Abstract
Previous work in culture has shown that basal forebrain (BF) oligodendrocyte (OLG) lineage cells respond to BDNF by increasing DNA synthesis and differentiation. Further, in the BF in vivo, reduced levels of BDNF as seen in BDNF+/− mice result in reduced numbers of NG2+ cells and deficits in myelin proteins throughout development and in the adult, suggesting that BDNF impacts the proliferating population of OLGs as well as differentiation in vivo. In this study, to investigate the roles BDNF may play in the repair of a demyelinating lesion, the cuprizone model was used and the corpus callosum was examined. BDNF protein levels were reduced after cuprizone treatment, suggesting that the demyelinating lesion itself elicits a decrease in BDNF. To analyze the effects of a further reduction of BDNF on OLG lineage cells following cuprizone, BDNF+/− mice were evaluated. These mice exhibited a blunted increase in the NG2 response at 4 and 5 weeks of cuprizone treatment. In addition, BDNF+/− mice exhibited decreased levels of myelin proteins during the demyelination and remyelination processes with no change in the total number of OLGs. These effects appear to be relatively specific to OLG lineage cells as comparable changes in CD11b+ microglia, GFAP+ astrocytes, and SMI32+ injured axons were not observed. These data indicate that BDNF may play a role following a demyelinating lesion by regulating the numbers of progenitors and the abilities of demyelinating and differentiating cells to express myelin proteins.
Introduction
An inherent capacity within the CNS to remyelinate denuded axons is evident in animal models of demyelination (Ludwin, 1987) and is also apparent in multiple sclerosis (MS) (Prineas et al., 1989; Raine and Wu, 1993). However, most remyelination is unsuccessful. Deficits in remyelination may depend, at least partially, on the ability of progenitors within the lesion area to proliferate and replace dying oligodendrocytes (OLGs) as well as the ability of OLGs (new or surviving) to express myelin proteins. Evidence suggests that remyelinating OLGs are derived from the pool of precursors present in the adult CNS (Ffrench-Constant and Raff, 1986; Wolswijk and Noble, 1989; Woodruff and Franklin, 1997; Watanabe et al., 2002), and that these progenitors are able to proliferate and differentiate into myelinating OLGs as in development (Gensert and Goldman, 1997; Di Bello et al., 1999). These progenitors are present in areas of active demyelination and remyelination in MS (Wilson et al., 2006) and may serve as potential sources of remyelinating OLGs. Factors that increase the survival, proliferation, and migration of progenitors and enhance OLG differentiation and synthesis of myelin, therefore, may be important to affect remyelination.
Multiple growth factors are candidates to affect the remyelination/demyelination processes. For example, platelet-derived growth factor (PDGF), insulin-like growth factor-1 (IGF-1), and epidermal growth factor have been shown to enhance remyelination (Mason et al., 2000a; Murtie et al., 2005; Aguirre et al., 2007; Vana et al., 2007). Other studies suggest that the neurotrophins may also impact recovery from demyelination. Following a lysolecithin-induced lesion, neurotrophin-3 (NT-3) decreases the demyelinated volume and increases myelin basic protein (MBP)+ OLGs in the lesion site (Jean et al., 2003). Similarly, nerve growth factor infusion into a lysolecithin-induced lesion enhances remyelination (Althaus, 2004). The present study investigates the role that a related neurotrophin, brain-derived neurotrophic factor (BDNF), may play in the repair of a cuprizone-elicited demyelinating lesion.
Previous culture studies indicate that BDNF enhances DNA synthesis and differentiation of basal forebrain (BF) OLGs through the trkB receptor (Du et al., 2006a; Van't Veer et al., 2009). In addition, mice with reduced levels of BDNF (BDNF+/− mice) exhibit deficits in numbers of BF NG2+ progenitors and myelin proteins throughout development and in adults (Vondran et al., 2010), suggesting that BDNF is important for the development of BF OLG lineage cells in vivo. Using BDNF+/− mice, we now evaluate whether BDNF may impact OLG progenitors and enhance the expression of myelinated traits in the corpus callosum following a cuprizone-elicited demyelinating lesion. Mice fed cuprizone exhibit a consistent time course of demyelination and proliferation of progenitors in the midline region of the corpus callosum overlying the fornix and consistent remyelination following removal from the drug (Mason et al., 2001; Matsushima and Morell, 2001), making this an attractive model with which to examine demyelination and remyelination.
We report that BDNF+/− mice exhibit deficits in progenitor cell and myelin protein responses to cuprizone, suggesting that BDNF is critical to recovery from a demyelinating lesion.
Materials and Methods
Experimental animals.
Breeding pairs of BDNFTm1Jae mice on a 129/BalbC/C57 background were purchased from Jackson Laboratories. Food and water were available ad libitum. The animals were managed by the University of Medicine and Dentistry of New Jersey/Robert Wood Johnson Animal Facility, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. Animal maintenance, husbandry, transportation, and housing are in compliance with the Laboratory Animal Welfare Act (PL 89–544; PL-91–579). Moreover, our use of animals is in compliance with NIH guidelines (NIH Manual, Chapter 4206). Breeding pairs were maintained by crossing wild-type and heterozygous animals. Mouse genotypes were determined by PCR from tail-derived DNA as described by Ernfors et al. (1994).
Cuprizone treatment.
The 8–10-week-old male BDNF+/+ and BDNF+/− mice were fed cuprizone (0.2% milled into mouse feed; Harlan Teklad) or control feed continuously for 3–6 weeks to evaluate the effects of BDNF on demyelination/remyelination. Alternatively, 8–10-week-old male BDNF+/+ and BDNF+/− mice were fed cuprizone (0.3% milled into mouse feed; Harlan Teklad) or control feed for 6 weeks followed by 4 weeks of control feed (6 + 4) to evaluate the effects of BDNF on a purely remyelination phase.
Western blot.
The midline of the corpus callosum overlying the fornix of BDNF+/+ and BDNF+/− littermates was dissected and frozen at −80°C. Tissue was lysed with lysis buffer containing 50 mm Tris-Hcl, 150 mm NaCl, 10 mm EDTA, 2 mm EGTA, 1% CHAPS, 0.5% NP-40, 1% Triton, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 20 μg/ml soybean trypsin inhibitor, 50 mm NaF, 1 mm PMSF, 0.5 μm microcystin, and 1 mm orthovanadate. Protein concentration was quantified using a BCA protein assay kit (Pierce). Both BDNF+/+ and BDNF+/− samples contained 10 μg of protein plus loading buffer containing 20% glycerol, 12% 2-mercaptoethanol, 8.7% SDS, 0.1% bromophenol blue, and 0.35 m Tris-HCl. Protein was run on 12% Bis-Tris gels for BDNF, MBP, and proteolipid protein (PLP); 3–8% Tris-acetate gels for NG2 and SMI32; or 4–12% Tris-glycine gels for myelin-associated glycoprotein (MAG) and GFAP.
Protein was transferred to a PVDF membrane (Millipore). Membranes were blocked with 4% BSA/TBS-T for 1 h followed by overnight incubation with primary antibody, polyclonal anti-BDNF (1:200; Santa Cruz Biotechnology), monoclonal anti-MBP (1:200; Serotec), polyclonal anti-MAG (1:1000; Millipore Bioscience Research Reagents), polyclonal anti-PLP (1:500; Santa Cruz Biotechnology), polyclonal anti-NG2 (1:750; Millipore Bioscience Research Reagents), monoclonal anti-GFAP (1:1000; Millipore Bioscience Research Reagents), or monoclonal anti-SMI32 (1:1000; Covance). Membranes were then incubated in horseradish peroxidase-linked anti-rabbit antibody (1:3000; GE Healthcare) for BDNF, MAG, or NG2; or anti-mouse antibody (1:3000; GE Healthcare) for MBP, GFAP, or SMI32; or biotinylated anti-goat (1:500; Vector Laboratories) for PLP followed by horseradish peroxidase-linked streptavidin (1:3000; GE Healthcare). Bands were visualized with a chemiluminescence system (Pierce). Membranes were stripped and reprobed with an antibody serving as a loading control: anti-GAPDH (1:1000; Biodesign) for BDNF, MBP, MAG, PLP, and GFAP; or anti-β-tubulin (1:1000; Sigma) for NG2 and SMI32. Western blots were analyzed using Quantity One V 4.2.1 software (Bio-Rad).
