Abstract
Lung disease due to Mycobacterium avium complex (MAC) organisms is increasing. A greater understanding of the host immune response to MAC organisms will provide a foundation to develop novel therapies for these recalcitrant infections. IL-32 is a newly described pro-inflammatory cytokine that enhances host immunity against various microbial pathogens. Cytokines that induce IL-32 such as interferon-gamma, IL-18, IL-12 and tumor necrosis factor-alpha are of considerable importance to mycobacterial immunity. We performed immunohistochemistry and morphometric analysis to quantify IL-32 expression in the lungs of 11 patients with MAC lung disease and 10 controls with normal lung tissues. After normalizing for basement membrane length, there was a profound increase in IL-32 expression in the airway epithelial cells of the MAC-infected lungs compared with controls. Following normalization for alveolar surface area, there was a trend toward increased IL-32 expression in type II alveolar cells and alveolar macrophages in the lungs of MAC patients. Human airway epithelial cells (BEAS-2B) infected with M. avium produced IL-32 by a nuclear factor-kappa B-dependent mechanism. In both BEAS-2B cells and human monocyte-derived macrophages, exogenous IL-32γ significantly reduced the growth of intracellular M. avium. This finding was corroborated by an increase in the number of intracellular M. avium recovered from THP-1 monocytes silenced for endogenous IL-32 expression. The anti-mycobacterial effect of IL-32 may be due, in part, to increased apoptosis of infected cells. These findings indicate that IL-32 facilitates host defense against MAC organisms but may also contribute to the airway inflammation associated with MAC pulmonary disease.
Keywords: cytokine, immunohistochemistry, non-tuberculous mycobacteria
Introduction
Non-tuberculous mycobacteria (NTM) are opportunistic environmental pathogens that can cause devastating pulmonary, soft tissue and disseminated infections in humans (1). Mycobacterium avium complex (MAC) organisms, including M. avium hominissuis and Mycobacterium intracellulare, are the most common NTM to cause disease in humans (2). While MAC is not considered a species of NTM, we will use the acronym to describe disease caused by organisms within the complex. MAC gained notoriety as the cause of disseminated infections in patients with advanced acquired immunodeficiency syndrome. While the number of MAC cases in HIV-positive individuals has diminished considerably following the widespread use of highly active anti-retroviral therapy, NTM lung disease in seemingly immunocompetent hosts is an emerging public health problem. Unlike tuberculosis (TB), NTM lung disease is not a reportable illness, making it difficult to accurately calculate incidence (3, 4). From 1997 to 2003, the estimated annual incidence in Canada was two to four cases per 100 000 (5, 6). Around this same period (2000–03), the incidence of NTM lung disease in northern Manhattan was remarkably similar at approximately two cases per 100 000 (7). More recent studies from the USA estimate the incidence (from about 2004 to 2006) to be five to six cases per 100 000 (3, 8, 9). In some parts of the world, the incidence may be significantly higher (10). Based on various measures including skin test sensitivity to the M. intracellulare-derived Battey antigen (PPD-B) and increased rates of NTM isolation in clinical microbiology laboratories, there is compelling evidence that the incidence of NTM infections is rising and now surpasses that of TB in the USA (3, 10–13). Moreover, since the duration of NTM lung disease is often measured in years or decades, the prevalence of disease, estimated to be on the order of 10–40 cases per 100 000, is significantly higher than the incidence (6).
Following infection of the lungs with MAC organisms, airway epithelial cells, macrophages and dendritic cells are first responders to the invading bacilli. While activated phagocytes are capable of killing ingested mycobacteria, inactivated macrophages may permit growth of the organisms due to active bacillary inhibition of phagosome–lysosome fusion (14). It is well established that patients with defects in the interferon-gamma (IFNγ)- IL-12 signaling pathways are more susceptible to disseminated and extra-pulmonary NTM disease; however, there is controversy surrounding whether patients with isolated NTM lung disease have immune defects. Kim et al. (15) found that PBMC from patients with NTM lung disease produced similar levels of pro-inflammatory cytokines including IFNγ, IL-1, IL-6, IL-12 and tumor necrosis factor-alpha (TNFα) compared with control cells when stimulated with mitogens and cytokines. In contrast, others have found that PBMC isolated from pulmonary MAC patients stimulated with mitogens, anti-CD3 or lipopolysaccharide produced less IFNγ, TNFα and/or IL-12 compared with cells from healthy subjects (16–18). Similarly, PBMC from MAC patients stimulated with heat-killed M. avium showed suppressed levels of IFNγ, TNFα, IL-12 and IL-18 compared with similarly treated PBMC from healthy subjects with skin test positivity to M. avium sensitin (19). While the immune defects of patients with pulmonary NTM have yet to be clearly defined, it has been shown that IL-8, IL-17, IL-23, and more recently, IL-32, are important in the host protective immune response to TB (20–26).
The importance of IL-32 in the context of NTM infections has yet to be examined. IL-32 induces the production of pro-inflammatory cytokines such as TNFα, IL-1β, macrophage inflammatory protein-2 (MIP-2), IL-6 and IL-8 (25, 27–29). TNFα, IFNγ, IL-1β, IL-12 and IL-18 can also induce expression of IL-32 (27, 30). These cytokines are important mediators of the host immunity against NTM (31–36). Recently, IL-32γ has been shown to trigger maturation and activation of dendritic cells, key responders to mycobacterial infection in the lung (37). In addition, IL-32 is an important host defense molecule active against microbial pathogens including HIV, influenza and Mycobacterium tuberculosis (MTB) (25, 38–42). IL-32 has also been implicated in a number of inflammatory disorders (27–30, 43–46).
MAC pulmonary disease is often associated with devastating lung inflammation and tissue damage. Given the role of IL-32 in inflammation and TB infections, it is essential to examine the expression of IL-32 in MAC-infected lung tissues. We used immunohistochemistry and morphometric analysis to determine the expression and distribution of IL-32 in surgically removed lung tissues from MAC patients. In order to determine the relevance of IL-32 expression levels in MAC patient lung tissues, we also examined the role of IL-32 in M. avium infection of airway epithelial cells and macrophages in vitro.
Methods
Materials
Human THP-1 monocytic cells and BEAS-2B human bronchial epithelial cells were obtained from the American Type Culture Collection (Rockville, MD, USA). For cell culture medium, RPMI 1640 was purchased from Cambrex (East Rutherford, NJ, USA), fetal bovine serum (FBS) from Atlanta Biologicals (Norcross, GA, USA) and L-glutamine and phorbal 12-myristate 13-acetate (PMA) from Sigma–Aldrich (St Louis, MO, USA). Affinity-purified anti-IL-32α mouse monoclonal antibody used for immunohistochemistry was kindly provided by Dr Soo-Hyun Kim (University of Colorado Anschutz Medical Campus) (27). Reagents used to make Middlebrook 7H9 broth medium and 7H10 solid agar medium to grow M. avium were obtained from Difco (Detroit, MI, USA). The p38mapk inhibitor (SB203580), MEK1 inhibitor [PD98059, MEK1 being the upstream kinase of extracellular-signal regulated kinase (ERK)] and the IκBα kinase-nuclear factor-kappa B (NF-κB) inhibitor (BAY 11-7082) were purchased from Calbiochem (San Diego, CA, USA), New England Biolabs (Beverly, MA, USA) and Biomol Research Laboratories (Plymouth Meeting, PA, USA), respectively.
