SUMMARY
While mechanobiological processes employ diverse mechanisms, at their heart are force-induced perturbations in the structure and dynamics of molecules capable of triggering subsequent events. Among the best-characterized force-sensing systems are bacterial mechanosensitive channels. These channels reflect an intimate coupling of protein conformation with the mechanics of the surrounding membrane; the membrane serves as an adaptable sensor that responds to an input of applied force and converts it into an output signal, interpreted for the cell by mechanosensitive channels. The cell can exploit this information in a number of ways - ensuring cellular viability in the presence of osmotic stress and perhaps also serving as a signal transducer for membrane tension or other functions. This review focuses on the bacterial mechanosensitive channels of large (MscL) and small (MscS) conductance and their eukaryotic homologues with an emphasis on the outstanding issues surrounding the function and mechanism of this fascinating class of molecules.
INTRODUCTION TO MECHANOSENSITIVE CHANNELS
All organisms, from single-celled bacteria to multi-cellular animals and plants, must sense and respond to mechanical force in their external environment (for example, shear force, gravity, touch) and in their internal environment (including osmotic pressure and membrane deformation) for proper growth, development, and health (Gillespie and Walker, 2001; Hamill and Martinac, 2001; Sukharev and Corey, 2004; Kung, 2005; Vogel and Sheetz, 2006; Hamill, 2007; Sachs, 2010). A variety of proteins and protein complexes can sense and respond to such mechanical forces. Mechanosensitive (MS) channels provide an excellent opportunity to study the interplay between membrane mechanical properties and protein structure and function. It is important to emphasize that the membranes of cells and organelles do not behave as inert substrates that surround their associated membrane proteins. Rather, the membrane is a dynamic medium that directly affects the function and spatial distribution of the proteins embedded in it (Engelman, 2005; Andersen and Koeppe, 2007). Elucidating the molecular details of mechanosensation within the context of the membrane adds to our understanding of how mechanical force can generate biophysical alterations that in turn lead to adaptive changes in cellular physiology.
The interplay between lipid properties and protein function has been demonstrated in the case of two channel proteins found in the plasma membrane of E. coli, MscL (Mechanosensitive channel of Large conductance) and MscS (Mechanosensitive channel of Small conductance). MscL and MscS directly respond to changes in membrane tension by opening nanoscale protein pores; interaction with the membrane is thus an integral aspect of their function (Kung et al., 2010). The importance of the membrane is supported by the observation that variations in the thickness of the phospholipid bilayer or the addition of compounds that induce spontaneous membrane curvature directly impact the tension required to open, or gate, MscL (Perozo et al., 2002).
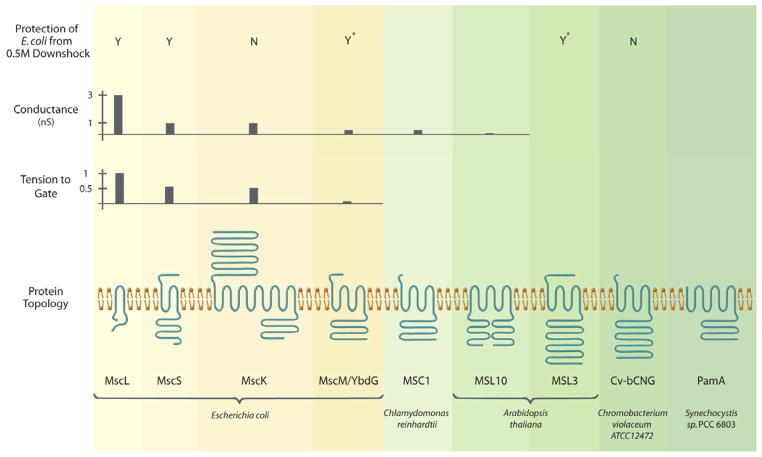
MscL and MscS were first identified from their membrane-stretch regulated electrophysiological activities in the plasma membrane of giant E. coli spheroplasts (Martinac et al., 1987; Sukharev et al., 1993; Cui et al., 1995). Subsequently, the mscL gene was cloned through a tour-de-force biochemical purification scheme (Sukharev et al., 1994). The gene encoding MscS, dubbed yggB, was identified through the use of a functional assay and reverse genetics (Levina et al., 1999). It is clear that MscS and MscL function redundantly to protect cells from lysis upon severe hypoosmotic shock; other functions for these channels are possible but have not been demonstrated. Proteins related to MscS are predicted to exist in all three kingdoms of life (Kung et al., 2010). MscS family members are widely distributed throughout bacterial and archaeal genomes, and multiple family members are often present (for example, the E. coli genome encodes five MscS-related proteins in addition to MscS itself (Booth et al., 2007; Schumann et al., 2010)). In the eukaryotes, MscS family members have been found in all plant genomes yet examined (for example, ten MscS-related proteins are present in the Arabidopsis thaliana genome), and in many (but not all) fungal genomes, but have not yet been identified in animals (Kloda and Martinac, 2002; Pivetti et al., 2003; Haswell, 2007). Figure 1 summarizes the functional and structural characteristics of the best-studied MscL and MscS channels from bacteria and eukaryotes.
Figure 1.
Functional and structural characteristics of MscL and MscS channels from bacteria and eukaryotes. (Top panel) Expression of MscS and MscL protects a bacterial strain lacking endogenous MscS and MscL from a 0.5 M osmotic downshock while expression of MscK and Cv-bCNG does not (Levina et al., 1999; Caldwell et al., 2010). Asterisks, over-expression of YbdG and MSL3 provides protection (Haswell et al., 2008; Schumann et al., 2010). (Top middle panel) Conductance of endogenous channels in giant E. coli spheroplasts (MscL, MscS, MscK, MscM, (reviewed in (Kung et al., 2010))), channels heterologously expressed in giant E. coli spheroplasts (MSC1, (Nakayama et al., 2007)) or endogenous channels in Arabidopsis thaliana root cells (MSL10, (Haswell et al., 2008)). (Bottom middle panel) Tension to gate expressed relative to MscL channels in the same patch (Berrier et al., 1996; Edwards et al., 2005; Li et al., 2007). (Bottom panel) Channel monomer topologies as predicted by TOPCONS (http://topcons.net/). Mature versions (after processing of chloroplast targeting sequences) of MSC1 and MSL3 are shown, and sequence loops connecting transmembrane helices were omitted for clarity.
MECHANOSENSITIVE CHANNELS ARE INTERPRETERS OF MEMBRANE TENSION
The essence of a mechanosensitive system is the ability to adopt conformational states with distinct functional properties in response to applied tension. From the standpoint of protein structure, the relevant parameters are the sensitivity of the conformational equilibrium to tension, and the differences in functional properties between these states. For MS channels, the relevant functional property is the ability to mediate the flow of solutes across the membrane in a tension-dependent fashion. MS channels may be most simply described as the tension-dependent equilibrium between two states, closed (C) and open (O), that differ in conductance (Sukharev et al., 1997) as exemplified by the kinetic scheme
where K is the equilibrium constant between closed and open states in the absence of tension. If there is a nonzero difference in cross-sectional area of the channel, ΔA = Aopen - Aclosed, then the contribution of membrane tension σ to the free energy of channel opening ΔG may be included through the expression
where ΔG° = −RT ln K is the standard free energy for channel opening in the absence of tension. On average, half the channels will be open at the applied tension σ1/2 where ΔG = 0, and
Analogous expressions may be used to describe the behavior of voltage-gated channels, where σ1/2 and ΔA for mechanosensitive channels correspond to V1/2, the transmembrane voltage drop where half the channels are open, and z, the number of charges moving across the voltage drop.
The sensitivity of the conformational equilibrium to tension is encoded in ΔA (= dΔG/dσ). The larger the value of ΔA, the greater the shift in equilibrium in response to tension, and the more “mechanosensitive” the channel. The relevant magnitudes of these quantities may be illustrated for a channel with ΔG° of 24 kJ mol−1 (10 times the average thermal energy (RT ~ 2.4 kJ mol−1 ~ 4.1 pN nm (per molecule))) and ΔA = 10 nm2 requiring a tension of 4 mN m−1 to open the channel half the time. It is worth noting that this behavior is not restricted to channels; any membrane protein capable of adopting multiple conformations with different cross-sectional areas will be mechanosensitive. Whether or not mechanosensitivity is a physiologically relevant property will depend on whether or not a particular membrane protein encounters tensions of sufficient magnitude to significantly shift the conformational equilibria between functionally distinct states.
The application of tension to a membrane can result in changes in its thickness or curvature that can in turn alter the energetic balance of embedded proteins, leading, for example, to the stabilization of the open state of a channel (reviewed in (Phillips et al., 2009)). In the case that a protein can adopt multiple conformations that differ in cross-sectional area, then application of membrane tension will increasingly favor the state of largest cross-sectional area by an amount − σΔA, just as the contribution of the more thermodynamically familiar −PΔV term for bulk ideal gases, for example, implies that increasing pressure will stabilize the state of smaller volume. Different conformational states of a membrane protein will likely exhibit different cross-sectional areas (with the larger areas favored by increasing σ), but also different interaction areas between the protein and membrane (with the larger interface disfavored by increasing σ), so that the interplay between tension and conformation can give rise to a rich variety of outcomes. The ability to stably transition between distinct conformational states at physiologically relevant tensions (i.e., where σΔA is approximately the energy difference between conformations in the absence of tension) is the key determinant of mechanosensitivity in a channel or other membrane protein.
The gating of MS channels can be experimentally monitored using patch clamp electrophysiology; the current flow through channels in a membrane patch at the end of a pipette is measured as a function of negative pressure (suction) to generate curvature and the associated tension in the patch (Guharay and Sachs, 1984; Hamill, 2006). From the dependence of the open probability on applied tension, ΔA and ΔG° for an MS channel may be evaluated. While this would appear to be a straightforward exercise in curve-fitting, there are two subtleties that complicate the analysis:
Membrane tension is not directly measured, but rather is calculated from ΔP, the negative pressure (suction) applied to the membrane patch, and r, the resulting curvature of the patch, through the Laplace-Young equation σ = 2rΔP (applications to MscL are described in (Sukharev et al., 1999; Moe and Blount, 2005)). As an estimate of the approximate values for these terms, a negative pressure of 5 × 103 Pa (0.05 atm) is required to generate a tension of 10 mN m−1 in a patch with a diameter of 1 μm (10−6 m).
If the channel population is non-uniform, with some channels activating at lower tensions and others at higher (perhaps due to heterogeneity in the local membrane environment, or variable extent of channel modification), the net effect is to broaden the transition from non-conducting to conducting states, which is equivalent to underestimating ΔA (Chiang et al., 2004).
