Abstract
The nucleotide receptor P2X7 is an attractive therapeutic target and potential biomarker for multiple inflammatory and neurologic disorders, and it is expressed in several immune, osteogenic and neurologic cell types. Aside from its role in the nervous system, it is activated by ATP released at sites of tissue damage, inflammation and infection. Ligand binding to P2X7 stimulates many cell responses, including calcium fluxes, MAPK activation, inflammatory mediator release, and apoptosis. Much work has centered on P2X7 action in cell death and mediator processing (e.g., pro-interleukin-1 cleavage by the inflammasome), but the contribution of P2X7 to transcriptional regulation is less well defined. In this review, we will focus on the growing evidence for the importance of nucleotide-mediated gene expression, we will highlight several animal model, human genetic, and clinical studies that support P2X7 as a therapeutic target, and we will discuss the latest developments in anti-P2X7 clinical trials.
Keywords: Nucleotide Receptors, P2X7, Gene Transcription, FosB, Macrophages, Inflammation
Introduction
Extracellular nucleotides serve as important signals in a variety of biological processes, including inflammation, tissue repair, bone remodeling, apoptosis and neurotransmission, and dysregulation of nucleotide-mediated signaling contributes to altered physiological responses and disease states (1, 2). In this regard, multiple nucleotides (i.e., ATP, ADP, UTP, UDP, etc.) can be released at sites of tissue injury, infection, platelet activation, mechanical stimulation, and in tumor microenvironments, and these molecules can bind to the P2 family of nucleotide receptors to stimulate a plethora of downstream events (3, 4). The levels of nucleotides in the extracellular space are normally low and are regulated by ectonucleotidases. However, in the event of localized trauma, high concentrations of nucleotides capable of activating the P2 receptors are achieved when damaged cells either release nucleotides and/or their ectonucleotidases are down-regulated (5). The P2 family is subdivided into the P2X ionotropic receptors (P2X1–7) and the P2Y metabotropic family members (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11–14). The P2X receptors are ion-gated calcium channels, and the P2Y receptors are seven transmembrane-spanning heterotrimeric G-protein coupled receptors (6, 7). The P2X and P2Y family members are expressed in numerous cell types and function in diverse processes including apoptosis, inflammatory mediator production, and platelet activation. Antagonists for P2X and P2Y receptors are being developed to treat numerous diseases, such as atherothrombosis, rheumatoid arthritis, neurodegenerative diseases and pain, by attenuating extracellular nucleotide-induced actions (8–12). Therefore, understanding the mechanisms by which P2 receptors function in both normal and disease states is critical for developing therapies that target these receptors.
One nucleotide receptor that is gaining interest as a potential therapeutic target and biomarker for an array of inflammatory, pathogenic, osteogenic and psychological diseases is P2X 7. The P2X7 nucleotide receptor is expressed in several cell types, including monocytes, macrophages, osteoblasts, osteoclasts, astrocytes and microglia (13–18). Activation of P2X7 by ATP stimulates multiple signaling processes, including ion fluxes (Ca2+ and Na+ influx, K+ efflux), the mitogen-activated protein kinases (MAPKs) ERK1/2, p38 and JNKs, the NADPH oxidase complex and reactive oxygen species (ROS) formation, phospholipase D, several caspases, and the formation of a non-specific pore permeable to small molecules (<900 Da). P2X7 enhances lipopolysaccharide (LPS)-initiated mediator production and can directly augment the expression, processing and/or secretion of many immuno-modulatory factors, such as interleukin-1β (IL-1β), IL-8, cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS) and vascular endothelial growth factor (VEGF) (12, 19–22). Because P2X7 action appears to be involved in the synthesis and release of multiple inflammatory mediators, it has been hypothesized that the production of a pharmacological inhibitor against P2X7 will reduce the magnitude of an inflammatory response under physiological conditions where an excessive immune reaction is unfavorable. In this regard, there are P2X7 antagonists in clinical trials for rheumatoid arthritis, and recent developments concerning anti-P2X7 therapies will be discussed below under “P2X7 As a Potential Therapeutic Target and Biomarker”.
The human P2X7 receptor is comprised of 595 amino acids, and numerous polymorphisms in its promoter, introns and exons have been identified, including several non-synonymous single nucleotide polymorphisms (SNPs) with links to disease (6, 23, 24). As illustrated in Fig. 1, P2X7 is predicted to possess a short intracellular N-terminal domain, two transmembrane domains, a large extracellular ligand-binding domain, and an intracellular C-terminal domain that is important for lipid binding and for proper plasma membrane expression of the protein (23, 25, 26). The receptor contains five N-linked glycosylation sites, and we have shown that glycosylation at N187 is critical for normal function and for receptor expression on the cell surface (13). Residue N187 is located near two SNPs (E186K and L191P) that have been reported to lead to attenuated P2X7 function (27). In addition, P2X7 contains intracellular palmitoylation sites that are important for its association with detergent-resistant membranes (28). Interestingly, P2X7 is unique from the other P2X family members because its C-terminal tail is at least 100 amino acids longer than the other P2X receptors, and P2X 7 is one of two P2X receptors reported to promote the formation of a non-specific pore (6, 7, 23, 29). Prolonged activation of P2X7 results in the formation of the reversible pore, leading to the passage of small molecules (<900 Da), and in certain circumstances, pore formation has been associated with the induction of apoptosis (12). In contrast, transient stimulation with P2X7 ligands, which may reflect a more physiological scenario given the rapid degradation of nucleotides in the extracellular environment, has been reported to lead to alterations in gene transcription rather than cell death (20, 30).
Fig. 1. Architecture of Human P2X7.
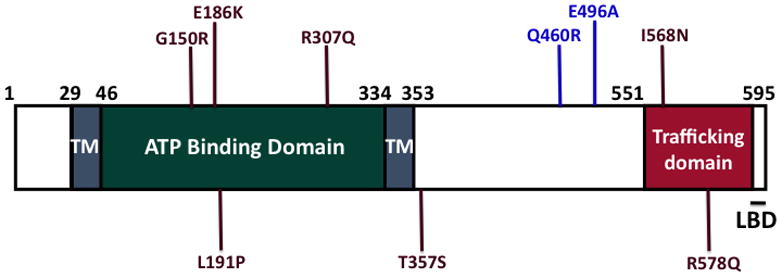
Human P2X7 possesses a predicted extracellular ATP binding domain, two transmembrane domains (TM), an intracellular trafficking domain, and a lipid binding domain (LBD). In addition, numerous P2X7 SNPs have been identified, including several with reduced function. Non-synonymous SNPs that exhibit reduced activity are represented in brown, and SNPs that have been correlated with human disease are shown in blue
In this review, we will highlight several of the recent reports that have demonstrated P2X7 may be an effective therapeutic target and biomarker for numerous inflammatory and neurological diseases by examining key animal model, human genetic, and clinical studies. We will also discuss the evidence that P2X7 can promote gene expression upon transient activation, spotlight our recent finding that P2X7 regulates gene transcription by inducing the expression of the activating protein-1 (AP-1) transcription factors FosB and ΔFosB, and consider the potential significance of the relationship between P2X7 and FosB/ΔFosB to immunological, osteogenic and neurologic disorders.
P2X7 As a Potential Therapeutic Target and Biomarker
The recent upsurge in P2X7 human genetic and animal studies has provided support to the idea that P2X7 represents a therapeutic target and biomarker for several diseases, including arthritis, asthma, papillary thyroid cancer, spinal cord injury, tuberculosis and mood disorders (8, 31–38). Several reports have correlated P2X7 SNPs to human disease, and many groups have performed P2X7 knockout or inhibitor studies in rodents to demonstrate the importance of P2X7 in immune, osteogenic and neurological regulation.
P2X7 Knockout Studies
Mice in which the P2X7 gene has been knocked out have been useful for the study of this receptor because these animals are viable and fertile, and upon initial examination, they do not appear to display any obvious physical or behavioral abnormalities (22, 39, 40). However, upon more detailed physical examination, P2X7 knockout mice have been found to present with skeletal deformities; specifically, these animals display excessive trabecular bone resorption and deficient periosteal bone formation (39). Examination of the skeletal system of P2X7-null mice also revealed that stimulation of periosteal bone growth by mechanical loading is markedly attenuated in these animals in comparison to their wild-type littermates (40). It should be noted that mechanical stimulation of osteblasts (the bone-forming cells of the skeletal system) has been linked to ATP release (41, 42), and P2X7 expression has been detected in both osteoblasts and osteoclasts (the cells responsible for bone resorption) (2). These skeletal observations will be discussed later in this review.
P2X7 knockout mice have been subjected to diverse immune challenges and psychological tests to identify additional phenotypic differences between P2X7 null animals and their wild-type counterpart controls, and these studies have provided several exciting results. A few selected studies utilizing P2X 7 knockout mice will be discussed below; in particular, we will focus on reports pertaining to lung inflammation, pain, glomerulonephritis and depression (43–52):
Using a cigarette smoke-induced lung inflammation mouse model, Lucattelli et al. tested the idea that P2X7 mediates lung inflammation (44). The premise for the study was based on data showing that the concentration of ATP (a P2X7 ligand) is higher in the lungs of smokers versus non-smokers (43). The authors also found that cigarette smoke increases P2X7 mRNA expression in alveolar macrophages and neutrophils, and mice treated with the P2X7 antagonist 1-[N, O-bis(5-isoquinolinesulfonyl)-N-methyl-L-tyrosyl]-4-phenylpiperazine (KN-62) and P2X7 knockout mice are protected from smoke-induced lung inflammation (44). With respect to pain research, several reports have shown that P2X7 is involved in promoting inflammatory and neuropathic pain (45, 46). Using various models to examine pain, P2X7 knockout mice and other rodents treated with P2X7 antagonists were found to experience reduced allodynia and hyperalgesia in comparison to control animals (45, 46). Chessell et al. also provided intriguing preliminary data related to P2X7 function and pain (45). In this study, peripheral nerves proximal to a patient’s site of injury were extracted during surgery, and the control nerves were obtained during limb amputations that were unrelated to a neurological issue (45). It was observed that P2X7 protein expression is higher in extracts from human injured nerves that cause pain in comparison to control nerves (45). With respect to renal disease, Taylor et al. examined P2X7 knockout mice in an immune mediated-glomerulonephritis model and reported that glomerular thrombosis is decreased in the knockout animals when compared to wild-type controls (47). Glomerulonephritis can be an acute or chronic problem and is a common cause of end-stage kidney disease. Taylor et al. observed that macrophage infiltration into glomeruli is decreased in P2X7 knockout mice and that the P2X7 inhibitor A-438079 decreases glomerulonephritis in rats (47). Thus, modulation of P2X7 status may serve as an effective therapeutic approach in several settings.
Although the majority of P2X7 studies involve an examination of receptor function in inflammation, several groups have provided evidence concerning the potential importance of this receptor from a psychiatric perspective. A dual role for P2X7 in both inflammation and psychiatry is not unexpected given the intimate interplay between immunological dysfunction and affective disorders (48). For example, depression often coincides with elevated levels of pro-inflammatory cytokines including factors that are regulated by P2X7 (e.g., IL-1β, TNF-α, IL-6), it has a high prevalence in individuals who have infectious or autoimmune diseases, and pro-inflammatory cytokines may produce “sickness behavior”, which is a set of adaptive behavioral changes that occur during an infection to help the organism cope with their disease (49–51). Because IL-1β, which is a well-established target of P2X7 action, can induce behavioral changes that are similar to depression, Basso et al. hypothesized that P2X7 can promote depressant symptoms (52). In these studies, P2X7 knockout mice were subjected to two well-characterized tests for depression-like behaviors, namely tail suspension and forced swim tests. In these assessments, the time an animal remains immobile during an inescapable stressor is recorded and is indicative of depressive behavior. Basso et al. observed that P2X7 knockout mice were significantly more mobile than the wild-type controls in both tests, suggesting that attenuation of P2X7 is associated with anti-depressive behavior (52). Further research on the role of P2X7 in the context of mood disorders appears warranted given the connection between cytokine production and neurological disorders, as well as recent genetic analyses linking P2X7 SNPs to mood disorders (27, 52, see below).
P2X7 Polymorphisms
To date, there are over twenty non-synonymous P2X7 SNPs that have been entered in the National Center for Biotechnology Information (NCBI) database. Information concerning the location of these SNPs within the P2X7 protein, their correlation with human disease, and relevant functional data are summarized in Fig. 1 and Table 1. The P2X7 non-synonymous SNPs identified thus far are located throughout the protein, including the predicted extracellular domain, the second transmembrane domain, and the intracellular C-terminal domain. The two most widely studied P2X7 SNPs are E496A and Q460R, and both of these polymorphisms are located in the intracellular C-terminal domain. The E496A polymorphism has been correlated with susceptibility to tuberculosis in several populations, and homozygosity of this polymorphism reportedly correlates with papillary thyroid cancer (32, 53–57). Mutation of P2X7 E496 to Ala results in attenuated ligand-stimulated pore formation, but conflicting data exists about whether it maintains normal cation channel activity (58–60). However, it has been observed that monocytes from subjects with the E496A polymorphism release significantly less IL-1β and IL-18, which are key immuno-modulatory factors that are regulated by P2X7 (61). Of note, we have detected that the E496A polymorphism exhibits attenuated P2X 7 ligand-stimulated induction of the AP-1 transcription factors FosB and ΔFosB (unpublished observations). Thus, these results are the first to suggest that a P2X7 polymorphism with a known linkage to human disease confers altered regulation of transcription factors and potentially the expression of other genes.
