Dear Editor,
Nerve injury-induced neuropathic pain is difficult to treat in clinic. Lack of comprehensive understanding of the mechanism underlying such chronic pain hypersensitivity delays the development of more effective therapy. Accumulated evidence shows that peripheral nerve injury alters the expression of many neurotransmitters, receptors, ion channels and signaling molecules in the dorsal root ganglion (DRG) and the dorsal horn of spinal cord 1. Some of these molecular changes in the pain pathway are correlated with the current therapy for neuropathic pain. For example, the up-regulation of the α2δ1 subunit of voltage-gated Ca2+ channel, a target of analgesic gabapentin, in DRG neurons and the dorsal spinal cord could be a molecular basis for using gabapentin in neuropathic pain treatment 1, 2. Down-regulated expression of μ-opioid receptors in DRG neurons partially explains the reduced effectiveness of morphine in neuropathic pain treatment 1. We recently found that the nociceptive afferent transmission is suppressed by follistatin-like 1 (FSTL1), a member of the follistatin gene family 3. Furthermore, genetic deletion of FSTL1 in DRG neurons results in pain hypersensitivity 4.
Both in situ hybridization and immunostaining (see Supplementary information, Data S1) showed that in the DRGs of adult male rats, FSTL1 was expressed in ∼52% of DRG neurons and most of them were small-diameter neurons (Figure 1A and 1B) 4, which transmit nociceptive signals generated at the peripheral nerve endings to the afferent terminals in laminae I-II of the spinal cord. FSTL1 is secreted from nociceptive afferent terminals and suppresses the excitatory synaptic transmission by activating the α1 subunit-containing Na+, K+-ATPase (α1NKA) 4, which plays a crucial role in maintaining the Na+ and K+ gradient across the plasma membrane and the excitable properties of neurons 5. However, the role of FSTL1 in chronic pain, such as nerve injury-induced neuropathic pain, remains unknown.
Figure 1.
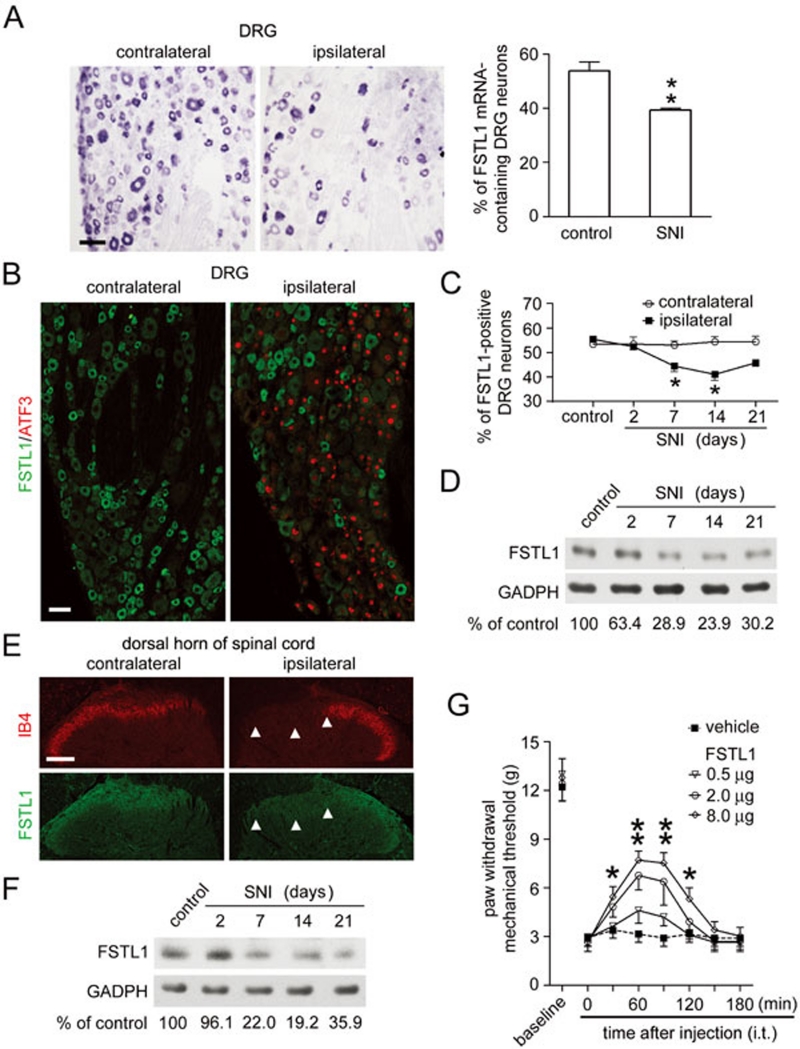
Decreased FSTL1 expression in a neuropathic pain model. (A) In situ hybridization showed that the FSTL1 mRNA level was decreased in the ipsilateral rat L5 DRG 14 days after unilateral spared nerve injury (SNI). Scale bar, 50 μm. *P < 0.05 versus the control DRGs (mean ± s.e.m., Student's t-test, n = 3). (B) Immunostaining showed a reduction in number of FSTL1-positive neurons in the ipsilateral L5 DRG 14 days after unilateral SNI. Double-immunofluorescent staining showed the absence of FSTL1 in the injured DRG neurons labeled by ATF3 in the nucleus. Scale bar, 50 μm. (C) Quantitative analysis showed a reduction of FSTL1-immunoreactive neurons in the ipsilateral L5 DRG after SNI. *P < 0.05 versus the control DRGs (mean ± s.e.m., Student's t-test, n = 3). (D) Immunoblotting showed a reduction in the amount of FSTL1 protein in L5 DRG after SNI. Immunoblot intensity of FSTL1 was normalized to GAPDH and compared with the level in control DRGs (n = 3). (E) Fourteen days after SNI, FSTL1 immunostaining was reduced in the region innervated by the tibial and common peroneal nerves (arrowheads) in the ipsilateral laminae I-II of the L5 spinal segment. IB4 labeling was also reduced in the same region. Scale bar, 100 μm. (F) Immunoblotting showed a reduction of FSTL1 protein in the ipsilateral dorsal horn of the L5 spinal segment after SNI (n = 3). (G) Intrathecal (i.t.) application of FSTL1 inhibits mechanical allodynia 14 days after SNI. *P < 0.05, **P < 0.01 versus SNI rats treated with vehicle (mean ± s.e.m., two-way ANOVA with a post hoc Bonferroni's test, n = 12).