The upper limit on the tension required to gate MS channels is set by the rupture point of membrane patches, typically 20 – 30 mN m−1. Different MS channels have characteristic tension thresholds for opening, with MscL, MscS and MscM requiring ~10, 5, and 1 mN m−1 (Kung et al., 2010) respectively, perhaps allowing a graded response in cells to imposed tension gradients (Figure 1; see also the discussion on “Physiological Functions of MscS and MscL type channels” below). MscL has been extensively characterized, with the most recent results yielding σ1/2 = 10.4 mN m−1 and ΔA = 20.4 nm2 (Chiang et al., 2004). The corresponding values for MscS reconstituted into liposomes are 5.5 mN m−1 and 8.4 nm2, respectively (Sukharev, 2002), and in spheroplasts are 7.8 mN m−1 and 15 nm2, respectively (Belyy et al., 2010b). Interestingly, it has been found that the critical gating tension depends upon the properties of the membrane itself, such as its thickness and the composition, including the presence or absence of curvature-inducing lipids. Using the representative values described above, ΔG° for the free energy difference between closed and open states in the absence of applied tension may be calculated as 120 kJ mol−1 for MscL, and 30 or 70 kJ mol−1 for MscS in liposomes and spheroplasts, respectively. ΔG° for MscL is large compared to other channels; for example, ΔGº between closed and open states of a voltage-gated channel in the absence of applied voltage may be calculated as ~50 kJ mol−1 (Schoppa et al., 1992), while the closed state of the acetylcholine receptor is favored by ~35 kJ mol−1 relative to the open state in the absence of ligand (Jadey et al., 2011). An earlier analysis of the gating behavior of MscL based on a two-state model with a uniform channel population yielded smaller values (σ1/2 = 11.8 mN m−1 and ΔA = 6.5 nm2, corresponding to ΔG° = 46 kJ mol−1 (Sukharev et al., 1999)) more consistent with the ΔG° values observed for non-mechanosensitive channels.
In addition to ΔA and ΔG°, which determine the sensitivity of the tension-dependent response, the other functionally relevant property of MS channels is their conductance. Conductance (G) is defined as the proportionality between the voltage drop (V) across the membrane and the ionic current flowing through the channel (I) using the Ohm’s law type equation
When I and V are in Amperes and Volts, respectively, the unit of conductance is the Siemen (the reciprocal of the Ohm, the unit of resistance). For bacterial channels, the conductances are in the 10−9 S, or nanoSiemen (nS), range, with MscL ~ 3 nS, MscS ~ 1 nS and MscM ~ 0.4 nS (see Figure 1). For a conductance of 1 nS, the current flow through the channel at an applied voltage of 0.1 V would be 100 pA or ~6 × 108 ions sec−1. The conductances of bacterial MS channels are several orders of magnitude larger than that typically observed for ion-selective channels and are consistent with the formation of a large diameter pore in the open state that serves as a non-selective channel. The pore diameter of MscL in the open state, for example, has been estimated from sieving experiments to exceed ~3 nm (Cruickshank et al., 1997), accommodating the passages of small proteins up to 9 kD (van den Bogaart et al., 2007). While ionic conductance is a useful proxy for function, neither MscL nor MscS exhibit significant specificity for any particular ion and so a more functionally relevant parameter would be the water flux or volume flow through the channel. To our knowledge, this quantity has not yet been experimentally determined for either MscL or MscS; a flux of ~ 4 waters per ns through an open MscL channel was indicated in the computational studies of Louhivuori et al. (2010), which was sufficient to relax an osmotically stressed liposome in microseconds.
Membranes are adaptable sensors of tension; no trapdoors required!
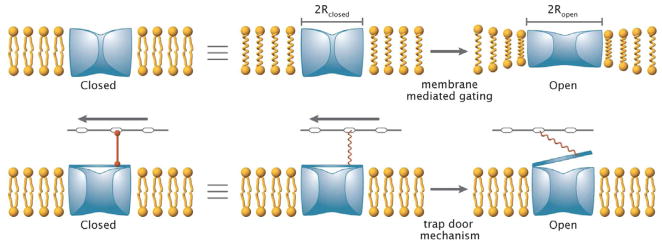
One of the main outstanding questions concerning the behavior of MS channels is the mechanistic basis of their function. That is, how is it that tension in the surrounding membrane can be communicated to the channel itself resulting in the conformational change to the open state? The schematics used to illustrate current thinking on these questions often depict physical linkages that pull on particular parts of the membrane protein in much the same way that pulling on an attached spring can open a trapdoor (Figure 2). However, as will be discussed below, direct physical linkages between a MS channel and the membrane are not a prerequisite to channel gating and one competing class of hypotheses invokes no direct physical linkages at all.
Figure 2.
Schematic representations of two models of gating for mechanosensitive channels. (A) The membrane mediated mechanism and (B) the trapdoor mechanism. (A) The closed state of a channel embedded in a bilayer will be at equilibrium in the absence of applied tension (left). The application of tension to a membrane (right) will serve to stabilize conformational states with greater cross-sectional areas of an embedded channel, with the larger areas favored by increasing tension. However, the change in protein conformation will perturb the membrane - protein interactions (schematically indicated by the compressed bilayer dimensions adjacent to the protein), which will contribute an unfavorable free energy term proportional to the interaction area between protein and membrane. As a consequence of these two competing effects, the interplay between tension and protein conformation can give rise to a rich variety of outcomes for the effect of tension on channel function, without the membrane pulling directly on the channel. (B) In the trapdoor mechanism, tension is coupled to the channel through an extramembrane component, such as the cytoskeleton or peptidoglycan (depicted by the bar with embedded ovals above the membrane), connected to a gate (trapdoor) covering the channel in the closed state (left). Movement of the extramembrane component relative to the membrane stretches the connecting spring, resulting in opening of the trapdoor (right). In this simplified mechanism, the membrane and channel are more passive participants with the applied tension doing work outside the membrane. It should be emphasized that these representations depict idealized mechanisms that are not mutually exclusive; activation barriers could be present that require direct interaction between components even when the overall transition is energetically favorable.
To illustrate the logic of the latter argument, we consider an analogy based upon the force-induced unfolding of DNA. The field of single-molecule biophysics has developed as a fascinating and useful tool for exploring the molecules of life. One of the premier tools in this field is the optical trap, which makes it possible to hold onto individual molecules as they perform their functions. For example, it is now nearly routine to hold onto individual molecular motors as they step along their cytoskeletal tracks (see the discussion in (Phillips et al., 2008), chapter 17). But the field has gone even farther in the development of single-molecule force spectroscopy, which allows the investigator to “read out” the signature of a given molecule on the basis of the relation between the applied force and the resulting displacement. Indeed, this has become so commonplace that the force-extension characteristics of DNA are now used to calibrate instruments such as optical and magnetic traps (Strick et al., 2000; Nelson, 2003; Phillips et al., 2008).
For the purposes of our argument, we consider the free energy competition that leads to extension of DNA molecules when subjected to pN-scale forces. What are the dynamical origins of the resistive force offered by a DNA molecule such as the 48.5 kbp genome of bacteriophage lambda as it is progressively stretched using an optical trap? The answer is that this resistive force arises from the decreased entropy of the DNA molecule as it is stretched from a blob into an extended conformation. It is only in the very high-force limit that we should think of the forces on a DNA molecule as resulting from stretching the “springs” of the individual atomic bonds. At low forces, it is entropic elasticity that dictates the measured force-response with no requirement for bond stretching (Smith et al., 1996; Nelson, 2003; Phillips et al., 2008).
To continue the analogy as it pertains to the gating of channels such as MscL, we need to consider the free energy cost of deforming the membrane surrounding the channel of interest. A simple estimate reveals that, as in the case of the force-induced unfolding of DNA, there is a free energy competition between the applied load and the free energy cost of inducing conformational changes in the molecule, in this case, the MS channel and the lipids that surround it (Wiggins and Phillips, 2004). For the combined system of channel, surrounding membrane and loading device, opening of the channel results in a favorable reduction in the free energy of the loading device and a concomitant increase in the free energy of the membrane surrounding the channel. If we imagine the perimeter of the channel to induce membrane deformation (as a result of hydrophobic mismatch or midplane bending), then the open conformation, which has a larger radius than the closed conformation, has a higher deformation free energy because the larger channel perimeter induces a larger region of deformed membrane. It is thus the interplay between two competing free energies--the energy of the deformed membrane and the reduced energy of the loading device upon channel opening--that determines whether the open or closed state is most favorable. Clearly, as the applied load increases, the competition tilts in favor of the open state. The key point is that this composite free energy results in channel gating without the need for any physical links to tug on particular parts of the protein (see Figure 2). However, as is clear from our discussion of parameters such as the area change and the hydrophobic mismatch, a prerequisite to such physical model building is an understanding of the structure of these proteins in both open and closed states, in the context of the lipid bilayer.
MOLECULAR ARCHITECTURE OF MSCL AND MSCS
Crystal Structures of MscL and MscS
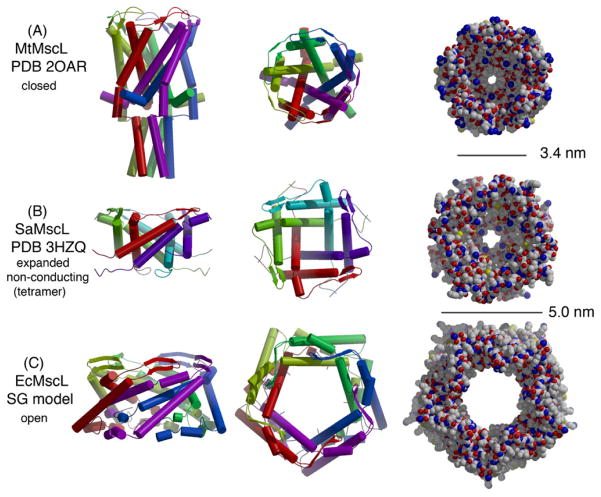
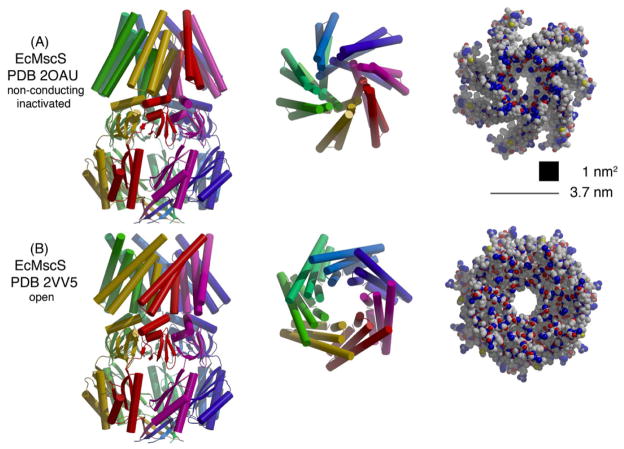
Some general architectural elements of relevance to mechanosensitivity may be identified from the existing crystal structures of MscLs from M. tuberculosis (MtMscL, Figure 3A (Chang et al., 1998)) and S. aureus (SaMscL, Figure 3B (Liu et al., 2009)) and structures of E. coli MscS (EcMscS) in distinct conformational states (Figures 4A (Bass et al., 2002; Steinbacher et al., 2007) and B (Wang et al., 2008)). These channels are organized as symmetric oligomers with the permeation pathway formed by the packing of subunits around the axis of rotational symmetry. MscL and MscS are likely pentameric and heptameric, respectively, as originally observed in the MtMscL and EcMscS structures. The more recent SaMscL structure is a tetramer, however, and there is clear evidence for oligomeric state variability of MscL, as least when over-expressed and detergent extracted (see below).