Table 1. P2X7 Non-synonymous SNPs.
Several P2X7 polymorphisms are listed including their location, reported functionality, and links with disease
| SNP | Characteristics |
|---|---|
| V76A | Reduced channel and pore activities (27) |
| R117W | Reduced channel and pore activities (27) |
| G150R | Reduced channel and pore activities (27) |
| H155Y | Increased channel and pore activities (27, 60) |
| A176V | Within the extracellular domain |
| E186K | Next to critical N-linked glycosylation site (13); reduced channel and pore activities (27) |
| L191P | Near critical N-linked glycosylation site (13); reduced channel and pore activites (27) |
| R270H | No change in function (66) |
| R270C | Within the extracellular domain |
| R276H | Within the extracellular domain |
| R307Q | Reduced channel and pore activities; within the ATP binding pocket; expresses on the cell surface (66, 168) |
| L320P | Within the extracellular domain |
| A348T | Increased channel and pore activities; correlated with T. gondii infection (27, 67) |
| T357S | Reduced channel and pore activities (27, 66) |
| I367T | Located in the intracellular domain near the second transmembrane domain |
| P430R | Within the intracellular domain |
| A433V | Within the intracellular domain |
| Q460R | Functionality is unclear; correlated with major depressive and bipolar disorders in some studies (62, 63, 65) |
| E496A | Reduced pore activity but may possess normal channel function; correlated with papillary thyroid cancer, tuberculosis (27, 33, 54, 58–60) |
| H521Q | Within the intracellular domain |
| V522I | Normal channel and pore activities (27) |
| R544Q | Our preliminary data suggests no change in function |
| I568N | Reduced channel and pore activity; within the C-terminal trafficking domain; does not express on the cell surface (27, 169) |
| R578Q | Previous studies and our preliminary data suggest it has reduced pore and signaling functions; within the C-terminal trafficking domain (12, 26) |
The P2X7 Q460R SNP has been examined in the context of mood disorders, and a correlation with bipolar and major depression disorders has been reported in some studies, whereas others have shown no statistically significant associations (62–65). Also, it is currently unclear whether the Q460R polymorphism results in altered ion channel or pore functions. For example, in one study it was reported that the Q460R mutation does not exhibit altered channel or pore function, whereas it was reported in another study that monocytes from patients with the Q460R polymorphism display enhanced pore function (27, 66). In addition to the E496A and Q460R SNPs, there is one report demonstrating that the A348T polymorphism located within the putative second transmembrane domain is protective against T. gondii infection (67). This P2X7 A348T polymorphism reportedly exhibits enhanced ion channel and pore activity (27). In sum, although it is evident that more studies are needed to determine if P2X7 polymorphisms may be used as clinical markers for disease risk, the potential exists for P2X7 to be used as a novel biomarker for diverse diseases.
Denlinger et al. have reported that P2X7 loss-of-function polymorphisms correlate with the likelihood of experiencing an asthma exacerbation following viral infection (31). Although the mechanisms by which these P2X7 SNPs contribute to loss of asthma control have not been defined, results from preliminary studies suggest the number of neutrophils that infiltrate into the airway is modulated in individuals with specific P2X7 genetic variations (31). To facilitate the possible use of P2X7 status as a novel biomarker for diverse diseases such as asthma, our group has developed a relatively inexpensive and non-invasive blood test to identify patients that possess P2X7 loss-of-function polymorphisms (68). In this test, <1 ml of whole blood is used in a flow cytometry-based P2X7 pore formation assay. The blood cells are stimulated with the P2X7 agonist 2′-3′-O-(4-benzoyl)benzoyl-ATP (BzATP) in the presence of the fluorescent dye YO-PRO®-1, which passes through the P2X7-stimulated pore. The pore is then closed via the addition of MgCl2, and the uptake of YO-PRO®-1 is measured using a flow cytometer. Pore activity below a specified threshold is considered “low” P2X7 activity, and “low” activity is generally predictive of a P2X7 loss-of-function polymorphism (66, 68). If P2X7 antagonists pass clinical trials and/or P2X7 becomes an established biomarker, the whole blood pore assay could be useful for identifying patients with attenuated P2X7 function using commonly available clinical equipment.
Anti-P2X7 Clinical Trials
Because of growing interest in the contribution of P2X7 to various diseases, numerous pharmaceutical companies have developed P2X7 antagonists. A few recent reviews offer a comprehensive listing of the P2X7 antagonist patents that have been documented up to November 2009 (8, 11, 69). In addition to the development of P2X7-directed compounds, many companies are testing these compounds in clinical trials for the treatment of conditions ranging from rheumatoid arthritis to neurological disorders. AstraZeneca’s P2X7 antagonist AZD-9056 has successfully passed Phase I trials and has recently completed a six month Phase IIb study for the treatment of rheumatoid arthritis (70). AZD-9056 was also examined for the treatment of osteoarthritis, chronic obstructive pulmonary disorder and irritable bowel syndrome, but these studies have since been terminated due to a lack of efficacy. Pfeizer’s P2X7 antagonist CE-224535 is currently in Phase II trials for the treatment of rheumatoid arthritis in patients who do not improve after methotrexate treatment (70, 71). As with AZD-9056, CE-224535 was tested as a treatment for osteoarthritis, but it was also terminated due to a lack of efficacy. Evotec is pursuing a “P2X7 Antagonist Program”, and their antagonist EVT 401 has successfully passed Phase I toxicology testing and will be entering Phase II studies for the treatment of rheumatoid arthritis. Additionally, GlaxoSmithKline’s P2X7 antagonist GSK1482160 is currently undergoing toxicology testing for treatment of inflammatory pain, such as that observed in arthritis (70).
Besides the current clinical trials testing P2X7 antagonists for the treatment of rheumatoid arthritis, several trials have been initiated to investigate the link between neurological disorders and P2X7 activation. Affectis Pharmaceuticals has synthesized a central nervous system (CNS)-penetrant P2X7 antagonist, AFC-5128, that is currently being tested as a potential treatment for neuroinflammatory and neurodegenerative diseases (72). Affectis Pharmaceuticals also recently patented two P2X7 antagonists for the treatment of depression and bipolar affective disorder (73). Furthermore, it has been reported that AstraZeneca’s AZD-9056 may also be used as treatment for mood disorders such as depression (11). P2X7 antagonists are not the only method being used in clinical trials to target P2X7 activity, e.g., studies in the Netherlands are currently underway to determine the effect of mechanical stimulation of bone using a Juvent 1000 vibration platform in order to promote ATP release in vivo (70). Mechanical stimuli are important for maintaining normal bone structure, and ATP is released from bone cells upon mechanical stress and contributes to bone remodeling by binding to P2 receptors (2, 74). Thus, increasing mechanical stimulation and activating P2 receptors may be a novel therapeutic target for the treatment of skeletal disorders such as osteoporosis. The motivation behind this study is also partially based on the observation that P2X7 loss-of-function SNPs are associated with fracture risk in a cohort of postmenopausal women (75).
IL-1β - A Critical Mediator of P2X7 and Regulator of Inflammatory and Neurologic Functions
One of the most well characterized functions of P2X7 is its promotion of the processing of IL-1β (and the related IL-18) into its biologically active form. This process is mediated by the assembly of an inflammasome comprised of caspase-1, Nacht Domain-, Leucine-Rich Repeat-, and PYD-Containing Protein 3 (NALP3), and Apoptosis-associated Speck-like containing a CARD (ASC) (76). Interestingly, many of the P2X7 drugs in clinical trials were screened for their ability to attenuate IL-1β release, a process used to identify compounds with the highest therapeutic potential. IL-1β is a pro-inflammatory cytokine that plays a role in inflammatory and neurologic disorders such as arthritis, sepsis, Alzheimer’s disease, depression and chronic neuropathic pain (77).
The biosynthesis of IL-1β involves two steps: 1) synthesis of pro-IL-1β and 2) proteolytic cleavage of pro-IL-1β by caspase-1 to form the active cytokine. The current consensus is that K+ efflux induced by activated P2X7 stimulates the cleavage of pro-caspase-1 to caspase-1, which consequently can convert IL-1β to its bioactive form (76). Qu et al. proposed that the rapid export of IL-1β upon stimulation of P2X7 involves the formation of multivesicular bodies that contain exosomes with bioactive IL-1β and inflammasome components (78). A role for P2X7 in IL-1β processing is well documented in monocytic cells treated with extracellular nucleotides and various Toll-like receptor (TLR) agonists, with LPS being the most extensively examined (79, 80). Macrophages from P2X7 null mice are deficient in ATP-stimulated IL-1β release (81), and human monocytic cells primed with LPS release mature IL-1β via a P2X7-dependent mechanism (19). In addition to extracellular nucleotides and LPS, other endogenous stimuli have been shown to induce P2X7-dependent IL-1β maturation. The human cathelicidin-derived peptide LL37, a potent antibacterial peptide produced by neutrophils and epithelial cells, has been shown to promote the release of mature IL-1β from LPS-primed monocytes in a P2X7-dependent manner (82). There is also compelling evidence for a role of the pannexins, which are a family of transmembrane channels, in P2X7-dependent IL-1β release (79, 83, 84). In terms of a disease model, LPS-treated monocytes from patients with rheumatoid arthritis produce significantly higher levels of IL-1β in response to ATP when compared to control patients (85). Thus, P2X7 plays a critical role in IL-1β processing and is a potential point of therapeutic intervention for the attenuation of IL-1β action.
Dysregulation of the processing and release of IL-1β has been extensively studied in neurological diseases, including Alzheimer’s disease. There is accumulating evidence suggesting that the key component in the development of the pathophysiology of Alzheimer’s disease is the inflammatory cycle encompassing IL-1β and other pro-inflammatory factors. IL-1β affects many aspects of neurodegeneration, such as the formation of β-amyloid precursor protein, activation of astrocytes, and increased inducible nitric oxide synthase (iNOS) production (77, 86). Multiple studies have revealed that P2X7 is involved in the processing/release of IL-1β and the generation of ROS from microglia, macrophages and other monocytic cells (87–90). In mixed glial cell cultures from P2X7 knockout mice, LPS-induced IL-1β release, but not TNFα release, is largely suppressed in comparison to wild-type controls (91), supporting the contention that P2X7 is a critical stimulator of IL-1β release. In addition, P2X7 has been reported to regulate the release of IL-1β and cysteinyl leukotrienes in certain astrocyte cultures (92, 93). Notably, IL-1β can potentiate its own mRNA expression and P2X7 expression in microglia and astrocytes, respectively (94, 95). This positive feedback loop continues to drive the inflammatory cycle and results in a reactive glial environment. Ultimately, sustained activation of microglia and astrocytes can lead to an increase in cell death, inflammation, neuronal stress, and cell damage, which positively promote the inflammatory cycle (77, 96).
As discussed above, several animal studies have linked P2X7 to neuropathic pain, and this connection is attributed to altered IL-1β production (97). In the study by Chessell et al., P2X7-deficient mice exhibited a decreased level of systemic IL-1β following an adjuvant challenge and were protected from inflammatory and neuropathic pain caused by both mechanical and thermal stimuli (45). In animal arthritic models, a P2X antagonist, oATP, has been reported to exert anti-nociceptive effects in rats, and mice lacking P2X7 display a decrease in the incidence and severity of monoclonal anti-collagen-induced arthritis (36, 81). Collectively, there is considerable data supporting the concept that P2X7-mediated IL-1β release plays a role in both chronic inflammatory and neuropathic pain.
P2X7-Mediated Gene Expression: A Recently Recognized Function
The release of mature IL-1β is not the only mechanism by which P2X7 is proposed to regulate inflammation; there is increased appreciation for the idea that P2X7 activates transcription factors and the synthesis of multiple immuno-modulatory factors in immune cells (12). It is a relatively recently recognized concept that P2X7 regulates gene transcription. To date, it has been reported that P2X7 ligands also promote the production of numerous immune mediators, including VEGF, COX-2, IL-2, IL-6, IL-8 and iNOS (20–22, 98–100). In addition, we and others have reported that P2X7 ligands can stimulate the expression, activation and/or nuclear translocation of several transcription factors, including members of the early growth response (Egr-1, -2 and -3) family, the nuclear factor of activated T cells (NFAT), nuclear factor-κB (NF-κB) family members, the cyclic-AMP response element (CRE)-binding protein (CREB), and the AP-1 family members c-Fos, FosB and JunB (20, 30, 100–104). Details about these findings in the context of immune regulation are provided below (also see Fig. 2).
