Abstract
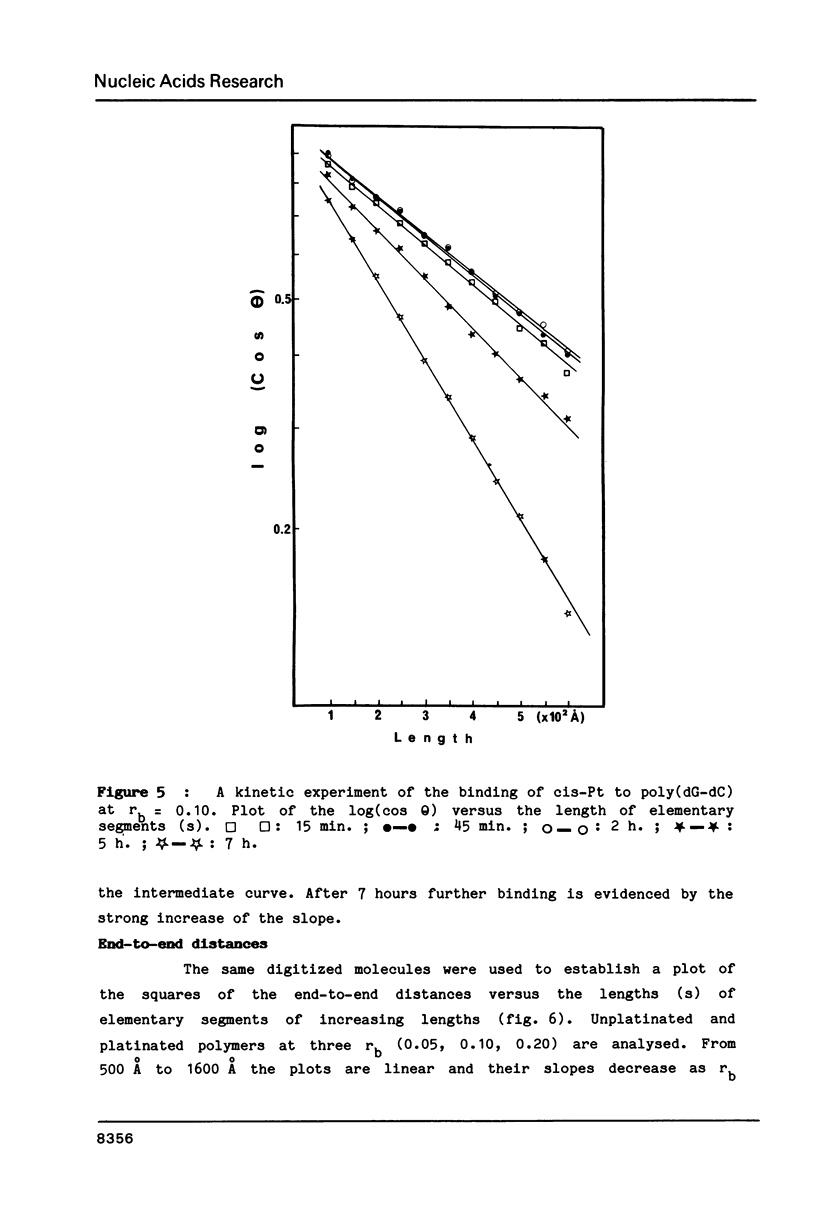
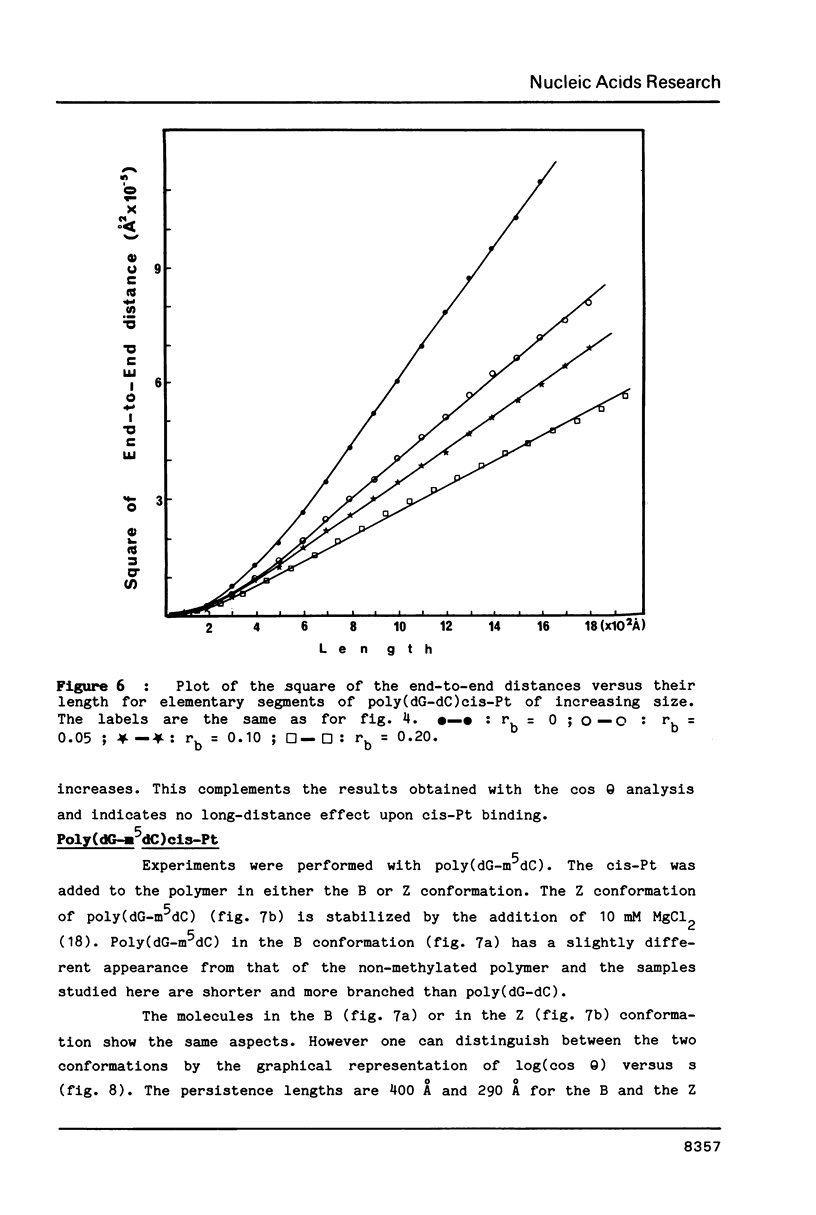
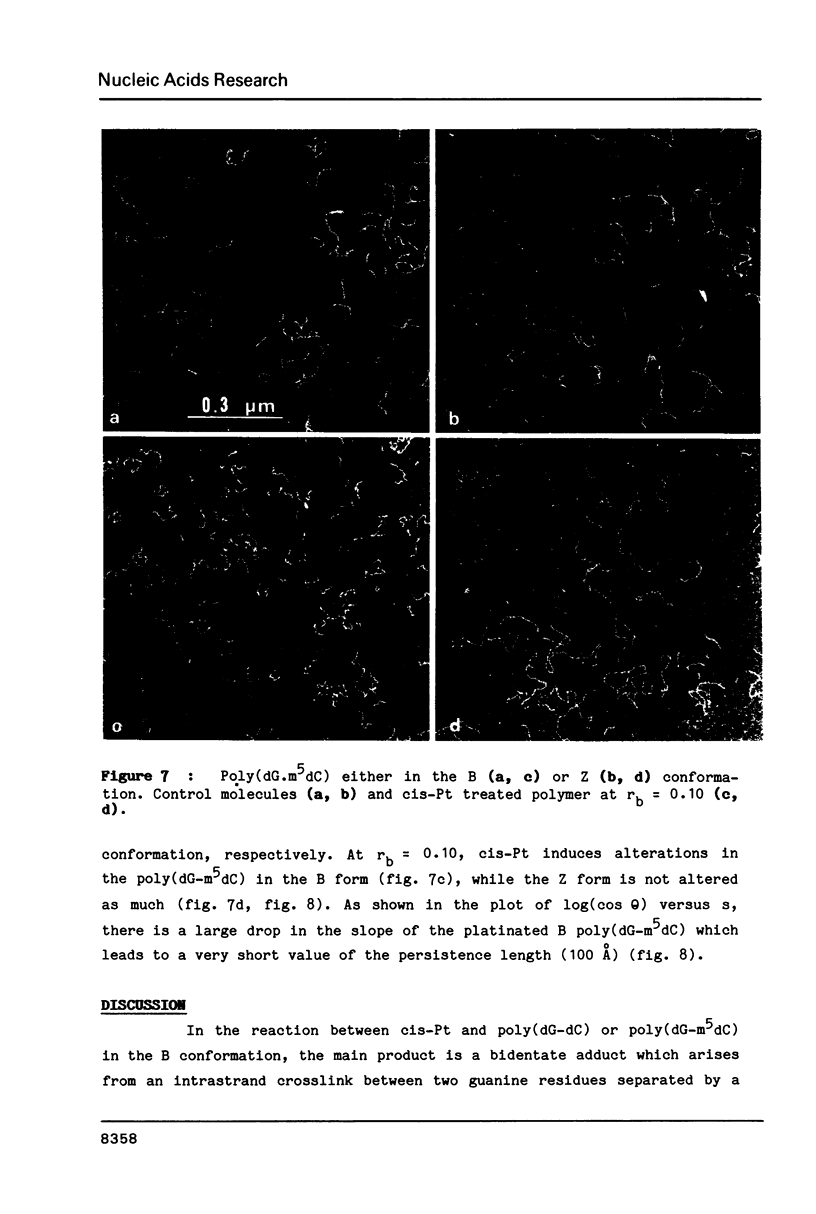
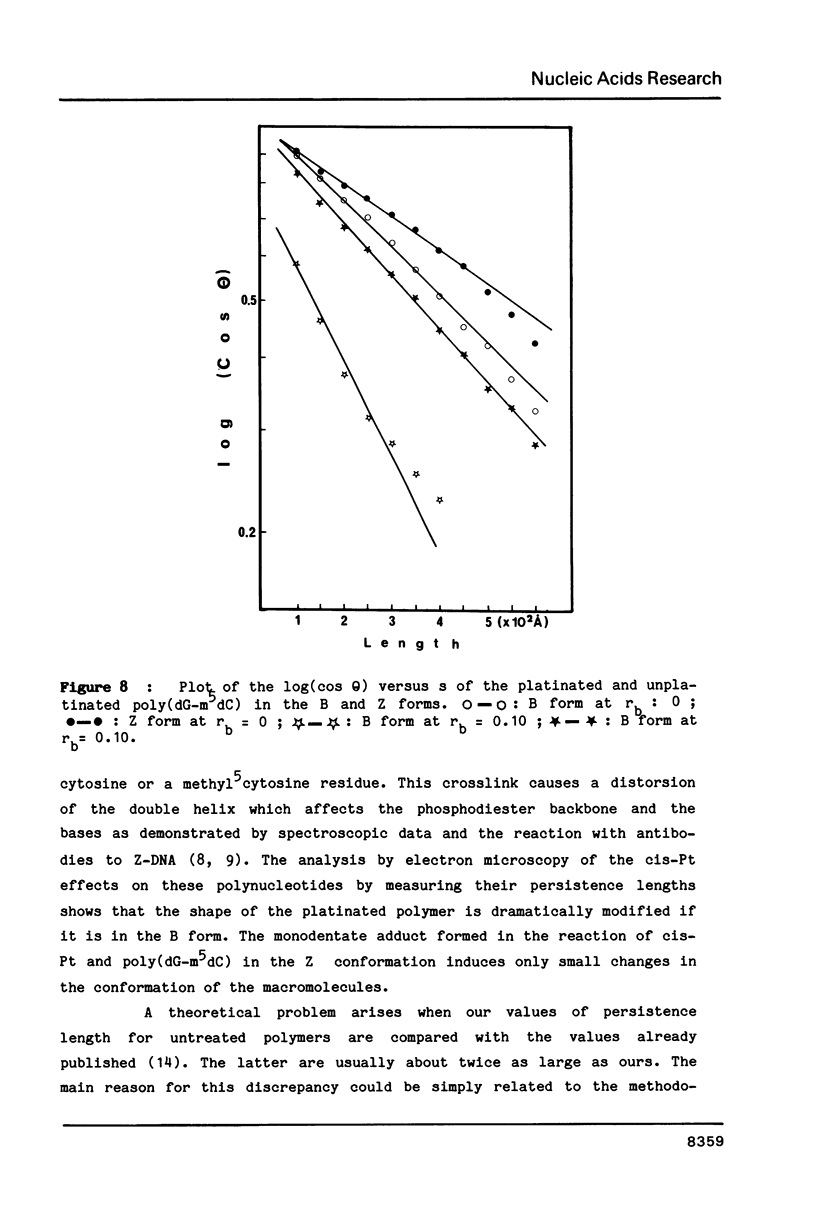
The antitumor drug cis-diamminedichloroplatinum (II) (cis-Pt) forms bidentate adducts with guanine residues of poly(dG-dC).poly(dG-dC). The secondary structure of the polymer is altered. In this work, high resolution pictures of naked molecules, obtained by dark field electron microscopy reveal DNA chain distortions with radii as small as 30 A. The extent of distortion increases with the drug/nucleotide ratio (rb). These alterations of the secondary structure are responsible for the apparent shortening of the molecules. Measurements of the persistence