Figure 3.
Structurally characterized forms of MscL as observed in the crystal structures of (A) M. tuberculosis MscL (Chang et al., 1998) and (B) S. aureus MscL (Liu et al., 2009), and (C) the model of the open state of EcMscL developed by Sukharev and Guy (“SG-model”; (Sukharev et al., 2001b)). For each structure, three representations are provided:
(left) Chain traces of the subunits in each oligomeric channel with subunits depicted in different colors and α-helices and β-sheets shown as cylinders and arrows, respectively. The symmetry axis of each channel, assumed to be parallel to the membrane normal, is oriented vertically, with the cytoplasmic region positioned at the bottom.
(middle) Chain traces of the transmembrane region of each channel. The same coloring scheme is used as in the left panel, and the view (down the membrane normal) is rotated 90° about the horizontal axis. The cytoplasmic helical bundle of MtMscL has been omitted.
(right) Space filling representation of the structures depicted in the middle panel. Scale bars of 3.4 nm and 5.0 nm are indicated. Although admittedly a crude estimate, the cross-sections of the membrane spanning region of each channel may be approximated as regular polygons (pentagon or square), giving values for the corresponding areas of MtMscL, SaMscL and the SG-model as 20, 25 and 43 nm2, respectively. Figure prepared with Molscript (Kraulis, 1991) and Raster-3D (Merritt and Bacon, 1997).
Figure 4.
Structurally characterized forms of E. coli MscS as observed in the crystal structures of (A) non-conducting/inactivated (Bass et al., 2002; Steinbacher et al., 2007) and (B) open (Wang et al., 2008) states. The organization of this figure parallels that represented in Figure 3 for MscL. The scale bar between the space-filling models equals 3.7 nm; assuming that the cross-sections of each structure are depicted as regular heptagons with this side length, the corresponding areas are 50 nm2. If lipids can intercalate between the splayed TM1-TM2 of adjacent subunits in the non-conducting/inactivated structure (Figure 4A), the cross-sectional area will be reduced from this value (see text); as a rough guide to the magnitude of this effect, a square of area 1 nm2 is depicted, which corresponds approximately to the gap between adjacent subunits
Although they share a common organization of an N-terminal transmembrane (TM) domain and a C-terminal cytoplasmic domain, the overall arrangements of the polypeptide folds in MscL and MscS are distinct, indicating that they do not share a common evolutionary ancestor. MscL and MscS contain two and three TM helices, respectively, packed in a relatively simple up-down/nearest neighbor topology. The packing of symmetry-related helices, either TM1 in MscL or TM3 in MscS, into a right-handed bundle generates the permeation pathway across the membrane. The striking pattern of conserved Gly and Ala residues noted in MscL and MscS (Moe et al., 1998; Levina et al., 1999) correspond to residues localized at these helix-helix packing interfaces. The permeation pathways of both channels are roughly funnel shaped with the larger opening facing the periplasmic surface of the membrane and the narrowest point near the cytoplasm. At their narrowest point, the pores are constricted by the side chains of symmetry-related residues: Leu19 and Val23 in MscL, and Leu105 and Leu109 in MscS. (unless otherwise specified, residue numbers refer to the E. coli MscL or MscS sequences as appropriate). Hydrophobic plugs have been similarly noted in the acetylcholine receptor (Miyazawa et al., 2003) and potassium channels (Doyle et al., 1998; Kuo et al., 2003), and may allow the channel to maintain a closed state without being completely shut geometrically (Anishkin and Sukharev, 2004; Beckstein and Sansom, 2004). In both MscL and MscS, the pore-forming helix connects directly to a helix likely to be positioned near the cytoplasmic surface of the membrane.
Experimental approaches to characterizing the gating transition
Given the substantial pore diameters present in their open states, MscL and MscS present attractive targets for characterizing the structural details underlying the conformational transitions that accompany channel gating; even low resolution techniques can provide important insights. A major experimental challenge to these studies, however, is the stabilization of defined conformational states (closed, open, inactive, etc.) of the channel. The successful realization of this objective absolutely requires the availability of a robust functional assay to characterize the state of the channel. For MS channels, patch clamp electrophysiology represents the “gold standard” for monitoring stretch-activated channel current, since this provides quantitative information on the tension-dependence and conductance (Guharay and Sachs, 1984; Sukharev et al., 1994; Hamill, 2006). The downshock assay provides another approach based on measurement of the survival to an osmotic downshock protocol of cells expressing defined sets of MS channels (Levina et al., 1999). The efflux of differently sized solutes through MscL has been assessed using a fluorescence assay with channels reconstituted into proteoliposomes (van den Bogaart et al., 2007). However, in our view, these assays should be complemented with creative new ways to look for as-yet undiscovered functional consequences of these channels and with a more complete characterization of how these channels are regulated.
Two constraints to the experimental study of MS channels are (i) it is difficult to continuously subject purified channels to applied tension, and (ii) no pharmacology has been described that could serve to trap either MscL or MscS in state-specific form (as is possible with voltage- or ligand-gated channels). These constraints notwithstanding, a variety of approaches have been developed to deduce the conformational changes underlying gating transitions; although these have been applied specifically to MS channels, they parallel efforts aimed at dealing with the broader challenges of trapping conformationally variable macromolecular systems in defined states. Here we highlight some of those approaches:
Crosslinking
A conceptual breakthrough in understanding the open state of MscL came from an approach developed by Sukharev and Guy based on disulfide trapping, electrophysiology and modeling studies (Sukharev et al., 2001a; Sukharev et al., 2001b). Cysteines were introduced at specific sites to form disulfides upon downshock conditions, demonstrating the proximity of these residues in the presumptive open state. This analysis defined an “iris”-type mechanism (Figure 3C) for the interconversion of open and closed states involving substantial changes in helical tilt away from the membrane normal, coupled to a sliding motion along the helix-helix packing interfaces such that the pore is primarily composed of residues from TM1 in both states. In the Sukharev-Guy model of the open state (“SG-model”), these rearrangements result in the formation of a pore of diameter ~3.5 nm, consistent with that anticipated for a channel of conductance ~ 3 nS.
Spectroscopic probes of structure
Based on an inspired analysis of the gating transition, Perozo and co-workers were able to stabilize open conformations of MscL and MscS using cone-shaped lysophospholipids to perturb the energetics of lipid packing around a channel (Perozo et al., 2002; Vasquez et al., 2008). Through the use of site-directed spin-labeling based electron paramagnetic resonance methodologies, the environments of the residues in the TM helices of MscL and MscS were characterized and converted into three-dimensional structures through computational approaches. These studies provided direct support for a mechanism that involves the expansion of the channel in the open state and lining of the permeation pathway predominantly by residues in TM1 (MscL) and TM3 (MscS), although some details differ from those proposed by Sukarev and Guy for MscL. More recently, fluorescence resonance energy transfer-based methods have been employed by Martinac and coworkers to study lysophospholipid-driven gating of MscL in liposomes (Corry et al., 2010).
Random mutagenesis and screening
Residues critical for the gating of MscL were initially identified through the isolation of “gain of function” mutations by Kung’s group through random mutagenesis and screening for variants that exhibited a slow or no-growth phenotype due to leakage of cytoplasmic solutes, even when grown under little or no hypo-osmotic stress (Ou et al., 1998). The most severe of these mutants were subsequently observed to map to the TM1-TM1 interface in the MtMscL structure. Studies by the Dougherty group characterizing the phenotypes of strains following random mutagenesis identified the periplasmic loop region as contributing to the gating transition (Maurer and Dougherty, 2003). A genetic screen identifying MscS variants leaky to potassium highlighted the importance of interactions between TM3 and the cytoplasmic domain for stabilizing non-conductive and inactivated conformations (Koprowski et al., 2011).
Site-directed mutagenesis and accessibility
The systematic mutagenesis of residues in the membrane-spanning helices of MscL and MscS has provided major insights into the gating transition, particularly the demonstration that increasing the polarity of specific residues near the constriction point facilitates channel opening for MscL (Yoshimura et al., 1999) MscS (Miller et al., 2003a; Edwards et al., 2008; Vasquez et al., 2008) and MscK (Li et al., 2007). Mutagenesis of conserved Gly and Ala in MscS TM3 has been used to address helical rearrangements during channel opening (Edwards et al., 2005; Akitake et al., 2007). In combination with chemical modification, including alkylation (Levin and Blount, 2004) or metal binding (Iscla et al., 2004), applied during downshock, these approaches provide powerful approaches for the study of conformational rearrangements or residue interactions that help define the pore architecture in the open state.
Lipid-protein interactions
The interface between MS channels and the membrane have been probed in various ways, by exploring the consequences of reconstituting MscL into proteoliposomes of differing fatty acid acyl chain length and head group (Perozo et al., 2002), probing the exposure of residues to the hydrophobic membrane environment through the site directed substitution of TM residues with asparagine (Yoshimura et al., 2004; Nomura et al., 2006), and the application of tryptophan-scanning mutagenesis with fluorescence spectroscopy to define the extent of the hydrophobic interface (Powl et al., 2003; Rasmussen et al., 2007). These studies indicate that there is efficient hydrophobic matching between MscL and the membrane, such that decreasing membrane thickness facilitates channel opening.