Lung tissue collection and preparation
After approval by the Institutional Review Board at National Jewish Health (NJH), fixed lung tissues were obtained from 11 patients diagnosed with MAC lung disease who had undergone pulmonary resection. The main indication for lung surgery was severe localized disease that was recalcitrant to antibiotic treatment. The clinical staff recommended surgery following extensive pre-operative evaluations. Recovered lung tissues were fixed with formalin and embedded in paraffin. Ten similarly preserved histologically normal lung tissues from organ donors were obtained post-mortem. All control donors had previously consented for organ research and had died from stroke or head trauma. One block per patient was randomly selected for analysis and all sections were stained with hematoxylin and eosin.
Immunohistochemistry of lung sections
Slides bearing lung sections were deparaffinized and labeled for IL-32 using the En Vision horseradish peroxidase detection system (DAKO) as described previously (43). Briefly, sections were treated with 10 mM sodium citrate (pH 6.0) for antigen retrieval and 3% hydrogen peroxide to block staining from endogenous peroxidase reactions. Sections were then incubated sequentially with the primary anti-IL-32α antibody (1:5000 dilution) for 30 min, the En Vision complex for 30 min and the En Vision chromogen reagent for 5 min with buffer rinse of the sections in between. Sections were counterstained with 1% methyl green and mounted using cover glass. As a negative control, tissue sections were stained using a non-immune mouse IgG isotype antibody.
Photomicrographic sampling of tissues
Photographic fields from the lung sections were selected using a uniform random sampling scheme with a motorized sampling stage controlled by the StagePro software (Media Cybernatics). Each slide was scanned to determine the total section area. In order to study the distribution of the various anatomical regions in the lung samples, slides were surveyed using the ×4 objective lens. Photographs were taken with a fixed interval of fields (determined by the size of the section, the size of the photographic field at the magnification used and the desired number of pictures), but with a random start (random number generated between zero and the numeral of sampling interval) from the upper left corner of the section. An average of 15 micrographs were obtained from each section. The same process was performed for sampling of the immunolabeled sections to determine the distribution of IL-32 expression, except that a ×20 lens was used and an average of 40 micrographs were obtained from each section. Airways were photographed in their entirety by overlapping micrographs.
Morphometric analysis
The morphometric methods used in this study have been detailed previously (47). Point count—an overlay of 13 × 10 lines—was superimposed on each micrograph. For characterization of the tissue sections, points on airways (including lumina, connective tissue, cartilage, mucous glands, etc.), vessels (including vessel lumen, intima, media, etc.), parenchyma (including alveolar airspace, alveolar septa, airspace cells) and granulomas were tallied for all micrographs of one specimen. The percent area of the section occupied by each anatomical region was calculated. Points positively labeled for IL-32 were counted and categorized according to location, e.g. airway epithelia, type II alveolar cells, alveolar macrophages or granulomas. Percent of IL-32 expression was calculated for each tissue type. Additionally, the volume density (Vv) of IL-32-positive airway epithelium, type II alveolar cells and alveolar macrophages were determined Vv = Pa/PT, where Pa = total number of points of interest and PT = total number of points counted for one section).
The surface densities (Sv) of alveolar epithelium and airway basement membrane were estimated by intercept counting. A set of 10 horizontal lines was overlaid on each micrograph. The number of times the test line intercept with the surface of interest was recorded. Sv was calculated by the formula Sv = 2Ia/LT, where Ia denotes intercepts with the surface of interest and LT signifies the total test line length.
The level of specific cell labeling of IL-32 was determined by dividing the Vv of IL-32 labeling on the cell type of interest with the related Sv (airway basement membrane for airway epithelium; alveolar surface area for type II cells and alveolar macrophage). Normalization of Vv by Sv was used to correct for overestimation of IL-32 staining due to atelectasis or changes in the diameter of the airway.
Culture and infection of BEAS-2B airway epithelial cells
BEAS-2B cells, an adenovirus 12-SV40 hybrid of a transformed human bronchial epithelial cell line, were cultured at 37°C, 5% CO2 in 100 cm2 tissue culture plates in RPMI 1640 medium containing 10% heat-inactivated FBS and 2 mM L-glutamine. Cells were utilized between 1 and 15 passages for all experiments. Cells were plated and grown to 90% confluence before infection with live M. avium.
Culture and differentiation of THP-1 cells
THP-1 cells were cultured in RPMI 1640 containing 10% heat-inactivated FBS and 2 mM L-glutamine. The cells were plated at a concentration of 5 × 105 cells per well of a 24-well flat-bottom tissue culture plate and incubated overnight (37°C, humidified, 5% CO2) in 15 ng/ml PMA in order to allow the cells to differentiate and adhere to the plate. After overnight incubation, PMA-containing medium was removed and replaced with fresh PMA-free medium (RPMI 1640 + 10% FBS + 2 mM glutamine).
Knockdown of IL-32 in THP-1 cells using stable expression of short-hairpin RNA-IL-32 sequences
Using short-interfering RNA (siRNA) technology, wild-type THP-1 cells were knocked down for IL-32γ by stable transfection with a plasmid that contained targeted sense and anti-sense sequences of the IL-32γ gene as well as the PUROMYCIN-RESISTANT gene, as described previously (25). The transfected cells encode for specific short-hairpin RNA (shRNA) sequences that contain anti-sense sequences to IL-32γ mRNA. THP-1 cells were used because they behave similarly to primary human macrophages in terms of immune responses to MTB (48–50). We created three stable THP-1 clones (clones 5, 6 and 7) knocked down for IL-32 expression as confirmed by reverse transcription (RT)–PCR and western blot analysis for IL-32 mRNA and protein (25). The THP-1 clone resulting in the greatest inhibition of IL-32 protein expression (clone 7) was used for the in vitro experiments. Wild-type THP-1 cells and cells transfected with a plasmid that encoded for a scrambled siRNA sequence were used as negative controls.