lengths of the polymer as well as the end-to-end distances of elementary segments of various lengths, are obtained from digitized electron micrographs. The measurements are used to monitor and quantify the observed modifications of polymer structure upon cis-Pt binding at various rb or incubation times. Poly(dG-m5dC).poly(dG-m5dC) in the B and Z forms have different persistence lengths. In the B form, this polymer is more altered by cis-Pt than in the Z one.
Full text
PDF


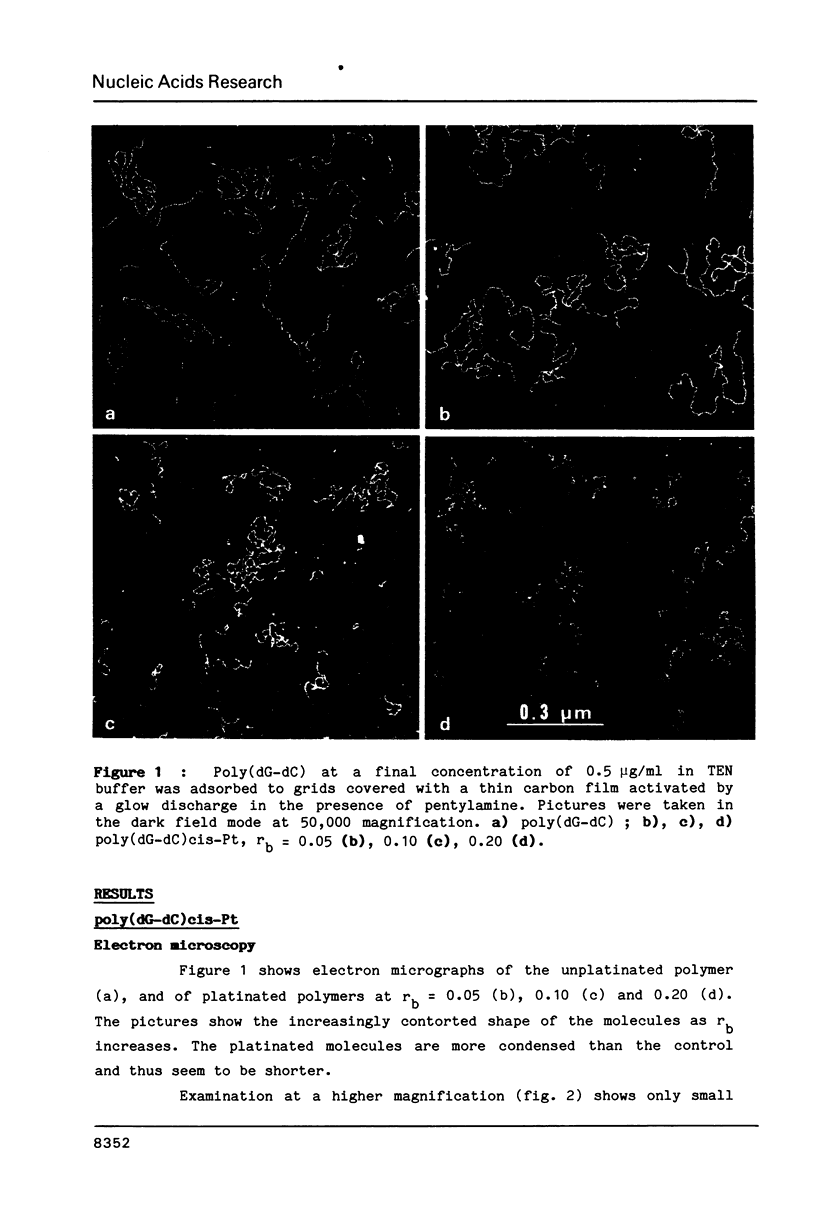
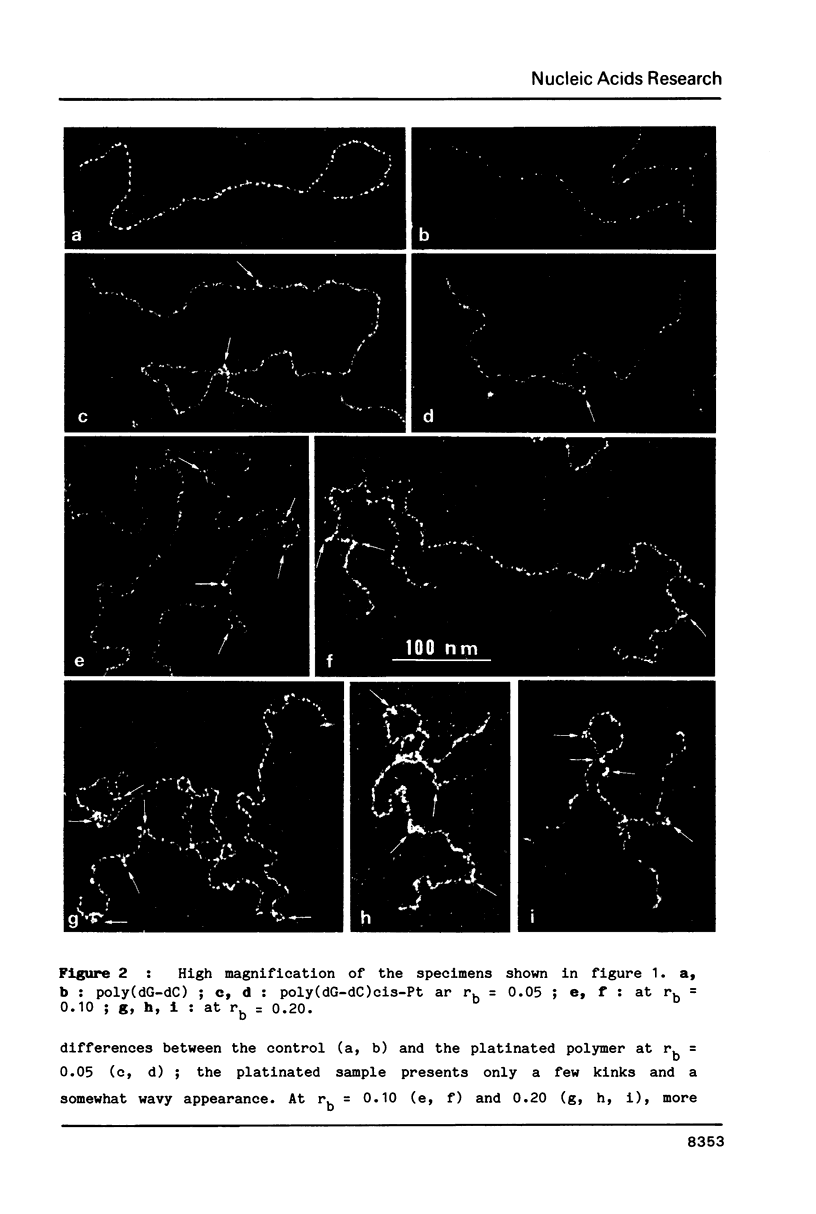
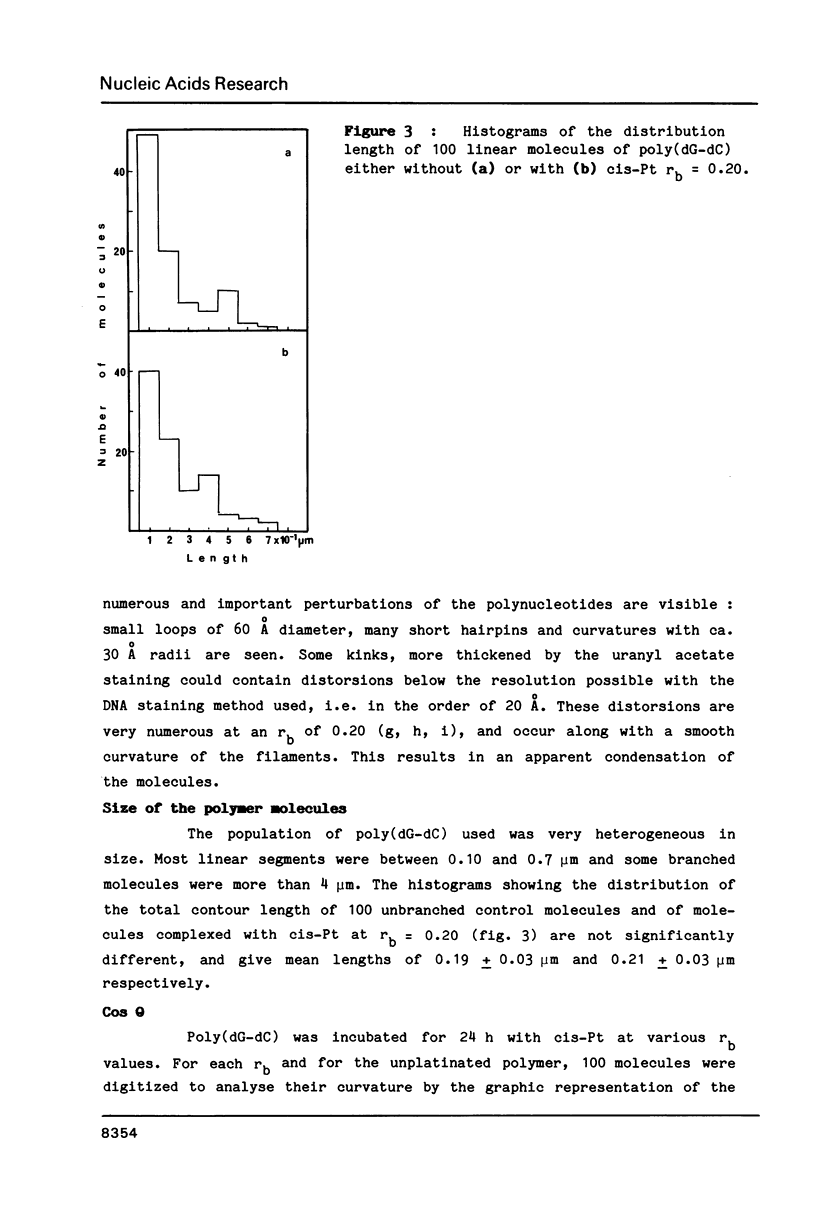
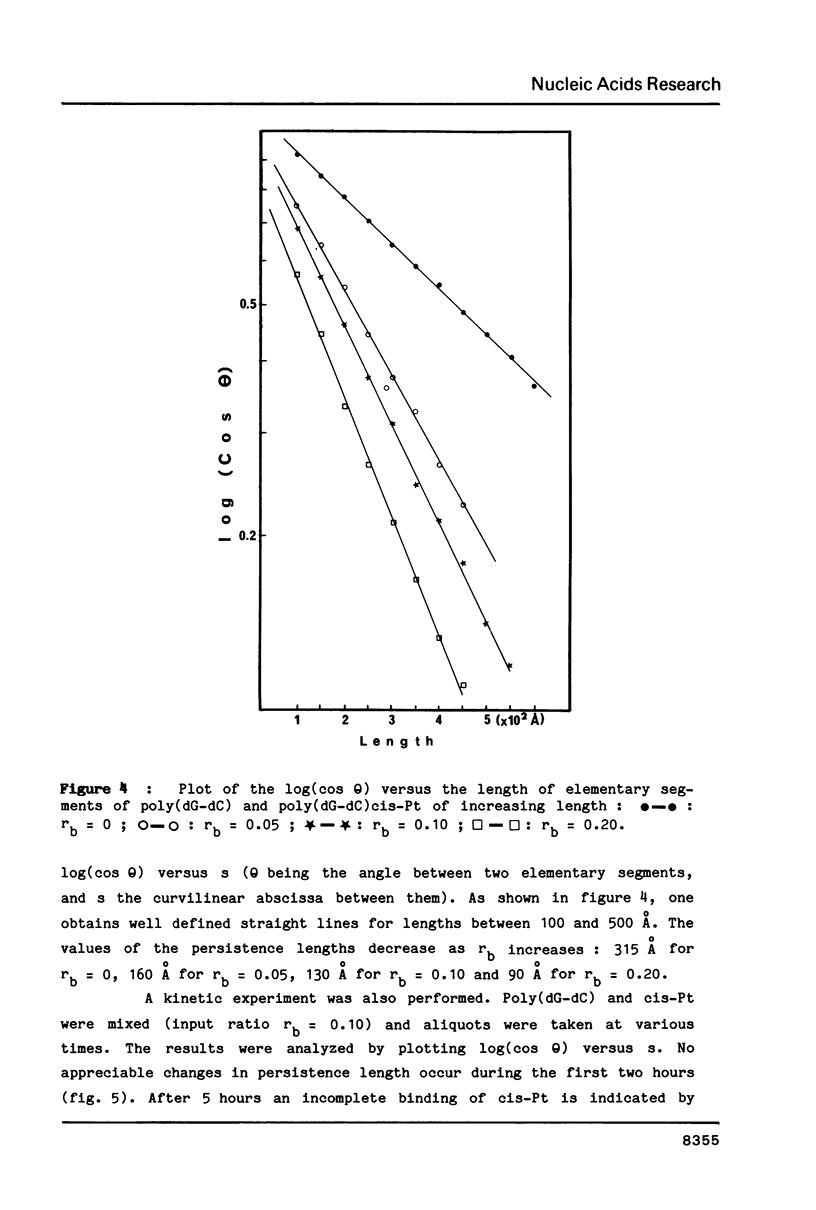