Immunohistochemistry.
Mice were perfused transcardially with 4% paraformaldehyde in PBS and post-fixed for 24 h in the same fixative followed by 48 h in 30% sucrose/PBS. Brains were embedded in OCT and frozen at −80°C for immunohistochemistry.
For MBP and FluoroMyelin staining, mouse brains were sectioned coronally in 14 μm serial sections onto slides using a Leica cryostat. For CC1, NG2, and CD11b staining, mouse brains were sectioned coronally in 30 μm serial sections using a Leica cryostat and kept free floating. The corpus callosum that was assessed includes the area at the midline (between the cinguli) overlying the fornix. For NG2 and CD11b staining, sections were blocked with 3% H2O2 for 30 min and 30% goat serum/0.3% Triton/PBS for 1 h, and incubated with polyclonal anti-NG2 (1:750; Millipore Bioscience Research Reagents) or CD11b (1:50; Millipore Bioscience Research Reagents) for 72 h. For CC1 staining, sections were microwaved in 0.01 m citrate buffer, blocked with 10% goat serum/PBS, and incubated with monoclonal anti-CC1 (1:200; Calbiochem). For MBP staining, sections were blocked with 10% goat serum/PBS, and incubated with monoclonal anti-MBP (Serotec; 1:50) for 72 h. After removing each of these primary antibodies, sections were incubated with biotinylated anti-rabbit IgG (1:200; Vector Laboratories) for NG2, anti-mouse IgG (1:200; Vector Laboratories) for CC1 and MBP, or anti-rat IgG (1:200; Vector Laboratories) for CD11b and visualized using the avidin-biotin complex (ABC) technique (Vectastain Elite Kit; Vector Laboratories) and the 3,3′-diaminobenzidine tablet set (DAB; Sigma). For FluoroMyelin staining, sections were treated as per the manufacturer's instructions (Invitrogen). Sections from BDNF+/+ and BDNF+/− mice from specific time points were treated identically side by side.
For double immunostaining for CC1 and trkB, sections were microwaved in 0.01 m citrate buffer, blocked in 33% goat serum/1% bovine serum albumin/0.1% Triton/PBS, and incubated with anti-CC1 for 72 h. Sections were reblocked with 33% goat serum/1% bovine serum albumin/0.1% Triton/PBS and incubated with polyclonal anti-trkB for 72 h. Sections were incubated with Alexa Fluor 594 anti-mouse antibody (1:750; Invitrogen) for 2 h. After washing with PBS, sections were incubated with biotinylated anti-rabbit antibody for 1 h followed by Alexa Fluor 488 streptavidin (1:800; Invitrogen) for 2 h.
Quantification.
Estimates for volumes and cell numbers in the corpus callosum were obtained by unbiased stereological morphometric analysis (West et al., 1991) using a Zeiss Axiophot and the Bioquant Image Analyzer Program, version 6.90.10. This method uses a 3D probe for counting cell nuclei, the optical dissector, and a systematic sampling scheme, the fractionator. The thickness is sampled so the density is measured as the number of cells per cubic millimeter. The 3D counting frame, the optical dissector, was set to 15 × 15 × 30 μm using a sampling grid of 125 × 125 μm. Nine 30 μm callosal sections separated by 120 μm were counted for each animal. Approximately 20 sampling sites were counted for each section.
For subventricular zone (SVZ) counts, images of 14 μm serial sections separated by 56 μm 1.10–0 mm from bregma of unlesioned adult BDNF+/+ and BDNF+/− littermates were captured and analyzed at 400× using a Leica microscope. NG2+ cells along the outer wall of the lateral ventricles were counted in a total of 20 sections for each animal. SVZ volumes of BDNF+/+ and BDNF+/− mice were quantitated.
Data analysis.
For each experiment, BDNF+/+ and BDNF+/− mice fed cuprizone were compared with their own control-fed mice for the same time points. Differences between controls and cuprizone mice were then evaluated. Statistical analysis was performed with StatView software, and the data are presented as the mean ± SEM. Statistical differences were determined using ANOVA followed by Fisher's protected least significant post hoc test or Student's t test, as appropriate. Conditions were considered significant at p < 0.05.
Results
Expression of trkB in corpus callosum OLGs
Work in culture has shown that BDNF enhances DNA synthesis and differentiation of BF OLG lineage cells through the trkB receptor (Du et al., 2006a; Van't Veer et al., 2009). To assess the role of BDNF in the corpus callosum following demyelination in vivo, we first evaluated whether OLGs of this region express trkB. Colabeling of postmitotic CC1+ OLGs with an anti-trkB antibody (which we confirmed recognizes only the full-length receptor) (Klein et al., 1989; Vondran et al., 2010) indicated that OLGs in the adult mouse corpus callosum express the receptor (Fig. 1), suggesting that corpus callosum OLGs may be responsive to BDNF.
Figure 1.
OLGs of the corpus callosum express full-length trkB. Immunohistochemistry shows that CC1+ cells (red) colocalize with trkB (green) in the adult corpus callosum of BDNF+/+ mice. The arrow shows a colocalized cell, and the arrowhead indicates a cell positive for only one antigen. Scale bar, 50 μm.
BDNF protein is reduced following cuprizone treatment
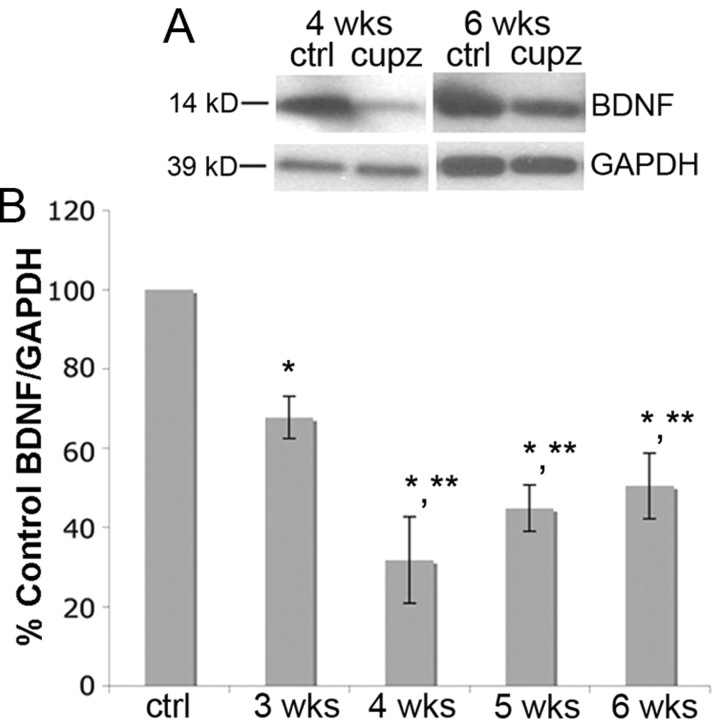
Cuprizone-induced demyelination is associated with changes in several factors in the local area. For example, proinflammatory cytokines tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), and anti-inflammatory cytokines transforming growth factor-β (TGF-β) and IL-10 as well as IGF-1 (Mason et al., 2000b; Biancotti et al., 2008) are elevated following cuprizone treatment. Other growth factors, such as PDGF, fibroblast growth factor, and NT-3 remain unchanged (Mason et al., 2000b). Changes in factors that can influence OLG development may lead to the effects seen following cuprizone, including OLG loss, demyelination, and spontaneous remyelination. In the present study, we evaluated BDNF levels by Western blot to determine whether cuprizone-induced demyelination affects the protein. Mature BDNF protein levels were decreased at 3 weeks and were then further reduced to a similar level at 4, 5, and 6 weeks of cuprizone treatment (Fig. 2), and even 4 weeks after removal of cuprizone (see Fig. 6A), suggesting that the demyelinating lesion elicits a reduction in BDNF synthesis that appears to be long lasting.
Figure 2.
BDNF protein is reduced in the corpus callosum following cuprizone treatment. A, Western blots of mature BDNF (14 kDa) in control animals and in animals treated with cuprizone for 4 or 6 weeks. Anti-GAPDH was used to normalize to total protein levels. B, The graph represents densitometric analysis of the blots at 3, 4, 5, and 6 weeks of cuprizone treatment. Results are shown as percentage control BDNF/GAPDH. Data were analyzed by ANOVA. *Significant difference from control (no cuprizone) at p < 0.05; **significant difference from animals treated with cuprizone for 3 weeks, but not from other time points. (N = 4 for all time points).