Computational studies
Computational studies have been informative in defining the closed-to-open transition, particularly in illuminating the helical rearrangements, the lining of the pore primarily by TM1 for MscL and TM3 for MscS and probing conformations beyond closed and open (subconducting and inactivated states) (Elmore and Dougherty, 2001; Gullingsrud et al., 2001; Kong et al., 2002; Gullingsrud and Schulten, 2003; Sotomayor and Schulten, 2004; Spronk et al., 2005; Sotomayor et al., 2007; Anishkin et al., 2008a; Anishkin et al., 2008b; Tang et al., 2008). A recurring implication of these calculations is the existence of strikingly non-symmetric conformations, which should be kept in mind when interpreting the results of crystallographic and biophysical investigations (see also (Shapovalov et al., 2003)). An important development is the simulation of solute release (40 μs) through a channel embedded in a liposomal membrane (Louhivuori et al., 2010). One may anticipate that simulation of the response of bacterial cells to osmotic downshock on physiological timescales (~100 ms, (Boer et al., 2011)) will become feasible in the not too distant future.
Synthetic proteins/non-natural amino acids
Although experimentally challenging, the successful chemical synthesis of MscL (Clayton et al., 2004) provides a foundation for the future generation of variants containing site-specific biophysical (fluorescence) or chemical (crosslinking) probes. In non-MS channel systems, similar objectives have been achieved with suppressor technology to incorporate non-natural amino acids (Wang et al., 2006), or by protein ligation to integrate synthetic peptides within a ribosomally synthesized framework (Flavell and Muir, 2009). Conceptually similar approaches have been used to generate light- and pH-activated forms of MscL (Kocer et al., 2005; Kocer et al., 2006).
Structural methods
While it has been difficult to trap a single type of channel in distinct states, the judicious use of mutants provided an approach successfully used to trap an open form of MscS ((Wang et al., 2008); Figure 4B). In these studies, the wild-type Ala at position 106—which is conserved in the TM3-TM3 interface—was replaced with Val. Electrophysiological characterization indicated that the channel harboring this substitution requires greater tension to open, but once open, it forms a stable subconducting state, which presumably facilitated crystallization in an open state. The use of homologs provided another approach to trapping different conformational states, as illustrated through the expanded intermediate observed for SaMscL (Liu et al., 2009), although the interpretation is complicated by a change in oligomeric state. Non-crystallographic structural methods have also been applied to MscL, including the use of electron microscopy (Yoshimura et al., 2008) and atomic force microscopy (Ornatska et al., 2003) to image open conformations. Intriguingly, the overall dimensions of the channels observed in these structures (20–30 nm) are two to three times larger than those proposed for the open state model (Sukharev et al., 2001a).
Structural models for the gating transition
While there is no universally conserved gating mechanism for MS channels, several general comments can be made. In both MscL and MscS, the gating transition is associated with changes in helix-helix packing around the pore such that one particular helix - TM1 of MscL or TM3 of MscS - appears to dominate the permeation pathway in all conformational states. In the closed state, the channels are characterized by a plug of hydrophobic residues that seal the pore. The transition between closed and open states is often described in terms of an iris-type motion of helices; in MscL, the open state is likely associated with an increase in helix tilt (Sukharev et al., 2001a), while in the open state of MscS, the helices appear to be more oriented along the membrane normal (Wang et al., 2008). In both channels, an antiparallel pair of helices (TM1 and TM2 from different subunits in MscL, and TM1-TM2 from the same subunits in MscS) reposition during the gating transition. In addition to changes in helix tilt, these transitions may also involve changes in helix kinking (Akitake et al., 2007).
As noted in the previous section, a key property of a mechanosensitive channel is ΔA, the change of cross-sectional area in the membrane between two conformational states. From the available structural models, the corresponding cross-sectional areas may be calculated; from these values, the changes in area between different functional states may then be obtained for comparison to the experimental determined values. From the space-filling models of MscL depicted in the right hand side of Figure 3, the cross-sectional areas of MtMscL and the Sukharev and Guy model may be estimated as ~20 and ~43 nm2. If these structures are assigned to the closed and open states, respectively, ΔA is calculated to be ~23 nm2, close to the latest experimental value of ~20 nm2. (While the difference in oligomeric state precludes a direct comparison of SaMscL to MtMscL and the SG model, the side length (5 nm) of the cross-sectional area of SaMscL (approximated as a square) is close to that of the SG model, supporting the interpretation of SaMscL as corresponding to an expanded conformational state). For the two EcMscS structures (non-conducting/inactivated and open; Figures 4A and B, respectively), the cross-sectional areas modeled as a heptagon are both ~50 nm2. This value excludes the spaces between TM1-TM2 helices of adjacent subunits in the non-conducting/inactivated model (Figure 4A); if these spaces are actually accessible to lipids, the cross-sectional area should be reduced by an amount 7 times the area of these gaps/subunit (estimated as ~1 nm2) ~7 nm2. This estimate for ΔA is in qualitative agreement with that observed experimentally (10.6 nm2) between the open and inactivated states of MscS (Akitake et al., 2005).
Establishing the relationship between structurally characterized forms and functionally assigned states remains challenging, as is identifying the effects of those perturbations required to make these studies experimentally accessible. Over-expression, detergent solubilization, crystallization, probe introduction, and mutagenesis perturb channels in ways that are poorly understood, and our ability to predict function from structure is still primitive. The functional state corresponding to the original EcMscS structure is an excellent example - while it was originally interpreted as open (Bass et al., 2002), molecular dynamics studies subsequently indicated it represents a “vapor-plugged desensitized or closed state” (Anishkin and Sukharev, 2004) and now perhaps it is best described as a non-conductive, tension insensitive, inactivated conformation (Belyy et al., 2010a).
A second example of these considerations is provided by the physiologically relevant oligomeric state(s) of MscL. Originally, MscL was believed to be a hexamer until the MtMscL structure revealed a pentamer (Chang et al., 1998). The tetramer observed in the C-terminal truncated variant of SaMscL (Liu et al., 2009) conclusively demonstrates that MscL adopts multiple alternative oligomeric states, although it does not address their functional relevance. It has been demonstrated that detergents can alter the apparent subunit stoichiometry of various MscLs (Dorwart et al., 2010; Gandhi et al., 2011; Iscla et al., 2011), and mutations, truncations, affinity tags, protein fusions, chemical modifications, over-expression, etc. may also perturb concentration- and conformation-dependent association equilibria. For MscL, it has not to our knowledge been established whether all the MscL channels in a biological membrane are electrophysiologically active, or whether additional, non-conducting forms with distinct oligomeric or alternative conformations (such as the precise pairing of TM1 and TM2 between subunits) could be present under certain conditions. While the recent in vivo crosslinking studies of Iscla et al. (2011) support the predominance of pentameric MscL species in membranes, small amounts of other species are also apparent under many conditions that presumably arise from incomplete crosslinking, but could also reflect the presence of alternative oligomeric states. Such considerations highlight the challenges of determining the oligomeric state(s) of membrane proteins in the native lipid bilayer environment, particularly if the population contains a mixture of oligomeric states.
PHYSIOLOGICAL FUNCTIONS OF MSCS AND MSCL TYPE CHANNELS
While it is clear that MscL and MscS channels allow solutes to flow across the cell membrane in response to membrane tension, the full range of physiological applications of this type of channel activity is still in the beginning stages of investigation. In the section below we consider how bacterial and eukaryotic cells may use MscL and MscS homologs to sense physiologically relevant changes in membrane tension, convert tension into solute flow across the membrane, and subsequently turn these transmembrane fluxes into useful action.
Osmotic shock protection
Both gram-negative and gram-positive bacteria are acutely attuned to osmotic shifts in their surrounding environment, and under conditions of hypo- or hyperosmotic stress, must adjust their cytosolic osmolarity to avoid lysis or plasmolysis (see (Poolman et al., 2004) for a review). As the osmolarity of the environment increases, bacteria actively import and/or metabolize potassium glutamate and a suite of osmoregulatory organic solutes, including glycine betaine and proline (summarized in (Wood et al., 2001)). The accumulation of such solutes helps maintain cellular turgor in hyperosmotic conditions, but presents a liability upon osmotic downshock, when bacterial cells must release these osmolytes or risk lysis (Levina et al., 1999).
It was predicted almost twenty years ago that MS channels might mediate the release of osmotic metabolites and ions (Berrier et al., 1992) and early experiments supported this idea. The exit of lactose and ATP from shocked cells was shown to be prevented by treatment with Gd3+ ions, which inhibit MscL activity in E. coli spheroplasts (Berrier et al., 1992). Later, the mscL gene was shown to be required for the shock-induced release of several cytosolic proteins (Ajouz et al., 1998; Berrier et al., 2000). However, the critical breakthrough and link to cell viability came with the identification of yggB as the gene encoding MscS; bacteria lacking both MscS and MscL activities are almost completely unable to survive an osmotic downshock of 0.5 M (Levina et al., 1999). A recent report adds complexity in that YbdG, a MscS-like channel that contributes to MscM activity in E. coli, is required for survival of less severe osmotic shocks (Schumann et al., 2010). It has also been demonstrated that homologs of MscL and MscS are required for survival of osmotic shock in B. subtilis (Wahome and Setlow, 2006; Hoffmann et al., 2008; Wahome and Setlow, 2008), indicating that this function is conserved in gram-positive bacteria.
A currently accepted model is that bacterial membranes contain multiple MS channels with distinct tension thresholds and conductances in order to provide a gradient of responses (Berrier et al., 1992; Batiza et al., 2002; Li et al., 2002; Balleza et al., 2010; Schumann et al., 2010) According to this argument, the more tension-sensitive channels, with smaller conductances (like MscS and YbdG, see Figure 1), are the first line of defense, opening early in response to osmotic shock and “buffering” MscL from opening until completely necessary. This strategy would have the advantage of opening a pore only as large as needed to save the cell from rupture, while preserving as much as possible cellular metabolites and membrane gradients. However, there does not appear to be a simple relationship between the gating tension of a particular channel and the level of osmotic shock protection it provides. For example the endogenous copy of ybdG protects mscL- mscS- mscK- mutant cells from a 0.15 M, but not a 0.25 M osmotic downshock, while over-expressing wild type YbdG in the same background provides protection from a 0.5 M shock (Schumann et al., 2010). These data suggest that the critical threshold to open a channel may depend upon the total number of channels present as well as the structure of the channel itself.
Osmotic Stress Response: Tip of the MscS Family Iceberg?