Isolation of human blood monocytes and differentiation into monocyte-derived macrophages
The study design and consent procedure were approved by the NJH Institutional Review Board. After informed consent was obtained, venous blood was collected in CPT vacutainer tubes (cell preparation tubes with sodium citrate; Becton Dickinson, Franklin Lakes, NJ, USA) and centrifuged at 1800 × g for 30 min at 25°C. The cloudy monolayer containing mononuclear cells was removed, re-suspended in sterile 1× PBS and centrifuged at 300 × g for 10 min at 25°C to pellet the cells. The cells were washed again in PBS, centrifuged at 300 × g for 10 min at 25°C to pellet the cells and re-suspended in RPMI medium supplemented with 10% FBS. Cells were counted, plated in a 24-well plate (∼3 × 106 cells per well), and 20 ng/ml of granulocyte macrophage colony-stimulating factor was added to each well. Monocytes were incubated overnight at 37°C and ambient 5% CO2 in order to allow for cellular adherence. After overnight incubation, non-adherent cells were removed and fresh medium (RPMI + 10% FBS) was added to the remaining cells. The human monocyte-derived macrophages (hMDM) were incubated for an additional 2 h before infection with M. avium.
Infection of BEAS-2B airway epithelial cells, THP-1 macrophages and hMDM with M. avium
M. avium 9141, a patient isolate, was kindly provided by Dr Leonid Heifets (NJH, Denver, CO, USA). M. avium was grown in liquid Middlebrook 7H9 supplemented with albumin dextrose complex (Remel) and incubated under rotation at 37°C over the period of 1 week. At late log phase, aliquots were adjusted to 1 McFarland (1 × 108 bacilli/ml), frozen and stored at −70°C until use.
THP-1 macrophages, hMDM and BEAS-2B cells were separately infected with M. avium at a multiplicity of infection (MOI) of 10 bacilli to 1 cell. After 1 h of incubation at 37°C and 5% CO2, the cells were washed twice with a 1:1 mixture of RPMI 1640:1× PBS. Adherent cells were lysed with 250 μl of 0.25% SDS for 5 min and lysis was confirmed visually using an inverted microscope. An equal amount (250 μl) of 7H9 plating solution (7H9 + OADC enrichment + Tween 80) was added to each lysed well in order to neutralize the SDS. Lysates were serially diluted in 7H9 broth and 5 μl of the diluted cell lysates were plated on solid 7H10 agar. The plates were incubated for ∼1 week at 37°C prior to determination of colony forming units (CFU) of M. avium. Cells incubated for an additional 2, 4 or 8 days were also washed twice following the 1-h infection followed by the addition of fresh medium to each well. The remaining cells were incubated for an additional 2, 4 or 8 days prior to lysing the cells and plating of M. avium-containing lysates on 7H10 agar. At all time points except for day 0, the cell culture supernatant was centrifuged at 600 × g for 5 min in order to pellet non-adherent cells. The cell monolayer in the well was subsequently lysed and the lysate was added to the pellet after decanting the supernatant. This whole cell lysate was used for serial dilution and plating of mycobateria.
RNA isolation and RT–PCR
BEAS-2B cells were plated into each well of six-well tissue culture plates (1 × 106 cells per well). Total RNA was extracted using the TRIzol reagent, and cDNA was prepared using reverse transcriptase, according to the manufacturer’s instructions (Invitrogen Life Technologies) and as previously described (25). Single-stranded cDNA products were used as templates in a 30 μl PCR amplification reaction according to the instructions of the Iscript cDNA Synthesis Kit and the PCR kit (Bio-Rad, Hercules, CA, USA). The following primers were used: IL-32 sense, 5′-GTGGACAGGTGAGTCGAGC-3′ and IL-32 anti-sense primer: 5′-GCCTGCACTGCATGGACCAG-3′. GAPDH sense primer: 5′-TCCATGACAACTTTGGTATCGTG-3′ and the GAPDH anti-sense: 5′-TGTCGCTGTTGAAGTCAGAGGA-3′. Amplifications were done by initial denaturation at 94°C for 4 min, followed by 30 cycles of amplification (94°C for 30 s, 55°C for 45 s, 72°C for 1 min), terminated at 72°C for 4 min and kept at 4°C until use. PCR products were separated on 1.5% agarose gels containing ethidium bromide.
Western blotting
Briefly, BEAS-2B cells were lysed using a phosphotyrosine lysis buffer that contains 50 mM Tris–HCl (pH 8.0), 137 mM NaCl, 10% (v/v) glycerol, 1% (v/v) NP-40, 2 mM Na3VO4, 1mM NaF, 1mM PMSF, 2 mg/ml leupeptin and 2 mg/ml aprotinin. The cell lysates were vortexed, sonicated and centrifuged at 10 000 × g for 10 min at 4°C. Protein concentrations of the cell lysates were determined using the Bradford protein assay (Bio-Rad). The lysate supernatants were mixed with equal volumes of 2× Laemmli sample buffer containing 40 mM dithiothreitol and boiled for 5 min. Twenty micrograms of protein for each condition were resolved by 12% SDS–PAGE and transferred onto a nitrocellulose membrane (Schleicher and Schuell, Bioscience GmbH, Dassel, Germany). After the membranes were blocked in 5% milk for 1 h, they were probed with IL-32α antibody at a dilution of 1:500 (v/v), followed by detection with HRP-conjugated anti-rabbit IgG (1:2000 dilution). Bands were visualized by chemiluminescence using the HRP western Blot Detection System (Cell Signaling Technology) as described in the manufacturer’s protocol.
TUNEL assay
Control (scrambled shRNA-transfected) and shRNA-IL-32γ transfected THP-1 cells and BEAS-2B cells were plated in each well of a four-chamber culture slide, followed by differentiation with PMA of the THP-1 cells and infection of both cell types with M. avium as described previously (51). For each condition, triplicate slides were prepared. The in situ cell death detection kit (Roche) was used to assay for apoptosis at the single cell level based on labeling of DNA strand breaks and followed according to manufacturer’s instructions and as previously described (25). Enumeration of apoptotic cells was performed by light microscopy, counting ∼5000 cells per condition per experiment.
Statistical analysis
Values for all measurements are expressed as mean ± SEM unless otherwise specified, and all statistical analyses were performed using GraphPad Prism software. Percent distribution of tissues and the differential expression of IL-32 within various cell types were compared between the control and MAC patient groups by the unpaired, two-tailed Student's t-test. For the CFU data, groups were compared using a two-way analysis of variance and Bonferroni’s post-hoc test. Differences were considered significant when P ≤ 0.05.
Results
Patient population
The 11 MAC patients were predominantly female (82%) and Caucasian (91%). The mean age ± SD at the time of surgery was 66 ± 9.5 years. Five of the 11 (45%) were never-smokers, five (45%) were former smokers and one (10%) was a current smoker. The 10 control patients who donated normal lung tissues were predominantly male (70%) and all were Caucasians. The mean age ± SD of the control donors at the time of death was 45.5 ± 14.6 years. All control patients were HIV negative and had no prior history of respiratory disease. Of the 10 control donors, 7 (70%) had a history of smoking, 1 (10%) chewed tobacco and 2 (20%) were never smokers.