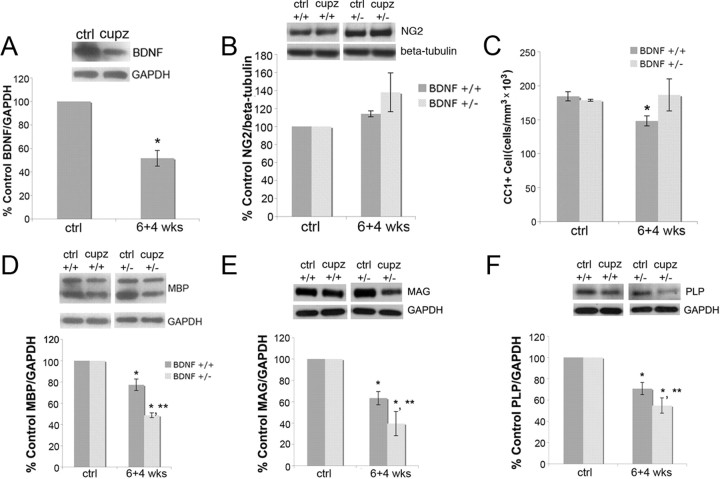
Figure 6.
Four weeks after cuprizone removal, BDNF remains reduced, and BDNF+/− mice exhibit a continued decrease of MBP, MAG, and PLP compared with BDNF+/+ mice, while the numbers of NG2 and CC1+ cells are equal to those of wild-type controls. A, BDNF protein is reduced in the corpus callosum during remyelination. Western blot is of mature BDNF (14 kDa) in control and 6 + 4 week cuprizone-treated animals. Anti-GAPDH was used to normalize to total protein levels. The graph represents densitometric analysis of the blots. Results are shown as a percentage of control BDNF/GAPDH. Data were analyzed by Student's t test. *Significant difference from control (no cuprizone) mice at p < 0.05 (N = 3). B, NG2 protein is unaffected in the corpus callosum during remyelination. Western blot evaluated NG2 in control and 6 + 4 week cuprizone-treated animals. β-Tubulin was used to normalize to total protein levels. The graph represents densitometric analysis of the blots. Results are shown as a percentage of control NG2/β-tubulin. Data were analyzed by ANOVA (N = 3). C, BDNF+/+ and BDNF+/− mice exhibit recovery of numbers of CC1+ OLGs after 6 + 4 weeks of cuprizone treatment. Coronal sections of the corpus callosum overlying the fornix of BDNF+/+ and BDNF+/− mice fed control feed or cuprizone for 6 + 4 weeks were stained with an antibody for CC1. Estimates for volumes and cell numbers were obtained by unbiased stereological morphometric. Data were analyzed by ANOVA. *Significant difference from control (no cuprizone) mice at p < 0.05 (N = 3). D–F, BDNF+/− mice exhibit a more severe decrease in MBP, MAG, and PLP following cuprizone treatment for 6 + 4 weeks than do BDNF+/+ mice. Anti-GAPDH was used to normalize to total protein. The graphs represent densitometric analysis of the blots. Results are shown as a percentage of control (no cuprizone) MBP/GAPDH, MAG/GAPDH, and PLP/GAPDH. Data were analyzed by ANOVA. *Significant difference from control (no cuprizone) mice at p < 0.05; **significant difference from BDNF+/+ mice at p < 0.05 (N = 3).
BDNF+/− mice exhibit a blunted increase in OLG progenitors in response to cuprizone
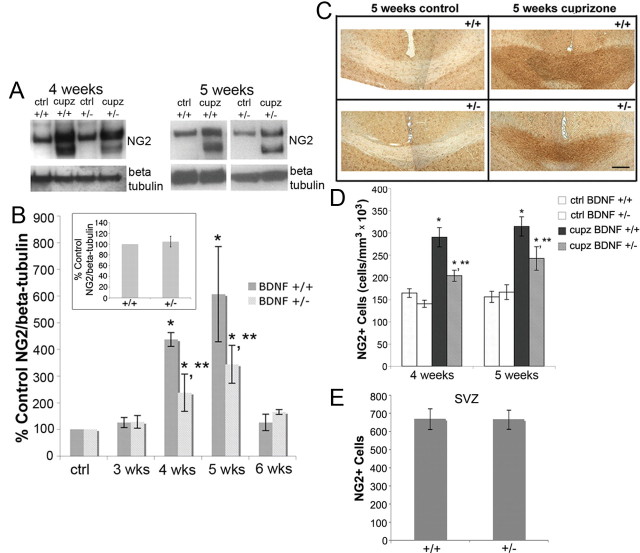
OLG progenitor cells, monitored with NG2, respond to the cuprizone lesion by increasing in number (Mason et al., 2000b). To evaluate the impact of reduced levels of BDNF on the cuprizone-elicited increase in NG2, NG2 protein as well as cell number were assessed in the corpus callosum. While NG2 protein levels were similar when BDNF+/+ and BDNF+/− mice were compared in the unlesioned adult corpus callosum (Fig. 3B, inset), distinct differences were noted under lesion conditions. BDNF+/+ mice exhibited an increase in NG2 protein at 4 and 5 weeks, as shown by Western blot, which return to control levels by 6 weeks (Fig. 3A,B). BDNF+/− mice also exhibited increases in NG2 protein following cuprizone feeding compared with BDNF+/− mice fed control feed. However, the NG2 increases were significantly reduced compared with those seen in BDNF+/+ mice (Fig. 3A,B).
Figure 3.
BDNF+/− mice exhibit a blunted increase in NG2 progenitors following cuprizone treatment compared with BDNF+/+ mice. A, Western blots of NG2 in control animals and animals treated with cuprizone for 4 or 5 weeks. Anti-β-tubulin was used to normalize to total protein levels. B, The graph represents densitometric analysis of the blots at 3, 4, 5, and 6 weeks of cuprizone treatment. Each result (BDNF+/+ or BDNF+/−) is shown as a percentage of its own control (no cuprizone) NG2/β-tubulin for each time point. Data were analyzed by ANOVA. *Significant difference from control (no cuprizone) mice at p < 0.05; **significant difference from BDNF+/+ mice at p < 0.05 (N = 4 at 3 and 6 weeks for BDNF+/+ and BDNF+/− mice, and at 5 weeks for BDNF+/− mice; N = 3 at 4 weeks for BDNF+/+ and BDNF+/− mice, and at 5 weeks for BDNF+/+ mice). Inset represents densitometric analysis of NG2 blots of 8–12-week-old BDNF+/+ and BDNF+/− unlesioned corpus callosum. Data were analyzed by Student's t test (N = 7). C, Representative sections of NG2-stained BDNF+/+ and BDNF+/− corpus callosum following 5 weeks of control or cuprizone feeding. D, Coronal sections of the corpus callosum overlying the fornix of BDNF+/+ and BDNF+/− mice fed control feed or cuprizone for 4 or 5 weeks were stained with an antibody for NG2. Estimates for volumes and cell numbers were obtained by unbiased stereological morphometric analysis using the Bioquant Image Analyzer Program, version 6.90.10. Data were analyzed by ANOVA. *Significant difference from control (no cuprizone) mice at p < 0.05; **significant difference from BDNF+/+ mice at p < 0.05 (N = 3 for both time points). E, Coronal sections of the SVZ 1.10–0 mm from bregma of adult BDNF+/+ and BDNF+/− mice were stained with an antibody for NG2. Total numbers of NG2 cells per assessed SVZ area were counted in BDNF+/+ and BDNF+/− mice and analyzed using the Student's t test (N = 3).
This observation was confirmed when NG2+ cells were evaluated immunohistochemically. As was the case with NG2 protein, when unlesioned BDNF+/+ and BDNF+/− mice were compared, the numbers of NG2 cells were similar in the corpus callosum (Fig. 3C,D), as well as the subventricular zone (a region thought to provide progenitors in response to demyelination (Nait-Oumesmar et al., 1999; Picard-Riera et al., 2002) (Fig. 3E). This finding is consistent with that of others who found that mice in which BDNF was excised in postmitotic neurons exhibited similar numbers of olig2+ cells in the striatum as wild-type mice (Rauskolb et al., 2010).