As outlined above, the primary role assigned to MscL and MscS type channels is the protection of bacterial cells from osmotic shock. However, the presence of multiple MscS-like proteins in bacterial genomes, as well as their presence in the genomes of multicellular eukaryotic organisms (Kloda and Martinac, 2002; Pivetti et al., 2003; Haswell, 2007; Porter et al., 2009) opens up numerous opportunities for functional specialization. In animal systems, diverse types of MS channels are implicated in osmosensing and osmotic regulation (for example, see (Colbert et al., 1997; Liedtke et al., 2000; Palmer et al., 2001; Liedtke and Friedman, 2003)), but also the perception of mechanical stimuli such as sound, pain, and touch (Sidi et al., 2003; Tracey et al., 2003; O’Hagan et al., 2005); for a complete overview, see (Arnadottir and Chalfie, 2010)). Below, we consider the evidence that MscS family members from bacteria and plants might i) sense and respond to sources of membrane tension other than internal osmotic stress; ii) be regulated by mechanisms in addition to membrane tension; and iii) signal in ways that are separable from ion flux.
Bacterial and plant species have multiple MscS family members with diverse expression and sub-cellular localization profiles
In both E. coli and B. subtilis the expression of MscL- and some MscS-type channels is induced in response to high salt and upon entry to stationary phase (Stokes et al., 2003; Wahome and Setlow, 2006, 2008; Wahome et al., 2009; Schumann et al., 2010), http://genexpdb.ou.edu/main), leading to the suggestion that MS channels are expressed in anticipation of upcoming osmotic challenges. However, in B. subtilis, MscS and MscL are no longer essential for osmotic shock protection once cells are in stationary phase (Wahome and Setlow, 2006, 2008). It thus seems equally plausible that MS channels are instead important for the process of exiting stationary phase and re-entering the growth cycle.
The genome of the model flowering plant Arabidopsis thaliana encodes ten MscS-Like (MSL) proteins with diverse expression and subcellular localization profiles (Pivetti et al., 2003; Haswell, 2007). MSL1-3 are targeted to subcellular organelles, while MSL4-10 are targeted to the plasma and vacuolar membranes (Haswell and Meyerowitz, 2006), Haswell et al 2008). Furthermore, MSL genes are differentially regulated by a variety of biotic and abiotic factors, including but not limited to salt and other osmotica, and are expressed in a wide variety of cells and tissues, including guard cells, reproductive structures, and the vasculature (http://www.weigelworld.org/, http://bar.utoronto.ca/efp_arabidopsis/cgi-bin/efpWeb.cgi). These data are consistent with functional specialization of the ten different channels.
Structural variation implies functional variation
The portion of MscS that is conserved among both prokaryotic and eukaryotic relatives includes the pore-lining helix (TM3) and the upper portion of the cytoplasmic domain; little conservation is seen outside of this region (Kloda and Martinac, 2002; Pivetti et al., 2003; Haswell, 2007; Balleza and Gomez-Lagunas, 2009). As shown in Figure 1, most family members are substantially larger than MscS, which may be considered the minimal size for this type of channel (Miller et al., 2003a). The number of TM helices N-terminal to the conserved pore-lining helix varies from 2 (as in MscS) to 10 or more (as in MscK) among MscS homologs. The structural and functional relevance of these additional TM helices is not clear, but may provide an opportunity to regulate the interaction between those helices that interact with surrounding lipids and those that form the channel pore (Booth et al., 2011).
The size of the C-terminus is also highly variable among MscS family members, as is the presence of conserved domains. For example, the members of a recently described MscS subfamily from bacteria harbor a cyclic nucleotide-binding domain at the extreme C-terminus, and several show cAMP-dependent channel gating (Caldwell et al., 2010). The C-termini of MSL2 and MSL3, two closely related MscS-Like proteins localized to the Arabidopsis chloroplast (Haswell and Meyerowitz, 2006), are unusually large and harbor a 22-amino acid domain of unknown function that is highly conserved among chloroplast-targeted MSLs from seed plants (Haswell, unpublished data). Though the exact physiological roles of these additional domains are not yet known, their presence illustrates the potential for undiscovered functions and regulatory mechanisms for MscS family members.
Eukaryotic MscS-Like proteins have complex functions
In eukaryotes, a clear functional link between MscS-type MS channels and osmotic stress response has not yet been established. Reduced expression of a MscS homolog from Chlamydomonas, MSC1, results in the restriction of chlorophyll fluorescence to small regions near the periphery of the cell, presumably due to a loss of chloroplast integrity (Nakayama et al., 2007). Arabidopsis MSL2 and MSL3 are required for normal chloroplast size and fission, as well as normal leaf morphology (Haswell and Meyerowitz, 2006; Wilson et al., 2011). There is evidence that MSC1 and MSL3 function as bona fide MS channels, and the appearance of enlarged, spherical plastids observed in plants deficient for MSL2 and MSL3 is consistent with an osmotically stressed plastid (Haswell and Meyerowitz, 2006; Nakayama et al., 2007). However, given the large C-termini of MSL2 and MSL3 described above and the complex plastid and whole-plant phenotypes observed when their function is compromised, MSC1, MSL2 and MSL3 may serve additional functions, perhaps even signaling osmotic stress from the plastid to the cytoplasm. No physiological function has yet been ascribed to any other eukaryotic MscS family member.
MscS-like proteins are implicated in cellular signal transduction pathways
The C-terminus of the cyanobacterial MscS homolog PamA was found to interact with the signaling protein PII, a scaffolding protein that coordinates several signal transduction pathways associated with nitrogen and carbon status (Osanai et al., 2005). Furthermore, a pamA-deficient mutant shows abnormal expression of genes involved in sugar and nitrogen signaling and is glucose-sensitive. The exact regulatory relationship between PamA and PII has not been established; PII could regulate PamA (Osanai and Tanaka, 2007) or vice versa, but it seems clear that PamA plays a role in cellular metabolism that is independent of osmotic shock.
Several MscS homologs do not contribute to osmotic shock survival
E. coli MscK is an intriguing example of a MscS homolog that may have evolved novel functions. Though MscK has a similar conductance to MscS and is non-selective, it does not significantly contribute to osmotic shock resistance (Levina et al., 1999; Li et al., 2002). MscK may be required for survival under high-K+ conditions, as its activation requires external potassium ions (Li et al., 2002). Removal of the large N-terminal periplasmic domain produces a MscK derivative capable of partially rescuing a mscS- mscL- mutant from osmotic shock (Li et al., 2002; Miller et al., 2003a). Similarly, several members of the bacterial cyclic nucleotide-gated (bCNG) channel subfamily of MscS homologs do not protect E. coli from osmotic shock and do not show tension-sensitivity in electrophysiological experiments, though they are activated by cAMP (Caldwell et al., 2010). Removal of the cyclic nucleotide monophosphate-binding domain at the extreme C-terminus produces channels that are partially protective in the osmotic shock assay (J.A. Maurer, personal communiation). These two examples suggest that additional protein domains found in MscS family members may impose regulatory control on a channel pore that otherwise retains its MS character. A similar function has been ascribed to the “gatekeeper” domains which regulate a subset of voltage-activated potassium channels (reviewed in (Armstrong, 2003)).
Are All MscS Homologs Channels?
MS channels have been considered to date as essentially sensor proteins, serving to link membrane tension to the transmembrane flux of water, ions and other solutes. However, the observed variations in structure and expression patterns among bacterial members and the distinct subcellular localizations of eukaryotic MSLs imply that some MscS homologs may have diverged so much as to constitute a different kind of membrane tension sensor or to have functions unrelated to mechanosensation. It is exciting to consider that some members of the MscS family may no longer serve as channels, instead linking membrane tension to a biochemical signal distinct from solute flux. Several lines of evidence indicate that gating of the MscS pore is accompanied by a large change in the conformation of its C-terminal domain (Koprowski and Kubalski, 2003; Miller et al., 2003b; Grajkowski et al., 2005; Machiyama et al., 2009). Perhaps in some MSLs, this conformational change regulates interactions with other proteins or functional domains, effectively communicating membrane tension to components of a signal transduction pathway; this would be analogous to the ability of the voltage sensor domain to be coupled with diverse functional elements, including channels and enzymes (Miller, 2006). That the C-terminal domains of certain MSL subfamilies are significantly larger than that of MscS, contain strongly conserved motifs, and/or have previously been shown to interact with signaling proteins (as described above) is consistent with this possibility.
CONCLUSIONS
Mechanosensation is one of the most ubiquitous phenomena in the living world, with consequences for molecules, organelles, cells and the organisms that harbor them. Studies of mechanosensation in bacteria have served as primary model systems for dissecting mechanoresponse and have led to the identification of a class of membrane proteins that gate in response to membrane tension. Subsequent work on this fascinating class of channels has resulted in the discovery of a host of MscS-like proteins in a diverse variety of organisms, raising the possibility of undiscovered functional roles for this family of channels.
It is our hope that many of the exciting questions raised in this review will be answered in the near future. Revealing experiments could include: i) a careful examination of the biochemical and biophysical consequences of osmotic shock, measured simultaneously, and/or in a single cell; ii) the development of new biophysical assays for channel activity as alternatives to electrophysiology for measurement of water or pH flux, or for monitoring conformational changes in real time; iii) evaluation of the role played by MS channels in cellular processes that alter membrane tension other than osmotic stress, such as rehydration of bacterial and pollen spores, membrane remodeling during cell or organelle fission, changes in cell morphology or size, and altered membrane synthesis; and iv) the development of tools to explore the regulatory architecture of MS channel expression, potentially providing significant insights into their function. Finally, MS channels may prove to be useful tools for biologists as well as for the organisms in which they reside when engineered to provide a direct readout of membrane tension in living cells.