Fig. 2. Summary Model of the Proposed Mechanisms of P2X7-mediated Control of Transcriptional Regulators.
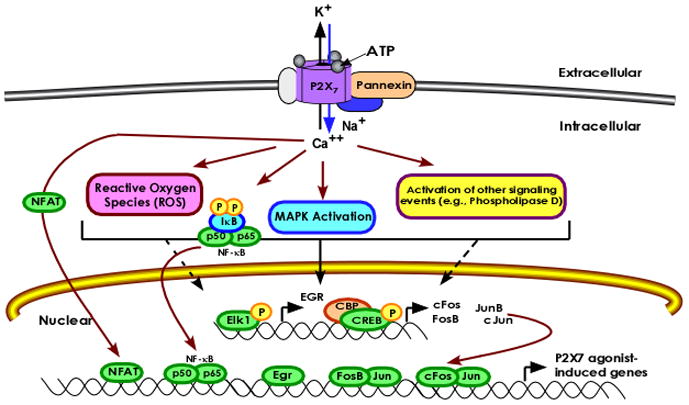
Stimulation of the ionotropic nucleotide receptor P2X7 with extracellular ATP induces the efflux of K+ and the influx of Ca++ and Na+. Following prolonged receptor stimulation, there is the formation of a nonselective pore that has been reported to involve the pannexins. Upon ligand binding, P2X7 can initiate multiple signaling events, including the production of reaction oxygen species, the activation of multiple MAP kinase (MAPK) cascades, and the expression/activation of numerous transcription factors (shown in green). The role of the various P2X 7-initiated signaling cascades in the activation of these transcription factors is still poorly understood, but many reports support a central role for intracellular Ca++ fluxes and MAPK signaling cascades. For example, Ca++ fluxes appear linked to the nuclear translocation of the NFAT, whereas reactive oxygen species production and Ca++ fluxes have been linked to NF-κB nuclear translocation, and MAPK-dependent events appear essential for the activation of the CREB and Egr transcription factors. In addition, the transcriptional regulation and expression of only a small number of P2X7-dependent genes has been elucidated to date, including the P2X7-mediated expression of several Egr and AP-1 (cFos, FosB, JunB) transcription factors. Pannexins: family of transmembrane channels; important in P2X7-mediated IL-1β processing and release. I-κB: NF-κB regulatory protein that is degraded after phosphorylation, allowing NF-κB translocation into the nucleus. p50/p65: NF-κB subunits proposed to be P2X7 agonist-induced. Elk-1 (Ets LiKe gene-1): Ets transcription factor of the ternary complex factor subfamily that binds to the serum response element in the promoter of genes; linked to the expression of Egr1 after P2X7 activation. CBP (CREB-binding protein): required for CREB-mediated gene expression; P2X7 agonists induce CREB/CBP complex formation. P: phospho-group; regulates the activation of various proteins. Jun (JunB, cJun): members of the AP-1 family of transcription factors; JunB and cJun expression is upregulated after P2X7 stimulation
Selected Immune Mediators Regulated by P2X7
One immune factor that has recently been shown to be regulated at the mRNA level in response to P2X7 signaling is VEGF, which is a pro-angiogenic factor and a drug target for multiple types of cancer (21, 105). This factor is released from monocytic cells during inflammatory wound repair to promote the formation of new blood vessels and immune cell migration (105, 106). Because both VEGF and high levels of ATP are released from cells upon shear stress (2, 107), we tested the idea that P2X7 is involved in the release of VEGF. We observed that P2X7 ligands induce VEGF mRNA synthesis and VEGF protein release from monocytic cells, and that a P2X7 antagonist attenuates ligand-stimulated VEGF secretion (21). In support of these data, Wei et al. showed that the P2X7 agonist BzATP induces VEGF mRNA expression in rat C6 glioma cells (99). Thus, it is possible that extracellular nucleotides regulate the resolution of inflammation by stimulating this pro-angiogenic factor.
IL-8 is an important chemokine that attracts immune cells such as neutrophils to sites of infection (108), and it has been demonstrated that P2X7 ligands induce the expression of this factor. Specifically, Wei et al. reported that BzATP stimulates IL-8 mRNA production in the C6 glioma cell line, and Idzko et al. showed that ATP and BzATP stimulate IL-8 release from primary human blood eosinophils (99, 109). In addition, Schneider et al. reported that human peripheral blood mononuclear cells release IL-8 upon ATP stimulation (110). Because ATP reportedly stimulates the recruitment of neutrophils to sites of tissue injury, and P2X7 pore function reportedly correlates with the change in nasal lavage neutrophil counts during a cold, it is interesting to speculate that these processes are partially mediated by P2X7 ligand-induced IL-8 expression (31, 111).
IL-2 is a well characterized cytokine that is involved in the development of memory and regulatory T cells (112), and it is considered an attractive therapeutic target for cancer and autoimmune diseases. Recombinant IL-2 has been used to treat melanoma and renal cell carcinoma, and several immuno-suppressive drugs function by inhibiting the IL-2 signaling cascade (113–115). In terms of a connection between extracellular nucleotides and IL-2 generation, several groups have reported that P2X7 ligands can induce the expression of IL-2. For example, Budagian et al. showed that ATP induces IL-2 mRNA expression in Jurkat T cells, and Yip et al. validated this finding in the same cell line (100, 116). Also, Woehrle et al. reported that siRNA against P2X7 attenuates ATP-induced IL-2 expression in Jurkat cells (117). These results support the idea that extracellular nucleotides may regulate adaptive immunity.
Another protein involved in mediating many inflammatory and vascular responses is iNOS (118), which is an enzyme that catalyzes the synthesis of NO from L-arginine. During an inflammatory response, iNOS promotes the production of high NO concentrations that are capable of stimulating microbial killing (118). In this regard, we have reported that BzATP enhances LPS-induced iNOS expression in macrophages (89, 102), and Gendron et al. presented evidence that BzATP enhances IFNγ-stimulated iNOS expression and NO production in BV-2 microglial cells (119). Thus, the production of iNOS by extracellular nucleotides may be one mechanism by which these ligands stimulate the killing of intracellular pathogens such as M. tuberculosis (120).
The pro-inflammatory enzyme COX-2 catalyzes a key step in the conversion of arachidonic acid into biologically active prostaglandins, and it is the target of several anti-inflammatory drugs (121). We have found that P2X7 ligands induce the expression of COX-2 in an osteoblast cell line and in human peripheral blood mononuclear cells (15, 20). Interestingly, COX-2 is a proposed regulator of bone turnover, and Panupinthu et al. reported that two COX-2 inhibitors reduce BzATP-stimulated mineralization and gene expression in calvarial cultures (122, 123). In accordance with the observation that COX-2 expression can be regulated by P2X7 stimulation, the production of prostaglandins, particularly prostaglandin E2 (PGE2), by ATP has also been attributed to P2X7 stimulation. Specifically, extracellular ATP-induced PGE2 release from osteoblasts appears to be P2X7-dependent (40). Furthermore, fluid shear stress, which is known to induce the release of ATP, significantly increases PGE2 release in calvarial osteoblasts from P2X7 wild-type mice but has no significant effect on PGE2 release in osteoblasts isolated from P2X7 knockout mice (40). Osteoblast stimulation has been linked to PGE2 release, which is important for osteoclast differentiation and bone resorption, as well as the pathogenesis of rheumatoid arthritis (124, 125). Therefore, P2X7 regulation of COX-2 expression appears important in the mediation of both inflammation and bone formation/remodelling.
Transcriptional Regulators and P2X7 Action
It is probable that additional immuno-modulatory gene targets of P2X7 signaling will soon be identified and that the mechanisms by which these targets are induced will be deciphered. In this regard, the activation of transcription factors (Egr, NFAT, NF-κB, CREB, AP-1) are likely to be key transcriptional regulators mediating the actions of P2X7 ligands, and these factors are discussed below (also see Fig. 2).
Egr
Egr-1, Egr-2 and Egr-3 are immediate early genes that play important roles in immunity, and the expression of these zinc finger transcription factors is induced by multiple mitogenic signals in various cell types. Egr-1 is involved in the activation of B and T cells while Egr-2 and Egr-3 are thought to promote anergy in T cells (126). Interestingly, it has been recently reported that P2X7 ligands induce the expression of these factors (103, 104). Specifically, Stefano et al. used HEK293 cells that express P2X7 to demonstrate that BzATP can promote the transcriptional up-regulation of Egr-1 via the action of ERK1/2 and the transcription factor Elk-1, supporting the notion that P2X7 activation influences gene transcription (104). In addition, Friedle et al. showed that BzATP induces Egr-1, Egr-2, and Egr-3 expression in N9 microglia and primary microglia cells (103). In these studies, it was also noted that the introduction of Egr RNAi into N9 microglia attenuates BzATP-stimulated IL-6 production, as well as the production of TNF-α induced by co-stimulation with LPS and BzATP (103). Furthermore, it has been reported that Egr-1 potentiates VEGF expression in lung cancer cells (127), suggesting that the P2X7-dependent VEGF release we observed with human monocytes may arise via Egr-1 activation.
NFAT
The NFAT transcription factor family (NFAT1–5) regulates the expression of many immune mediators. NFAT is activated by a rise in cytoplasmic Ca++ levels achieved by the release of Ca++ stores from the endoplasmic reticulum and the opening of Ca++-release-activated Ca++ channels. This rise in intracellular Ca++ activates calmodulin, which activates the serine/threonine phosphatase calcineurin. Calcineurin then dephosphorylates NFAT and exposes a nuclear import sequence, allowing the transcription factor to translocate into the nucleus and promote gene transcription (128, 129). Several groups have provided evidence that NFAT is regulated by P2X7; thus, activation of this transcription factor family by nucleotides may a key mechanism by which the expression of immune mediators is regulated by P2X7. Yip et al. showed that P2X7 RNAi in Jurkat T cells attenuates NFAT activity and IL-2 transcription induced by a T cell activating factor (100). Adinolfi et al. demonstrated that the P2X inhibitor oxidized ATP (oATP) decreases NFAT1 nuclear expression in HEK293 cells exogenously expressing P2X7, and Kataoka et al. showed ATP activates NFAT1 in MG-5 microglial cells (101, 130). Additionally, Ferrari et al. reported that ATP and BzATP activate NFAT in N9 microglial cells (131).
NF-κB
NF-κB is a widely distributed transcription factor that modulates the induction of numerous immuno-modulatory genes, including IL-6, IL-8, TNF-α, iNOS and COX-2 (132, 133). This factor exists in an inactive state in the cytoplasm through its association with inhibitor of κB (IκB). Upon phosphorylation of IκB by IκB kinase (IKK), IκB becomes marked for degradation by the proteasome and NF-κB translocates into the nucleus where it can promote gene transcription (132, 133). We and others have reported that P2X7 agonists stimulate NF-κB translocation and DNA binding activity in multiple cell types. For example, Ferrari et al. showed ATP stimulates NF-κB binding to DNA in the mouse microglial cell lines N9 and N13, and that this binding was inhibited by antioxidant treatment, whereas Budagian et al. reported that ATP stimulates NF-κB binding to DNA in Jurkat T cells (116, 134). Similarly, Korcok et al. demonstrated BzATP induces the nuclear translocation of NF-κB in mouse osteoclasts but not in osteoclasts from P2X7 knockout mice (135), and we showed that BzATP induces NF-κB DNA binding in RAW 264.7 macrophages and in human peripheral blood mononuclear cells (15, 98, 102).
CREB
The leucine zipper transcription factor CREB functions to control many cell processes, including glucose homeostasis, cell survival, and inflammatory mediator production. Phosphorylation of CREB on serine-133 stimulates co-activation by CREB-binding protein (CBP) or p300, allowing CREB to bind to conserved CREB response elements (CREs; 5′-TGACGTCA-3′) in the promoters of cAMP-responsive genes. Several immuno-modulatory genes including IL-2, IL-6, IL-10 and TNF-α contain CRE sites in their promoters (136). We have reported that P2X7 activation can stimulate CREB function in monocytic cells (30). We found that stimulation with the P2X7 agonist BzATP results in phosphorylation of CREB at serine-133 and that this signaling event is dependent upon ERK1/2 and Ca++ (30). Ligands for P2X7 can lead to CREB activation in human peripheral blood mononuclear cells, the human monocytic cell line THP-1, the murine macrophage cell line RAW 264.7, and in HEK293 cells that exogenously express P2X7. We also showed that RAW 264.7 cells that express a loss-of-function P2X7 mutation, i.e., RAW S342F cells, do not exhibit CREB phosphorylation in response to BzATP but do exhibit activation of CREB in response to LPS or anisomycin (an activator of p38 MAPK) (30). In addition, Potucek et al. reported that BzATP activates CREB in BV-2 microglia cells (137). In further support of a role for P2X7 in the transcriptional regulation of gene expression, we have shown that inhibition of CREB activation using dominant negative CREB constructs significantly attenuates the expression of the AP-1 genes c-Fos and FosB (20, 30).