In contrast, when NG2 cells were evaluated under lesion conditions, the numbers of NG2+ cells in the corpus callosum of BDNF+/+ and BDNF+/− mice following 4 and 5 weeks cuprizone were distinct. Counting, using unbiased stereological morphometric analysis, revealed that numbers of NG2+ cells increased in both BDNF+/+ and BDNF+/− mice at 4 and 5 weeks. However, BDNF+/− mice exhibited a blunted increase in cell number compared with BDNF+/+ mice (Fig. 3C,D). BDNF, then, impacts the numbers of OLG progenitors that respond to a demyelinating insult, thereby influencing the response of this proliferating population to injury.
BDNF+/− mice compared with wild-type mice exhibit exacerbated deficits in myelin proteins during demyelination, but do not exhibit increased losses of mature OLGs
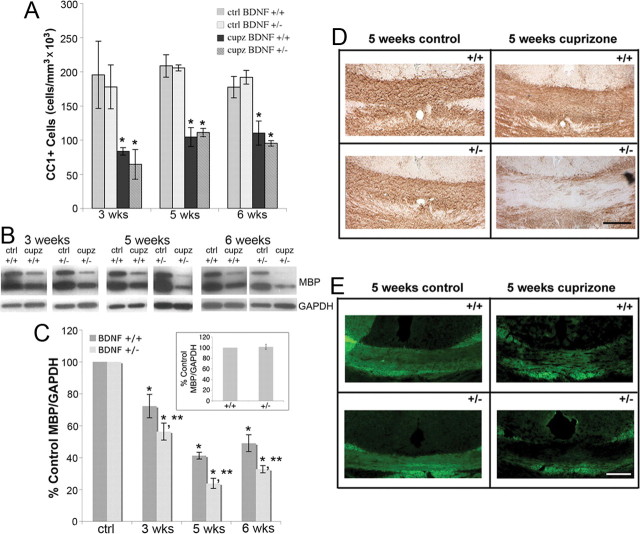
OLG loss and demyelination occur in the corpus callosum over 6 weeks of cuprizone treatment (Matsushima and Morell, 2001). The effects of BDNF on the loss of OLGs following injury were evaluated using immunohistochemistry for CC1. The numbers of CC1+ cells in the corpus callosum of BDNF+/+ and BDNF+/− mice following 3, 5, and 6 weeks of cuprizone treatment were counted using unbiased stereological morphometric analysis. BDNF+/+ and BDNF+/− mice exhibited similar numbers of CC1+ OLGs before the lesion and exhibited similar losses of CC1+ OLGs following cuprizone treatment (Fig. 4A), suggesting that BDNF does not impact the numbers of OLGs in the adult or the survival of OLGs after injury.
Figure 4.
CC1+ cell numbers are similarly reduced in the corpus callosum of BDNF+/+ and BDNF+/− mice following cuprizone treatment, while BDNF+/− mice exhibit a more severe decrease in MBP following cuprizone treatment than do BDNF+/+ mice. A, Coronal sections of the corpus callosum overlying the fornix of BDNF+/+ and BDNF+/− mice fed control feed or cuprizone for 3, 5, or 6 weeks were stained with an antibody for CC1. Estimates for volumes and cell numbers were obtained by unbiased stereological morphometric analysis using the Bioquant Image Analyzer Program, version 6.90.10. Data were analyzed by ANOVA. *Significant difference from control (no cuprizone) mice at p < 0.05 (N = 3 for all time points). B, Western blots of MBP in the corpus callosum of control animals and animals treated with cuprizone for 3, 5, and 6 weeks. Anti-GAPDH was used to normalize to total protein levels. C, The graph represents densitometric analysis of the blots at 3, 5, and 6 weeks of cuprizone treatment. Each result (BDNF+/+ or BDNF+/−) is shown as a percentage of its own control (no cuprizone) MBP/GAPDH for each time point. Data were analyzed by ANOVA. *Significant difference from control (no cuprizone) mice at p < 0.05; **significant difference from BDNF+/+ mice at p < 0.05 (N = 3 at 3 weeks for BDNF+/+ and BDNF+/− mice; N = 4 at all other time points). Inset represents densitometric analysis of MBP blots of 8–12-week-old BDNF+/+ and BDNF+/− unlesioned corpus callosum. Data were analyzed by Student's t test (N = 7). D, E, Sections from BDNF+/+ and BDNF+/− mice fed control feed or cuprizone for 5 weeks were stained with an antibody for MBP and visualized with DAB (D) or FluoroMyelin (E). Sections are coronal over the fornix.
During the course of cuprizone-elicited demyelination, myelin protein mRNA as well as myelin proteins, such as MBP, MAG, and PLP, also decrease (Morell et al., 1998; Matsushima and Morell, 2001; Lindner et al., 2008). Thus, myelin proteins may provide evidence of myelin dysfunction during demyelination. In the present study, MBP protein levels were analyzed in the unlesioned and lesioned brain. As noted above with respect to NG2, there were no differences in levels of myelin protein when BDNF+/+ and BDNF+/− mice were compared in the unlesioned brain (Fig. 4C, insert). On the other hand, following cuprizone treatment, BDNF+/− mice exhibited a greater decrease in MBP at 3, 5, and 6 weeks of cuprizone treatment than did BDNF+/+ mice fed cuprizone (Fig. 4B,C).
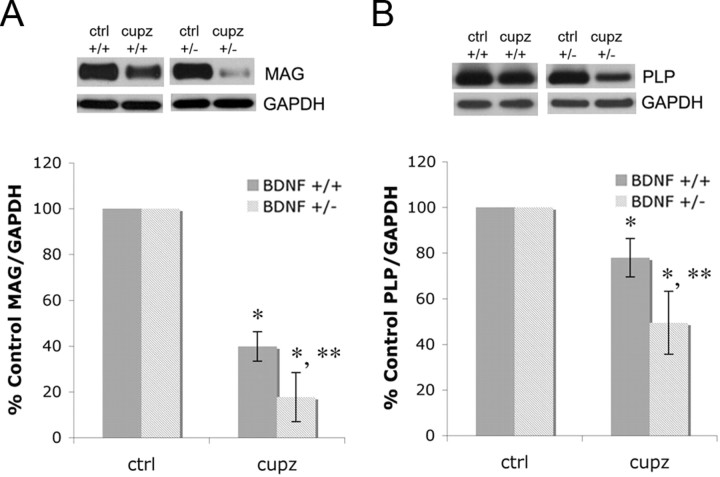
Previous studies in vivo and in culture indicate that BDNF impacts multiple myelin proteins (Du et al., 2006a,b; Vondran et al., 2010). To determine whether multiple myelin proteins are affected in the cuprizone model, MAG and PLP were evaluated at the peak of demyelination at 5 weeks. Similar to results found with MBP, BDNF+/− mice exhibited a more severe decrease in both MAG and PLP compared with BDNF+/+ mice (Fig. 5). These data complement previous observations suggesting that BDNF influences levels of differentiated traits in, but not survival of postmitotic BF OLGs (Du et al., 2003, 2006a; Vondran et al., 2010). In the corpus callosum, BDNF impacts levels of differentiated traits found in OLGs, but not their number during the demyelinating phase of the cuprizone injury, suggesting that the effect of BDNF is on the demyelination process.
Figure 5.
BDNF+/− mice exhibit a more severe decrease in MAG and PLP following cuprizone treatment than do BDNF+/+ mice. A, B, Western blots of MAG (A) and PLP (B) in control animals and animals treated with cuprizone for 5 weeks in the corpus callosum. Anti-GAPDH was used to normalize to total protein levels. The graphs represent densitometric analysis of the blots of samples from animals treated with cuprizone for 5 weeks. Each result (BDNF+/+ or BDNF+/−) is shown as a percentage of its own control (no cuprizone) MAG/GAPDH or PLP/GAPDH. Data were analyzed by ANOVA. *Significant difference from control (no cuprizone) mice at p < 0.05; **significant difference from BDNF+/+ mice at p < 0.05 (N = 3).