Acknowledgments
We thank present and past members of our groups for dealing so constructively with tension, and Henry Lester for stimulating discussions. Our research on mechanosensitive channels is supported by NIH grant GM084211.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ajouz B, Berrier C, Garrigues A, Besnard M, Ghazi A. Release of thioredoxin via the mechanosensitive channel MscL during osmotic downshock of Escherichia coli cells. J Biol Chem. 1998;273:26670–26674. doi: 10.1074/jbc.273.41.26670. [DOI] [PubMed] [Google Scholar]
- Akitake B, Anishkin A, Liu N, Sukharev S. Straightening and sequential buckling of the pore-lining helices define the gating cycle of MscS. Nat Struct Mol Biol. 2007;14:1141–1149. doi: 10.1038/nsmb1341. [DOI] [PubMed] [Google Scholar]
- Akitake B, Anishkin A, Sukharev S. The “dashpot” mechanism of stretch-dependent gating in MscS. J Gen Physiol. 2005;125:143–154. doi: 10.1085/jgp.200409198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen OS, Koeppe RE. Bilayer thickness and membrane protein function: An energetic perspective. Ann Rev Biophys Biomol Struct. 2007;36:107–130. doi: 10.1146/annurev.biophys.36.040306.132643. [DOI] [PubMed] [Google Scholar]
- Anishkin A, Akitake B, Sukharev S. Characterization of the resting MscS: Modeling and analysis of the closed bacterial mechanosensitive channel of small conductance. Biophys J. 2008a;94:1252–1266. doi: 10.1529/biophysj.107.110171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anishkin A, Kamaraju K, Sukharev S. Mechanosensitive channel MscS in the open state: Modeling of the transition, explicit simulations, and experimental measurements of conductance. J Gen Physiol. 2008b;132:67–83. doi: 10.1085/jgp.200810000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anishkin A, Sukharev S. Water dynamics and dewetting transitions in the small mechanosensitive channel MscS. Biophys J. 2004;86:2883–2895. doi: 10.1016/S0006-3495(04)74340-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong CM. Voltage-gated K channels. Science STKE. 2003;2003:re10. doi: 10.1126/stke.2003.188.re10. [DOI] [PubMed] [Google Scholar]
- Arnadottir J, Chalfie M. Eukaryotic mechanosensitive channels. Annu Rev Biophys. 2010;39:111–137. doi: 10.1146/annurev.biophys.37.032807.125836. [DOI] [PubMed] [Google Scholar]
- Balleza D, Gomez-Lagunas F. Conserved motifs in mechanosensitive channels MscL and MscS. Eur Biophys J. 2009;38:1013–1027. doi: 10.1007/s00249-009-0460-y. [DOI] [PubMed] [Google Scholar]
- Balleza D, Gomez-Lagunas F, Quinto C. Cloning and functional expression of an MscL ortholog from Rhizobium etli: characterization of a mechanosensitive channel. J Memb Biol. 2010;234:13–27. doi: 10.1007/s00232-010-9235-8. [DOI] [PubMed] [Google Scholar]
- Bass RB, Strop P, Barclay M, Rees DC. Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science. 2002;298:1582–1587. doi: 10.1126/science.1077945. [DOI] [PubMed] [Google Scholar]
- Batiza AF, Kuo MM, Yoshimura K, Kung C. Gating the bacterial mechanosensitive channel MscL in vivo. Proc Natl Acad Sci USA. 2002;99:5643–5648. doi: 10.1073/pnas.082092599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstein O, Sansom MSP. The influence of geometry, surface character, and flexibility on the permeation of ions and water through biological pores. Phys Biol. 2004;1:42–52. doi: 10.1088/1478-3967/1/1/005. [DOI] [PubMed] [Google Scholar]
- Belyy V, Anishkin A, Kamaraju K, Liu NL, Sukharev S. The tension-transmitting ‘clutch’ in the mechanosensitive channel MscS. Nat Struct Mol Biol. 2010a;17:451–U492. doi: 10.1038/nsmb.1775. [DOI] [PubMed] [Google Scholar]
- Belyy V, Kamaraju K, Akitake B, Anishkin A, Sukharev S. Adaptive behavior of bacterial mechanosensitive channels is coupled to membrane mechanics. J Gen Physiol. 2010b;135:641–652. doi: 10.1085/jgp.200910371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrier C, Besnard M, Ajouz B, Coulombe A, Ghazi A. Multiple mechanosensitive ion channels from Escherichia coli, activated at different thresholds of applied pressure. J Memb Biol. 1996;151:175–187. doi: 10.1007/s002329900068. [DOI] [PubMed] [Google Scholar]
- Berrier C, Coulombe A, Szabo I, Zoratti M, Ghazi A. Gadolinium ion inhibits loss of metabolites induced by osmotic shock and large stretch-activated channels in bacteria. Eur J Biochem. 1992;206:559–565. doi: 10.1111/j.1432-1033.1992.tb16960.x. [DOI] [PubMed] [Google Scholar]
- Berrier C, Garrigues A, Richarme G, Ghazi A. Elongation factor Tu and DnaK are transferred from the cytoplasm to the periplasm of Escherichia coli during osmotic downshock presumably via the mechanosensitive channel mscL. J Bacteriol. 2000;182:248–251. doi: 10.1128/jb.182.1.248-251.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boer M, Anishkin A, Sukharev S. Adaptive MscS gating in the osmotic permeability response in E. coli: the question of time. Biochem. 2011;50:4087–4096. doi: 10.1021/bi1019435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth IR, Edwards MD, Miller S, Li C, Black S, Bartlett W, Schumann U. Structure-function relations of MscS. In: Hamill OP, editor. Mechanosensitive Ion Channels, Part A. 2007. pp. 269–294. [Google Scholar]
- Booth IR, Rasmussen T, Edwards MD, Black S, Rasmussen A, Bartlett W, Miller S. Sensing bilayer tension: bacterial mechanosensitive channels and their gating mechanisms. Biochem Soc Trans. 2011;39:733–740. doi: 10.1042/BST0390733. [DOI] [PubMed] [Google Scholar]
- Caldwell DB, Malcolm HR, Elmore DE, Maurer JA. Identification and experimental verification of a novel family of bacterial cyclic nucleotide-gated (bCNG) ion channels. Biochim Biophys Acta - Biomembranes. 2010;1798:1750–1756. doi: 10.1016/j.bbamem.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Chang G, Spencer RH, Lee AT, Barclay MT, Rees DC. Structure of the MscL homolog from Mycobacterium tuberculosis: a gated mechanosensitive ion channel. Science. 1998;282:2220–2226. doi: 10.1126/science.282.5397.2220. [DOI] [PubMed] [Google Scholar]
- Chiang CS, Anishkin A, Sukharev S. Gating of the large mechanosensitive channel in situ: estimation of the spatial scale of the transition from channel population responses. Biophys J. 2004;86:2846–2861. doi: 10.1016/S0006-3495(04)74337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D, Shapovalov G, Maurer JA, Dougherty DA, Lester HA, Kochendoerfer GG. Total chemical synthesis and electrophysiological characterization of mechanosensitive channels from Escherichia coli and Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2004;101:4764–4769. doi: 10.1073/pnas.0305693101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert HA, Smith TL, Bargmann CI. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci. 1997;17:8259–8269. doi: 10.1523/JNEUROSCI.17-21-08259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corry B, Hurst AC, Pal P, Nomura T, Rigby P, Martinac B. An improved open-channel structure of MscL determined from FRET confocal microscopy and simulation. J Gen Physiol. 2010;136:483–494. doi: 10.1085/jgp.200910376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruickshank CC, Minchin RF, Le Dain AC, Martinac B. Estimation of the pore size of the large-conductance mechanosensitive ion channel of Escherichia coli. Biophys J. 1997;73:1925–1931. doi: 10.1016/S0006-3495(97)78223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C, Smith DO, Adler J. Characterization of mechanosensitive channels in Escherichia coli cytoplasmic membrane by whole-cell patch clamp recording. J Memb Biol. 1995;144:31–42. doi: 10.1007/BF00238414. [DOI] [PubMed] [Google Scholar]
- Dorwart MR, Wray R, Brautigam CA, Jiang YX, Blount P. S. aureus MscL Is a pentamer in vivo but of variable stoichiometries in vitro: Implications for detergent-solubilized membrane proteins. Plos Biol. 2010:8. doi: 10.1371/journal.pbio.1000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle DA, Cabral JM, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Edwards MD, Bartlett W, Booth IR. Pore mutations of the Escherichia coli MscS channel affect desensitization but not ionic preference. Biophys J. 2008;94:3003–3013. doi: 10.1529/biophysj.107.123448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards MD, Li Y, Kim S, Miller S, Bartlett W, Black S, Dennison S, Iscla I, Blount P, Bowie JU, et al. Pivotal role of the glycine-rich TM3 helix in gating the MscS mechanosensitive channel. Nat Struct Mol Biol. 2005;12:113–119. doi: 10.1038/nsmb895. [DOI] [PubMed] [Google Scholar]
- Elmore DE, Dougherty DA. Molecular dynamics simulations of wild-type and mutant forms of the Mycobacterium tuberculosis MscL channel. Biophys J. 2001;81:1345–1359. doi: 10.1016/S0006-3495(01)75791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman DM. Membranes are more mosaic than fluid. Nature. 2005;438:578–580. doi: 10.1038/nature04394. [DOI] [PubMed] [Google Scholar]
- Flavell RR, Muir TW. Expressed Protein Ligation (EPL) in the study of signal transduction, ion conduction, and chromatin biology. Accounts of Chemical Research. 2009;42:107–116. doi: 10.1021/ar800129c. [DOI] [PubMed] [Google Scholar]
- Gandhi CS, Walton TA, Rees DC. OCAM: A new tool for studying the oligomeric diversity of MscL channels. Prot Sci. 2011;20:313–326. doi: 10.1002/pro.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie PG, Walker RG. Molecular basis of mechanosensory transduction. Nature. 2001;413:194–202. doi: 10.1038/35093011. [DOI] [PubMed] [Google Scholar]
- Grajkowski W, Kubalski A, Koprowski P. Surface changes of the mechanosensitive channel MscS upon its activation, inactivation, and closing. Biophys J. 2005;88:3050–3059. doi: 10.1529/biophysj.104.053546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guharay F, Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol-London. 1984;352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullingsrud J, Kosztin D, Schulten K. Structural determinants of MscL gating studied by molecular dynamics simulations. Biophys J. 2001;80:2074–2081. doi: 10.1016/S0006-3495(01)76181-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullingsrud J, Schulten K. Gating of MscL studied by steered molecular dynamics. Biophys J. 2003;85:2087–2099. doi: 10.1016/s0006-3495(03)74637-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP. Twenty odd years of stretch-sensitive channels. Pflugers Archiv-Eur J Physiol. 2006;453:333–351. doi: 10.1007/s00424-006-0131-0. [DOI] [PubMed] [Google Scholar]