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behe M., Felsenfeld G. Effects of methylation on a synthetic polynucleotide: the B--Z transition in poly(dG-m5dC).poly(dG-m5dC). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1619–1623. doi: 10.1073/pnas.78.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castleman H., Erlanger B. F. Electron microscopy of "Z-DNA". Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):133–142. doi: 10.1101/sqb.1983.047.01.018. [DOI] [PubMed] [Google Scholar]
- Cohen G. L., Bauer W. R., Barton J. K., Lippard S. J. Binding of cis- and trans-dichlorodiammineplatinum(II) to DNA: evidence for unwinding and shortening of the double helix. Science. 1979 Mar 9;203(4384):1014–1016. doi: 10.1126/science.370979. [DOI] [PubMed] [Google Scholar]
- Dubochet J., Ducommun M., Zollinger M., Kellenberger E. A new preparation method for dark-field electron microscopy of biomacromolecules. J Ultrastruct Res. 1971 Apr;35(1):147–167. doi: 10.1016/s0022-5320(71)80148-x. [DOI] [PubMed] [Google Scholar]
- Frontali C., Dore E., Ferrauto A., Gratton E., Bettini A., Pozzan M. R., Valdevit E. An absolute method for the determination of the persistence length of native DNA from electron micrographs. Biopolymers. 1979 Jun;18(6):1353–1373. doi: 10.1002/bip.1979.360180604. [DOI] [PubMed] [Google Scholar]
- Hofmann H., Voss T., Kühn K., Engel J. Localization of flexible sites in thread-like molecules from electron micrographs. Comparison of interstitial, basement membrane and intima collagens. J