To support the Western blot data and explore the effects on myelin within the lesioned region, the corpus callosum was evaluated using immunohistochemistry of MBP and FluoroMyelin to detect myelin. Coronal sections of the corpus callosum clearly indicated the difference between genotypes following 5 weeks of cuprizone treatment. BDNF+/− mice exhibited reduced MBP immunoreactivity compared with BDNF+/+ mice (Fig. 4D). These results are consistent with our findings that mice with reduced levels of BDNF exhibit a greater deficit in MBP in response to a demyelinating lesion than do wild-type mice. Sections also were stained with FluoroMyelin, which stains myelin due to its lipophilic affiliation, to determine whether myelin itself was affected. Similar to results seen with MBP, BDNF+/− mice exhibited reduced FluoroMyelin reactivity compared with BDNF+/+ mice (Fig. 4E), indicating that BDNF impacts myelin integrity as well as myelin proteins following a cuprizone lesion.
BDNF+/− mice continue to exhibit deficits in MBP during remyelination
As shown above, reduced levels of BDNF impact the proliferating population of NG2 cells and myelin proteins following demyelination. To examine the effects of BDNF on remyelination, mice were fed cuprizone for 6 weeks and then returned to control feed for an additional 4 weeks (6 + 4 weeks), a protocol that has been shown to result in remyelination of the corpus callosum (Matsushima and Morell, 2001).
The BDNF+/+ and BDNF+/− mice used in our study differ from mice used by others with different genetic backgrounds because they do not fully demyelinate at 6 weeks when fed 0.2% cuprizone (Fig. 4). Therefore, to enhance our ability to assess remyelination, the mice were fed 0.3% cuprizone. This does not elicit toxicity as measured by weight loss but does result in an almost complete loss of MBP. Four weeks following cuprizone removal, BDNF levels remained decreased in wild-type mice (Fig. 6A). NG2 was not affected 4 weeks after cuprizone removal (Fig. 6B), and the numbers of mature OLGs returned almost to control levels in both BDNF+/+ and BDNF+/− mice (Fig. 6C). However, while MBP, MAG, and PLP deficits were evident in both the BDNF+/− and BDNF+/+ mice, BDNF+/− mice exhibit even more severe deficits than were noted in wild-type mice (Fig. 6D–F). The data suggest that the differentiation of the mature OLGs is impaired in the BDNF+/− mice.
Effects on the OLG lineage cells appear to be relatively specific to that cell population
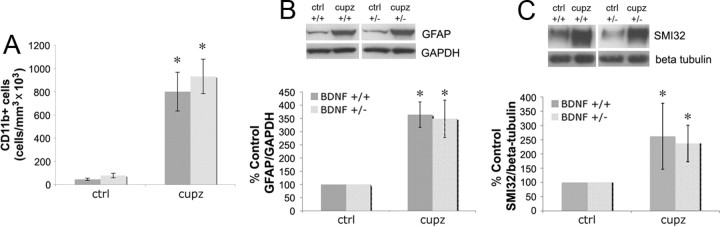
In addition to OLG lineage cells, microglia and astrocytes infiltrate the cuprizone-elicited demyelinated lesion (Hiremath et al., 1998). To evaluate how levels of BDNF may impact these proliferating cells within the lesion, the numbers of microglia were monitored using CD11b and the effects on astrocytes were monitored using GFAP. Both CD11b+ cells and GFAP protein levels increased following 4 weeks of cuprizone treatment (Fig. 7A,B). However, there were no significant differences in the numbers of CD11b+ cells or in GFAP protein levels when BDNF+/+ mice were compared with BDNF+/− mice. This is in contrast to differences in NG2 and myelin proteins, and suggests that the loss of BDNF impacts the OLG lineage cell population specifically.
Figure 7.
BDNF+/+ and BDNF+/− mice exhibit similar increases in CD11b+ cells, as well as GFAP and SMI32 protein following cuprizone treatment. A, Coronal sections of the corpus callosum overlying the fornix of BDNF+/+ and BDNF+/− mice fed control feed or cuprizone for 4 weeks were stained with an antibody for CD11b. Estimates for volumes and cell numbers were obtained by unbiased stereological morphometric analysis using the Bioquant Image Analyzer Program, version 6.90.10. Data were analyzed by ANOVA. *Significant difference from control (no cuprizone) mice at p < 0.05 (N = 3). B, Western blot evaluated GFAP in control animals and animals treated with cuprizone for 4 weeks. GAPDH was used to normalize to total protein levels. The graph represents densitometric analysis of the blots after 4 weeks of cuprizone treatment. Results (BDNF+/+ or BDNF+/− mice) are shown as a percentage of its own control (no cuprizone) GFAP/GAPDH. Data were analyzed by ANOVA. *Significant difference from control (no cuprizone) mice at p < 0.05 (N = 4 for BDNF+/+ mice and N = 3 for BDNF+/− mice). C, Western blot evaluated SMI32 in control animals and animals treated with cuprizone for 4 weeks. Anti-β tubulin was used to normalize to total protein levels. The graph represents densitometric analysis of the blots after 4 weeks of cuprizone treatment. Results (BDNF+/+ or BDNF+/− mice) are shown as a percentage of its own control (no cuprizone) SMI32/β tubulin. Data were analyzed by ANOVA. *Significant difference from control (no cuprizone) mice at p < 0.05 (N = 4 for BDNF+/+ and N = 3 for BDNF+/− mice).
BDNF has been reported to impact several neuronal populations, including those that project in the corpus callosum (Catapano et al., 2004). It is possible that BDNF may elicit effects on the OLG lineage cells following demyelination through the mediation of these axons. To explore this possibility, levels of SMI32, a marker of dephosphorylated neurofilaments and an indicator of axonal damage, were measured. Following 4 weeks of cuprizone feed, both BDNF+/+ and BDNF+/− mice exhibited similar increases in SMI32 levels (Fig. 7C), suggesting that axonal damage is similar despite differences in levels of BDNF at the lesion site.
In toto, while marked differences are observed in the manner in which OLG lineage cells from BDNF+/− and BDNF+/+ mice respond to a lesion, effects on microglia, astrocytes, and damaged axons are not readily apparent. Future studies are necessary, however, to determine whether more subtle differences that may contribute to effects on OLG lineage cells are detected in these populations.
Discussion
Previous work in culture and in vivo suggests that BDNF impacts OLG lineage cell development in the BF (Du et al., 2006a; Van't Veer et al., 2009; Vondran et al., 2010). This study evaluated whether BDNF impacts OLG lineage cells following a cuprizone-elicited demyelinating lesion of the corpus callosum. In the corpus callosum following this lesion, BDNF is reduced during demyelination and remyelination, suggesting it as a factor important in the progression of the pathology. BDNF+/− mice exhibit a blunted increase in NG2+ cells compared with BDNF+/+ mice following cuprizone, suggesting that a loss of BDNF impacts the response of these cells to demyelination. BDNF+/+ and BDNF+/− mice exhibit similar losses of postmitotic OLGs. However, compared with BDNF+/+ mice, myelin proteins are reduced in BDNF+/− mice during demyelination and remained reduced in BDNF+/− mice during remyelination. Effects appear to be somewhat specific to OLGs since levels of GFAP, SMI32, and microglia are unaffected when the BDNF+/+ and BDNF+/− mice are compared.
The role of BDNF on OLGs in vivo during development
Our past work and the work of others suggest that BDNF plays a role in the development of OLGs in vivo. BDNF−/− mice exhibit decreased numbers of myelinated axons in the postnatal optic nerve, decreased PLP and MBP mRNA in the postnatal hippocampus and cortex (Cellerino et al., 1997), as well as decreased MBP in the hippocampus and raphe (Djalali et al., 2005). BDNF+/− mice exhibit delayed myelination in the spinal cord and optic nerve during development (Xiao et al., 2010) and decreases in NG2 progenitors as well as myelin proteins in the BF throughout development and into adulthood (Vondran et al., 2010). Thus, a loss of BDNF appears to impair OLG development in multiple brain regions. Further, in mice with a conditional deletion of trkB from the embryonic forebrain, levels of MBP and numbers of myelinated axons in the corpus callosum and hippocampus are reduced (Medina et al., 2004), suggesting that trkB signaling may be involved in OLG development and myelination.