Distribution of tissue types
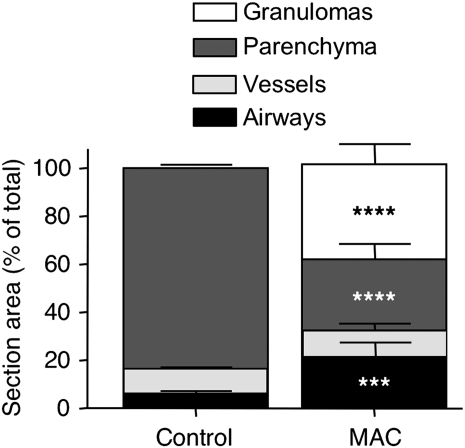
There was a differential distribution of tissue types in the lungs of controls as compared with MAC patients (Fig. 1). Control lungs were predominantly comprised of parenchymal tissue (83.5 ± 1.7%), with a smaller contribution by airways (6.2 ± 1.4%) and vessels (10.3 ± 1.0%) (Fig. 1). As expected, granulomas were absent from control lung tissues. The lung tissues from MAC patients had proportionately less parenchyma (29.6 ± 6.5%), likely due to the fact that nearly 40% of the ‘resected’ lung tissue was granulomatous (Fig. 1). In the MAC patients, there was significantly more area designated as airways, probably due to a combination of dilated airways, i.e. bronchiectasis and bronchioloectasis, as well as greater cellular inflammation in the airway walls compared with control lungs (Fig. 1).
Fig. 1.
Distribution of tissue types in normal and MAC-infected lung samples. Data shown are the mean percent section area ± SEM. n = 10 for histologically normal lungs and n = 11 for MAC lung samples. Parenchyma, vessel and airway designations include airspaces and luminae. ****P < 0.0001, ***P < 0.001 compared with the corresponding tissue in the histologically normal lungs (control).
Distribution of IL-32-positive cell types in control and MAC-infected lungs
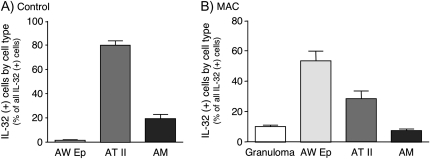
In control lungs, type II alveolar cells accounted for the greatest percentage (78%) of all identified IL-32-positive cells, followed by alveolar macrophages (20%) and airway epithelial cells (∼2%) (Fig. 2A). The relative number of IL-32-positive airway epithelial cells in normal lung tissues was relatively low (Fig. 2A). In MAC-infected lungs, airway epithelial cells accounted for 53% of all IL-32-positive cells, followed by type II alveolar cells (30%), cells in the granulomas (10%) and alveolar macrophages (7%) (Fig. 2B).
Fig. 2.
The percent contribution of specific IL-32-positive cell types proportionate to all IL-32-positive cells. Data shown are the distribution (mean percentage ± SEM) of each cell type expressing IL-32 compared with total IL-32-positive cells for (A) normal lungs (n = 10) and (B) MAC lungs (n = 11). AW Ep = airway epithelial cells; AT II = type II alveolar cells; AM = alveolar macrophages.
IL-32 expression is markedly increased in airway epithelial cells of MAC patients
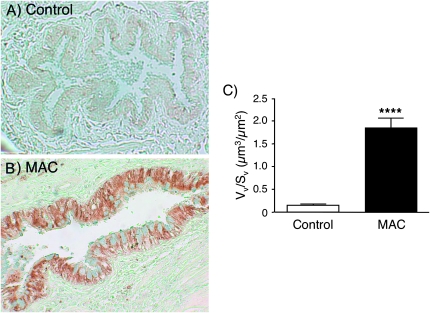
We quantified the distribution of IL-32 within the different cell types of control and MAC lung tissues by morphometric analysis, beginning with airway epithelial cells. Whereas there was minimal IL-32 staining within the airway epithelial cells of control lungs (Figs 2A and 3A), there was robust IL-32 expression in these cells in the MAC lung tissues (Figs 2B and 3B). The increased IL-32 expression in airway epithelial cells of the MAC lungs was significant after normalization for the length of the epithelial basement membrane (Fig. 3C).
Fig. 3.
Relative expression of IL-32 in airway epithelial cells in normal and MAC-infected lung samples. (A) Representative normal lung showing little or no IL-32 staining in the airway epithelium. (B) Representative lung tissue sample of a patient with MAC pulmonary disease showing widespread IL-32 staining in the airway epithelial cells. (C) Quantification of IL-32 staining by morphometric analysis, normalized to the length of epithelial basement membrane. Mean Vv/Sv ± SEM for 10 normal lungs = 0.1477 ± 0.0330. Mean Vv/Sv ± SEM for 11 MAC lungs = 1.8440 ± 0.2214. Vv/Sv denotes the ratio of the volume density to the surface density. ****P < 0.0001 compared with control lungs.
IL-32 is expressed in type II alveolar cells in control and MAC lungs
Human type II alveolar cells were one of the first cell types described to express IL-32 (27). Immunohistochemical analysis revealed that IL-32 was present in type II alveolar cells of both normal and MAC-diseased lungs (Fig. 2). Although the ‘proportion’ of IL-32-positive type II alveolar cells compared with all cell types was actually lower in the MAC patients (Fig. 2A versus Fig. 2B), the ‘number’ of type II alveolar cells expressing IL-32 was visibly increased in MAC lungs compared with the normal lungs (Fig. 4B and C versus Fig. 4A). This finding was corroborated by morphometric analysis showing a trend toward increased IL-32 expression in type II alveolar cells of lungs infected with MAC following normalization for the alveolar surface area (Fig. 4D).
Fig. 4.
Relative expression of IL-32 in type II alveolar cells in normal and MAC-infected lung samples. Immunohistochemical staining of IL-32 in type II alveolar cells (arrows) of representative (A) normal and (B and C) two MAC-diseased lungs. (D) Quantification of IL-32 staining of type II alveolar cells following morphometric analysis, normalized to the alveolar surface area. Mean Vv/Sv ± SEM for 10 normal lungs = 0.0607 ± 0.0105. Mean Vv/Sv ± SEM for 11 MAC lung samples = 0.0917 ± 0.0138. Vv/Sv denotes the ratio of the volume density to the surface density.
IL-32-positive alveolar macrophages are modestly increased in MAC patients
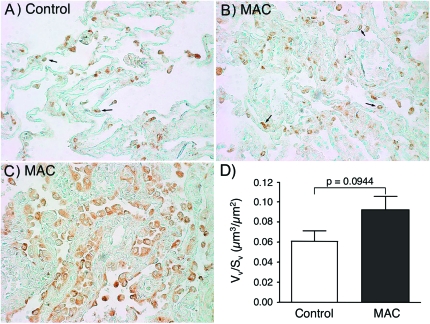
IL-32 was also detected in the alveolar macrophages of both control and MAC diseased lungs (Fig. 2). While the proportion of IL-32-positive alveolar macrophages compared wth all cell types was greater in normal lung samples (Fig. 2A versus Fig. 2B), the number of IL-32-positive alveolar macrophages was visibly greater in the inflamed MAC-infected lungs (Fig. 5B and C versus Fig. 5A). This finding was confirmed by morphometric analysis following normalization for alveolar surface area, wherein there was an increase in the number of IL-32-positive macrophages in the lungs of MAC patients, a difference that approached statistical significance (Fig. 5D).
