Abstract
Background. Circulating immune complexes (CIC) containing galactose (Gal)-deficient IgA1 from adults with IgA nephropathy (IgAN) induce proliferation of cultured mesangial cells, but activities of CIC from pediatric patients with the disease have not been studied.
Methods. CIC of different sizes were isolated from sera of pediatric and adult IgAN patients and their effects on cultured human mesangial cells (MC) were assessed by measuring cellular proliferation, expression of IL-6 and IL-8 and laminin and phosphotyrosine signaling.
Results. Large CIC from pediatric IgAN patients (>800 kDa) containing Gal-deficient IgA1 stimulated cellular proliferation, whereas in some patients, smaller CIC were inhibitory. Addition of stimulatory and inhibitory CIC to MC differentially altered phosphorylation patterns of three major tyrosine-phosphorylated proteins of molecular mass 37, 60 and 115 kDa. The stimulatory CIC transiently increased tyrosine-phosphorylation of the 37-kDa protein and decreased phosphorylation of the other two proteins, whereas the inhibitory CIC increased phosphorylation of all three proteins. Furthermore, we investigated the influence of IgA1-containing CIC from sera of children with IgAN with clinically active disease (i.e., abnormal urinalysis and/or serum creatinine concentration) or inactive disease (i.e., normal urinalysis and serum creatinine concentration) on the expression of IL-6 and IL-8 genes by mesangial cells. Real-time reverse transcription–polymerase chain reaction results showed that the CIC from a patient with active disease stimulated MC to express the two cytokine genes at higher levels than did the CIC from a patient with inactive disease. Moreover, stimulatory CIC increased production of the extracellular matrix protein laminin.
Conclusion. These data indicate that sera of pediatric IgAN patients contain biologically active CIC with Gal-deficient IgA1.
Keywords: autoimmunity, circulating immune complexes, glycosylation, immunoglobulin, O-linked glycans
Introduction
IgA nephropathy (IgAN) is characterized by the predominant or co-dominant presence of IgA1 in mesangial immunodeposits [1–4]. The incidence of IgAN is highest among young adults [1, 4, 5] and slightly lower in children and adolescents [5]. Proliferation of mesangial cells (MC) and expansion of extracellular matrix are found in patients with mild clinical disease, but progressive glomerular sclerosis and interstitial fibrosis are associated with end-stage renal disease (ESRD) in 10–20% of patients having onset during childhood, within 20 years after diagnosis [6].
IgA1 in the circulation and in the mesangial deposits displays an abnormal glycosylation pattern [7–19]. Specifically, O-linked glycans of the IgA1 hinge region have a reduced content of galactose (Gal). In the absence of Gal, the terminal sugar is N-acetylgalactosamine (GalNAc), to which sialic acid may be attached [13, 14]. The truncated sugar moieties and/or hinge-region glycopeptides [20, 21] are recognized by circulating anti-glycan antibodies [14, 21, 22] and immune complexes are formed [13, 14, 18, 23, 24]. Due to their physicochemical properties, some of these circulating immune complexes (CIC) apparently escape hepatic clearance and deposit in the renal mesangium to induce an inflammatory response [14, 23, 25–28].
We have shown that CIC containing Gal-deficient IgA1 from adult IgAN patients bind to cultured human MC more efficiently than does uncomplexed Gal-deficient IgA1 [29]. These complexes were of two types: those that stimulated cultured human MC to proliferate and those that inhibited cellular proliferation [30].
In this study, we examined properties of IgA1-containing CIC from sera of pediatric patients with IgAN. Our results support the hypothesis that aberrantly glycosylated IgA1-containing CIC are involved in the pathogenesis of IgAN in children.
Materials and methods
IgAN patients and controls
For proliferation experiments, serum samples from eight patients with biopsy-proven IgAN were collected during an acute episode of macroscopic hematuria [four pediatric patients P1–4 (age ≤18 years) and four adult patients A1–4, Table 1]. Estimated glomerular filtration rate was calculated with the Schwartz formula for pediatric patients [31] and by the four-variable Modification of Diet in Renal Disease formula for adults [32]. Renal histology was graded according to the Haas system [33]. Two additional sera from pediatric patients were used for the real-time reverse transcription–polymerase chain reaction (RT–PCR) assays because no RNA was available from the initial experiment. Subject P2 was studied again when she was 12 years old and had inactive disease, as manifested by a normal urinalysis and normal serum creatinine concentration. An 8-year-old boy (P5, Table 1) was studied within 1 month of diagnostic biopsy. He had active disease with macroscopic hematuria at the time of study. Serum levels of Gal-deficient IgA1 for subjects P1–5 and A1–4 are shown in Supplementary Table 1. Blood samples were collected from four other adult IgAN patients (mean age 24.5 years; two men; serum creatinine ranged from 0.8 to 2.7 mg/dL; dipstick urinary protein ranged from 1+ to 4+ and blood, from 1+ to 4+). As additional controls, blood samples were collected from four healthy adults (mean age 37 years; two men) and two healthy boys (mean age 8.5 years). All healthy controls had normal serum creatinine concentrations and urinalysis negative for protein and blood by dipstick.
Table 1.