- Hamill OP, editor. Mechanosensitive Ion Channels, Part A. San Diego: Academic Press; 2007. [Google Scholar]
- Hamill OP, Martinac B. Molecular basis of mechanotransduction in living cells. Physiol Rev. 2001;81:685–740. doi: 10.1152/physrev.2001.81.2.685. [DOI] [PubMed] [Google Scholar]
- Haswell ES. MscS-like proteins in plants. In: Hamill OP, editor. Mechanosensitive Ion Channels, Part A. 2007. pp. 329–359. [Google Scholar]
- Haswell ES, Meyerowitz EM. MscS-like proteins control plastid size and shape in Arabidopsis thaliana. Curr Biol. 2006;16:1–11. doi: 10.1016/j.cub.2005.11.044. [DOI] [PubMed] [Google Scholar]
- Haswell ES, Peyronnet R, Barbier-Brygoo H, Meyerowitz EM, Frachisse JM. Two MscS homologs provide mechanosensitive channel activities in the Arabidopsis root. Curr Biol. 2008;18:730–734. doi: 10.1016/j.cub.2008.04.039. [DOI] [PubMed] [Google Scholar]
- Hoffmann T, Boiangiu C, Moses S, Bremer E. Responses of Bacillus subtilis to hypotonic challenges: physiological contributions of mechanosensitive channels to cellular survival. Appl Environ Microbiol. 2008;74:2454–2460. doi: 10.1128/AEM.01573-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iscla I, Levin G, Wray R, Reynolds R, Blount P. Defining the physical gate of a mechanosensitive channel, MscL, by engineering metal-binding sites. Biophys J. 2004;87:3172–3280. doi: 10.1529/biophysj.104.049833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iscla I, Wray R, Blount P. The oligomeric state of the truncated mechanosensitive channel of large conductance shows no variance in vivo. Prot Sci. 2011 doi: 10.1002/pro.686. e-pub in advance of publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadey SV, Purohit P, Bruhova I, Gregg TM, Auerbach A. Design and control of acetylcholine receptor conformational change. Proc Natl Acad Sci USA. 2011;108:4328–4333. doi: 10.1073/pnas.1016617108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloda A, Martinac B. Common evolutionary origins of mechanosensitive ion channels in Archaea, Bacteria and cell-walled Eukarya. Archaea. 2002;1:35–44. doi: 10.1155/2002/419261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocer A, Walko M, Bulten E, Halza E, Feringa BL, Meijberg W. Rationally designed chemical modulators convert a bacterial channel protein into a pH-sensory valve. Ang Chemie-Intl Ed. 2006;45:3126–3130. doi: 10.1002/anie.200503403. [DOI] [PubMed] [Google Scholar]
- Kocer A, Walko M, Meijberg W, Feringa BL. A light-actuated nanovalve derived from a channel protein. Science. 2005;309:755–758. doi: 10.1126/science.1114760. [DOI] [PubMed] [Google Scholar]
- Kong Y, Shen Y, Warth TE, Ma J. Conformation pathways in the gating of Escherichia coli mechanosensitive channel. Proc Natl Acad Sci USA. 2002;99:5999–6004. doi: 10.1073/pnas.092051099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprowski P, Grajkowski W, Isacoff EY, Kubalski A. Genetic screen for potassium leaky small mechanosensitive channels (MscS) in Escherichia coli: recognition of cytoplasmic beta domain as a new gating element. J, Biol Chem. 2011;286:877–888. doi: 10.1074/jbc.M110.176131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprowski P, Kubalski A. C termini of the Escherichia coli mechanosensitive ion channel (MscS) move apart upon the channel opening. J Biol Chem. 2003;278:11237–11245. doi: 10.1074/jbc.M212073200. [DOI] [PubMed] [Google Scholar]
- Kraulis PJ. Molscript - a program to produce both detailed and schematic plots of protein structures. J Appl Cryst. 1991;24:946–950. [Google Scholar]
- Kung C. A possibly unifying principle for mechanosensation. Nature. 2005;436:647–654. doi: 10.1038/nature03896. [DOI] [PubMed] [Google Scholar]
- Kung C, Martinac B, Sukharev S. Mechanosensitive channels in microbes. Annu Rev Microbiol. 2010;64:313–329. doi: 10.1146/annurev.micro.112408.134106. [DOI] [PubMed] [Google Scholar]
- Kuo A, Gulbis JM, Antcliff JF, Rahman T, Lowe ED, Zimmer J, Cuthbertson J, Ashcroft FM, Ezaki T, Doyle DA. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science. 2003;300:1922–1926. doi: 10.1126/science.1085028. [DOI] [PubMed] [Google Scholar]
- Levin G, Blount P. Cysteine scanning of MscL transmembrane domains reveals residues critical for mechanosensitive channel gating. Biophys J. 2004;86:2862–2870. doi: 10.1016/S0006-3495(04)74338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levina N, Totemeyer S, Stokes NR, Louis P, Jones MA, Booth IR. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J. 1999;18:1730–1737. doi: 10.1093/emboj/18.7.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Edwards MD, Jeong H, Roth J, Booth IR. Identification of mutations that alter the gating of the Escherichia coli mechanosensitive channel protein, MscK. Mol Microbiol. 2007;64:560–574. doi: 10.1111/j.1365-2958.2007.05672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Moe PC, Chandrasekaran S, Booth IR, Blount P. Ionic regulation of MscK, a mechanosensitive channel from Escherichia coli. EMBO J. 2002;21:5323–5330. doi: 10.1093/emboj/cdf537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4−/− mice. Proc Natl Acad Sci USA. 2003;100:13698–13703. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZF, Gandhi C, Rees DC. Structure of a tetrameric MscL in an expanded intermediate state. Nature. 2009;461:120–124. doi: 10.1038/nature08277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louhivuori M, Risselada HJ, van der Giessen E, Marrink SJ. Release of content through mechano-sensitive gates in pressurized liposomes. Proc Natl Acad Sci USA. 2010;107:19856–19860. doi: 10.1073/pnas.1001316107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machiyama H, Tatsumi H, Sokabe M. Structural changes in the cytoplasmic domain of the mechanosensitive channel MscS during opening. Biophys J. 2009;97:1048–1057. doi: 10.1016/j.bpj.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinac B, Buechner M, Delcour AH, Adler J, Kung C. Pressure-sensitive ion channel in Escherichia coli. Proc Natl Acad Sci USA. 1987;84:2297–2301. doi: 10.1073/pnas.84.8.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer JA, Dougherty DA. Generation and evaluation of a large mutational library from the Escherichia coli mechanosensitive channel of large conductance, MscL - Implications for channel gating and evolutionary design. J Biol Chem. 2003;278:21076–21082. doi: 10.1074/jbc.M302892200. [DOI] [PubMed] [Google Scholar]
- Merritt EA, Bacon DJ. Raster3D: Photorealistic molecular graphics. Meth Enzym. 1997;277:505–524. doi: 10.1016/s0076-6879(97)77028-9. [DOI] [PubMed] [Google Scholar]
- Miller C. Lonely voltage sensor seeks protons for permeation. Science. 2006;312:534–535. doi: 10.1126/science.1127186. [DOI] [PubMed] [Google Scholar]
- Miller S, Bartlett W, Chandrasekaran S, Simpson S, Edwards M, Booth IR. Domain organization of the MscS mechanosensitive channel of Escherichia coli. EMBO J. 2003a;22:36–46. doi: 10.1093/emboj/cdg011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S, Edwards MD, Ozdemir C, Booth IR. The closed structure of the MscS mechanosensitive channel. Cross-linking of single cysteine mutants. J Biol Chem. 2003b;278:32246–32250. doi: 10.1074/jbc.M303188200. [DOI] [PubMed] [Google Scholar]
- Miyazawa A, Fujiyoshi Y, Unwin N. Structure and gating mechanism of the acetylcholine receptor pore. Nature. 2003;423:949–955. doi: 10.1038/nature01748. [DOI] [PubMed] [Google Scholar]
- Moe P, Blount P. Assessment of potential stimuli for mechano-dependent gating of MscL: effects of pressure, tension, and lipid headgroups. Biochem. 2005;44:12239–12244. doi: 10.1021/bi0509649. [DOI] [PubMed] [Google Scholar]
- Moe PC, Blount P, Kung C. Functional and structural conservation in the mechanosensitive channel MscL implicates elements crucial for mechanosensation. Mol Microbiol. 1998;28:583–592. doi: 10.1046/j.1365-2958.1998.00821.x. [DOI] [PubMed] [Google Scholar]
- Nakayama Y, Fujiu K, Sokabe M, Yoshimura K. Molecular and electrophysiological characterization of a mechanosensitive channel expressed in the chloroplasts of Chlamydomonas. Proc Natl Acad Sci USA. 2007;104:5883–5888. doi: 10.1073/pnas.0609996104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson P. Biological Physics. New York: W.H. Freeman; 2003. [Google Scholar]
- Nomura T, Sokabe M, Yoshimura K. Lipid-protein interaction of the MscS mechanosensitive channel examined by scanning mutagenesis. Biophys J. 2006;91:2874–2881. doi: 10.1529/biophysj.106.084541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hagan R, Chalfie M, Goodman MB. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat Neurosci. 2005;8:43–50. doi: 10.1038/nn1362. [DOI] [PubMed] [Google Scholar]
- Ornatska M, Jones SE, Naik RR, Stone MO, Tsukruk VV. Biomolecular stress-sensitive gauges: Surface mediated immobilization of mechanosensitive membrane protein. J Amer Chem Soc. 2003;125:12722–12723. doi: 10.1021/ja037686q. [DOI] [PubMed] [Google Scholar]
- Osanai T, Sato S, Tabata S, Tanaka K. Identification of PamA as a PII-binding membrane protein important in nitrogen-related and sugar-catabolic gene expression in Synechocystis sp PCC 6803. J Biol Chem. 2005;280:34684–34690. doi: 10.1074/jbc.M507489200. [DOI] [PubMed] [Google Scholar]
- Osanai T, Tanaka K. Keeping in touch with PII: PII-interacting proteins in unicellular cyanobacteria. Plant Cell Physiol. 2007;48:908–914. doi: 10.1093/pcp/pcm072. [DOI] [PubMed] [Google Scholar]
- Ou X, Blount P, Hoffman RJ, Kung C. One face of a transmembrane helix is crucial in mechanosensitive channel gating. Proc Natl Acad Sci USA. 1998;95:11471–11475. doi: 10.1073/pnas.95.19.11471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CP, Zhou XL, Lin J, Loukin SH, Kung C, Saimi Y. A TRP homolog in Saccharomyces cerevisiae forms an intracellular Ca(2+)-permeable channel in the yeast vacuolar membrane. Proc Natl Acad Sci USA. 2001;98:7801–7805. doi: 10.1073/pnas.141036198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perozo E, Kloda A, Cortes DM, Martinac B. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nature Struct Biol. 2002;9:696–703. doi: 10.1038/nsb827. [DOI] [PubMed] [Google Scholar]