AP-1
The AP-1 transcription factors are stimulated by many factors, including UV light, growth factors and oxidative stress, and they regulate the transcription of genes important for metastasis, invasion, differentiation, hypoxia, angiogenesis, proliferation, and apoptosis (138). This family of transcription factors includes c-Jun, JunB, JunD, Fra-1, Fra-2, c-Fos, FosB and ΔFosB, and various dimers of these factors bind to AP-1 sites in gene promoters (the AP-1 consensus sequence is TGAC/GTCA). AP-1 family members contain a basic leucine zipper region that is important for dimerization with another AP-1 protein and for DNA binding. Transcriptionally active AP-1 proteins include a Jun protein and a Fos family member (138). We and other groups have reported that P2X7 ligands can induce the expression and activation of AP-1 transcription factors (20, 116, 139). We found that P2X7 ligands modestly induce c-Fos and JunB expression, but can robustly promote the rapid (i.e., hrs) induction of FosB and ΔFosB in macrophages and osteoblasts, and we also observed that these agonists stimulate the binding of FosB proteins to DNA (20). FosB and ΔFosB are two of the lesser-studied AP-1 family members, but increasing evidence supports a role for these transcription factors in inflammation, bone turnover, and neuronal function. The biology of FosB and ΔFosB and the potential significance of our observation in the context of immune, osteogenic, and neuronal regulation will be discussed below.
FosB and ΔFosB
The AP-1 transcription factors FosB and ΔFosB are poorly understood, but the further investigation of their contributions to neurobiology and immunology appears warranted. These factors are most well known for their roles in adaptive changes in the brain in response to stimulation with drugs of abuse, and ΔFosB is hypothesized to be a “molecular switch” in the development of drug addiction (140). Overall, much less is known about FosB and ΔFosB in comparison to the other AP-1 family members, but it is known that FosB and ΔFosB possess a PEST domain (a sequence rich in proline, glutamate, serine, threonine), a basic region, and a leucine zipper. The leucine zipper is the major site of interaction between FosB and its AP-1 binding partner Jun. ΔFosB is a truncated splice variant of FosB that lacks 101 amino acids from the C-terminus of FosB and a portion of the transactivation domain found in full-length FosB. ΔFosB mRNA is spliced from full-length FosB by the excision of 140 nucleotides of “intronic” sequence in the open reading frame in FosB. This splicing event induces a frameshift that creates a stop codon in ΔFosB (141). ΔFosB is quite stable because it lacks two destabilizing elements (a proteasome-dependent and a proteasome-independent degron) that are present in full-length FosB; thus, its half-life is longer than FosB in many systems (142).
There is considerable interest in ΔFosB in psychiatric research because ΔFosB accumulates in region-specific areas of the brain after chronic stimuli with an array of drugs or following stress, and its expression persists for weeks to months. It is thought that ΔFosB regulates long-term adaptive changes in the brain by altering transcriptional profiles and by controlling natural and drug-induced reward (143). Previous reports have shown that ΔFosB can be induced by cocaine, amphetamine, morphine, nicotine, alcohol, antidepressants, anti-psychotic drugs, chronic exercise, sucrose, and sexual reward. Furthermore, ΔFosB remains expressed in the brain for several weeks or more after cessation of the stimulus (143–149). Nestler et al and McClung et al offer comprehensive reviews on the long-term persistence of ΔFosB in the brain (140, 143), and numerous animal model studies support the idea that ΔFosB regulates behavioral plasticity and is critical for normal psychiatric behavior. One of the first FosB knockout studies demonstrated that FosB knockout mice exhibit normal cognitive and sensory functions but they do not nurture their offspring; thus, their pups require surrogate parents (150). As for regulating behavioral plasticity, it was discovered early that FosB knockout mice display exaggerated responses to initial cocaine exposure but fail to become sensitized after repeated exposure (151). Subsequent studies have demonstrated the importance of ΔFosB in regulating reward by both natural stimuli and drugs of abuse. For example, mice that over-express ΔFosB in the nucleus accumbens exhibit increased sensitivity to drugs of abuse and drug-seeking behavior (152, 153).
Several reports have provided evidence that FosB and ΔFosB may possess broader functions, including the capacity to modulate the inflammatory response and bone formation. In terms of inflammation, it has been reported that FosB becomes transcriptionally active in mouse lungs after mechanical ventilation (154), and ΔFosB accumulates and persists in lumbar segments of rat spinal cords after injection of λ-carrageenan type IV, a molecule used to induce inflammatory pain (155). In addition, studies have shown oxidative stress induced by H2O2 promotes the expression of FosB (156). Furthermore, FosB expression is higher in inflammatory breast cancer tissue in comparison to non-inflammatory tissue controls (157). As for bone formation, FosB knockout mice do not display any skeletal abnormalities (158), whereas ΔFosB transgenic mice exhibit increased progressive osteosclerosis, or augmented bone formation, that appears to be osteoblast-dependent (159). The mechanisms by which ΔFosB regulates bone turnover are not well defined, but it has been reported that FosB/ΔFosB is required for mechanical stress-induced production of IL-11 in osteoblasts (160). As noted above, mechanical stress stimulates ATP release from bone cells and contributes to bone remodeling (2). As for IL-11, this factor regulates normal bone remodeling through the regulation of both osteoclasts and osteoblasts, and it has been demonstrated that IL-11 promotes osteoblast differentiation in vitro (161). It is possible that ATP induces the expression of FosB and ΔFosB via the action of P2X7, and that these events result in the transcription of osteogenic genes such as IL-11.
Examination of the Evidence for an ATP/P2X7/FosB Pathway
We have reported the existence of a previously unrecognized ATP/P2X7/FosB pathway in monocytic and osteoblast cells (20) and propose that this signaling network contributes to the transcriptional regulation of multiple immuno-modulatory factors. We have found that transient stimulation with P2X7 ligands (BzATP, ATP) induces the expression of FosB and ΔFosB in human peripheral blood mononuclear cells, RAW 264.7 macrophages, mouse bone marrow-derived macrophages, the preosteoblast cell line MC3T3-E1, and HEK293 cells that stably express P2X7 (20 and data not shown). In addition to showing P2X7 ligands induce the expression of FosB and ΔFosB, we also used electrophoretic mobility shift assays (EMSA) to demonstrate that BzATP activates FosB proteins in RAW 264.7 and MC3T3-E1 cells (20). In these studies, cells were treated with agonist for 5 min, the media was replaced, and the cells were harvested 0.5–3 h later. The short-term (5 min) treatment with agonist may be more representative of an in vivo microenvironment where bursts of high levels of nucleotides are achieved upon tissue damage or platelet degranulation, and these high concentrations are normalized after nucleotide breakdown by ectonucleotidases. Strikingly, low levels of BzATP (5–30 μM) that do not stimulate most P2X7-mediated events, such as ion channel activation and pore formation (7), can induce the expression of FosB and ΔFosB (ref. (20) and data not shown). These data support the idea that FosB and ΔFosB are two of the most sensitive known mediators of P2X 7 action. We reported that both FosB and ΔFosB become expressed 1 h after BzATP treatment, and that the FosB proteins persist for at least 3 h. Recent work from our laboratory has shown that FosB and ΔFosB are substantially degraded in RAW 264.7 cells at a time 8 h post-BzATP treatment (data not shown), which is consistent with results in rat adrenal pheochromocytoma (PC12) cells showing FosB and ΔFosB are degraded within 8 h following treatment with 20% serum (142). Therefore, it is unlikely that nucleotides stimulate long-term persistence of FosB and ΔFosB in macrophages as observed in models of drug addiction where ΔFosB persists for days to weeks in the brain (143).
To determine the biological significance of the robust induction of FosB and ΔFosB in response to nucleotides, we tested the idea that FosB and ΔFosB are required for P2X7-mediated induction of pro-inflammatory proteins. To test our hypothesis, we used RNAi to knock down FosB expression in MC3T3-E1 osteoblasts, treated the cells with BzATP for 5 min, replaced the media, and prepared cell lysates after 4 h to assess COX-2 expression using immunoblotting (20). We observed that P2X7 ligand-mediated COX-2 induction in MC3T3-E1 cells is dependent upon FosB proteins (20), revealing that at least one key endpoint of P2X7 signaling is linked to FosB expression, Accordingly, it is of interest to ascertain whether the ATP/P2X7/FosB signaling axis functions in monocytic cells to regulate the expression of other immuno-modulatory factors.
It is known that the P2X7 agonists BzATP and ATP can activate other P2X family members (7); therefore, to verify that P2X7 mediates the induction of FosB and ΔFosB, we performed several control experiments and utilized a recently developed P2X7 inhibitor (20). We observed that BzATP-mediated FosB and ΔFosB induction is substantially attenuated in RAW 264.7 cells that express the loss-of-function P2X7 S342F mutant, and BzATP is unable to induce FosB-DNA binding activity (as indicated by an EMSA) in the P2X7-defective cell line. Furthermore, it was noted that the P2X7 antagonist A438079 reduces BzATP-stimulated FosB and ΔFosB induction in RAW 264.7 macrophages (20). Thus, we conclude that P2X7 stimulates FosB and ΔFosB expression in response to nucleotides, but it is currently unknown whether other P2X family members may also induce the expression of these transcription factors.
Future Studies
The nucleotide receptor P2X7 is a key mediator of the inflammatory response, and increasing evidence implicates that it also performs important functions in bone and neuronal regulation. Numerous studies support the idea that P2X7 is an attractive therapeutic target for inflammatory conditions; thus, P2X7 inhibitors are in clinical trials for rheumatoid arthritis, and several groups are attempting to develop additional P2X7 antagonists (162–166). Furthermore, recent reports support the hypothesis that P2X7 induces the transcription of immuno-modulatory genes, and that this action is likely a key mechanism by which P2X7 mediates inflammatory, osteogenic and neuronal functions.
Our recent analyses of P2X7-stimulated transcriptional responses have revealed that P2X7 activates CREB and the intriguing, yet poorly understood, AP-1 transcription factors FosB and ΔFosB. In addition, we have found that FosB/ΔFosB is involved in P2X7 ligand-stimulated COX-2 induction in osteoblast cells, supporting the idea that this signaling axis controls the induction of inflammatory mediators. The finding that extremely low doses of P2X7 ligands can promote the potent induction of FosB/ΔFosB in macrophages and osteoblasts suggests that this system is responsible for some of the most essential, and perhaps unrecognized, actions of P2X7. Little is known regarding the role of FosB and ΔFosB in the inflammatory response when compared to the amount of data establishing their role in behavioral plasticity, and thus defining the significance of this transcriptional regulatory system in immune function is of great interest. Similarly, it is unknown whether P2X7 polymorphisms contribute to altered inflammatory mediator production via dysregulated FosB/ΔFosB production/action, and this topic is also an area of active investigation. Many disorders with chronic inflammation such as asthma and rheumatoid arthritis are attributed, at least in part, to genetic factors, but the genes and polymorphisms that promote these disorders have not been clearly identified. Because FosB and ΔFosB are stimulated by low concentrations of nucleotides, it is possible that P2X7 is constitutively being activated to induce these transcription factors and regulate basal production of immuno-modulatory proteins.
As briefly discussed above, ATP, P2X7, and FosB proteins are thought to regulate bone formation. Two major cell types that regulate bone formation are osteoclasts (resorb mineralized bone) and osteoblasts (form bone), and these cell types coordinately function together to ensure normal bone turnover. Although FosB knockout mice do not display detectable skeletal deformities (158), mice that over-express ΔFosB appear to exhibit osteoblast-dependent osteosclerosis (159), whereas P2X7 knockout mice display excessive trabecular bone resorption and deficient periosteal bone formation (39). The role of P2X7 in osteoclast formation and regulation is complex; therefore, additional studies are required to determine whether the skeletal deformities observed in P2X7 knockout mice are attributed to altered osteoclast function (2). However, it has been reported that P2X7 ligands stimulate osteoblast differentiation and mineralization; thus, altered osteoblast function may be a mechanism by which P2X 7 knockout animals display skeletal defects. Because we have shown P2X7 ligands induce FosB and ΔFosB in the osteoblast cell line MC3T3-E1, it is possible that the ATP/P2X7/FosB pathway is activated in this cell type to regulate bone formation and normal bone turnover. The identification of transcriptional targets of the ATP/P2X7/FosB pathway in osteoblasts will facilitate the identification of the mechanisms by which extracellular nucleotides regulate this cell type.