Clinical features of IgAN patientsa
| Diagnosis |
Episode of macroscopic hematuria study sample |
Last follow-up |
|||||||||||||||
| Subject | Age | Gender | Biopsy grade | BP | ACEi | eGFR | UP/C | Age | BP | ACEi | eGFR | UP/C | Age | BP | ACEi | eGFR | UP/C |
| P1 | 4.6 | M | 4 | 106/56 | No | 120 | 0.81 | 4.9 | ND | No | ND | 1+b | 8.0 | 115/69 | No | 120 | Traceb |
| P2 | 8.7 | F | 4 | 120/62 | No | 100 | 1.00 | 8.7 | 101/64 | Yes | 95 | 1.44 | 15.4 | 113/64 | No | 113 | 0.14 |
| P3 | 11.6 | F | 4 | 126/67 | No | 106 | 3.65 | 11.9 | 114/74 | Yes | 97 | 3.48 | 18.2 | 119/71 | Yes | 110 | 0.42 |
| P4 | 11.6 | M | 3 | 127/77 | No | 138 | 0.35 | 16.0 | 137/62 | Yes | 131 | 217c | 20.2 | 125/73 | No | 18 | ND |
| P5 | 8.6 | M | 2 | 104/62 | No | 101 | 1.44 | 8.6 | 91/56 | Yes | 101 | 1.93 | 11.2 | 104/62 | Yes | 156 | 0.82 |
| A1 | 22.6 | F | 4 | 116/69 | No | 131 | 0.34 | 23.6 | 101/71 | No | 94 | 0.68 | 26.7 | 120/84 | No | 58 | 0.07 |
| A2 | 25.9 | F | 4 | 102/64 | ARB | 67 | 0.72 | 26.0 | 108/80 | Yes | 71 | 0.72 | 34.8 | 126/86 | Yes | 115 | 0.20 |
| A3 | 18.9 | M | 4 | 150/85 | Yes | 95 | 1.85 | 19.4 | 141/84 | Yes | 102 | 3.49 | 19.5 | 148/80 | Yes | 86 | 1.65 |
| A4 | 38.4 | M | 1 | 155/63 | No | 39 | ND | 40.2 | 155/70 | No | 51 | 0.47 | 41.8 | 119/72 | Yes | 74 | ND |
UP/C, urinary protein/creatinine ratio (milligram protein per milligram creatinine); P, pediatric patient; A, adult patient; ND, not determined; eGFR, estimated glomerular filtration rate (mL/min/1.73m2); MH, macroscopic hematuria. Biopsy grading by the Haas criteria (ref. [33]).
Semi-quantitative protein on a Bayer multi-stick; 1+ is equivalent to 30 mg protein/dL—usually a UP/C ratio <0.5.
Urine creatinine not done, protein expressed in mg/dL urine.
This study was approved by the Institutional Review Boards at the University of Alabama at Birmingham and the University of Tennessee Health Sciences Center. All participants ≥18 years of age provided informed consent. For children under the age of 18 years, informed consent was obtained from a parent or legally authorized representative and all children ≥8 years of age provided signed assent.
Cell cultures and proliferation assays
Human MC were purchased from BioWhittaker (Walkersville, MD) and passages 3–4 were maintained in RPMI 1640 with 20% fetal calf serum (FCS) and other supplements [29, 30].
Proliferation experiments were performed in 24-well tissue culture plates; MC at 85–95% confluence were serum starved in a medium containing 0.5% FCS for 24 h [29]. Serum fractions were filter sterilized, mixed with an equal volume of the medium containing 1% FCS and incubated in duplicate with MC in humidified 5% CO2 atmosphere at 37°C for 20 h [30]. The culture medium alone and medium supplemented with platelet-derived growth factor (PDGF, 10 ng/mL; R&D Systems, Minneapolis, MN) were used as negative and positive controls, respectively. For the last 4 h of incubation, 100 μL 3H-thymidine (1 μCi; PerkinElmer, Wellesley, MA) in the culture medium was added to each well. MC in each well were washed two times with phosphate-buffered saline (PBS) to remove free 3H-thymidine [each wash was filtered using MultiScreen-HA filter plates (Millipore, Bedford, MA) to capture detached cells]. Washed cells were lysed with 0.2 mL 0.3 M NaOH, then each well was washed with 0.1 mL 5% acetic acid; all washes and the filter were combined and added to 5 mL scintillation liquid. The radioactivity was determined using a Wallac liquid scintillation counter 1409DSA (PerkinElmer). Average values were calculated from duplicates for each serum fraction and expressed directly as counts per minute (c.p.m.) or relative to the negative control (c.p.m. of sample/c.p.m. of the control) as relative proliferation. Ratios >1.0 indicate stimulation, while ratios <1.0 indicate inhibition [30].
Isolation of serum fractions enriched with CIC
Serum fractions enriched with CIC were obtained by size-exclusion chromatography [14, 29, 30] with 0.5 mL serum filtered (0.45 μm pore size; Pall Corporation, Ann Arbor, MI) and fractionated on a calibrated Superose 6 column (600 × 12 mm; Amersham Biosciences Corporation, Piscataway, NJ) in PBS [14, 29]. Fractions containing proteins of large molecular mass (from void volume to ∼700 kDa) were collected (0.25 mL per fraction) and each two fractions were pooled. Aliquot of each pooled fraction was filter sterilized and added to MC. Remaining aliquots were analyzed for IgA, IgG, Gal-deficient IgA1 and IgA–IgG complexes [14, 22].