Our present study shows that FSTL1 expression in rat DRG neurons is markedly decreased following peripheral nerve injury. To examine the nerve injury-induced changes in mRNA and protein levels of FSTL1, we prepared the spared nerve injury (SNI) model as described for rats 6. To confirm the effect of nerve damage on FSTL1 expression, we also prepared the sciatic nerve transection (SNT) model. The lumbar (L) 4 and L5 DRGs from rats (200 g, male) with unilateral SNI or SNT and control rats were processed for in situ hybridization. The number of FSTL1 mRNA-containing DRG neurons in the ipsilateral L4 and L5 DRGs was markedly reduced after peripheral nerve injury, as compared with that in the contralateral DRGs (Figure 1A; Supplementary information, Figure S1A). A similar number of FSTL1 mRNA-positive neurons was found in the contralateral DRGs and the DRGs of control rats. Thus, the expression of FSTL1 in DRG neurons is down-regulated after peripheral nerve injury.
Both immunostaining and immunoblotting showed that the nerve injury-induced reduction in FSTL1 mRNA resulted in a decrease in the FSTL1 protein level in the cell bodies of DRG neurons (Figure 1B-1D). Reduction in FSTL1 occurred directly in injured neurons, as evidenced by a loss of FSTL1 in DRG neurons expressing activated transcription factor 3 (Figure 1B), a marker of DRG neurons with injured peripheral axons 7. Fourteen days after unilateral SNI, the number of FSTL1-immunoreactive DRG neurons was reduced from ∼53% in the control rat DRGs to ∼39% in the ipsilateral DRGs (Figure 1C). Immunoblot analysis (Supplementary information, Data S1) showed that the FSTL1 protein level in the ipsilateral DRG neurons was reduced to ∼23.9% of that in the control DRGs (Figure 1D). A similar reduction in the FSTL1 level was found in the ipsilateral L4 and L5 DRGs after unilateral SNT (Supplementary information, Figure S1B and S1C).
Small DRG neurons give rise to unmyelinated (C-type) and thinly myelinated (Aδ-type) afferent fibers, which project to the laminae I-II of the spinal cord of rats. Our recent study revealed that FSTL1 is transported to C- and Aδ-afferent terminals in the superficial dorsal horn of rat spinal cord, and secreted in response to stimulations 4. To determine the spinal region innervated by the tibial and common peroneal nerves, we used isolectin B4 (IB4) as a marker, because IB4 is specifically present in a subset of small DRG neurons and strongly reduced after peripheral nerve injury 8. Using double-immunofluorescent staining and immunoblot analysis (Supplementary information, Data S1), we found a marked reduction of FSTL1 immunoreactivity and IB4 labeling in the region innervated by the tibial and common peroneal nerves in the ipsilateral laminae I-II of the L4-5 spinal segments (Figure 1E and 1F). A similar reduction in FSTL1 was found in the ipsilateral dorsal horn of rat spinal cord after unilateral sciatic nerve axotomy (Supplementary information, Figure S1D).