In terms of neuronal function, there is accumulating evidence that both P2X 7 and FosB proteins play critical roles in a psychiatric context. P2X7 function has been attributed to depression, and a P2X7 non-synonymous SNP has been correlated with affective mood disorders (52, 62, 63, 65). Furthermore, the roles of FosB and ΔFosB in drug addiction and reward from various stimuli (natural and drugs of abuse) are well established. In a depression study using P2X7 knockout mice, it was demonstrated that animals deficient in P2X7 have less depressive symptoms than wild-type controls, suggesting that P2X7 can contribute to depressive behavior (52). If the depressive behavior mediated by P2X7 is linked to the induction of ΔFosB, one would expect that animals over-expressing ΔFosB would also exhibit symptoms of depression. However, Berton et al. reported the opposite effect in mice over-expressing ΔFosB in the ventrolateral periaqueductal gray; the authors found that mice with the strongest induction of ΔFosB in this region were the most resilient to inescapable stress, which is an animal model for affective disorder (167). Thus, P2X7 likely promotes depressive behaviors using a non-FosB-dependent mechanism, e.g., P2X7 knockout mice may have coped better with inescapable stress because of a deficiency in IL-1β processing (52). Nonetheless, it is reasonable to speculate that extracellular nucleotides and FosB/ΔFosB play a role in drug addiction. It is not known whether P2X 7 is expressed in reward pathways in the brain or whether P2X7 induces FosB and ΔFosB expression in neuronal cells. If extracellular nucleotides do induce ΔFosB in region-specific areas of the brain important for drug tolerance and reward, it would be fascinating to test the hypothesis that polymorphisms in P2X 7 can contribute to individual patterns of drug abuse.
In sum, the field of P2X7 biology is rapidly growing, yet much is left to be determined concerning the mechanisms by which this receptor regulates immunity, bone formation, and neurologic function. Recent evidence supporting a function for extracellular nucleotides in the regulation of gene transcription offers new insight into the mechanisms by which multiple physiological responses are controlled by P2X7.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants 1 U19 AI070503, 2 R01 HL069116, 5 R01 CA108467 and 1 P01 HL0885940 to PJB.
References
- 1.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 2.Orriss IR, Burnstock G, Arnett TR. Purinergic signalling and bone remodelling. Curr Opin Pharmacol. 2010;10:322–330. doi: 10.1016/j.coph.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Burnstock G. Purinergic signaling and vascular cell proliferation and death. Arterioscler Thromb Vasc Biol. 2002;22:364–373. doi: 10.1161/hq0302.105360. [DOI] [PubMed] [Google Scholar]
- 4.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 5.Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol. 2003;64:785–795. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- 6.North R, Surprenant A. Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- 7.North R. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 8.Guile S, Alcaraz L, Birkinshaw T, Bowers K, Ebden M, Furber M, Stocks M. Antagonists of the P2X(7) receptor. From lead identification to drug development. J Med Chem. 2009;52:3123–3141. doi: 10.1021/jm801528x. [DOI] [PubMed] [Google Scholar]
- 9.Angiolillo D, Ueno M, Goto S. Basic principles of platelet biology and clinical implications. Circ J. 2010;74:597–607. doi: 10.1253/circj.cj-09-0982. [DOI] [PubMed] [Google Scholar]
- 10.Abergel E, Nikolsky E. Ticagrelor. An investigational oral antiplatelet treatment for reduction of major adverse cardiac events in patients with acute coronary syndrome. Vasc Health Risk Manag. 2010;6:963–977. doi: 10.2147/VHRM.S13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunosewoyo H, Kassiou M. P2X purinergic receptor ligands: recently patented compounds. Expert Opin Ther Pat. 2010;20:625–646. doi: 10.1517/13543771003702424. [DOI] [PubMed] [Google Scholar]
- 12.Lenertz LY, Gavala M, Hill LM, Bertics PJ. Cell signaling via the P2X(7) nucleotide receptor: linkage to ROS production, gene transcription, and receptor trafficking. Purinergic Signal. 2009;5:175–187. doi: 10.1007/s11302-009-9133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lenertz LY, Wang Z, Guadarrama AG, Hill LM, Gavala ML, Bertics PJ. Mutation of putative N-linked glycosylation sites on the human nucleotide receptor P2X(7) reveals a key residue important for receptor function. Biochemistry. 2010;49:4611–4619. doi: 10.1021/bi902083n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boumechache M, Masin M, Edwardson J, Górecki D, Murrell-Lagnado R. Analysis of assembly and trafficking of native P2X4 and P2X7 receptor complexes in rodent immune cells. J Biol Chem. 2009;284:13446–13454. doi: 10.1074/jbc.M901255200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aga M, Johnson CJ, Hart AP, Guadarrama AG, Suresh M, Svaren J, Bertics PJ, Darien BJ. Modulation of monocyte signaling and pore formation in response to agonists of the nucleotide receptor P2X(7) J Leukoc Biol. 2002;72:222–232. [PubMed] [Google Scholar]
- 16.Bianco F, Colombo A, Saglietti L, Lecca D, Abbracchio M, Matteoli M, Verderio C. Different properties of P2X(7) receptor in hippocampal and cortical astrocytes. Purinergic Signal. 2009;5:233–240. doi: 10.1007/s11302-009-9137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grol MW, Panupinthu N, Korcok J, Sims SM, Dixon SJ. Expression, signaling, and function of P2X7 receptors in bone. Purinergic Signal. 2009;5:205–221. doi: 10.1007/s11302-009-9139-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou D, Chen ML, Zhang YQ, Zhao ZQ. Involvement of spinal microglial P2X7 receptor in generation of tolerance to morphine analgesia in rats. J Neurosci. 2010;30:8042–8047. doi: 10.1523/JNEUROSCI.5377-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L, Baricordi O, Di Virgilio F. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997;159:1451–1458. [PubMed] [Google Scholar]
- 20.Gavala ML, Hill LM, Lenertz LY, Karta MR, Bertics PJ. Activation of the transcription factor FosB/activating protein-1 (AP-1) is a prominent downstream signal of the extracellular nucleotide receptor P2RX7 in monocytic and osteoblastic cells. J Biol Chem. 2010;285:34288–34298. doi: 10.1074/jbc.M110.142091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill LM, Gavala ML, Lenertz LY, Bertics PJ. Extracellular ATP may contribute to tissue repair by rapidly stimulating purinergic receptor X7-dependent vascular endothelial growth factor release from primary human monocytes. J Immunol. 2010;185:3028–3034. doi: 10.4049/jimmunol.1001298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, Griffiths RJ, Gabel CA. Altered cytokine production in mice lacking P2X7 receptors. J Biol Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- 23.Denlinger LC, Fisette PL, Sommer JA, Watters JJ, Prabhu U, Dubyak GR, Proctor RA, Bertics PJ. The nucleotide receptor P2X7 contains multiple protein- and lipid-interaction motifs including a potential binding site for bacterial lipopolysaccharide. J Immunol. 2001;167:1871–1876. doi: 10.4049/jimmunol.167.4.1871. [DOI] [PubMed] [Google Scholar]
- 24.Fuller S, Stokes L, Skarratt K, Gu B, Wiley JS. Genetics of the P2X7 receptor and human disease. Purinergic Signal. 2009;5:257–262. doi: 10.1007/s11302-009-9136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smart M, Gu B, Panchal R, Wiley J, Cromer B, Williams D, Petrou S. P2X7 receptor cell surface expression and cytolytic pore formation are regulated by a distal C-terminal region. J Biol Chem. 2003;278:8853–8860. doi: 10.1074/jbc.M211094200. [DOI] [PubMed] [Google Scholar]
- 26.Denlinger LC, Sommer JA, Parker K, Gudipaty L, Fisette PL, Watters JJ, Proctor RA, Dubyak GR, Bertics PJ. Mutation of a dibasic amino acid motif within the C terminus of the P2X7 nucleotide receptor results in trafficking defects and impaired function. J Immunol. 2003;171:1304–1311. doi: 10.4049/jimmunol.171.3.1304. [DOI] [PubMed] [Google Scholar]
- 27.Roger S, Mei ZZ, Baldwin JM, Dong L, Bradley H, Baldwin SA, Surprenant A, Jiang LH. Single nucleotide polymorphisms that were identified in affective mood disorders affect ATP-activated P2X(7) receptor functions. J Psychiatr Res. 2009;44:347–355. doi: 10.1016/j.jpsychires.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Gonnord P, Delarasse C, Auger R, Benihoud K, Prigent M, Cuif M, Lamaze C, Kanellopoulos J. Palmitoylation of the P2X7 receptor, an ATP-gated channel, controls its expression and association with lipid rafts. FASEB J. 2009;23:795–805. doi: 10.1096/fj.08-114637. [DOI] [PubMed] [Google Scholar]
- 29.Shinozaki Y, Sumitomo K, Tsuda M, Koizumi S, Inoue K, Torimitsu K. Direct Observation of ATP-Induced Conformational Changes in Single P2X4 Receptors. PLoS Biol. 2009;7:e103. doi: 10.1371/journal.pbio.1000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gavala ML, Pfeiffer ZA, Bertics PJ. The nucleotide receptor P2RX7 mediates ATP-induced CREB activation in human and murine monocytic cells. J Leukoc Biol. 2008;84:1159–1171. doi: 10.1189/jlb.0907612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Denlinger LC, Shi L, Guadarrama AG, Schell K, Green D, Morrin A, Hogan K, Sorkness R, Busse WW, Gern JE. Attenuated P2X7 pore function as a risk factor for virus-induced loss of asthma control. Am J Respir Crit Care Med. 2009;179:265–270. doi: 10.1164/rccm.200802-293OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dardano A, Falzoni S, Caraccio N, Polini A, Tognini S, Solini A, Berti P, Di Virgilio F, Monzani F. 1513A>C polymorphism in the P2X7 receptor gene in patients with papillary thyroid cancer: correlation with histological variants and clinical parameters. J Clin Endocrinol Metab. 2009;94:695–698. doi: 10.1210/jc.2008-1322. [DOI] [PubMed] [Google Scholar]
- 33.Solini A, Cuccato S, Ferrari D, Santini E, Gulinelli S, Callegari M, Dardano A, Faviana P, Madec S, Di Virgilio F, Monzani F. Increased P2X7 receptor expression and function in thyroid papillary cancer: a new potential marker of the disease? Endocrinology. 2008;149:389–396. doi: 10.1210/en.2007-1223. [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Arcuino G, Takano T, Lin J, Peng W, Wan P, Li P, Xu Q, Liu Q, Goldman S, Nedergaard M. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med. 2004;10:821–827. doi: 10.1038/nm1082. [DOI] [PubMed] [Google Scholar]
- 35.Peng W, Cotrina M, Han X, Yu H, Bekar L, Blum L, Takano T, Tian G, Goldman S, Nedergaard M. Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc Natl Acad Sci U S A. 2009;106:12489–12493. doi: 10.1073/pnas.0902531106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dell’Antonio G, Quattrini A, Dal Cin E, Fulgenzi A, Ferrero M. Antinociceptive effect of a new P(2Z)/P2X7 antagonist, oxidized ATP, in arthritic rats. Neurosci Lett. 2002;327:87–90. doi: 10.1016/s0304-3940(02)00385-3. [DOI] [PubMed] [Google Scholar]