Fig. 5.
Relative expression of IL-32 in alveolar macrophages in normal and MAC-infected lung samples. Immunohistochemical staining of IL-32 in alveolar macrophages (arrows) of representative (A) normal lung and (B and C) two MAC-diseased lungs. (C) A cluster of alveolar macrophages (arrow) from a MAC lung tissue sample. (D) Quantification of IL-32 staining by morphometric analysis, normalized for the alveolar surface area. Mean Vv/Sv ± SEM for 10 normal lungs = 0.0313 ± 0.0053. Mean Vv/Sv ± SEM for 11 MAC lungs = 0.0494 ± 0.0069. Vv/Sv denotes the ratio of the volume density to the surface density.
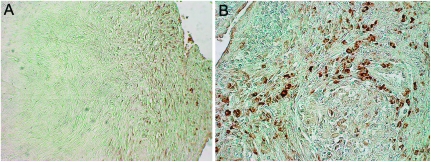
IL-32 is expressed in granulomas of MAC lung disease
Intact, well-formed lung granulomas are considered to help contain mycobacteria during chronic infections. We qualitatively determined the expression of IL-32 in the granulomas of MAC-diseased lungs. IL-32 expression was found in the periphery of the granulomas, as shown by representative lung tissue sections of two MAC patients (Fig. 6A and B). There was no detectable IL-32 in the central necrotic regions of the granulomas.
Fig. 6.
Expression of IL-32 in lung granulomas of patients with pulmonary MAC disease. (A) Representative lung tissue from a patient with MAC pulmonary disease showing modest staining for IL-32 in cells located along the periphery of a granuloma, with no staining centrally. (B) A granuloma from another MAC patient showing increased IL-32 staining confined to the non-necrotic periphery.
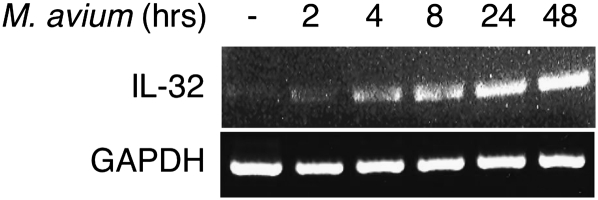
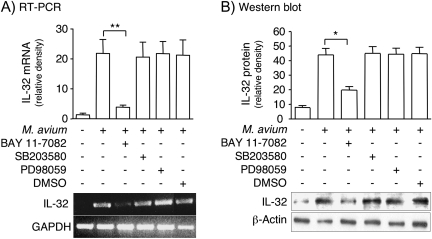
M. avium induces IL-32 production in airway epithelial cells by NF-κB activation
Since IL-32 was highly expressed in the airway epithelial cells of MAC-diseased lungs, we examined whether M. avium induces IL-32 expression in a human bronchial epithelial cell line (BEAS-2B). BEAS-2B cells were infected with M. avium for 2–48 h, total RNA isolated and RT–PCR performed with sense and anti-sense primers for IL-32. M. avium infection induced the production of IL-32 mRNA, with the strongest induction occurring after 48 h of stimulation (Fig. 7). Because MAC organisms have been shown to activate the mitogen-activated protein kinase (MAPK) and IκBα kinase-NF-κB signaling pathways (52, 53), we investigated whether these pathways are involved in the induction of IL-32 by M. avium using pertinent inhibitors. BEAS-2B cells were incubated for 1 h with either 30 μM SB203580 (a p38mapk inhibitor), 30 μM PD98059 (an inhibitor of the upstream kinase of ERK), 10 μM BAY 11-7082 (inhibitor of IκBα kinase) or 0.075% (v/v) of the vehicle DMSO before infection with M. avium at a MOI of 10:1 for 24 h. Blocking IκBα kinase, the kinase which is essential for NF-κB activation, significantly inhibited M. avium-induced IL-32 mRNA expression, whereas inhibition of p38mapk and ERK pathways had no effect (Fig. 8A). To determine whether IL-32 protein was induced by M. avium and altered by the inhibitors, we performed similar experiments in which BEAS-2B cells were infected for 24 h in the absence or presence of the same inhibitors, followed by immunoblotting of the nuclear-free whole cell lysates for IL-32. As shown in Fig. 8B, M. avium induced IL-32 protein production was significantly attenuated following the addition of BAY 11-7082, whereas inhibiting p38mapk and ERK had no significant effect. We conclude that M. avium infection induces IL-32 production in human airway epithelial cells and that the NF-κB signaling pathway is an important mediator of this induction.
Fig. 7.
IL-32 mRNA production in BEAS-2B cells infected with M. avium. BEAS-2B cells were infected with M. avium at the indicated times and RT–PCR performed using primers for IL-32. Data shown are representative of three independent experiments.
Fig. 8.
IL-32 mRNA and protein production in BEAS-2B cells infected with M. avium. (A) BEAS-2B cells were infected with M. avium with or without MAPK and IκBα kinase inhibitors and RT-PCR performed using primers for IL-32. RT–PCR image shown is representative of three independent experiments. The bar graph represents the mean density ± SD of the IL-32 product; **P < 0.01. (B) BEAS-2B cells were infected with M. avium in the presence or absence of MAPK and IκBα kinase inhibitors. Whole-cell lysates were prepared, separated by SDS–PAGE and immunoblotted for IL-32α. The western blot shown is representative of three independent experiments. The bar graph represents the mean density ± SD of the IL-32α immunoband; *P < 0.05.
IL-32γ modestly inhibits the growth of intracellular M. avium in BEAS-2B cells
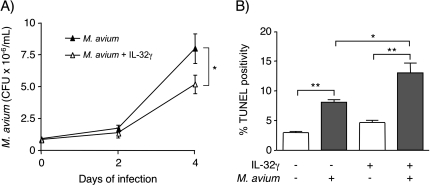
Previous studies have found that mycobacteria can infect airway epithelial cells (54–57). To determine whether exogenous IL-32 inhibits mycobacterial growth within human bronchial epithelial cells, BEAS-2B cells were infected with M. avium for 1 h (day 0), 2 or 4 days with or without 50 ng/ml IL-32γ, the IL-32 isoform with the greatest biological activity (25, 58). After the indicated times of infection, the cells were washed to remove extracellular mycobacteria and lysed. The lysates were serially diluted and cultured to quantify intracellular M. avium. BEAS-2B cells were successfully infected with M. avium and IL-32 significantly reduced the number of intracellular bacilli after 4 days of infection (Fig. 9A).
Fig. 9.