The role of BDNF on OLGs in vivo in the adult and after a lesion
These effects in some brain regions during development may differ from those in other brain regions in the adult. Our data indicate that the numbers of corpus callosum and SVZ OLG lineage cells and the levels of myelin proteins are similar in BDNF+/+ and BDNF+/− mice in adulthood, a result consistent with that of others (Rauskolb et al., 2010; Xiao et al., 2010), and in contrast to observations made by us in the adult medial septum-BF (Vondran et al., 2010). Interestingly, this difference in cortical versus BF responses to BDNF was noticed previously in culture, where we reported that BF OLGs respond to BDNF, while cortical OLGs do not (Du et al., 2003). We argued at that time that this regional difference may be another example of the heterogeneity of OLGs, which are reported to differ with respect to morphology (Del Rio-Hortega, 1928; Bjartmar et al., 1994), biochemistry (Hartman et al., 1982; Butt et al., 1995), and responsivity to the environment (Kettenmann et al., 1991; Takeda et al., 1995; Power et al., 2002).
Importantly, while we did not detect differences in OLG characters in comparing BDNF+/+ and BDNF+/− unlesioned corpus callosum, our studies indicate that differences are apparent after a lesion. When OLGs are exposed to a demyelinating lesion, they become sensitive to BDNF levels. These results are reminiscent of those by others implicating a role for BDNF in OLG repair. For example, following spinal cord injury, fibroblasts engineered to produce BDNF increase myelinated axons and the proliferation of OLG progenitors in the lesion area (McTigue et al., 1998). Intrathecal delivery of BDNF following spinal cord injury decreases apoptosis of OLGs and promotes recovery of MBP (Ikeda et al., 2002; Koda et al., 2002). In an animal model of immune-mediated demyelination, experimental allergic encephalomyelitis (EAE), BDNF delivery via transformed bone marrow stem cells significantly delays onset and severity of clinical symptoms, reduces the area of demyelination, and reduces the numbers of apoptotic cells (Makar et al., 2008, 2009). BDNF, therefore, may be useful in ameliorating deficits following a demyelinating lesion in a number of brain regions. This study is the first to indicate that BDNF may impact OLG lineage cells of the corpus callosum following a cuprizone-elicited demyelinating lesion using BDNF+/− mice.
Lesioned OLG lineage cells appear to be particularly sensitive to BDNF
Our studies suggest that effects of BDNF may preferentially affect OLGs following demyelination. While OLG lineage cells are differentially affected in the BDNF+/− mouse, as opposed to the BDNF+/+ mouse, after cuprizone, microglia, astrocytes, and axons appear not to be. However, it should be noted that while we report that BDNF may preferentially affect OLG lineage cells after a cuprizone lesion, we do not know whether this effect is directly on OLGs. BDNF may still have subtle effects that we did not detect on non-OLG lineage cells that may contribute to the OLG response to cuprizone. Data in the literature, in fact, have argued that effects of BDNF may be indirect and mediated, for example, by neurons that also express trkB and are responsive to BDNF (Cellerino et al., 1997; but, see Xiao et al., 2010). In addition, BDNF may elicit effects on OLGs by affecting the immune response elicited by T cells or macrophages/microglia (Makar et al., 2009). For example, Makar et al. (2009) suggest that BDNF elicits its protective effects in EAE by modulating the expression of cytokines from proinflammatory to anti-inflammatory. Astrocytes and microglia are reported to express trkB receptors in culture (Condorelli et al., 1995), and astrocytes have been shown to express trkB after CNS injury in rats (McKeon et al., 1997) and in MS lesions (Stadelmann et al., 2002). Future studies that delete trkB specifically on OLGs, neurons, astrocytes, or microglia will most directly address the roles played by these other cell populations on the OLG response to cuprizone. Nevertheless, our studies support the possibility that BDNF may be a factor critical to the development of OLGs and the recovery of OLGs from a demyelinating lesion, whether or not the effect involves other cell types.
The loss of BDNF following a cuprizone lesion
We have found that BDNF is decreased in the lesion site following cuprizone. Why might this occur? One possible reason for this decrease may be the loss of OLGs that have the capacity to normally produce the trophin (Dai et al., 2003). Alternatively, the environment of the lesion may change the expression of hosts of factors produced by other glial cells, such as astrocytes and microglia. After CNS injury, proinflammatory cytokines, TNF-α and IL-1β, and anti-inflammatory cytokines, TGFβ and IL-10, as well as IGF-1 (Mason et al., 2000b; Biancotti et al., 2008) are increased. Interestingly, BDNF expression can be modulated by cytokines, and at least one class of these molecules, the Th1 cytokines, downregulate the expression of BDNF (Lisak et al., 2006, 2007), supporting the possibility that multiple factors collaborate to affect the response to a lesion.
Relevance to MS
The present study suggests that BDNF may provide protection during demyelination, as occurs in MS, and is consistent with an increasing literature that links BDNF with repair in MS. In another animal model of MS-like demyelination, EAE, treatment with the MS drug glatiramer acetate (GA) increased BDNF, which was sustained over the disease course and was correlated with the amelioration of clinical symptoms (Aharoni et al., 2005). In addition, mice receiving transformed bone marrow stromal cells producing BDNF exhibited delayed onset of disease, enhanced clinical recovery, reduced apoptosis, and reduced demyelination following EAE (Makar et al., 2008, 2009).
Clinical data of MS patients also implicates BDNF as a therapeutic target for the disease. BDNF levels are decreased in the blood of MS patients compared with healthy controls, and BDNF increases in MS patients after recovery from the relapsing phase of relapsing-remitting MS (RRMS), suggesting that BDNF may contribute to the recovery (Azoulay et al., 2005; Frota et al., 2009). Further, BDNF is increased in the serum and CSF of patients with RRMS during the recovery phase of the disease compared with patients with secondary progressive MS (Sarchielli et al., 2002). Current MS therapies may act to enhance the production of BDNF in MS patients. For example, peripheral blood mononuclear cells treated with GA exhibit increased secretion of BDNF (Azoulay et al., 2005). Further, mononuclear cells from MS patients treated with GA exhibited higher levels of BDNF than cells from patients treated with other drugs (Sarchielli et al., 2007). RRMS patients treated with interferon-β, another MS therapy with no further exacerbation of the disease, also exhibited increased production of BDNF by peripheral blood mononuclear cells (Caggiula et al., 2006). Thus, one mechanism of action of current MS therapies may be to increase BDNF in MS patients, leading to protective effects.
Footnotes
This project is supported by NIH Grant NS036647 and the National Multiple Sclerosis Society. We thank Lauren D. Lercher for excellent technical assistance.