Enzyme-linked immunosorbent assay
Serum fractions were analyzed by enzyme-linked immunosorbent assay (ELISA) for levels of total IgA, IgG, IgG specific for Gal-deficient IgA1 [using Gal-deficient IgA1 (Ale) myeloma protein as antigen] and Gal-deficient IgA1 [using Helix aspersa agglutinin, (HAA), a lectin that binds terminal GalNAc in Gal-deficient IgA1] and for IgA–IgG-containing immune complexes [14, 29, 30]. Biotin-labeled HAA was purchased from Sigma–Aldrich (St. Louis, MO), biotin-labeled goat IgG F(ab’)2 human IgG- and IgA-specific antibodies were purchased from BioSource International (Camarillo, CA) and F(ab’)2 fragment of anti-human IgA antibody (heavy chain-specific) was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). IgA–IgG complexes were determined using capture ELISA [14, 22]. IgG subclasses were determined by ELISA with subclass-specific monoclonal antibodies [13].
Immunohistochemical staining
MC were grown on Lab-Tech chambered slides (Nalge/Nunc, now Thermo Fisher Scientific, Pittsburgh, PA), serum starved as described above and incubated with control medium, serum fractions containing CIC or PDGF for 24 h. MC were then washed, fixed in 3% paraformaldehyde and stained with antibody directed against proliferating cell nuclear antigen (PCNA; DAKO, Carpinteria, CA) to detect proliferating cells or stained with the DeadEnd colorimetric TUNEL (TdT-mediated dUTP Nick-End Labeling) system (Promega, Madison, WI) to detect apoptotic cells. For PCNA staining, antigen heat retrieval was used [34].
Expression of cytokines in MC
MC were grown on slides in RPMI 1640 + 20% FCS to 50–60% confluence and serum starved for 24 h before CIC-containing serum fractions were added. Control cells were cultured in the absence of CIC. After 24 h incubation, brefeldin A (Sigma–Aldrich) was added 3 h before fixation in 3% paraformaldehyde. After permeabilization with saponin, MC were stained for interleukin 6 (IL-6) and tumor growth factor- beta (TGF-β) proteins with goat IgG antibodies specific for human IL-6 and TGF-β (R&D Systems), followed by biotin-labeled anti-goat IgG antibodies. The ABC (Avidin: Biotinylated enzyme Complex; Vector Laboratories, Burlingame, CA) detection system [35] with DAB (3,3′-diaminobenzidine; Sigma–Aldrich) and biotinyl-tyramide reagent kit for signal amplification (PerkinElmer) were used for visualization and the cells were then counterstained with methyl green. MC staining was observed by microscope and the stained cells and total number of cells were counted.
Real-time RT–PCR measurement of transcription of IL-6 and IL-8 genes
Messenger RNA was isolated from serum-starved MC after 24-h stimulation with CIC using RNAStat60 [29]. MC with medium only and medium supplemented with PDGF (10 ng/mL) served as negative and positive controls, respectively. Additional controls included MC supplemented with uncomplexed Gal-deficient IgA1 myeloma protein (Ale). Reverse transcription was done with SuperScript II [29, 36] and real-time PCR was performed for 42 cycles of denaturation at 95°C, annealing at 58°C for IL-8 or at 60°C for IL-6 and extension at 72°C using LightCycler 480 (Roche, Indianapolis, IN) with SYBR Green I chemistry [36]. Primers for β-actin, IL-6 and IL-8 were purchased from R&D Systems.
MC stimulation with CIC for western blot analysis
MC were grown in T25 flasks to ∼90% confluence and then serum starved for 48 h with 0.5% FCS; the medium was changed after 24 h. Four hours before the incubation with CIC, fresh serum-free medium was added. CIC were added in RPMI 1640 for 15 min or 1 h (for phosphotyrosine analysis) or 2 days (for laminin determination).
After incubation with CIC, MC were lysed with lysis buffer (50 mM HEPES, 150 mM NaCl, 1 mM EDTA, 2.5 mM EGTA, 10% glycerol, 0.1% Tween-20, 1 mM dithiothreitol) supplemented with protease- and phosphatase-inhibitor cocktails (Sigma–Aldrich). The protease-inhibitor cocktail contained 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride (AEBSF), aprotinin, leupeptin, bestatin, pepstatin A and E-64. Phosphatase-inhibitor cocktail included cantharidin, bromotetramisole, microcytin LR, sodium orthovanadate, sodium molybdenate, sodium tartarate and imidazole. We also used additional inhibitors, phenylmethanesulfonyl fluoride (1 mM), NaF (1 mM) and β-glycerophosphate (10 mM) (Sigma–Aldrich). The resultant lysates were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis using 10% polyacrylamide gel (Bio-Rad, Hercules, CA).
Proteins were transferred to polyvinylidene difluoride (PVDF) membrane (0.45 μM, Millipore). After transfer, the PVDF membrane was blocked with SuperBlock Blocking Buffer (Pierce, Rockford, IL) and incubated overnight with mouse monoclonal anti-phosphotyrosine antibody (clone 4G10; Upstate, Charlottesville, VA) or polyclonal rabbit anti-human laminin antibody (Chemicon, Temecula, CA; specific for beta chains) at 4°C, followed by 1-h incubation with anti-mouse Ig horseradish peroxidase-conjugate (Upstate) at room temperature. The reaction was developed with SuperSignal West Pico Solution (Pierce).