We further tested whether the nerve injury-induced FSTL1 expression change contributed to the pain hypersensitivity. Adult male rats were housed under a 12:12 h light/dark cycle at 22-26 °C, and mechanical hyperalgesia was examined (Supplementary information, Supplementary information, Data S1). Mechanical stimuli were applied using ascending graded von Frey filaments (Supplementary information, Data S1). When nerve injury-induced loss of endogenous FSTL1 was compensated by intrathecal injection (i.t.) of FSTL1 protein, the mechanical allodynia induced by SNI was reduced in a FSTL1 dose-dependent manner (Figure 1G). Thus, the marked reduction of FSTL1 in the dorsal spinal cord could be a mechanism underlying the development of neuropathic pain.
We recently show that the conditional Fstl1 gene knockout mice exhibit a reduced threshold of somatic sensation and a hypersensitivity to noxious stimulations 4, suggesting that the loss of FSTL1-dependent NKA activation led to sensory hypersensitivity, including exaggerated pain. A reduction of endogenous FSTL1 in the dorsal spinal cord after peripheral nerve injury could be an intrinsic mechanism underlying neuropathic pain, because intrathecal application of FSTL1 substantially alleviated allodynia. This analgesic effect of FSTL1 was as strong as that induced by gabapentin (i.t., 200 μg), whereas only a weak effect could be induced by a high dose of morphine (i.t., 20 μg) (Supplementary information, Figure S1E). FSTL1 has been shown to serve as a target protein for auto-antibodies in human rheumatoid arthritis 9. Reduction in FSTL1-dependent homeostatic regulation under pathological conditions, such as that resulting from FSTL1 auto-antibodies in human rheumatoid arthritis, may contribute to abnormal sensation. Moreover, low α1NKA activity is considered to be partly responsible for the diabetic neuropathy that causes paraesthesias and pain 10. Thus, defective FSTL1-α1NKA interactions may underlie the abnormal sensations and pain associated with many diseases. Our results underscore the therapeutic potential of the FSTL1-α1NKA system in the treatment of intractable pain.
Acknowledgments
This work was supported by NNSFC (30630029 and 30621062), MOST (2011CBA00400 and 2009CB522005), and grants 06R214160 and 2007KIP402.
Footnotes
(Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary Information
Materials and Methods
Decreased FSTL1 expression in DRG neurons after peripheral axotomy.
References
- Zhang X, Xiao HS. Gene array analysis to determine the components of neuropathic pain signaling. Curr Opin Mol Ther. 2005;7:532–537. [PubMed] [Google Scholar]
- Luo ZD, Chaplan SR, Higuera ES, et al. Upregulation of dorsal root ganglion α2δ calcium channel subunit and its correlation with allodynia in spinal nerve-injured rats. J Neurosci. 2001;21:1868–1875. doi: 10.1523/JNEUROSCI.21-06-01868.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brekken RA, Sage EH. SPARC, a matricellular protein: at the crossroads of cell-matrix. Matrix Biol. 2000;19:569–580. doi: 10.1016/s0945-053x(00)00105-0. [DOI] [PubMed] [Google Scholar]
- Li KC, Zhang FX, Li CL, et al. Follistatin-like 1 suppresses sensory afferent transmission by activating Na+, K+-ATPase. Neuron. 2011;69:974–987. doi: 10.1016/j.neuron.2011.01.022. [DOI] [PubMed] [Google Scholar]
- Kaplan JH. Biochemistry of Na,K-ATPase. Annu Rev Biochem. 2002;71:511–535. doi: 10.1146/annurev.biochem.71.102201.141218. [DOI] [PubMed] [Google Scholar]
- Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- Tsujino H, Kondo E, Fukuoka T, et al. Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: a novel neuronal marker of nerve injury. Mol Cell Neurosci. 2000;15:170–182. doi: 10.1006/mcne.1999.0814. [DOI] [PubMed] [Google Scholar]
- Persson JK, Lindh B, Elde R, et al. The expression of different cytochemical markers in normal and axotomised dorsal root ganglion cells projecting to the nucleus gracilis in the adult rat. Exp Brain Res. 1995;105:331–344. doi: 10.1007/BF00233034. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Ozaki S, Osakada F, et al. Cloning of follistatin-related protein as a novel autoantigen in systemic rheumatic diseases. Int Immunol. 1998;10:1305–1314. doi: 10.1093/intimm/10.9.1305. [DOI] [PubMed] [Google Scholar]
- Krishnan AV, Kiernan MC. Altered nerve excitability properties in established diabetic neuropathy. Brain. 2005;128:1178–1187. doi: 10.1093/brain/awh476. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Materials and Methods
Decreased FSTL1 expression in DRG neurons after peripheral axotomy.