References
- Aguirre A, Dupree JL, Mangin JM, Gallo V. A functional role for EGFR signaling in myelination and remyelination. Nat Neurosci. 2007;10:990–1002. doi: 10.1038/nn1938. [DOI] [PubMed] [Google Scholar]
- Aharoni R, Eilam R, Domev H, Labunskay G, Sela M, Arnon R. The immunomodulator glatiramer acetate augments the expression of neurotrophic factors in brains of experimental autoimmune encephalomyelitis mice. Proc Natl Acad Sci U S A. 2005;102:19045–19050. doi: 10.1073/pnas.0509438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althaus HH. Remyelination in multiple sclerosis: a new role for neurotrophins? Prog Brain Res. 2004;146:415–432. doi: 10.1016/S0079-6123(03)46026-3. [DOI] [PubMed] [Google Scholar]
- Azoulay D, Vachapova V, Shihman B, Miler A, Karni A. Lower brain-derived neurotrophic factor in serum of relapsing remitting MS: reversal by glatiramer acetate. J Neuroimmunol. 2005;167:215–218. doi: 10.1016/j.jneuroim.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Biancotti JC, Kumar S, de Vellis J. Activation of inflammatory response by a combination of growth factors in cuprizone-induced demyelinated brain leads to myelin repair. Neurochem Res. 2008;33:2615–2628. doi: 10.1007/s11064-008-9792-8. [DOI] [PubMed] [Google Scholar]
- Bjartmar C, Hildebrand C, Loinder K. Morphological heterogeneity of rat oligodendrocytes: electron microscopic studies on serial sections. Glia. 1994;11:235–244. doi: 10.1002/glia.440110304. [DOI] [PubMed] [Google Scholar]
- Butt AM, Ibrahim M, Ruge FM, Berry M. Biochemical subtypes of oligodendrocyte in the anterior medullary velum of the rat as revealed by the monoclonal antibody Rip. Glia. 1995;14:185–197. doi: 10.1002/glia.440140304. [DOI] [PubMed] [Google Scholar]
- Caggiula M, Batocchi AP, Frisullo G, Angelucci F, Patanella AK, Sancricca C, Nociti V, Tonali PA, Mirabella M. Neurotrophic factors in relapsing remitting and secondary progressive multiple sclerosis patients during interferon beta therapy. Clin Immunol. 2006;118:77–82. doi: 10.1016/j.clim.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Catapano LA, Arlotta P, Cage TA, Macklis JD. Stage-specific and opposing roles of BDNF, NT-3 and bFGF in differentiation of purified callosal projection neurons toward cellular repair of complex circuitry. Eur J Neurosci. 2004;19:2421–2434. doi: 10.1111/j.0953-816X.2004.03303.x. [DOI] [PubMed] [Google Scholar]
- Cellerino A, Carroll P, Thoenen H, Barde YA. Reduced size of retinal ganglion cell axons and hypomyelination in mice lacking brain-derived neurotrophic factor. Mol Cell Neurosci. 1997;9:397–408. doi: 10.1006/mcne.1997.0641. [DOI] [PubMed] [Google Scholar]
- Condorelli DF, Salin T, Dell' Albani P, Mudo G, Corsaro M, Timmusk T, Metsis M, Belluardo N. Neurotrophins and their trk receptors in cultured cells of the glial lineage and in white matter of the central nervous system. J Mol Neurosci. 1995;6:237–248. doi: 10.1007/BF02736783. [DOI] [PubMed] [Google Scholar]
- Dai X, Lercher LD, Clinton PM, Du Y, Livingston DL, Vieira C, Yang L, Shen MM, Dreyfus CF. The trophic role of oligodendrocytes in the basal forebrain. J Neurosci. 2003;23:5846–5853. doi: 10.1523/JNEUROSCI.23-13-05846.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio-Hortega P. Tercera aportacion al conocimiento morfologico e interpretacion funcional de la oligodendroglia. Mem Real Soc Esp Hist Nat. 1928;14:40–122. [Google Scholar]
- Di Bello IC, Dawson MR, Levine JM, Reynolds R. Generation of oligodendroglial progenitors in acute inflammatory demyelinating lesions of the rat brainstemis associated with demyelination rather than inflammation. J Neurocytol. 1999;28:365–381. doi: 10.1023/a:1007069815302. [DOI] [PubMed] [Google Scholar]
- Djalali S, Höltje M, Grosse G, Rothe T, Stroh T, Grosse J, Deng DR, Hellweg R, Grantyn R, Hörtnagl H, Ahnert-Hilger G. Effects of brain-derived neurotrophic factor (BDNF) on glial cells and serotonergic neurones during development. J Neurochem. 2005;92:616–627. doi: 10.1111/j.1471-4159.2004.02911.x. [DOI] [PubMed] [Google Scholar]
- Du Y, Fischer TZ, Lee LN, Lercher LD, Dreyfus CF. Regionally specific effects of BDNF on oligodendrocytes. Dev Neurosci. 2003;25:116–126. doi: 10.1159/000072261. [DOI] [PubMed] [Google Scholar]
- Du Y, Fischer TZ, Clinton-Luke P, Lercher LD, Dreyfus CF. Distinct effects of p75 in mediating actions of neurotrophins on basal forebrain oligodendrocytes. Mol Cell Neurosci. 2006a;31:366–375. doi: 10.1016/j.mcn.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Du Y, Lercher LD, Zhou R, Dreyfus CF. Mitogen-activated protein kinase pathway mediates effects of brain-derived neurotrophic factor on differentiation of basal forebrain oligodendrocytes. J Neurosci Res. 2006b;84:1692–1702. doi: 10.1002/jnr.21080. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Lee KF, Jaenisch R. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature. 1994;368:147–150. doi: 10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant C, Raff MC. Proliferating bipotential glial progenitor cells in adult rat optic nerve. Nature. 1986;319:499–502. doi: 10.1038/319499a0. [DOI] [PubMed] [Google Scholar]
- Frota ER, Rodrigues DH, Donadi EA, Brum DG, Maciel DR, Teixeira AL. Increased plasma levels of brain derived neurotrophic factor (BDNF) after multiple sclerosis relapse. Neurosci Lett. 2009;460:130–132. doi: 10.1016/j.neulet.2009.05.057. [DOI] [PubMed] [Google Scholar]
- Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19:197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- Hartman BK, Agrawal HC, Agrawal D, Kalmbach S. Development and maturation of central nervous system myelin: comparison of immunohistochemical localization of proteolipid protein and basic protein in myelin and oligodendrocytes. Proc Natl Acad Sci U S A. 1982;79:4217–4220. doi: 10.1073/pnas.79.13.4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiremath MM, Saito Y, Knapp GW, Ting JP, Suzuki K, Matsushima GK. Microglial/macrophage accumulation during cuprizone-induced demyelination in C57BL/6 mice. J Neuroimmunol. 1998;92:38–49. doi: 10.1016/s0165-5728(98)00168-4. [DOI] [PubMed] [Google Scholar]
- Ikeda O, Murakami M, Ino H, Yamazaki M, Koda M, Nakayama C, Moriya H. Effects of brain-derived neurotrophic factor (BDNF) on compression-induced spinal cord injury: BDNF attenuates down-regulation of myelin basic protein expression. J Neuropathol Exp Neurol. 2002;61:142–153. doi: 10.1093/jnen/61.2.142. [DOI] [PubMed] [Google Scholar]
- Jean I, Lavialle C, Barthelaix-Pouplard A, Fressinaud C. Neurotrophin-3 specifically increases mature oligodendrocyte population and enhances remyelination after chemical demyelination of adult rat CNS. Brain Res. 2003;972:110–118. doi: 10.1016/s0006-8993(03)02510-1. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Blankenfeld GV, Trotter J. Physiological properties of oligodendrocytes during development. Ann N Y Acad Sci. 1991;633:64–77. doi: 10.1111/j.1749-6632.1991.tb15596.x. [DOI] [PubMed] [Google Scholar]
- Klein R, Parada LF, Coulier F, Barbacid M. trkB, a novel tyrosine protein kinase receptor expressed during mouse neural development. EMBO J. 1989;8:3701–3709. doi: 10.1002/j.1460-2075.1989.tb08545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koda M, Murakami M, Ino H, Yoshinaga K, Ikeda O, Hashimoto M, Yamazaki M, Nakayama C, Moriya H. Brain-derived neurotrophic factor suppresses delayed apoptosis of oligodendrocytes after spinal cord injury in rats. J Neurotrauma. 2002;6:777–785. doi: 10.1089/08977150260139147. [DOI] [PubMed] [Google Scholar]
- Lindner M, Heine S, Haastert K, Garde N, Fokuhl J, Linsmeier F, Grothe C, Baumgärtner W, Stangel M. Sequential myelin protein expression during remyelination reveals fast and efficient repair after central nervous system demyelination. Neuropathol Appl Neurobiol. 2008;34:105–114. doi: 10.1111/j.1365-2990.2007.00879.x. [DOI] [PubMed] [Google Scholar]
- Lisak RP, Benjamins JA, Bealmear B, Yao B, Land S, Nedelkoska L, Skundric D. Differential effects of Th1, monocyte/macrophage and Th2 cytokine mixtures on early gene expression for immune-related molecules by central nervous system mixed glial cell cultures. Mult Scler. 2006;12:149–168. doi: 10.1191/135248506ms1251oa. [DOI] [PubMed] [Google Scholar]