Effect of exogenous IL-32γ on the growth of intracellular M. avium in BEAS-2B airway epithelial cells. (A) BEAS-2B cells were infected with M. avium with or without 50 ng/ml IL-32γ. The number of cell-associated M. avium were quantified after 1 h (0 day), 2 and 4 days post-infection. Value are means ± SEM of two independent experiments; *P < 0.05. (B) BEAS-2B cells were stimulated with 50 ng/ml IL-32γ, infected with M. avium or both for 24 h. The amount of apoptosis was measured by quantifying percent TUNEL positivity and reported as the percentage of 5000 total cells per condition from three separate experiments (mean ± SEM); *P < 0.05, **P < 0.01.
Previous studies suggest that IL-32 induces apoptosis, which is known to inhibit the growth of intracellular mycobacteria (59–62). We measured induction of apoptosis following the addition of exogenous IL-32γ to M. avium-infected BEAS-2B cells using the TUNEL assay. As shown in Fig. 9(B), there was no significant increase in apoptosis after treating uninfected with IL-32γ. Following M. avium infection, there was a significant increase in apoptosis, which was further increased following the addition of 50 ng/ml IL-32γ (Fig. 9B). We conclude from these studies that IL-32γ modestly inhibits the proliferation of intracellular M. avium in airway epithelial cells. IL-32γ enhanced apoptosis of infected cells, suggesting that the decrease in cell-associated CFU recovered following incubation with IL-32 may be partially due to increased apoptosis (25).
Silencing endogenous IL-32 increases the intracellular burden of M. avium
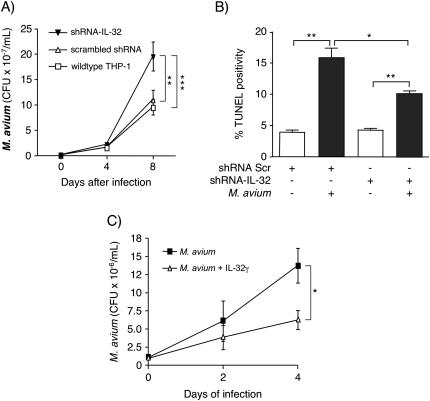
To corroborate the findings of the epithelial cell experiments, we infected THP-1 macrophages that were silenced for IL-32 expression by siRNA technology (25). Wild-type THP-1 cells, THP-1 cells transfected with a scrambled shRNA sequence and THP-1 cells transfected with shRNA-IL-32γ were infected with M. avium for 1 h (day 0), 4 or 8 days. After the indicated times of infection, the cells were washed, lysed and intracellular M. avium quantified. The number of intracellular M. avium was significantly increased after 8 days of infection in cells lacking IL-32 expression compared with the control THP-1 cells (Fig. 10A). Since cells lacking IL-32 were more burdened by mycobacteria, we conclude that endogenous IL-32 inhibits the proliferation of intracellular M. avium.
Fig. 10.
Effect of endogenous IL-32 on the growth of intracellular M. avium in human macrophages. (A) Wild-type THP-1 cells (open squares), THP-1 cells transfected with a scrambled shRNA (open triangles) or shRNA–IL-32 (clone 7) (closed triangles) were infected with M. avium. Intracellular burden of M. avium was quantified 1 h (0 days), 4 and 8 days after infection. Data represent mean ± SEM of two representative experiments, **P < 0.01, ***P < 0.001. (B) The amount of apoptosis was measured after 24 h of incubation by quantifying percent TUNEL positivity and reported as the percentage of 5000 total cells per condition from three separate experiments (mean ± SEM); *P < 0.05, **P < 0.01. (C) Human MDM cells were infected M. avium with or without 50 ng/ml IL-32γ. The number of cell-associated M. avium was quantified after 1 h (0 day), 2 and 4 days post-infection. Values are mean ± SEM from two independent experiments; *P < 0.05.
We previously reported that there was partial but significant abrogation of MTB-induced apoptosis in THP-1 cells silenced for IL-32 and infected with MTB (25). To determine if this is relevant for M. avium infection, we quantified apoptosis using the same cell conditions described above. Silencing IL-32 expression partially but significantly abrogated the increase in cell death seen in cells with intact IL-32 production following M. avium infection (Fig. 10B).
To determine whether exogenous IL-32γ inhibits the growth of intracellular M. avium in primary macrophages, hMDM were infected with M. avium for 1 h (day 0), 2 or 4 days with or without 50 ng/ml IL-32γ. At the indicated times following infection, the cells were lysed and M. avium cultured and quantified. The number of intracellular M. avium was significantly less after 4 days of infection in cells pre-treated with IL-32γ (Fig. 10C). These in vitro studies indicate that IL-32 facilitates host protective immunity against M. avium infection in airway epithelial cells and human macrophages.
Discussion
The long-term success rate in the treatment of pulmonary MAC remains relatively low (13). A greater understanding of the host immune response to MAC organisms may lead to novel treatment approaches that enhance protective immunity. IL-32 is increasingly recognized to possess a panoply of biological effects and is produced by a wide variety of cell types (27, 45). IL-32 is induced following stimulation by cytokines (IFNγ, IL-12, IL-18 and TNFα) and infection with a variety of pathogens (25–27, 30, 38–42). In turn, IL-32 induces production of pro-inflammatory cytokines and chemokines including TNFα, IL-1β, IL-6, IL-8 and MIP-2 (25, 27).
IL-32 has been implicated in the pathogenesis of chronic obstructive pulmonary disease (COPD) and of autoimmune disorders such as rheumatoid arthritis and non-familial forms of Crohn’s disease (30, 43, 44, 46, 63). While IL-32 contributes to the pathogenesis of the aforementioned disorders, it facilitates the host immune response against HIV (38, 42), influenza (39, 40) and MTB (25). One mechanism by which IL-32 antagonizes intracellular MTB in macrophages is through induction of caspase-3-dependent apoptosis although caspase-3-independent mechanisms are likely involved (25).
Given the significance of IL-32 in host defense against intracellular pathogens including MTB, we examined the expression of IL-32 in lung tissues of MAC patients. It is important to emphasize that the surgically resected lung tissues were severely diseased, as evinced by radiographically identified end-stage bronchiectasis, atelectasis and dense consolidation and by microscopically evident airway and parenchymal granulomatous inflammation. Thus, to find that only 30% of the resected MAC lung specimens were comprised of ‘parenchyma’ is not surprising. After visually identifying IL-32 within various cell types by immunohistochemistry, we quantified IL-32 expression using morphometric analysis. In normal lung tissues, low levels of IL-32 were observed in type II alveolar cells and to a lesser extent in alveolar macrophages. In MAC-infected lungs, there was a pronounced increase in the expression of IL-32 within airway epithelial cells. In addition, there was a trend toward an increase in the absolute number of IL-32-positive type II alveolar cells and macrophages in the lungs of the MAC patients. This is likely due to type II cell hyperplasia and influx of alveolar macrophages in response to the chronic MAC infection. IL-32-positive cells were also found in the periphery of MAC-induced lung granulomas. Since IL-32 induces TNFα (25, 27), a cytokine critical in maintaining granuloma integrity, we speculate that IL-32 may contribute to the maintenance of intact mycobacterial granulomas, lesions considered important in preventing mycobacterial dissemination (64, 65).