Results
Proliferation of MC after incubation with CIC from serum samples collected from IgAN patients during episodes of macroscopic hematuria
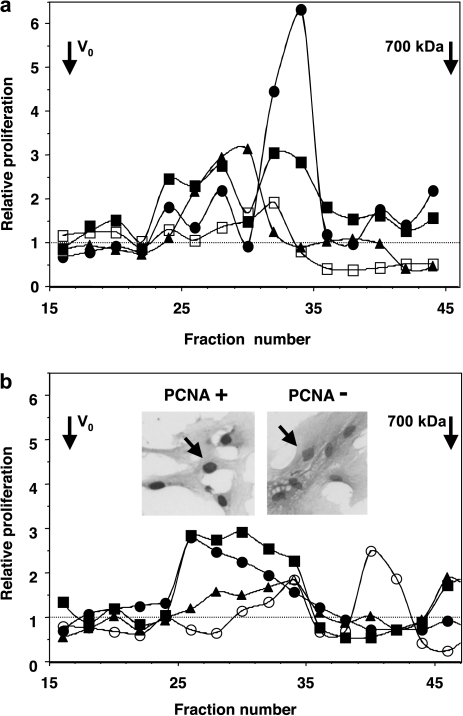
IgA1-containing CIC from sera of four pediatric IgAN patients collected at the time of acute disease (macroscopic hematuria, Table 1, subjects P1–P4) were fractionated based on their size. Individual fractions were added to serum-starved MC in culture, and cellular proliferation was measured. Two types of IgA1-containing CIC were observed: stimulatory CIC and inhibitory CIC (Figure 1a). Fractions with stimulatory activity (≥800–900 kDa) corresponded to CIC with large amounts of Gal-deficient IgA1 (detected by ELISA as HAA-reactive IgA1; [13, 14, 30]). Serum levels of Gal-deficient IgA1 are provided in Supplementary Table 1. Samples from all pediatric patients (Figure 1a) contained stimulatory complexes (≥800–900 kDa); samples from two of them also had inhibitory complexes (with molecular mass of 700–800 kDa). The increase in proliferation after stimulation with these complexes was 2- to 6-fold greater than baseline proliferation. Interestingly, Patient P4 whose complexes exhibited the greatest stimulatory activity (Figure 1a) later manifested sustained proteinuria over several years, as well as recurrent macroscopic hematuria, and eventually progressed to ESRD. Conversely, the individual (P3) who had low amounts of stimulatory CIC with Gal-deficient IgA1 (Figure 1a, empty symbols) also had low levels of serum Gal-deficient IgA1 (Supplementary table 1).
Fig. 1.
Proliferation of human MC measured by 3H-thymidine incorporation after stimulation with IgA1-containing CIC from sera of (a) four pediatric IgAN patients (P1, full square; P2, full triangle; P3, empty square; P4, full circle) and (b) four adult IgAN patients (A1, full circle; A2, full square; A3, full triangle; A4, empty circle), all collected at the time of macroscopic hematuria (Table 1). All samples exhibited stimulatory CIC of ≥800–900 kDa and some samples also had smaller, inhibitory CIC of ∼700–800 kDa. Staining for PCNA confirmed the results: stimulatory CIC increased expression of PCNA in MC. Inserts in (b) show staining of PCNA-positive and PCNA-negative MC in culture. Dashed lines mark background level of MC proliferation.
In a fashion similar to that for pediatric patients, IgA1-containing CIC in the samples from adult patients with IgAN collected also at the time of macroscopic hematuria (Table 1) showed stimulatory activity in the fractions containing CIC ≥800–900 kDa (Figure 1b). The individual (A4) who had low amounts of stimulatory CIC with Gal-deficient IgA1 (Figure 1b, empty symbols) also had low levels of serum Gal-deficient IgA1 (Supplementary table 1). MC incubated with stimulatory and non-stimulatory serum fractions were stained with anti-PCNA antibody (insert in Figure 1b). Serum-starved MC in the basal medium (RPMI 1640 + 0.5% FCS) showed cells modestly expressing PCNA, whereas incubation with Gal-deficient IgA1-containing CIC (800–900 kDa) markedly stimulated expression (93% of MC were PCNA-positive; left insert in Figure 1b). As a positive control, PDGF induced PCNA expression in 98% of the MC. We also examined cellular apoptosis using TUNEL staining. There was no change in the staining of MC stimulated with various serum fractions, including the inhibitory CIC, suggesting that inhibition of 3H-thymidine incorporation by 700–800 kDa CIC was due to decreased cellular proliferation rather than accelerated apoptosis.
Cultured human MC incubated with 800–900-kDa IgA1-containing CIC from IgAN patients exhibited enhanced cellular proliferation and spreading compared to MC incubated with corresponding fractions from sera of normal healthy controls (Supplementary figure 1). Furthermore, depletion of IgA from serum abolished the stimulatory and inhibitory properties of CIC-containing fractions, as described [30]. Uncomplexed Gal-deficient IgA1 myeloma protein did not alter MC proliferation or cell spreading. The magnitude of this proliferation depended on the type of immune complexes in the individual samples. By ELISA, the stimulatory complexes contained large amounts of Gal-deficient IgA1 complexed with IgG. IgG3 and IgG1 were the predominant IgG subclasses in both types of immune complexes [22].