- Lisak RP, Benjamins JA, Bealmear B, Nedelkoska L, Yao B, Land S, Studzinski D. Differential effects of Th1, monocyte/macrophage and Th2 cytokine mixtures on early gene expression for glial and neural-related molecules in central nervous system mixed glial cell cultures: neurotrophins, growth factors and structural proteins. J Neuroinflammation. 2007;4:30. doi: 10.1186/1742-2094-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwin SK. Remyelination in demyelinating diseases of the central nervous system. Crit Rev Neurobiol. 1987;3:1–28. [PubMed] [Google Scholar]
- Makar TK, Trisler D, Sura KT, Sultana S, Patel N, Bever CT. Brain derived neurotrophic factor treatment reduces inflammation and apoptosis in experimental allergic encephalomyelitis. J Neurol Sci. 2008;270:70–76. doi: 10.1016/j.jns.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Makar TK, Bever CT, Singh IS, Royal W, Sahu SN, Sura TP, Sultana S, Sura KT, Patel N, Dhib-Jalbut S, Trisler D. Brain-derived neurotrophic factor gene delivery in an animal model of multiple sclerosis using bone marrow stem cells as a vehicle. J Neuroimmunol. 2009;210:40–51. doi: 10.1016/j.jneuroim.2009.02.017. [DOI] [PubMed] [Google Scholar]
- Mason JL, Ye P, Suzuki K, D'Ercole AJ, Matsushima GK. Insulin-like growth factor-1 inhibits mature oligodendrocyte apoptosis during primary demyelination. J Neurosci. 2000a;20:5703–5708. doi: 10.1523/JNEUROSCI.20-15-05703.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JL, Jones JJ, Taniike M, Morell P, Suzuki K, Matsushima GK. Mature oligodendrocyte apoptosis precedes IGF-1 production and oligodendrocyte progenitor accumulation and differentiation during demyelination/remyelination. J Neurosci Res. 2000b;61:251–262. doi: 10.1002/1097-4547(20000801)61:3<251::AID-JNR3>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Mason JL, Langaman C, Morell P, Suzuki K, Matsushima GK. Episodic demyelination and subsequent remyelination within the murine central nervous system: changes in axonal calibre. Neuropathol Appl Neurobiol. 2001;27:50–58. doi: 10.1046/j.0305-1846.2001.00301.x. [DOI] [PubMed] [Google Scholar]
- Matsushima GK, Morell P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 2001;11:107–116. doi: 10.1111/j.1750-3639.2001.tb00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon RJ, Silver J, Large TH. Expression of full-length trkB receptors by reactive astrocytes after chronic CNS injury. Exp Neurol. 1997;148:558–567. doi: 10.1006/exnr.1997.6698. [DOI] [PubMed] [Google Scholar]
- McTigue DM, Horner PJ, Stokes BT, Gage FH. Neurotrophin-3 and brain-derived neurotrophic factor induce oligodendrocyte proliferation and myelination of regenerating axons in the contused adult rat spinal cord. J Neurosci. 1998;18:5354–5365. doi: 10.1523/JNEUROSCI.18-14-05354.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina DL, Sciarretta C, Calella AM, Von Bohlen Und Halbach O, Unsicker K, Minichiello L. TrkB regulates neocortex formation through the Shc/PLCgamma-mediated control of neuronal migration. EMBO J. 2004;23:3803–3814. doi: 10.1038/sj.emboj.7600399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell P, Barrett CV, Mason JL, Toews AD, Hostettler JD, Knapp GW, Matsushima GK. Gene expression in brain during cuprizone-induced demyelination and remyelination. Mol Cell Neurosci. 1998;12:220–227. doi: 10.1006/mcne.1998.0715. [DOI] [PubMed] [Google Scholar]
- Murtie JC, Zhou YX, Le TQ, Armstrong RC. In vivo analysis of oligodendrocyte lineage development in postnatal FGF2-null mice. Glia. 2005;49:542–554. doi: 10.1002/glia.20142. [DOI] [PubMed] [Google Scholar]
- Nait-Oumesmar B, Decker L, Lachapelle F, Avellana-Adalid V, Bachelin C, Van Evercooren AB. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur J Neurosci. 1999;11:4357–4366. doi: 10.1046/j.1460-9568.1999.00873.x. [DOI] [PubMed] [Google Scholar]
- Picard-Riera N, Decker L, Delarasse C, Goude K, Nait-Oumesmar B, Liblau R, Pham-Dinh D, Evercooren AB. Experimental autoimmune encephalomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. Proc Natl Acad Sci U S A. 2002;99:13211–13216. doi: 10.1073/pnas.192314199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J, Mayer-Pröschel M, Smith J, Noble M. Oligodendrocyte precursor cells from different brain regions express divergent properties consistent with the differing time courses of myelination in these regions. Dev Biol. 2002;245:362–375. doi: 10.1006/dbio.2002.0610. [DOI] [PubMed] [Google Scholar]
- Prineas JW, Kwon EE, Goldenberg PZ, Ilyas AA, Quarles RH, Benjamins JA, Sprinkle TJ. Multiple sclerosis. Oligodendrocyte proliferation and differentiation in fresh lesions. Lab Invest. 1989;61:489–503. [PubMed] [Google Scholar]
- Raine CS, Wu E. Multiple sclerosis: remyelination in acute lesions. J Neuropathol Exp Neurol. 1993;52:199–204. [PubMed] [Google Scholar]
- Rauskolb S, Zagrebelsky M, Dreznjak A, Deogracias R, Matsumoto T, Wiese S, Erne B, Sendtner M, Schaeren-Wiemers N, Korte M, Barde YA. Global deprivation of brain-derived neurotrophic factor in the CNS reveals an area-specific requirement for dendritic growth. J Neurosci. 2010;30:1739–1749. doi: 10.1523/JNEUROSCI.5100-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarchielli P, Greco L, Stipa A, Floridi A, Gallai V. Brain-derived neurotrophic factor in patients with multiple sclerosis. J Neuroimmunol. 2002;132:180–188. doi: 10.1016/s0165-5728(02)00319-3. [DOI] [PubMed] [Google Scholar]
- Sarchielli P, Zaffaroni M, Floridi A, Greco L, Candeliere A, Mattioni A, Tenaglia S, Di Filippo M, Calabresi P. Production of brain-derived neurotrophic factor by mononuclear cells of patients with multiple sclerosis treated with glatiramer acetate, interferon-beta 1a, and high doses of immunoglobulins. Mult Scler. 2007;13:313–331. doi: 10.1177/1352458506070146. [DOI] [PubMed] [Google Scholar]
- Stadelmann C, Kerschensteiner M, Misgeld T, Brück W, Hohlfeld R, Lassmann H. BDNF and gp145trkB in multiple sclerosis brain lesions: neuroprotective interactions between immune and neuronal cells? Brain. 2002;125:75–85. doi: 10.1093/brain/awf015. [DOI] [PubMed] [Google Scholar]
- Takeda M, Nelson DJ, Soliven B. Calcium signaling in cultured rat oligodendrocytes. Glia. 1995;14:225–236. doi: 10.1002/glia.440140308. [DOI] [PubMed] [Google Scholar]
- Vana AC, Flint NC, Harwood NE, Le TQ, Fruttiger M, Armstrong RC. Platelet-derived growth factor promotes repair of chronically demyelinated white matter. J Neuropathol Exp Neurol. 2007;66:975–988. doi: 10.1097/NEN.0b013e3181587d46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van't Veer A, Du Y, Fischer TZ, Boetig DR, Wood MR, Dreyfus CF. Brain-derived neurotrophic factor effects on oligodendrocyte progenitors of the basal forebrain are mediated through trkB and the MAP kinase pathway. J Neurosci Res. 2009;87:69–78. doi: 10.1002/jnr.21841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vondran MW, Clinton-Luke P, Honeywell JZ, Dreyfus CF. BDNF+/- mice exhibit deficits in oligodendrocyte lineage cells of the basal forebrain. Glia. 2010;58:848–856. doi: 10.1002/glia.20969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Toyama Y, Nishiyama A. Differentiation of proliferated NG2-positive glial progenitor cells in a remyelinating lesion. J Neurosci Res. 2002;69:826–836. doi: 10.1002/jnr.10338. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Wilson HC, Scolding NJ, Raine CS. Co-expression of PDGF alpha receptor and NG2 by oligodendrocyte precursors in human CNS and multiple sclerosis lesions. J Neuroimmunol. 2006;176:162–173. doi: 10.1016/j.jneuroim.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Wolswijk G, Noble M. Identification of an adult-specific glial progenitor cell. Development. 1989;105:387–400. doi: 10.1242/dev.105.2.387. [DOI] [PubMed] [Google Scholar]
- Woodruff RH, Franklin RJ. Growth factors and remyelination in the CNS. Histol Histopathol. 1997;12:459–466. [PubMed] [Google Scholar]
- Xiao J, Wong AW, Willingham MM, van den Buuse M, Kilpatrick TJ, Murray SS. Brain-derived neurotrophic factor promotes central nervous system myelination via a direct effect upon oligodendrocytes. Neurosignals. 2010;18:186–202. doi: 10.1159/000323170. [DOI] [PubMed] [Google Scholar]