- 37.Broom D, Matson D, Bradshaw E, Buck M, Meade R, Coombs S, Matchett M, Ford K, Yu W, Yuan J, Sun S, Ochoa R, Krause J, Wustrow D, Cortright D. Characterization of N-(adamantan-1-ylmethyl)-5-[(3R-amino-pyrrolidin-1-yl)methyl]-2-chloro-benzamide, a P2X7 antagonist in animal models of pain and inflammation. J Pharmacol Exp Ther. 2008;327:620–633. doi: 10.1124/jpet.108.141853. [DOI] [PubMed] [Google Scholar]
- 38.Dell’Antonio G, Quattrini A, Cin E, Fulgenzi A, Ferrero M. Relief of inflammatory pain in rats by local use of the selective P2X7 ATP receptor inhibitor, oxidized ATP. Arthritis Rheum. 2002;46:3378–3385. doi: 10.1002/art.10678. [DOI] [PubMed] [Google Scholar]
- 39.Ke H, Qi H, Weidema A, Zhang Q, Panupinthu N, Crawford D, Grasser W, Paralkar V, Li M, Audoly L, Gabel C, Jee W, Dixon S, Sims S, Thompson D. Deletion of the P2X7 nucleotide receptor reveals its regulatory roles in bone formation and resorption. Mol Endocrinol. 2003;17:1356–1367. doi: 10.1210/me.2003-0021. [DOI] [PubMed] [Google Scholar]
- 40.Li J, Liu D, Ke HZ, Duncan RL, Turner CH. The P2X7 nucleotide receptor mediates skeletal mechanotransduction. J Biol Chem. 2005;280:42952–42959. doi: 10.1074/jbc.M506415200. [DOI] [PubMed] [Google Scholar]
- 41.Romanello M, Pani B, Bicego M, D’Andrea P. Mechanically induced ATP release from human osteoblastic cells. Biochem Biophys Res Commun. 2001;289:1275–1281. doi: 10.1006/bbrc.2001.6124. [DOI] [PubMed] [Google Scholar]
- 42.Buckley KA, Golding SL, Rice JM, Dillon JP, Gallagher JA. Release and interconversion of P2 receptor agonists by human osteoblast-like cells. FASEB J. 2003;17:1401–1410. doi: 10.1096/fj.02-0940com. [DOI] [PubMed] [Google Scholar]
- 43.Lommatzsch M, Cicko S, Müller T, Lucattelli M, Bratke K, Stoll P, Grimm M, Dürk T, Zissel G, Ferrari D, Di Virgilio F, Sorichter S, Lungarella G, Virchow J, Idzko M. Extracellular adenosine triphosphate and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:928–934. doi: 10.1164/rccm.200910-1506OC. [DOI] [PubMed] [Google Scholar]
- 44.Lucattelli M, Cicko S, Müller T, Lommatzsch M, de Cunto G, Cardini S, Sundas W, Grimm M, Zeiser R, Dürk T, Zissel G, Sorichter S, Ferrari D, Di Virgilio F, Virchow J, Lungarella G, Idzko M. P2X7 receptor signalling in the pathogenesis of smoke-induced lung inflammation and emphysema. Am J Respir Cell Mol Biol. 2010 doi: 10.1165/rcmb.2010-0038OC. [DOI] [PubMed] [Google Scholar]
- 45.Chessell I, Hatcher J, Bountra C, Michel A, Hughes J, Green P, Egerton J, Murfin M, Richardson J, Peck W, Grahames C, Casula M, Yiangou Y, Birch R, Anand P, Buell G. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114:386–396. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 46.Clark A, Staniland A, Marchand F, Kaan T, McMahon S, Malcangio M. P2X7-dependent release of interleukin-1beta and nociception in the spinal cord following lipopolysaccharide. J Neurosci. 2010;30:573–582. doi: 10.1523/JNEUROSCI.3295-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor S, Turner C, Elliott J, McDaid J, Hewitt R, Smith J, Pickering M, Whitehouse D, Cook H, Burnstock G, Pusey C, Unwin R, Tam F. P2X7 deficiency attenuates renal injury in experimental glomerulonephritis. J Am Soc Nephrol. 2009;20:1275–1281. doi: 10.1681/ASN.2008060559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maes M. The cytokine hypothesis of depression: inflammation, oxidative & nitrosative stress (IO&NS) and leaky gut as new targets for adjunctive treatments in depression. Neuro Endocrinol Lett. 2008;29:287–291. [PubMed] [Google Scholar]
- 49.Smith RS. The macrophage theory of depression. Med Hypotheses. 1991;35:298–306. doi: 10.1016/0306-9877(91)90272-z. [DOI] [PubMed] [Google Scholar]
- 50.Anisman H, Merali Z, Poulter MO, Hayley S. Cytokines as a precipitant of depressive illness: animal and human studies. Curr Pharm Des. 2005;11:963–972. doi: 10.2174/1381612053381701. [DOI] [PubMed] [Google Scholar]
- 51.Pollak Y, Yirmiya R. Cytokine-induced changes in mood and behaviour: implications for ‘depression due to a general medical condition’, immunotherapy and antidepressive treatment. Int J Neuropsychopharmacol. 2002;5:389–399. doi: 10.1017/S1461145702003152. [DOI] [PubMed] [Google Scholar]
- 52.Basso A, Bratcher N, Harris R, Jarvis M, Decker M, Rueter L. Behavioral profile of P2X7 receptor knockout mice in animal models of depression and anxiety: relevance for neuropsychiatric disorders. Behav Brain Res. 2009;198:83–90. doi: 10.1016/j.bbr.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 53.Sharma S, Kumar V, Khosla R, Kajal N, Sarin B, Sehajpal P. Association of P2X7 receptor +1513 (A->C) polymorphism with tuberculosis in a Punjabi population. Int J Tuberc Lung Dis. 2010;14:1159–1163. [PubMed] [Google Scholar]
- 54.Xiao J, Sun L, Yan H, Jiao W, Miao Q, Feng W, Wu X, Gu Y, Jiao A, Guo Y, Peng X, Shen A. Metaanalysis of P2X7 gene polymorphisms and tuberculosis susceptibility. FEMS Immunol Med Microbiol. 2010;60:165–170. doi: 10.1111/j.1574-695X.2010.00735.x. [DOI] [PubMed] [Google Scholar]
- 55.Niño-Moreno P, Portales-Pérez D, Hernández-Castro B, Portales-Cervantes L, Flores-Meraz V, Baranda L, Gómez-Gómez A, Acuña-Alonzo V, Granados J, González-Amaro R. P2X7 and NRAMP1/SLC11 A1 gene polymorphisms in Mexican mestizo patients with pulmonary tuberculosis. Clin Exp Immunol. 2007;148:469–477. doi: 10.1111/j.1365-2249.2007.03359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tekin D, Kayaalti Z, Dalgic N, Cakir E, Soylemezoglu T, Isin Kutlubay B, Aydin Kilic B. Polymorphism in the p2x7 gene increases susceptibility to extrapulmonary tuberculosis in Turkish children. Pediatr Infect Dis J. 2010;29:779–782. doi: 10.1097/INF.0b013e3181d9932e. [DOI] [PubMed] [Google Scholar]
- 57.Fernando S, Saunders B, Sluyter R, Skarratt K, Goldberg H, Marks G, Wiley J, Britton W. A polymorphism in the P2X7 gene increases susceptibility to extrapulmonary tuberculosis. Am J Respir Crit Care Med. 2007;175:360–366. doi: 10.1164/rccm.200607-970OC. [DOI] [PubMed] [Google Scholar]
- 58.Gu B, Zhang W, Worthington R, Sluyter R, Dao-Ung P, Petrou S, Barden J, Wiley JS. A Glu-496 to Ala polymorphism leads to loss of function of the human P2X7 receptor. J Biol Chem. 2001;276:11135–11142. doi: 10.1074/jbc.M010353200. [DOI] [PubMed] [Google Scholar]
- 59.Boldt W, Klapperstück M, Büttner C, Sadtler S, Schmalzing G, Markwardt F. Glu496Ala polymorphism of human P2X7 receptor does not affect its electrophysiological phenotype. Am J Physiol Cell Physiol. 2003;284:C749–756. doi: 10.1152/ajpcell.00042.2002. [DOI] [PubMed] [Google Scholar]
- 60.Cabrini G, Falzoni S, Forchap S, Pellegatti P, Balboni A, Agostini P, Cuneo A, Castoldi G, Baricordi O, Di Virgilio F. A His-155 to Tyr polymorphism confers gain-of-function to the human P2X7 receptor of human leukemic lymphocytes. J Immunol. 2005;175:82–89. doi: 10.4049/jimmunol.175.1.82. [DOI] [PubMed] [Google Scholar]
- 61.Sluyter R, Shemon AN, Wiley JS. Glu496 to Ala polymorphism in the P2X7 receptor impairs ATP-induced IL-1 beta release from human monocytes. J Immunol. 2004;172:3399–3405. doi: 10.4049/jimmunol.172.6.3399. [DOI] [PubMed] [Google Scholar]
- 62.Barden N, Harvey M, Gagné B, Shink E, Tremblay M, Raymond C, Labbé M, Villeneuve A, Rochette D, Bordeleau L, Stadler H, Holsboer F, Müller-Myhsok B. Analysis of single nucleotide polymorphisms in genes in the chromosome 12Q24. 31 region points to P2RX7 as a susceptibility gene to bipolar affective disorder. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:374–382. doi: 10.1002/ajmg.b.30303. [DOI] [PubMed] [Google Scholar]
- 63.Hejjas K, Szekely A, Domotor E, Halmai Z, Balogh G, Schilling B, Sarosi A, Faludi G, Sasvari-Szekely M, Nemoda Z. Association between depression and the Gln460Arg polymorphism of P2RX7 gene: a dimensional approach. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:295–299. doi: 10.1002/ajmg.b.30799. [DOI] [PubMed] [Google Scholar]
- 64.Grigoroiu-Serbanescu M, Herms S, Mühleisen T, Georgi A, Diaconu C, Strohmaier J, Czerski P, Hauser J, Leszczynska-Rodziewicz A, Jamra R, Babadjanova G, Tiganov A, Krasnov V, Kapiletti S, Neagu A, Vollmer J, Breuer R, Rietschel M, Nöthen M, Cichon S, Propping P. Variation in P2RX7 candidate gene (rs2230912) is not associated with bipolar I disorder and unipolar major depression in four European samples. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:1017–1021. doi: 10.1002/ajmg.b.30952. [DOI] [PubMed] [Google Scholar]
- 65.McQuillin A, Bass N, Choudhury K, Puri V, Kosmin M, Lawrence J, Curtis D, Gurling H. Case-control studies show that a non-conservative amino-acid change from a glutamine to arginine in the P2RX7 purinergic receptor protein is associated with both bipolar- and unipolar-affective disorders. Mol Psychiatry. 2009;14:614–620. doi: 10.1038/mp.2008.6. [DOI] [PubMed] [Google Scholar]
- 66.Denlinger LC, Coursin D, Schell K, Angelini G, Green D, Guadarrama AG, Halsey J, Prabhu U, Hogan K, Bertics PJ. Human P2X7 pore function predicts allele linkage disequilibrium. Clin Chem. 2006;52:995–1004. doi: 10.1373/clinchem.2005.065425. [DOI] [PubMed] [Google Scholar]
- 67.Jamieson SE, Peixoto-Rangel AL, Hargrave AC, Roubaix LA, Mui EJ, Boulter NR, Miller EN, Fuller SJ, Wiley JS, Castellucci L, Boyer K, Peixe RG, Kirisits MJ, Elias LeS, Coyne JJ, Correa-Oliveira R, Sautter M, Smith NC, Lees MP, Swisher CN, Heydemann P, Noble AG, Patel D, Bardo D, Burrowes D, McLone D, Roizen N, Withers S, Bahia-Oliveira LM, McLeod R, Blackwell JM. Evidence for associations between the purinergic receptor P2X(7) (P2RX7) and toxoplasmosis. Genes Immun. 2010;11:374–383. doi: 10.1038/gene.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Denlinger LC, Angelini G, Schell K, Green D, Guadarrama AG, Prabhu U, Coursin D, Bertics PJ, Hogan K. Detection of human P2X7 nucleotide receptor polymorphisms by a novel monocyte pore assay predictive of alterations in lipopolysaccharide-induced cytokine production. J Immunol. 2005;174:4424–4431. doi: 10.4049/jimmunol.174.7.4424. [DOI] [PubMed] [Google Scholar]
- 69.Carroll WA, Donnelly-Roberts D, Jarvis MF. Selective P2X(7) receptor antagonists for chronic inflammation and pain. Purinergic Signal. 2009;5:63–73. doi: 10.1007/s11302-008-9110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. [accessed January 2, 2011];ClinicalTrials.gov. Home Page http://www.clinicaltrials.gov.
- 71. [accessed January 2, 2011];Pfizer Pipeline. http://media.pfizer.com/files/research/pipeline/2008_0930/pipeline_2008_0930.pdf.
- 72. [accessed January 2, 2011];Affectis Pharmaceuticals AG. Home Page. http://www.affectis.com.
- 73. [accessed January 2, 2011];Affectis Pharmaceuticals AG. http://www.affectis.com/attachments/011_Affectis%20-%20Patent%20Update_02Apr09.pdf.