Activation of MC by Gal-deficient IgA1-containing CIC: protein tyrosine phosphorylation
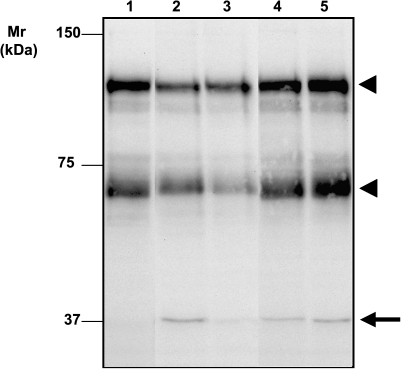
Tyrosine phosphorylation of cellular proteins is one of the major means for signal transduction and regulation of enzymatic activities. We used anti-phosphotyrosine antibody to assess potential effects on MC of Gal-deficient IgA1-containing CIC. Incubation of MC with stimulatory and inhibitory CIC resulted in different profiles of major tyrosine-phosphorylated proteins of Mr 37, 60 and 115 kDa. While the stimulatory CIC transiently increased phosphorylation of the 37-kDa protein and decreased phosphorylation of the other two proteins, the inhibitory CIC increased phosphorylation of all three proteins (Figure 2). These results indicated differential activities of the stimulatory and inhibitory complexes on protein tyrosine phosphorylation.
Fig. 2.
Western blot of MC lysates with anti-phosphotyrosine antibody. Human MC were incubated with stimulatory (Lanes 2 and 3) or inhibitory (Lanes 4 and 5) immune complexes for 15 min (Lanes 2 and 4) or 1 h (Lanes 3 and 5). Lysate sample from mock-treated control (untreated) MC is in Lane 1. Stimulatory CIC transiently increased phosphorylation of the 37-kDa protein and decreased phosphorylation of the other two proteins, whereas the inhibitory CIC increased phosphorylation of all three proteins. The arrow marks 37-kDa phosphoprotein; arrowheads, the other two phosphoproteins.
Activation of MC by Gal-deficient IgA1-containing CIC: induction of cytokine production
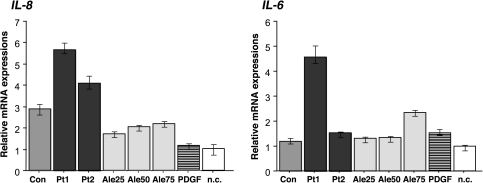
Our preliminary results, as well as results of other investigators, indicated that circulating IgA1-containing complexes from patients with IgAN affected production of cytokines [37, 38]. Therefore, we measured the effect of the stimulatory CIC on MC and their expression of IL-6 and IL-8 using real-time RT–PCR. CIC were isolated from sera of two pediatric patients, one with active and one with inactive disease, and from a healthy pediatric control. Aliquots of the isolated CIC samples were analyzed for total IgA, Gal-deficient IgA1 and IgG–IgA CIC and sera of the subjects were analyzed for total IgA, Gal-deficient IgA1 and anti-Gal-deficient-IgA1 IgG antibodies (Table 2). These analyses showed that the IgAN patient with active disease had higher amounts of Gal-deficient IgA1 and anti-Gal-deficient-IgA1 IgG antibodies compared to those in the patient with inactive disease and the healthy control. Similarly, the patient with active disease had higher amount of Gal-deficient IgA1 in the pooled CIC compared to the patient with inactive disease and the healthy control (Table 2). CIC from the patient with active disease stimulated MC to express the two cytokine genes at higher levels than did the CIC from the patient with inactive disease (Figure 3). MC incubated with the corresponding serum fractions from a healthy control increased transcription of IL-8 gene (P < 0.01) but only marginally for that of IL-6 gene compared to the mock-treated control. Additional controls included polymeric Gal-deficient IgA1 (Ale) myeloma protein and PDGF. The IgA1 (Ale) myeloma protein increased transcription of both cytokine genes in a dose-dependent fashion, but the stimulatory effect was significantly lower than that of CIC from the serum of the IgAN patient with active disease. In contrast, PDGF increased expression of IL-6 but not IL-8. In summary, IgA1-containing CIC from IgAN patients that stimulated proliferation of MC also exhibited strong stimulatory effect on expression of IL-6 and IL-8 genes and contained elevated amounts of Gal-deficient IgA1.
Table 2.
Concentration of IgA, Gal-deficient IgA1 (HAA-IgA1) and IgG-IgA CIC in the pooled stimulatory fractions and levels of IgA, Gal-deficient IgA1 (HAA-IgA1) and IgG specific for Gal-deficient IgA1 in serum samples
| Pooled fractions | Con | Pt1 | Pt2 |
| IgA (ng/mL) | 37.40 | 108.10 | 185.20 |
| HAA-IgA1 (OD @ 490 nm) | 0.86 | 3.59 | 2.44 |
| IgG-IgA CIC (OD @ 490 nm) | 0.88 | 1.29 | 1.38 |
| Serum | |||
| IgA (mg/mL) | 1.73 | 2.22 | 1.73 |
| HAA-IgA1 (U/mL) | 458 | 935 | 803 |
| IgG specific for Gal-deficient IgA1 (U/mg IgG) | 210 | 500 | 320 |
Fig. 3.
Transcription of IL-8 and IL-6 genes was determined by real-time RT–PCR using total RNA from human MC incubated for 20 h with stimulatory CIC from sera of two patients with IgAN (Pt columns) or corresponding fractions from a healthy control (Con columns). Pt1 had active disease, whereas Pt2 had an inactive disease (i.e., normal urinalysis). Medium without CIC served as the negative control (n.c. columns). PDGF (PDGF columns) and free uncomplexed Gal-deficient IgA1 myeloma protein (Ale) served as additional controls. IgA1 (Ale) was used in concentrations 25, 50 and 75 μg per well (Ale25, Ale50, Ale75 columns). Expression of specific genes was measured in triplicate and expressed relative to that of β-actin. Data comparisons were performed by the E-method [36] and calculated as mean expression in control MC to the expression in stimulated MC. Means and ranges are shown.