To the best of our knowledge, only one study has examined IL-32 expression in human lung tissues (46). Calebrese et al. (46) compared IL-32 mRNA and protein expression in the resected lungs of smokers with and without COPD and non-smoking control subjects. They found that IL-32 expression was significantly increased in macrophages, type I and type II alveolar cells and ciliated bronchiolar epithelia of COPD subjects compared with controls without COPD (46). In lung tissues of COPD patients, IL-32 co-localized with TNFα and the level of expression of both cytokines correlated directly with the degree of airflow obstruction (46). They also noted a qualitative increase in IL-32 expression in connective tissue-associated lung diseases but this increase was absent from both an asthmatic lung and a lung with acute viral pneumonia (46). While our study sought to determine IL-32 expression in the lungs of MAC patients, our results confirm the findings of the COPD study in that IL-32 expression was found to be increased in chronically inflamed lungs.
IL-32 was produced following infection of airway epithelial cells (BEAS-2B) with M. avium. This finding supports the enhanced IL-32 expression seen in the airway epithelial cells of resected lung tissues from MAC patients. In BEAS-2B cells, induction of IL-32 was mediated by NF-κB signaling and was not dependent on p38mapk or ERK signaling. These findings provide a potential functionality to the two NF-κB binding sites located in the 5′-flanking region of the IL-32 gene promoter (41). Additionally, we were unable to identify any reports of a cis-regulatory site for ATF-2, a transcription factor induced by p38mapk (66). These results indicate that IL-32 is induced in airway epithelial cells following M. avium infection in vitro and in vivo.
In the respiratory tract, airway epithelial cells are the first points of contact for inhaled environmental pollutants, cigarette smoke and microorganisms including mycobacteria. Inflammatory cellular bronchiolitis, a common clinical manifestation of MAC lung disease, contributes significantly to the cough and shortness of breath associated with this disorder. Given the robust expression of IL-32 in the airway epithelial cells, it is reasonable to posit that IL-32 contributes to bronchiolar inflammation by inducing pro-inflammatory cytokines and chemokines such as TNFα, IL-1β, IL-6, IL-8 and MIP-2 (25, 27). Other pro-inflammatory cytokines can also induce the production of IL-32, which may further lung inflammation (27, 30). Indeed, IL-32 production by airway and gastrointestinal epithelial cells have been implicated in the pathogenesis of various inflammatory diseases (27, 39, 40, 46, 63, 67, 68).
While airway inflammation contributes to the symptoms of MAC lung disease, the recruitment of phagocytes and lymphocytes to the site of infection is important for host immunity. After the engagement of Toll-like receptors, airway epithelial cells release cytokines and chemokines which are then able to recruit phagocytes and lymphocytes, resulting in a critically important host defense strategy against respiratory infections (69, 70). Airway epithelial cells may also contribute to host immunity by secreting anti-microbial peptides and by directly engulfing microbial organisms. Human epithelial cells derived from human epidermoid laryngeal carcinoma (HEp-2) and type II alveolar cells have been shown to internalize a wide variety of bacterial pathogens including M. avium (54, 56) and MTB (55, 57) through a process known as macropinocytosis (57). We found that IL-32 modestly inhibits the growth of M. avium in BEAS-2B cells; a possible mechanism for this is the increased induction of apoptosis and other forms of programmed cell death (PCD) by IL-32 in infected cells (25). While induction of apoptosis in the present studies was measured by determining the total number of TUNEL-positive cells, it is important to emphasize that TUNEL cannot distinguish different types of PCD. The TUNEL recognizes caspase-1-mediated pyroptosis and caspase-independent PCD as well as classic caspase-3-dependent apoptosis (71). Each of these PCD mechanisms can facilitate the clearance of intracellular pathogens. Future experiments are necessary to determine the relative contribution of each of these death pathways in IL-32 induced killing of intracellular M. avium. It also remains to be seen whether the observed increase IL-32 expression in airway epithelial cells of MAC-infected lungs directly contributes to the host defense against MAC organisms in vivo.
Following quantification and normalization to alveolar surface area, there was a clear trend in the increased number of IL-32-positive macrophages in the MAC-infected lungs (Fig. 5D). We performed in vitro experiments in order to determine the biological significance of IL-32 in MAC infection of macrophages using two complementary approaches. Firstly, we determined whether silencing endogenous IL-32 alters the ability of differentiated THP-1 macrophages to control M. avium infection, as we previously described for MTB infection (25). We found cells knocked down for IL-32 were more burdened by intracellular M. avium than cells with intact IL-32 expression. Secondly, we also found that exogenous IL-32γ modestly inhibited the growth of intracellular M. avium in primary hMDM.
Similar to the prototypical inflammatory cytokine TNFα, we speculate that while IL-32 may contribute to lung inflammation associated with MAC lung disease, the cytokine also facilitates host immunity against the offending pathogen (25, 27). We posit that a robust localized inflammatory response that includes IL-32 may be initially required to mitigate the infection. However, an anti-inflammatory response mediated by immunosuppressive cytokines such as IL-10 and transforming growth factor-beta may subsequently be needed to limit lung injury. Interestingly, in certain hematopoietic cell lines, IL-32 can induce IL-10 which then inhibits IL-32 production, providing a counter-regulatory mechanism against a relentless cycle of injurious inflammation (72, 73).
In summary, IL-32 is expressed in multiple cell types in the lungs but particularly in the airway epithelial cells of patients with MAC pulmonary disease. The marked increase in IL-32 expression in the airway epithelium and to a lesser extent in alveolar macrophages of MAC-infected lungs likely contributes to airway inflammation. However, we also found that IL-32 is a host protective mediator against MAC organisms in airway epithelial cells and macrophages. Future studies utilizing murine models are required to corroborate these findings.
Funding
Department of Veterans Affairs Veterans Health Administration, Office of Research and Development (E.D.C.) and National Institutes of Health (AI15614 to C.A.D.).
Acknowledgments
We thank Dr Leonid Heifets for kindly providing the M. avium isolate used in the in vitro experiments.