- 74.Amin S. Mechanical factors and bone health: effects of weightlessness and neurologic injury. Curr Rheumatol Rep. 2010;12:170–176. doi: 10.1007/s11926-010-0096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ohlendorff SD, Tofteng CL, Jensen JE, Petersen S, Civitelli R, Fenger M, Abrahamsen B, Hermann AP, Eiken P, Jørgensen NR, Jrgensen NR. Single nucleotide polymorphisms in the P2X7 gene are associated to fracture risk and to effect of estrogen treatment. Pharmacogenet Genomics. 2007;17:555–567. doi: 10.1097/FPC.0b013e3280951625. [DOI] [PubMed] [Google Scholar]
- 76.Ferrari D, Pizzirani C, Adinolfi E, Lemoli R, Curti A, Idzko M, Panther E, Di Virgilio F. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- 77.Griffin WS. Inflammation and neurodegenerative diseases. Am J Clin Nutr. 2006;83:470S–474S. doi: 10.1093/ajcn/83.2.470S. [DOI] [PubMed] [Google Scholar]
- 78.Qu Y, Franchi L, Nunez G, Dubyak GR. Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol. 2007;179:1913–1925. doi: 10.4049/jimmunol.179.3.1913. [DOI] [PubMed] [Google Scholar]
- 79.Di Virgilio F. Liaisons dangereuses: P2X(7) and the inflammasome. Trends Pharmacol Sci. 2007;28:465–472. doi: 10.1016/j.tips.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 80.Takenouchi T, Sugama S, Iwamaru Y, Hashimoto M, Kitani H. Modulation of the ATP-lnduced release and processing of IL-1beta in microglial cells. Crit Rev Immunol. 2009;29:335–345. doi: 10.1615/critrevimmunol.v29.i4.40. [DOI] [PubMed] [Google Scholar]
- 81.Labasi JM, Petrushova N, Donovan C, McCurdy S, Lira P, Payette MM, Brissette W, Wicks JR, Audoly L, Gabel CA. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol. 2002;168:6436–6445. doi: 10.4049/jimmunol.168.12.6436. [DOI] [PubMed] [Google Scholar]
- 82.Elssner A, Duncan M, Gavrilin M, Wewers MD. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1 beta processing and release. J Immunol. 2004;172:4987–4994. doi: 10.4049/jimmunol.172.8.4987. [DOI] [PubMed] [Google Scholar]
- 83.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. The EMBO J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pelegrin P, Barroso-Gutierrez C, Surprenant A. P2X7 receptor differentially couples to distinct release pathways for IL-1beta in mouse macrophage. J Immunol. 2008;180:7147–7157. doi: 10.4049/jimmunol.180.11.7147. [DOI] [PubMed] [Google Scholar]
- 85.Al-Shukaili A, Al-Kaabi J, Hassan B. A comparative study of interleukin-1beta production and p2x7 expression after ATP stimulation by peripheral blood mononuclear cells isolated from rheumatoid arthritis patients and normal healthy controls. Inflammation. 2008;31:84–90. doi: 10.1007/s10753-007-9052-0. [DOI] [PubMed] [Google Scholar]
- 86.Griffin WS, Sheng JG, Royston MC, Gentleman SM, McKenzie JE, Graham DI, Roberts GW, Mrak RE. Glial-neuronal interactions in Alzheimer’s disease: the potential role of a ‘cytokine cycle’ in disease progression. Brain Pathol. 1998;8:65–72. doi: 10.1111/j.1750-3639.1998.tb00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Parvathenani LK, Tertyshnikova S, Greco CR, Roberts SB, Robertson B, Posmantur R. P2X7 mediates superoxide production in primary microglia and is up-regulated in a transgenic mouse model of Alzheimer’s disease. J Biol Chem. 2003;278:13309–13317. doi: 10.1074/jbc.M209478200. [DOI] [PubMed] [Google Scholar]
- 88.Bernardino L, Balosso S, Ravizza T, Marchi N, Ku G, Randle JC, Malva JO, Vezzani A. Inflammatory events in hippocampal slice cultures prime neuronal susceptibility to excitotoxic injury: a crucial role of P2X7 receptor-mediated IL-1beta release. J Neurochem. 2008;106:271–280. doi: 10.1111/j.1471-4159.2008.05387.x. [DOI] [PubMed] [Google Scholar]
- 89.Guerra AN, Gavala ML, Chung HS, Bertics PJ. Nucleotide receptor signalling and the generation of reactive oxygen species. Purinergic Signal. 2007;3:39–51. doi: 10.1007/s11302-006-9035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pfeiffer ZA, Guerra AN, Hill LM, Gavala ML, Prabhu U, Aga M, Hall DJ, Bertics PJ. Nucleotide receptor signaling in murine macrophages is linked to reactive oxygen species generation. Free Radical Biol & Med. 2007;42:1506–1516. doi: 10.1016/j.freeradbiomed.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mingam R, De Smedt V, Amédée T, Bluthé RM, Kelley KW, Dantzer R, Layé S. In vitro and in vivo evidence for a role of the P2X7 receptor in the release of IL-1 beta in the murine brain. Brain Behav Immun. 2008;22:234–244. doi: 10.1016/j.bbi.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ballerini P, Ciccarelli R, Caciagli F, Rathbone MP, Werstiuk ES, Traversa U, Buccella S, Giuliani P, Jang S, Nargi E, Visini D, Santavenere C, Di Iorio P. P2X7 receptor activation in rat brain cultured astrocytes increases the biosynthetic release of cysteinyl leukotrienes. Int J Immunopathol Pharmacol. 2005;18:417–430. doi: 10.1177/039463200501800303. [DOI] [PubMed] [Google Scholar]
- 93.Bianco F, Colombo A, Saglietti L, Lecca D, Abbracchio MP, Matteoli M, Verderio C. Different properties of P2X(7) receptor in hippocampal and cortical astrocytes. Purinergic Signal. 2009;5:233–240. doi: 10.1007/s11302-009-9137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee SC, Liu W, Dickson DW, Brosnan CF, Berman JW. Cytokine production by human fetal microglia and astrocytes. Differential induction by lipopolysaccharide and IL-1 beta. J Immunol. 1993;150:2659–2667. [PubMed] [Google Scholar]
- 95.Narcisse L, Scemes E, Zhao Y, Lee SC, Brosnan CF. The cytokine IL-1beta transiently enhances P2X7 receptor expression and function in human astrocytes. Glia. 2005;49:245–258. doi: 10.1002/glia.20110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brough D, Le Feuvre RA, Iwakura Y, Rothwell NJ. Purinergic (P2X7) receptor activation of microglia induces cell death via an interleukin-1-independent mechanism. Mol Cell Neurosci. 2002;19:272–280. doi: 10.1006/mcne.2001.1054. [DOI] [PubMed] [Google Scholar]
- 97.Hughes J, Hatcher J, Chessell I. The role of P2X(7) in pain and inflammation. Purinergic Signal. 2007;3:163–169. doi: 10.1007/s11302-006-9031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hu Y, Fisette PL, Denlinger LC, Guadarrama AG, Sommer JA, Proctor RA, Bertics PJ. Purinergic receptor modulation of lipopolysaccharide signaling and inducible nitric-oxide synthase expression in RAW 264. 7 macrophages. J Biol Chem. 1998;273:27170–27175. doi: 10.1074/jbc.273.42.27170. [DOI] [PubMed] [Google Scholar]
- 99.Wei W, Ryu J, Choi H, McLarnon J. Expression and function of the P2X(7) receptor in rat C6 glioma cells. Cancer Lett. 2008;260:79–87. doi: 10.1016/j.canlet.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 100.Yip L, Woehrle T, Corriden R, Hirsh M, Chen Y, Inoue Y, Ferrari V, Insel PA, Junger WG. Autocrine regulation of T-cell activation by ATP release and P2X7 receptors. FASEB J. 2009;23:1685–1693. doi: 10.1096/fj.08-126458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kataoka A, Tozaki-Saitoh H, Koga Y, Tsuda M, Inoue K. Activation of P2X7 receptors induces CCL3 production in microglial cells through transcription factor NFAT. J Neurochem. 2009;108:115–125. doi: 10.1111/j.1471-4159.2008.05744.x. [DOI] [PubMed] [Google Scholar]
- 102.Aga M, Watters JJ, Pfeiffer ZA, Wiepz GJ, Sommer JA, Bertics PJ. Evidence for nucleotide receptor modulation of cross talk between MAP kinase and NF-kappa B signaling pathways in murine RAW 264. 7 macrophages. Am J Physiol Cell Physiol. 2004;286:C923–930. doi: 10.1152/ajpcell.00417.2003. [DOI] [PubMed] [Google Scholar]
- 103.Friedle S, Brautigam V, Nikodemova M, Wright M, Watters JJ. The P2X7-Egr pathway regulates nucleotide-dependent inflammatory gene expression in microglia. Glia. 2011;59:1–13. doi: 10.1002/glia.21071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stefano L, Rössler OG, Griesemer D, Hoth M, Thiel G. P2X(7) receptor stimulation upregulates Egr-1 biosynthesis involving a cytosolic Ca(2+) rise, transactivation of the EGF receptor and phosphorylation of ERK and Elk-1. J Cell Physiol. 2007;213:36–44. doi: 10.1002/jcp.21085. [DOI] [PubMed] [Google Scholar]
- 105.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 106.Dineen SP, Lynn KD, Holloway SE, Miller AF, Sullivan JP, Shames DS, Beck AW, Barnett CC, Fleming JB, Brekken RA. Vascular endothelial growth factor receptor 2 mediates macrophage infiltration into orthotopic pancreatic tumors in mice. Cancer Res. 2008;68:4340–4346. doi: 10.1158/0008-5472.CAN-07-6705. [DOI] [PubMed] [Google Scholar]
- 107.Thi MM, Suadicani SO, Spray DC. Fluid flow-induced soluble vascular endothelial growth factor isoforms regulate actin adaptation in osteoblasts. J Biol Chem. 2010;285:30931–30941. doi: 10.1074/jbc.M110.114975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kobayashi Y. The role of chemokines in neutrophil biology. Front Biosci. 2008;13:2400–2407. doi: 10.2741/2853. [DOI] [PubMed] [Google Scholar]
- 109.Idzko M, Panther E, Bremer HC, Sorichter S, Luttmann W, Virchow CJ, Di Virgilio F, Herouy Y, Norgauer J, Ferrari D. Stimulation of P2 purinergic receptors induces the release of eosinophil cationic protein and interleukin-8 from human eosinophils. Br J Pharmacol. 2003;138:1244–1250. doi: 10.1038/sj.bjp.0705145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schneider EM, Vorlaender K, Ma X, Du W, Weiss M. Role of ATP in trauma-associated cytokine release and apoptosis by P2X7 ion channel stimulation. Ann N Y Acad Sci. 2006;1090:245–252. doi: 10.1196/annals.1378.027. [DOI] [PubMed] [Google Scholar]
- 111.McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 112.Malek TR, Castro I. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity. 2010;33:153–165. doi: 10.1016/j.immuni.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Novak K. Swinging the vote for rapamycin. Nat Rev Cancer. 2002;2:75. doi: 10.1038/nrc739. [DOI] [PubMed] [Google Scholar]
- 114.Sun M, Lughezzani G, Perrotte P, Karakiewicz PI. Treatment of metastatic renal cell carcinoma. Nat Rev Urol. 2010;7:327–338. doi: 10.1038/nrurol.2010.57. [DOI] [PubMed] [Google Scholar]
- 115.Tawbi H, Nimmagadda N. Targeted therapy in melanoma. Biologics. 2009;3:475–484. doi: 10.2147/btt.2009.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Budagian V, Bulanova E, Brovko L, Orinska Z, Fayad R, Paus R, Bulfone-Paus S. Signaling through P2X7 receptor in human T cells involves p56lck, MAP kinases, and transcription factors AP-1 and NF-kappa B. J Biol Chem. 2003;278:1549–1560. doi: 10.1074/jbc.M206383200. [DOI] [PubMed] [Google Scholar]
- 117.Woehrle T, Yip L, Manohar M, Sumi Y, Yao Y, Chen Y, Junger WG. Hypertonic stress regulates T cell function via pannexin-1 hemichannels and P2X receptors. J Leukoc Biol. 2010;88:1181–1189. doi: 10.1189/jlb.0410211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guzik TJ, Korbut R, Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 2003;54:469–487. [PubMed] [Google Scholar]
- 119.Gendron FP, Chalimoniuk M, Strosznajder J, Shen S, González FA, Weisman GA, Sun GY. P2X7 nucleotide receptor activation enhances IFN gamma-induced type II nitric oxide synthase activity in BV-2 microglial cells. J Neurochem. 2003;87:344–352. doi: 10.1046/j.1471-4159.2003.01995.x. [DOI] [PubMed] [Google Scholar]
- 120.Kusner D, Adams J. ATP-induced killing of virulent Mycobacterium tuberculosis within human macrophages requires phospholipase D. J Immunol. 2000;164:379–388. doi: 10.4049/jimmunol.164.1.379. [DOI] [PubMed] [Google Scholar]