To verify and expand these data, we stained MC with IL-6-specific antibody and determined the number of positive cells before and after incubation with the stimulatory IgA1-containing complexes. In addition, we stained MC for TGF-β, a potent pro-fibrogenic factor involved in stimulating the production of extracellular matrix proteins. The large molecular mass IgA1-containing complexes increased the number of cells producing IL-6 (32% of the cells stained after stimulation versus 2% at baseline). An increase of TGF-β-positive cells was also observed: ∼20% cells were positive after the stimulation compared with 2% positive cells at baseline.
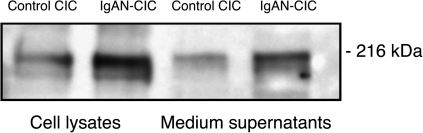
As the incubation of MC with IgA1-containing CIC induced production of TGF-β, we next determined whether synthesis of extracellular matrix components, such as laminin, was also affected. Human MC were incubated for 2 days with the fractions containing the large-molecular-mass complexes from an IgAN patient. As a control, we used comparable serum fractions from a healthy individual. Then, the cells and supernatants were harvested and analyzed by western blot using anti-laminin antibody (Figure 4). An increased amount of laminin was found in the cells and the supernatants from the cultured MC stimulated by the CIC.
Fig. 4.
Expression of laminin in human MC incubated for 48 h with stimulatory CIC from an IgAN patient or with serum fractions of similar size from a normal control. Lysed MC (same amount of protein was loaded) or culture supernatants (same volume of supernatant was loaded) were separated using 4–20% gradient sodium dodecyl sulfate–polyacrylamide gel electrophoresis under reducing conditions and the blots were probed with polyclonal anti-laminin antibody specific for beta chains.
Discussion
In this study, we showed that, for pediatric patients with IgAN, CIC of various sizes with aberrantly glycosylated IgA1 differentially altered proliferation of MC in vitro. Greater stimulatory activity by CIC from some pediatric patients compared to that for complexes from other patients may reflect a larger amount of these complexes or an accentuated biological activity. While it is not clear what role these CIC play in vivo, we postulate that their size and composition have some bearing on their biological activity. Factors such as molecular form and quantity of Gal-deficient IgA1 [36], number and localization of terminal GalNAc epitopes in the hinge region and concentration, isotype and affinity of the circulating anti-glycan antibodies [22] may influence the properties of the IgA1-CIC, their hepatic catabolism and deposition in the renal mesangium [29, 30]. Our results also revealed that CIC from a patient with active disease stimulated MC to express genes encoding IL-6 and IL-8 at higher levels than did the CIC from the patient with inactive disease. Others found that IgA1-stimulated MC produce soluble factors, such as TNFα or platelet-activating factor, that affect glomerular permselectivity through regulating expression of nephrin and other molecules in podocytes [39, 40].
Our findings demonstrated that CIC from both adult and pediatric IgAN patients contain aberrantly glycosylated IgA1. The role of aberrant glycosylation of IgA1 in the formation of the pathogenic CIC was further supported by the observation that one pediatric and one adult patient who had low serum levels of Gal-deficient IgA1 showed low proliferative activity of CIC (P3 and A4 in Figure 1, empty symbols and Supplementary table 1). Adding the large complexes (≥800–900 kDa) to cultures of human MC induced cellular proliferation and synthesis of inflammatory cytokines and laminin, a component of the mesangial extracellular matrix. Furthermore, the IgA1-containing CIC differentially induced a phosphotyrosine pattern of cellular proteins. The large stimulatory complexes transiently increased phosphorylation of the 37-kDa protein and decreased phosphorylation of the other two major phosphoproteins of 60 and 115 kDa, whereas small inhibitory CIC increased phosphorylation of all three proteins. Because protein-tyrosine kinases are often involved in cell signaling upon binding of ligand to its receptor, we interpret these results to be an additional manifestation of the differences in biological properties of the two types of IgA1-containing CIC.
In summary, patients with IgAN exhibit two types of IgA1-containing CIC: large complexes that stimulate proliferation of cultured human MC and small complexes that inhibit cellular proliferation. These complexes also differentially alter cellular protein tyrosine phosphorylation. Furthermore, the large stimulatory IgA1-containing CIC increase expression of cytokines and synthesis of an extracellular matrix protein by cultured human MC. The stimulatory complexes are abundant in Gal-deficient IgA1. Based on these findings, we contend that the pathogenesis of IgAN in children and adults is likely mediated by renal deposition of CIC containing abnormally glycosylated IgA1.
Supplementary data
Supplementary data are available online at http://ndt.oxfordjournals.org.
Acknowledgments
This work was supported by grants DK078244, DK082753, DK080301, DK061525, DK083663 and DK071802 from the National Institutes of Health, by General Clinical Research Centers of the University of Alabama at Birmingham (M01 RR00032) and the University of Tennessee Health Sciences Center (M01 RR00211), Research Projects of the Ministry of Health of the Czech Republic No. MSM 6198959216, MSM 0021620819 and MSM 0021620812 and a generous gift from Anna and Donald Waite. The authors express their appreciation to Catherine V. Barker and Sue Y. Woodford (University of Alabama at Birmingham) and Dr Kimberly Fisher and Sandra Grimes (University of Tennessee) for help with collecting clinical samples, Rose Kulhavy (University of Alabama at Birmingham) for providing IgA1 myeloma proteins and Candace Kirksey and Claretha Nichols for the processing of serum samples.