Mol Biol. 1984 Jan 25;172(3):325–343. doi: 10.1016/s0022-2836(84)80029-7. [DOI] [PubMed] [Google Scholar]
- Malfoy B., Hartmann B., Leng M. The B goes to Z transition of poly(dG-dC) . poly(dG-dC) modified by some platinum derivatives. Nucleic Acids Res. 1981 Nov 11;9(21):5659–5669. doi: 10.1093/nar/9.21.5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinge J. M., Leng M. Reaction of cis-diamminedichloroplatinum (II) and DNA in B or Z conformation. EMBO J. 1984 Jun;3(6):1273–1279. doi: 10.1002/j.1460-2075.1984.tb01962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinge J. M., Ptak M., Leng M. Immunological and spectroscopic studies of poly(dG-dC).poly(dG-dC) modified by cis-diamminedichloroplatinum(II). Nucleic Acids Res. 1984 Jul 25;12(14):5767–5778. doi: 10.1093/nar/12.14.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mong S., Daskal Y., Prestayko A. W., Crooke S. T. DNA supercoiling, shortening, and induction of single-strand regions by cis-diamminedichloroplatinum(II). Cancer Res. 1981 Oct;41(10):4020–4026. [PubMed] [Google Scholar]
- Revet B., Delain E., Dante R., Niveleau A. Three dimensional association of double-stranded helices are produced in conditions for Z-DNA formation. J Biomol Struct Dyn. 1983 Dec;1(4):857–871. doi: 10.1080/07391102.1983.10507489. [DOI] [PubMed] [Google Scholar]
- Revet B., Delain E. The drosophila X virus contains a 1-microM double-stranded RNA circularized by a 67-kd terminal protein: high-resolution denaturation mapping of its genome. Virology. 