [DOI] [PubMed] [Google Scholar]
- 29.Wong MG, Suzuki Y, Tomino Y, et al. Peritubular ischemia contributes more to tubular damage than proteinuria in immune-mediated glomerulonephritis. J Am Soc Nephrol. 2008;19:290–297. doi: 10.1681/ASN.2007020226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson CB. Immune models of glomerular injury. In: Neilson EG, Couser WG, editors. Immunologic Renal Diseases. Philadelphia, PA: Lippincott-Raven Publishers; 1997. pp. 729–773. [Google Scholar]
- 31.Suzuki Y, Gómez-guerrero C, Egido J, et al. Pre-existing glomerular immune complexes induce polymorphonuclear cell recruitment through an Fc receptor-dependent respiratory burst: potential role in the perpetuation of immune nephritis. J Immunol. 2003;170:3243–3253. doi: 10.4049/jimmunol.170.6.3243. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki Y, Gómez-guerrero C, Egido J, et al. Susceptibility to T cell-mediated injury in immune complex disease is linked to local activation of renin-angiotensin system: the role of NF-AT pathway. J Immunol. 2002;169:4136–4146. doi: 10.4049/jimmunol.169.8.4136. [DOI] [PubMed] [Google Scholar]
- 33.Hidaka T, Suzuki Y, Tomino Y, et al. Amelioration of crescentic glomerulonephritis by RhoA kinase inhibitor, Fasudil, through podocyte protection and prevention of leukocyte migration. Am J Pathol. 2008;172:603–614. doi: 10.2353/ajpath.2008.070196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sasaki H, Kamijo-Ikemori A, Sugaya T, et al. Urinary fatty acids and liver-type fatty acid binding protein in diabetic nephropathy. Nephron Clin Pract. 2009;112:c148–c156. doi: 10.1159/000214210. [DOI] [PubMed] [Google Scholar]
- 35.Lindner A, Hinds TR, Joly A, et al. Neutral lipid from proteinuric rat urine is a novel inhibitor of the red blood cell calcium pump. J Am Soc Nephrol. 1999;10:1170–1178. doi: 10.1681/ASN.V1061170. [DOI] [PubMed] [Google Scholar]
- 36.Maatman RG, van de Westerlo EM, van Kuppevelt TH, et al. Molecular identification of the liver- and the heart-type fatty acid-binding proteins in human and rat kidney. Use of the reverse transcriptase polymerase chain reaction. Biochem J. 1992;288:285–290. doi: 10.1042/bj2880285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Atshaves BP, McIntosh AM, Lyuksyutova OI, et al. Liver fatty acid-binding protein gene ablation inhibits branched-chain fatty acid metabolism in cultured primary hepatocytes. J Biol Chem. 2004;279:30954–30965. doi: 10.1074/jbc.M313571200. [DOI] [PubMed] [Google Scholar]
- 38.Erol E, Kumar LS, Cline GW, et al. Liver fatty acid binding protein is required for high rates of hepatic fatty acid oxidation but not for the action of PPARalpha in fasting mice. FASEB J. 2004;18:347–349. doi: 10.1096/fj.03-0330fje. [DOI] [PubMed] [Google Scholar]
- 39.Fels LM, Sanz-Altamira PM, Stolte H, et al. Filtration characteristics of the single isolated perfused glomerulus of Myxine glutinosa. Ren Physiol Biochem. 1993;16:276–284. doi: 10.1159/000173773. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki Y, Ruiz-Prtega M, Egido J, et al. Inflammation and angiotensin II. Int J Biochem Cell Biol. 2003;35:881–900. doi: 10.1016/s1357-2725(02)00271-6. [DOI] [PubMed] [Google Scholar]
- 41.Kamijo-Ikemori A, Sugaya T, Kimura K. Urinary fatty acid binding protein in renal disease. Clin Chim Acta. 2006;374:1–7. doi: 10.1016/j.cca.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 42.Kamijo-Ikemori A, Sugaya T, Kimura K, et al. Amelioration of diabetic tubulointerstitial damage in liver-type fatty acid binding protein transgenic mice. Nephrol Dial Transplant. 2009;24:788–800. doi: 10.1093/ndt/gfn573. [DOI] [PubMed] [Google Scholar]
- 43.Ravetch JV. Fc receptors: rubor redux. Cell. 1994;78:553–560. doi: 10.1016/0092-8674(94)90521-5. [DOI] [PubMed] [Google Scholar]
- 44.Sylvestre DL, Ravetch JV. Fc receptors initiate the Arthus reaction: redefining the inflammatory cascade. Science. 1994;265:1095–1098. doi: 10.1126/science.8066448. [DOI] [PubMed] [Google Scholar]
- 45.Boyce NW, Holdsworth SR. Anti-glomerular basement membrane antibody-induced experimental glomerulonephritis: evidence for dose-dependent, direct antibody and complement-induced, cell-independent injury. J Immunol. 1985;135:3918–3921. [PubMed] [Google Scholar]
- 46.Schrijver G, Schalkwijk J, Koene RA, et al. Antiglomerular basement membrane nephritis in beige mice. Deficiency of leukocytic neutral proteinases prevents the induction of albuminuria in the heterologous phase. J Exp Med. 1989;1:1435–1448. doi: 10.1084/jem.169.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boyce NW, Holdsworth SR. Intrarenal hemodynamic alterations induced by anti-GBM antibody. Kidney Int. 1987;31:8–14. doi: 10.1038/ki.1987.2. [DOI] [PubMed] [Google Scholar]