Conflict of interest statement. None declared.
References
- 1.Berger J, Hinglais N. Les dépôts intercapillaires d'IgA-IgG (Intercapillary deposits of IgA-IgG) J Urol Nephrol (Paris) 1968;74:694–695. [PubMed] [Google Scholar]
- 2.Conley ME, Cooper MD, Michael AF. Selective deposition of immunoglobulin A1 in immunoglobulin A nephropathy, anaphylactoid purpura nephritis, and systemic lupus erythematosus. J Clin Invest. 1980;66:1432–1436. doi: 10.1172/JCI109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Julian BA, Novak J. IgA nephropathy: an update. Curr Opin Nephrol Hypertens. 2004;13:171–179. doi: 10.1097/00041552-200403000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Emancipator SN. IgA nephropathy and Henoch-Schönlein syndrome. In: Jennette JC, Olson JL, Schwartz MM, Silva FG, editors. Heptinstall's Pathology of the Kidney. Philadelphia, PA: Lippincott-Raven Publishers; 1998. pp. 479–539. [Google Scholar]
- 5.Wyatt RJ, Julian BA, Baehler RW, et al. Epidemiology of IgA nephropathy in central and eastern Kentucky for the period 1975 through 1994. J Am Soc Nephrol. 1998;9:853–858. doi: 10.1681/ASN.V95853. [DOI] [PubMed] [Google Scholar]
- 6.Sanders JT, Wyatt RJ. IgA nephropathy and Henoch-Schönlein purpura nephritis. Curr Opin Pediatr. 2008;20:163–170. doi: 10.1097/MOP.0b013e3282f4308b. [DOI] [PubMed] [Google Scholar]
- 7.Andre PM, Le Pogamp P, Chevet D. Impairment of jacalin binding to serum IgA in IgA nephropathy. J Clin Lab Anal. 1990;4:115–119. doi: 10.1002/jcla.1860040208. [DOI] [PubMed] [Google Scholar]
- 8.Mestecky J, Tomana M, Crowley-Nowick PA, et al. Defective galactosylation and clearance of IgA1 molecules as a possible etiopathogenic factor in IgA nephropathy. Contrib Nephrol. 1993;104:172–182. doi: 10.1159/000422410. [DOI] [PubMed] [Google Scholar]
- 9.Allen AC, Harper SJ, Feehally J. Galactosylation of N- and O-linked carbohydrate moieties of IgA1 and IgG in IgA nephropathy. Clin Exp Immunol. 1995;100:470–474. doi: 10.1111/j.1365-2249.1995.tb03724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiki Y, Horii A, Iwase H, et al. O-linked oligosaccharide on IgA1 hinge region in IgA nephropathy. Fundamental study for precise structure and possible role. Contrib Nephrol. 1995;111:73–84. [PubMed] [Google Scholar]
- 11.Baharaki D, Dueymes M, Perrichot R, et al. Aberrant glycosylation of IgA from patients with IgA nephropathy. Glycoconj J. 1996;13:505–511. doi: 10.1007/BF00731436. [DOI] [PubMed] [Google Scholar]
- 12.Hiki Y, Kokubo T, Iwase H, et al. Underglycosylation of IgA1 hinge plays a certain role for its glomerular deposition in IgA nephropathy. J Am Soc Nephrol. 1999;10:760–769. doi: 10.1681/ASN.V104760. [DOI] [PubMed] [Google Scholar]
- 13.Tomana M, Matousovic K, Julian BA, et al. Galactose-deficient IgA1 in sera of IgA nephropathy patients is present in complexes with IgG. Kidney Int. 1997;52:509–516. doi: 10.1038/ki.1997.361. [DOI] [PubMed] [Google Scholar]
- 14.Tomana M, Novak J, Julian BA, et al. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest. 1999;104:73–81. doi: 10.1172/JCI5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung JCK, Poon PYK, Lai KN. Increased sialylation of polymeric immunoglobulin A1: mechanism of selective glomerular deposition in immunoglobulin A nephropathy? J Lab Clin Med. 1999;133:152–160. doi: 10.1016/s0022-2143(99)90008-2. [DOI] [PubMed] [Google Scholar]
- 16.Floege J, Feehally J. IgA nephropathy: recent developments. J Am Soc Nephrol. 2000;11:2395–2403. doi: 10.1681/ASN.V11122395. [DOI] [PubMed] [Google Scholar]
- 17.Novak J, Julian BA, Tomana M, et al. Progress in molecular and genetic studies of IgA nephropathy. J Clin Immunol. 2001;21:310–327. doi: 10.1023/a:1012284402054. [DOI] [PubMed] [Google Scholar]
- 18.Mestecky J, Novak J, Julian BA, et al. Pathogenic potential of galactose-deficient IgA1 in IgA nephropathy. Nephrology. 2002;7:S92–S99. [Google Scholar]
- 19.Coppo R, Amore A. Aberrant glycosylation in IgA nephropathy (IgAN) Kidney Int. 2004;65:1544–1547. doi: 10.1111/j.1523-1755.2004.05407.x. [DOI] [PubMed] [Google Scholar]
- 20.Kokubo T, Hiki Y, Iwase H, et al. Exposed peptide core of IgA1 hinge region in IgA nephropathy. Nephrol Dial Transplant. 1999;14:81–85. doi: 10.1093/ndt/14.1.81. [DOI] [PubMed] [Google Scholar]