- 121.Cha Y, Solnica-Krezel L, DuBois R. Fishing for prostanoids: deciphering the developmental functions of cyclooxygenase-derived prostaglandins. Dev Biol. 2006;289:263–272. doi: 10.1016/j.ydbio.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 122.Blackwell KA, Raisz LG, Pilbeam CC. Prostaglandins in bone: bad cop, good cop? Trends Endocrinol Metab. 2010;21:294–301. doi: 10.1016/j.tem.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Panupinthu N, Rogers JT, Zhao L, Solano-Flores LP, Possmayer F, Sims SM, Dixon SJ. P2X7 receptors on osteoblasts couple to production of lysophosphatidic acid: a signaling axis promoting osteogenesis. J Cell Biol. 2008;181:859–871. doi: 10.1083/jcb.200708037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7:292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- 125.McCoy JM, Wicks JR, Audoly LP. The role of prostaglandin E2 receptors in the pathogenesis of rheumatoid arthritis. J Clin Invest. 2002;110:651–658. doi: 10.1172/JCI15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gómez-Martín D, Díaz-Zamudio M, Galindo-Campos M, Alcocer-Varela J. Early growth response transcription factors and the modulation of immune response: implications towards autoimmunity. Autoimmun Rev. 2010;9:454–458. doi: 10.1016/j.autrev.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 127.Shimoyamada H, Yazawa T, Sato H, Okudela K, Ishii J, Sakaeda M, Kashiwagi K, Suzuki T, Mitsui H, Woo T, Tajiri M, Ohmori T, Ogura T, Masuda M, Oshiro H, Kitamura H. Early growth response-1 induces and enhances vascular endothelial growth factor-A expression in lung cancer cells. Am J Pathol. 2010;177:70–83. doi: 10.2353/ajpath.2010.091164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mancini M, Toker A. NFAT proteins: emerging roles in cancer progression. Nat Rev Cancer. 2009;9:810–820. doi: 10.1038/nrc2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gwack Y, Feske S, Srikanth S, Hogan PG, Rao A. Signalling to transcription: store-operated Ca2+ entry and NFAT activation in lymphocytes. Cell Calcium. 2007;42:145–156. doi: 10.1016/j.ceca.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 130.Adinolfi E, Callegari MG, Cirillo M, Pinton P, Giorgi C, Cavagna D, Rizzuto R, Di Virgilio F. Expression of the P2X7 receptor increases the Ca2+ content of the endoplasmic reticulum, activates NFATc1, and protects from apoptosis. J Biol Chem. 2009;284:10120–10128. doi: 10.1074/jbc.M805805200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ferrari D, Stroh C, Schulze-Osthoff K. P2X7/P2Z purinoreceptor-mediated activation of transcription factor NFAT in microglial cells. J Biol Chem. 1999;274:13205–13210. doi: 10.1074/jbc.274.19.13205. [DOI] [PubMed] [Google Scholar]
- 132.Wang S, Liu Z, Wang L, Zhang X. NF-kappaB signaling pathway, inflammation and colorectal cancer. Cell Mol Immunol. 2009;6:327–334. doi: 10.1038/cmi.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Luqman S, Pezzuto JM. NFkappaB: a promising target for natural products in cancer chemoprevention. Phytother Res. 2010;24:949–963. doi: 10.1002/ptr.3171. [DOI] [PubMed] [Google Scholar]
- 134.Ferrari D, Wesselborg S, Bauer MK, Schulze-Osthoff K. Extracellular ATP activates transcription factor NF-kappaB through the P2Z purinoreceptor by selectively targeting NF-kappaB p65. J Cell Biol. 1997;139:1635–1643. doi: 10.1083/jcb.139.7.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Korcok J, Raimundo LN, Ke HZ, Sims SM, Dixon SJ. Extracellular nucleotides act through P2X7 receptors to activate NF-kappaB in osteoclasts. J Bone Miner Res. 2004;19:642–651. doi: 10.1359/JBMR.040108. [DOI] [PubMed] [Google Scholar]
- 136.Wen AY, Sakamoto KM, Miller LS. The role of the transcription factor CREB in immune function. J Immunol. 2010;185:6413–6419. doi: 10.4049/jimmunol.1001829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Potucek YD, Crain JM, Watters JJ. Purinergic receptors modulate MAP kinases and transcription factors that control microglial inflammatory gene expression. Neurochem Int. 2006;49:204–214. doi: 10.1016/j.neuint.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 138.Milde-Langosch K. The Fos family of transcription factors and their role in tumourigenesis. Eur J Cancer. 2005;41:2449–2461. doi: 10.1016/j.ejca.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 139.Padeh S, Cohen A, Roifman CM. ATP-induced activation of human B lymphocytes via P2-purinoceptors. J Immunol. 1991;146:1626–1632. [PubMed] [Google Scholar]
- 140.Nestler EJ, Barrot M, Self DW. DeltaFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci U S A. 2001;98:11042–11046. doi: 10.1073/pnas.191352698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nakabeppu Y, Nathans D. A naturally occurring truncated form of FosB that inhibits Fos/Jun transcriptional activity. Cell. 1991;64:751–759. doi: 10.1016/0092-8674(91)90504-r. [DOI] [PubMed] [Google Scholar]
- 142.Carle T, Ohnishi Y, Ohnishi Y, Alibhai I, Wilkinson M, Kumar A, Nestler E. Proteasome-dependent and -independent mechanisms for FosB destabilization: identification of FosB degron domains and implications for DeltaFosB stability. Eur J Neurosci. 2007;25:3009–3019. doi: 10.1111/j.1460-9568.2007.05575.x. [DOI] [PubMed] [Google Scholar]
- 143.McClung C, Ulery P, Perrotti L, Zachariou V, Berton O, Nestler E. DeltaFosB: a molecular switch for long-term adaptation in the brain. Brain Res Mol Brain Res. 2004;132:146–154. doi: 10.1016/j.molbrainres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 144.Greenwood BN, Foley TE, Le TV, Strong PV, Loughridge AB, Day HE, Fleshner M. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behav Brain Res. 2010;217:354–362. doi: 10.1016/j.bbr.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hope BT, Nye HE, Kelz MB, Self DW, Iadarola MJ, Nakabeppu Y, Duman RS, Nestler EJ. Induction of a long-lasting AP-1 complex composed of altered Fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron. 1994;13:1235–1244. doi: 10.1016/0896-6273(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 146.Nye HE, Nestler EJ. Induction of chronic Fos-related antigens in rat brain by chronic morphine administration. Mol Pharmacol. 1996;49:636–645. [PubMed] [Google Scholar]
- 147.Pich EM, Pagliusi SR, Tessari M, Talabot-Ayer D, Hooft van Huijsduijnen R, Chiamulera C. Common neural substrates for the addictive properties of nicotine and cocaine. Science. 1997;275:83–86. doi: 10.1126/science.275.5296.83. [DOI] [PubMed] [Google Scholar]
- 148.Werme M, Messer C, Olson L, Gilden L, Thorén P, Nestler EJ, Brené S. Delta FosB regulates wheel running. J Neurosci. 2002;22:8133–8138. doi: 10.1523/JNEUROSCI.22-18-08133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wallace DL, Vialou V, Rios L, Carle-Florence TL, Chakravarty S, Kumar A, Graham DL, Green TA, Kirk A, Iñiguez SD, Perrotti LI, Barrot M, DiLeone RJ, Nestler EJ, Bolaños-Guzmán CA. The influence of DeltaFosB in the nucleus accumbens on natural reward-related behavior. J Neurosci. 2008;28:10272–10277. doi: 10.1523/JNEUROSCI.1531-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Brown J, Ye H, Bronson R, Dikkes P, Greenberg M. A defect in nurturing in mice lacking the immediate early gene fosB. Cell. 1996;86:297–309. doi: 10.1016/s0092-8674(00)80101-4. [DOI] [PubMed] [Google Scholar]
- 151.Hiroi N, Brown JR, Haile CN, Ye H, Greenberg ME, Nestler EJ. FosB mutant mice: loss of chronic cocaine induction of Fos-related proteins and heightened sensitivity to cocaine’s psychomotor and rewarding effects. Proc Natl Acad Sci U S A. 1997;94:10397–10402. doi: 10.1073/pnas.94.19.10397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zachariou V, Bolanos CA, Selley DE, Theobald D, Cassidy MP, Kelz MB, Shaw-Lutchman T, Berton O, Sim-Selley LJ, Dileone RJ, Kumar A, Nestler EJ. An essential role for DeltaFosB in the nucleus accumbens in morphine action. Nat Neurosci. 2006;9:205–211. doi: 10.1038/nn1636. [DOI] [PubMed] [Google Scholar]
- 153.Kelz MB, Chen J, Carlezon WA, Whisler K, Gilden L, Beckmann AM, Steffen C, Zhang YJ, Marotti L, Self DW, Tkatch T, Baranauskas G, Surmeier DJ, Neve RL, Duman RS, Picciotto MR, Nestler EJ. Expression of the transcription factor deltaFosB in the brain controls sensitivity to cocaine. Nature. 1997;401:272–276. doi: 10.1038/45790. [DOI] [PubMed] [Google Scholar]
- 154.Gharib S, Liles W, Klaff L, Altemeier W. Noninjurious mechanical ventilation activates a proinflammatory transcriptional program in the lung. Physiol Genomics. 2009;37:239–248. doi: 10.1152/physiolgenomics.00027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Luis-Delgado O, Barrot M, Rodeau J, Ulery P, Freund-Mercier M, Lasbennes F. The transcription factor DeltaFosB is recruited by inflammatory pain. J Neurochem. 2006;98:1423–1431. doi: 10.1111/j.1471-4159.2006.03970.x. [DOI] [PubMed] [Google Scholar]
- 156.Chaum E, Yin J, Yang H, Thomas F, Lang J. Quantitative AP-1 gene regulation by oxidative stress in the human retinal pigment epithelium. J Cell Biochem. 2009;108:1280–1291. doi: 10.1002/jcb.22358. [DOI] [PubMed] [Google Scholar]
- 157.Bièche I, Lerebours F, Tozlu S, Espie M, Marty M, Lidereau R. Molecular profiling of inflammatory breast cancer: identification of a poor-prognosis gene expression signature. Clin Cancer Res. 2004;10:6789–6795. doi: 10.1158/1078-0432.CCR-04-0306. [DOI] [PubMed] [Google Scholar]
- 158.Gruda MC, van Amsterdam J, Rizzo CA, Durham SK, Lira S, Bravo R. Expression of FosB during mouse development: normal development of FosB knockout mice. Oncogene. 1996;12:2177–2185. [PubMed] [Google Scholar]
- 159.Sabatakos G, Sims NA, Chen J, Aoki K, Kelz MB, Amling M, Bouali Y, Mukhopadhyay K, Ford K, Nestler EJ, Baron R. Overexpression of DeltaFosB transcription factor(s) increases bone formation and inhibits adipogenesis. Nat Med. 2000;6:985–990. doi: 10.1038/79683. [DOI] [PubMed] [Google Scholar]
- 160.Kido S, Kuriwaka-Kido R, Imamura T, Ito Y, Inoue D, Matsumoto T. Mechanical stress induces Interleukin-11 expression to stimulate osteoblast differentiation. Bone. 2009;45:1125–1132. doi: 10.1016/j.bone.2009.07.087. [DOI] [PubMed] [Google Scholar]
- 161.Suga K, Saitoh M, Fukushima S, Takahashi K, Nara H, Yasuda S, Miyata K. Interleukin-11 induces osteoblast differentiation and acts synergistically with bone morphogenetic protein-2 in C3H10T1/2 cells. J Interferon Cytokine Res. 2001;21:695–707. doi: 10.1089/107999001753124435. [DOI] [PubMed] [Google Scholar]
- 162.Gleave RJ, Walter DS, Beswick PJ, Fonfria E, Michel AD, Roman SA, Tang SP. Synthesis and biological activity of a series of tetrasubstituted-imidazoles as P2X(7) antagonists. Bioorg Med Chem Lett. 2010;20:4951–4954. doi: 10.1016/j.bmcl.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 163.Chambers LJ, Stevens AJ, Moses AP, Michel AD, Walter DS, Davies DJ, Livermore DG, Fonfria E, Demont EH, Vimal M, Theobald PJ, Beswick PJ, Gleave RJ, Roman SA, Senger S. Synthesis and structure-activity relationships of a series of (1H-pyrazol-4-yl)acetamide antagonists of the P2X7 receptor. Bioorg Med Chem Lett. 2010;20:3161–3164. doi: 10.1016/j.bmcl.2010.03.096. [DOI] [PubMed] [Google Scholar]
- 164.Chen X, Pierce B, Naing W, Grapperhaus ML, Phillion DP. Discovery of 2-chloro-N-((4,4-difluoro-1-hydroxycyclohexyl)methyl)-5-(5-fluoropyrimidin-2-yl)benzamide as a potent and CNS penetrable P2X7 receptor antagonist. Bioorg Med Chem Lett. 2010;20:3107–3111. doi: 10.1016/j.bmcl.2010.03.094. [DOI] [PubMed] [Google Scholar]
- 165.Lee JY, Yu J, Cho WJ, Ko H, Kim YC. Synthesis and structure-activity relationships of pyrazolodiazepine derivatives as human P2X7 receptor antagonists. Bioorg Med Chem Lett. 2009;19:6053–6058. doi: 10.1016/j.bmcl.2009.09.053. [DOI] [PubMed] [Google Scholar]
- 166.Lee G, Joshi B, Chen W, Jeong L, Moon H, Jacobson K, Kim Y. Synthesis and structure-activity relationship studies of tyrosine-based antagonists at the human P2X7 receptor. Bioorg Med Chem Lett. 2008;18:571–575. doi: 10.1016/j.bmcl.2007.11.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Berton O, Covington HE, Ebner K, Tsankova NM, Carle TL, Ulery P, Bhonsle A, Barrot M, Krishnan V, Singewald GM, Singewald N, Birnbaum S, Neve RL, Nestler EJ. Induction of deltaFosB in the periaqueductal gray by stress promotes active coping responses. Neuron. 2007;55:289–300. doi: 10.1016/j.neuron.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 168.Gu B, Sluyter R, Skarratt K, Shemon A, Dao-Ung L, Fuller S, Barden J, Clarke A, Petrou S, Wiley J. An Arg307 to Gln polymorphism within the ATP-binding site causes loss of function of the human P2X7 receptor. J Biol Chem. 2004;279:31287–31295. doi: 10.1074/jbc.M313902200. [DOI] [PubMed] [Google Scholar]
- 169.Wiley J, Dao-Ung L, Li C, Shemon A, Gu B, Smart M, Fuller S, Barden J, Petrou S, Sluyter R. An Ile-568 to Asn polymorphism prevents normal trafficking and function of the human P2X7 receptor. J Biol Chem. 2003;278:17108–17113. doi: 10.1074/jbc.M212759200. [DOI] [PubMed] [Google Scholar]