1982 Nov;123(1):29–44. doi: 10.1016/0042-6822(82)90292-6. [DOI] [PubMed] [Google Scholar]
- Roberts J. J., Thomson A. J. The mechanism of action of antitumor platinum compounds. Prog Nucleic Acid Res Mol Biol. 1979;22:71–133. doi: 10.1016/s0079-6603(08)60799-0. [DOI] [PubMed] [Google Scholar]
- Thomas T. J., Bloomfield V. A. Chain flexibility and hydrodynamics of the B and Z forms of poly(dG-dC).poly(dG-dC). Nucleic Acids Res. 1983 Mar 25;11(6):1919–1930. doi: 10.1093/nar/11.6.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushay H. M., Santella R. M., Caradonna J. P., Grunberger D., Lippard S. J. Binding of [(dien)PtCl] Cl to poly(dG-dC)-poly(dG-dC) facilitates the B goes to Z conformational transition. Nucleic Acids Res. 1982 Jun 11;10(11):3573–3588. doi: 10.1093/nar/10.11.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider H. J., Sogo J. M., Koller T. A routine method for protein-free spreading of double- and single-stranded nucleic acid molecules. Proc Natl Acad Sci U S A. 1975 Jan;72(1):83–87. doi: 10.1073/pnas.72.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Sande J. H., McIntosh L. P., Jovin T. M. Mn2+ and other transition metals at low concentration induce the right-to-left helical transformation of poly[d(G-C)]. EMBO J. 1982;1(7):777–782. doi: 10.1002/j.1460-2075.1982.tb01247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]