- 21.Kokubo T, Hashizume K, Iwase H, et al. Humoral immunity against the proline-rich peptide epitope of the IgA1 hinge region in IgA nephropathy. Nephrol Dial Transplant. 2000;15:28–33. doi: 10.1093/ndt/15.1.28. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki H, Fun R, Zhang Z, et al. Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest. 2009;119:1668–1677. doi: 10.1172/JCI38468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Czerkinsky C, Koopman WJ, Jackson S, et al. Circulating immune complexes and immunoglobulin A rheumatoid factor in patients with mesangial immunoglobulin A nephropathies. J Clin Invest. 1986;77:1931–1938. doi: 10.1172/JCI112522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coppo R, Basolo B, Piccoli G, et al. IgA1 and IgA2 immune complexes in primary IgA nephropathy and Henoch-Schönlein nephritis. Clin Exp Immunol. 1984;57:583–590. [PMC free article] [PubMed] [Google Scholar]
- 25.Coppo R, Basolo B, Martina G, et al. Circulating immune complexes containing IgA, IgG and IgM in patients with primary IgA nephropathy and with Henoch-Schönlein nephritis. Correlation with clinical and histologic signs of activity. Clin Nephrol. 1982;18:230–239. [PubMed] [Google Scholar]
- 26.Allen AC, Bailey EM, Brenchley PEC, et al. Mesangial IgA1 in IgA nephropathy exhibits aberrant O-glycosylation: observations in three patients. Kidney Int. 2001;60:969–973. doi: 10.1046/j.1523-1755.2001.060003969.x. [DOI] [PubMed] [Google Scholar]
- 27.Hiki Y, Odani H, Takahashi M, et al. Mass spectrometry proves under-O-glycosylation of glomerular IgA1 in IgA nephropathy. Kidney Int. 2001;59:1077–1085. doi: 10.1046/j.1523-1755.2001.0590031077.x. [DOI] [PubMed] [Google Scholar]
- 28.Levinsky RJ, Barratt TM. IgA immune complexes in Henoch-Schönlein purpura. Lancet. 1979;2:1100–1103. doi: 10.1016/s0140-6736(79)92505-4. [DOI] [PubMed] [Google Scholar]
- 29.Novak J, Vu HL, Novak L, Julian BA, et al. Interactions of human mesangial cells with IgA and IgA-containing circulating immune complexes. Kidney Int. 2002;62:465–475. doi: 10.1046/j.1523-1755.2002.00477.x. [DOI] [PubMed] [Google Scholar]
- 30.Novak J, Tomana M, Matousovic K, et al. IgA1-containing immune complexes in IgA nephropathy differentially affect proliferation of mesangial cells. Kidney Int. 2005;67:504–513. doi: 10.1111/j.1523-1755.2005.67107.x. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz GJ, Haycock GB, Edelmann CM, Jr., et al. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–263. [PubMed] [Google Scholar]
- 32.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 33.Haas M. Histologic subclassification of IgA nephropathy: a clinicopathologic study of 244 cases. Am J Kidney Dis. 1997;29:829–842. doi: 10.1016/s0272-6386(97)90456-x. [DOI] [PubMed] [Google Scholar]
- 34.Kerby JD, Verran DJ, Luo KL, et al. Immunolocalization of FGF-1 and receptors in human renal allograft vasculopathy associated with chronic rejection. Transplantation. 1996;62:467–475. doi: 10.1097/00007890-199608270-00008. [DOI] [PubMed] [Google Scholar]
- 35.Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki H, Moldoveanu Z, Hall S, et al. IgA1-secreting cell lines from patients with IgA nephropathy produce aberrantly glycosylated IgA1. J Clin Invest. 2008;118:629–639. doi: 10.1172/JCI33189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duque N, Gomez-Guerrero C, Egido J. Interaction of IgA with Fcα receptors of human mesangial cells activates transcription factor nuclear factor-κB and induces expression and synthesis of monocyte chemoattractant protein-1, IL-8, and IFN-inducible protein 10. J Immunol. 1997;159:3474–3482. [PubMed] [Google Scholar]
- 38.Moura IC, Arcos-Fajardo M, Gdoura A, et al. Engagement of transferrin receptor by polymeric IgA1: evidence for a positive feedback loop involving increased receptor expression and mesangial cell proliferation in IgA nephropathy. J Am Soc Nephrol. 2005;16:2667–2676. doi: 10.1681/ASN.2004111006. [DOI] [PubMed] [Google Scholar]
- 39.Lai KN, Leung JC, Chan LY, et al. Podocyte injury induced by mesangial-derived cytokines in IgA nephropathy. Nephrol Dial Transplant. 2009;24:62–72. doi: 10.1093/ndt/gfn441. [DOI] [PubMed] [Google Scholar]
- 40.Coppo R, Fonsato V, Balegno S, et al. Aberrantly glycosylated IgA1 induces mesangial cells to produce platelet-activating factor that mediates nephrin loss in cultured podocytes. Kidney Int. 2010;77:417–427. doi: 10.1038/ki.2009.473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.