- Phillips R, Kondev J, Theriot J. Physical Biology of the Cell. New York, NY: Garland Science; 2008. [Google Scholar]
- Phillips R, Ursell T, Wiggins P, Sens P. Emerging roles for lipids in shaping membrane-protein function. Nature. 2009;459:379–385. doi: 10.1038/nature08147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivetti CD, Yen MR, Miller S, Busch W, Tseng YH, Booth IR, Saier MH., Jr Two families of mechanosensitive channel proteins. Microbiol Mol Biol Rev. 2003;67:66–85. doi: 10.1128/MMBR.67.1.66-85.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B, Spitzer JJ, Wood JM. Bacterial osmosensing: roles of membrane structure and electrostatics in lipid-protein and protein-protein interactions. Biochim Biophys Acta. 2004;1666:88–104. doi: 10.1016/j.bbamem.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Porter BW, Zhu YJ, Webb DT, Christopher DA. Novel thigmomorphogenetic responses in Carica papaya: touch decreases anthocyanin levels and stimulates petiole cork outgrowths. Ann Bot. 2009;103:847–858. doi: 10.1093/aob/mcp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powl AM, East JM, Lee AG. Lipid-protein interactions studied by introduction of a tryptophan residue: the mechanosensitive channel MscL. Biochem. 2003;42:14306–14317. doi: 10.1021/bi034995k. [DOI] [PubMed] [Google Scholar]
- Rasmussen A, Rasmussen T, Edwards MD, Schauer D, Schumann U, Miller S, Booth IR. The role of tryptophan residues in the function and stability of the mechanosensitive channel MscS from Escherichia coli. Biochem. 2007;46:10899–10908. doi: 10.1021/bi701056k. [DOI] [PubMed] [Google Scholar]
- Sachs F. Stretch-activated ion channels: what are they? Physiology. 2010;25:50–56. doi: 10.1152/physiol.00042.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppa NE, McCormack K, Tanouye MA, Sigworth FJ. The size of gating charge in wild-type and mutant Shaker potassium channels. Science. 1992;255:1712–1715. doi: 10.1126/science.1553560. [DOI] [PubMed] [Google Scholar]
- Schumann U, Edwards MD, Rasmussen T, Bartlett W, van West P, Booth IR. YbdG in Escherichia coli is a threshold-setting mechanosensitive channel with MscM activity. Proc Natl Acad Sci USA. 2010;107:12664–12669. doi: 10.1073/pnas.1001405107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapovalov G, Bass R, Rees DC, Lester HA. Open-state disulfide crosslinking between Mycobacterium tuberculosis mechanosensitive channel subunits. Biophys J. 2003;84:2357–2365. doi: 10.1016/S0006-3495(03)75041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidi S, Friedrich RW, Nicolson T. NompC TRP channel required for vertebrate sensory hair cell mechanotransduction. Science. 2003;301:96–99. doi: 10.1126/science.1084370. [DOI] [PubMed] [Google Scholar]
- Smith SB, Cui Y, Bustamante C. Overstretching B-DNA: the elastic response of individual double-stranded and single-stranded DNA molecules. Science. 1996;271:795–799. doi: 10.1126/science.271.5250.795. [DOI] [PubMed] [Google Scholar]
- Sotomayor M, Schulten K. Molecular dynamics study of gating in the mechanosensitive channel of small conductance MscS. Biophys J. 2004;87:3050–3065. doi: 10.1529/biophysj.104.046045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotomayor M, Vasquez V, Perozo E, Schulten K. Ion conduction through MscS as determined by electrophysiology and simulation. Biophys J. 2007;92:886–902. doi: 10.1529/biophysj.106.095232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spronk SA, Dougherty DA, Lester HA. Hydration of the pore of the mechanosensitive channel of small conductance (MscS) studied by molecular dynamics. Biophys J. 2005;88:149A–149A. [Google Scholar]
- Steinbacher S, Bass RB, Strop P, Rees DC. Structures of the prokaryotic mechanosensitive channels MscL and MscS. In: Hamill OP, editor. Mechanosensitive Ion Channels, Part A. 2007. pp. 1–24. [Google Scholar]
- Stokes NR, Murray HD, Subramaniam C, Gourse RL, Louis P, Bartlett W, Miller S, Booth IR. A role for mechanosensitive channels in survival of stationary phase: regulation of channel expression by RpoS. Proc Natl Acad Sci USA. 2003;100:15959–15964. doi: 10.1073/pnas.2536607100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick T, Allemand J, Croquette V, Bensimon D. Twisting and stretching single DNA molecules. Prog Biophys Mol Biol. 2000;74:115–140. doi: 10.1016/s0079-6107(00)00018-3. [DOI] [PubMed] [Google Scholar]
- Sukharev S. Purification of the small mechanosensitive channel of Escherichia coli (MscS): the subunit structure, conduction, and gating characteristics in liposomes. Biophys J. 2002;83:290–298. doi: 10.1016/S0006-3495(02)75169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukharev S, Betanzos M, Chiang CS, Guy HR. The gating mechanism of the large mechanosensitive channel MscL. Nature. 2001a;409:720–724. doi: 10.1038/35055559. [DOI] [PubMed] [Google Scholar]
- Sukharev S, Corey DP. Mechanosensitive channels: multiplicity of families and gating paradigms. Science STKE. 2004:2004. doi: 10.1126/stke.2192004re4. [DOI] [PubMed] [Google Scholar]
- Sukharev S, Durell SR, Guy HR. Structural models of the MscL gating mechanism. Biophys J. 2001b;81:917–936. doi: 10.1016/S0006-3495(01)75751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukharev SI, Blount P, Martinac B, Blattner FR, Kung C. A large-conductance mechanosensitive channel in E. coli encoded by mscL alone. Nature. 1994;368:265–268. doi: 10.1038/368265a0. [DOI] [PubMed] [Google Scholar]
- Sukharev SI, Blount P, Martinac B, Kung C. Mechanosensitive channels of Escherichia coli: The MscL gene, protein, and activities. Annu Rev of Physiol. 1997;59:633–657. doi: 10.1146/annurev.physiol.59.1.633. [DOI] [PubMed] [Google Scholar]
- Sukharev SI, Martinac B, Arshavsky VY, Kung C. Two types of mechanosensitive channels in the Escherichia coli cell envelope: solubilization and functional reconstitution. Biophys J. 1993;65:177–183. doi: 10.1016/S0006-3495(93)81044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukharev SI, Sigurdson WJ, Kung C, Sachs F. Energetic and spatial parameters for gating of the bacterial large conductance mechanosensitive channel, MscL. J Gen Physiol. 1999;113:525–540. doi: 10.1085/jgp.113.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YY, Yoo J, Yethiraj A, Cui Q, Chen X. Gating mechanisms of mechanosensitive channels of large conductance, II: Systematic study of conformational transitions. Biophys J. 2008;95:581–596. doi: 10.1529/biophysj.107.128496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey WD, Jr, Wilson RI, Laurent G, Benzer S. Painless, a Drosophila gene essential for nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- van den Bogaart G, Krasnikov V, Poolman B. Dual-color fluorescence-burst analysis to probe protein efflux through the mechanosensitive channel MscL. Biophys J. 2007;92:1233–1240. doi: 10.1529/biophysj.106.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez V, Sotomayor M, Cordero-Morales J, Schulten K, Perozo E. A structural mechanism for MscS gating in lipid bilayers. Science. 2008;321:1210–1214. doi: 10.1126/science.1159674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- Wahome PG, Cowan AE, Setlow B, Setlow P. Levels and localization of mechanosensitive channel proteins in Bacillus subtilis. Arch Microbiol. 2009;191:403–414. doi: 10.1007/s00203-009-0465-z. [DOI] [PubMed] [Google Scholar]
- Wahome PG, Setlow P. The synthesis and role of the mechanosensitive channel of large conductance in growth and differentiation of Bacillus subtilis. Arch Microbiol. 2006;186:377–383. doi: 10.1007/s00203-006-0152-2. [DOI] [PubMed] [Google Scholar]
- Wahome PG, Setlow P. Growth, osmotic downshock resistance and differentiation of Bacillus subtilis strains lacking mechanosensitive channels. Arch Microbiol. 2008;189:49–58. doi: 10.1007/s00203-007-0292-z. [DOI] [PubMed] [Google Scholar]
- Wang L, Xie J, Schultz PG. Expanding the genetic code. Annu Rev Biophys Biomolec Struct. 2006;35:225–249. doi: 10.1146/annurev.biophys.35.101105.121507. [DOI] [PubMed] [Google Scholar]
- Wang WJ, Black SS, Edwards MD, Miller S, Morrison EL, Bartlett W, Dong CJ, Naismith JH, Booth IR. The structure of an open form of an E. coli mechanosensitive channel at 3.45 Å resolution. Science. 2008;321:1179–1183. doi: 10.1126/science.1159262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins P, Phillips R. Analytic models for mechanotransduction: gating a mechanosensitive channel. Proc Natl Acad Sci U S A. 2004;101:4071–4076. doi: 10.1073/pnas.0307804101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ME, Jensen GS, Haswell ES. Two mechanosensitive channel homologs influence division ring placement in Arabidopsis chloroplasts. Plant Cell. 2011 doi: 10.1105/tpc.111.088112. e-pub in advance of publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JM, Bremer E, Csonka LN, Kraemer R, Poolman B, van der Heide T, Smith LT. Osmosensing and osmoregulatory compatible solute accumulation by bacteria. Comp Biochem Physiol A Mol Integr Physiol. 2001;130:437–460. doi: 10.1016/s1095-6433(01)00442-1. [DOI] [PubMed] [Google Scholar]
- Yoshimura K, Batiza A, Schroeder M, Blount P, Kung C. Hydrophilicity of a single residue within MscL correlates with increased channel mechanosensitivity. Biophys J. 1999;77:1960–1972. doi: 10.1016/S0006-3495(99)77037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura K, Nomura T, Sokabe M. Loss-of-function mutations at the rim of the funnel of mechanosensitive channel MscL. Biophys J. 2004;86:2113–2120. doi: 10.1016/S0006-3495(04)74270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura K, Usukura J, Sokabe M. Gating-associated conformational changes in the mechanosensitive channel MscL. Proc Natl Acad Sci USA. 2008;105:4033–4038. doi: 10.1073/pnas.0709436105. [DOI] [PMC free article] [PubMed] [Google Scholar]