References
- 1.Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 2007;175:367. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 2.Kasperbauer SH, Daley CL. Diagnosis and treatment of infections due to Mycobacterium avium complex. Semin. Respir. Crit. Care Med. 2008;29:569. doi: 10.1055/s-0028-1085708. [DOI] [PubMed] [Google Scholar]
- 3.Prevots DR, Shaw PA, Strickland D, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated healthcare delivery systems. Am. J. Respir. Crit. Care Med. 2010;182:970. doi: 10.1164/rccm.201002-0310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taiwo B, Glassroth J. Nontuberculous mycobacterial lung diseases. Infect. Dis. Clin. North Am. 2004;24:769. doi: 10.1016/j.idc.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Marras TK, Chedore P, Ying AM, Jamieson F. Isolation prevalence of pulmonary non-tuberculous mycobacteria in Ontario, 1997–2003. Thorax. 2007;62:661. doi: 10.1136/thx.2006.070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iseman MD, Marras TK. The importance of nontuberculous mycobacterial lung disease. Am. J. Respir. Crit. Care Med. 2008;178:999. doi: 10.1164/rccm.200808-1258ED. [DOI] [PubMed] [Google Scholar]
- 7.Bodle EE, Cunningham JA, Della-Latta P, Schluger NW, Saiman L. Epidemiology of nontuberculous mycobacteria in patients without HIV infection, New York City. Emerg. Infect. Dis. 2008;14:390. doi: 10.3201/eid1403.061143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffith DE, Stout JE. It is better to light a candle … than to repeat the opinions of experts. Am. J. Respir. Crit. Care Med. 2010;182:865. doi: 10.1164/rccm.201008-1251ED. [DOI] [PubMed] [Google Scholar]
- 9.Winthrop KL, McNelley E, Kendall B, et al. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am. J. Respir. Crit. Care Med. 2010;182:977. doi: 10.1164/rccm.201003-0503OC. [DOI] [PubMed] [Google Scholar]
- 10.Lai CC, Tan CK, Chou CH, et al. Increasing incidence of nontuberculous mycobacteria, Taiwan, 2000–2008. Emerg. Infect. Dis. 2010;16:294. doi: 10.3201/eid1602.090675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan K, Wang J, Marras TK. Nontuberculous mycobacterial sensitzation in the United States: national trends over three decades. Am. J. Respir. Crit. Care Med. 2007;176:306. doi: 10.1164/rccm.200702-201OC. [DOI] [PubMed] [Google Scholar]
- 12.Marras TK, Daley CL. Epidemiology of human pulmonary infection with nontuberculous mycobacteria. Clin. Chest Med. 2002;23:553. doi: 10.1016/s0272-5231(02)00019-9. [DOI] [PubMed] [Google Scholar]
- 13.Daley CL, Griffith DE. Pulmonary non-tuberculous mycobacterial infections. Int. J. Tuberc. Lung Dis. 2010;14:665. [PubMed] [Google Scholar]
- 14.Frehel C, de Chastellier C, Lang T, Rastogi N. Evidence for inhibition of fusion of lysosomal and prelysosomal compartments with phagosomes in macrophages infected with pathogenic Mycobacterium avium. Infect. Immun. 1986;52:252. doi: 10.1128/iai.52.1.252-262.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim RD, Greenberg DE, Ehrmantraut ME, et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am. J. Respir. Crit. Care Med. 2008;178:1066. doi: 10.1164/rccm.200805-686OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwon YS, Kim EJ, Lee S-H, et al. Decreased cytokine production in patients with nontuberculous mycobacterial lung disease. Lung. 2007;185:337. doi: 10.1007/s00408-007-9040-z. [DOI] [PubMed] [Google Scholar]
- 17.Safdar A, Atrmstrong D, Murray HW. A novel defect in interferon-γ secretion in patients with refractory nontuberculous pulmonary mycobacteriosis. Ann. Intern. Med. 2003;138:521. doi: 10.7326/0003-4819-138-6-200303180-00030. [DOI] [PubMed] [Google Scholar]
- 18.Safdar A, White DA, Stover D, Armstrong D, Murray HW. Profound interferon gamma deficiency in patients with chronic pulmonary nontuberculous mycobacteriosis. Am. J. Med. 2002;113:756. doi: 10.1016/s0002-9343(02)01313-x. [DOI] [PubMed] [Google Scholar]
- 19.Vankayalapati R, Wizel B, Samten B, et al. Cytokine profiles in immunocompetent persons infected with Mycobacterium avium complex. J. Infect. Dis. 2001;183 doi: 10.1086/318087. 478–484. [DOI] [PubMed] [Google Scholar]
- 20.Ma X, Reich RA, Wright JA, et al. Association between interleukin-8 gene alleles and human susceptibility to tuberculosis disease. J. Infect. Dis. 2003;188:349. doi: 10.1086/376559. [DOI] [PubMed] [Google Scholar]
- 21.Khader SA, Cooper AM. IL-23 and IL-17 in tuberculosis. Cytokine. 2008;41:79. doi: 10.1016/j.cyto.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khader SA, Bell GK, Pearl JE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 2007;8:369. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 23.Cooper AM, Khader SA. The role of cytokines in the initiation, expansion, and control of cellular immunity to tuberculosis. Immunol. Rev. 2008;226:191. doi: 10.1111/j.1600-065X.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torrado E, Cooper AM. IL-17 and Th17 cells in tuberculosis. Cytokine Growth Factor Rev. 2010;21:455. doi: 10.1016/j.cytogfr.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bai X, Kim S, Azam T, et al. IL-32 is a host protective cytokine against Mycobacterium tuberculosis in differentiated THP-1 human macrophages. J. Immunol. 2010;184:3830. doi: 10.4049/jimmunol.0901913. [DOI] [PubMed] [Google Scholar]
- 26.Netea MG, Azam T, Lewis EC, et al. Mycobacterium tuberculosis induces interleuckin-32 production through a caspase-1/IL-18/interferon-gamma-dependent mechanism. PLoS Med. 2006;3:e277. doi: 10.1371/journal.pmed.0030277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SH, Han SY, Azam T, Yoon DY, Dinarello CA. Interleukin-32: a cytokine and inducer of TNFα. Immunity. 2005;22 doi: 10.1016/j.immuni.2004.12.003. 131–142. [DOI] [PubMed] [Google Scholar]
- 28.Shoda HK, Fujio Y, Yamaguchi A, et al. Interactions between IL-32 and tumur necrosis factor alpha contribute to the exacerbation of immune-inflammatory diseases. Arthritis Res. Ther. 2006;8:R166. doi: 10.1186/ar2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong J, Bae S, Kang Y, et al. Suppressing IL-32 in monocytes impairs the induction of the proinflammatory cytokines TNFalpha and IL-1beta. Cytokine. 2010;49:171. doi: 10.1016/j.cyto.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Heinhuis B, Koenders MI, van Riel PL, et al. Tumour necrosis factor alpha-driven IL-32 expression in rheumatoid arthritis synovial tissue amplifies an inflammatory cascade. Ann. Rheum Dis. 2010;70 doi: 10.1136/ard.2010.139196. 660–667. [DOI] [PubMed] [Google Scholar]
- 31.Denis M, Ghadirian E. Interleukin-1 is involved in mouse resistance to Mycobacterium avium. Infect. Immun. 1994;62:457. doi: 10.1128/iai.62.2.457-461.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bermudez LE, Wu M, Young L. Interleukin-12-stimulated natural killer cells can activate human macrophages to inhibit growth of Mycobacterium avium. Infect. Immun. 1995;63:4099. doi: 10.1128/iai.63.10.4099-4104.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fieschi C, Dupuis S, Picard C, Casanova JL. Human interleukin-12-interferon-γ axis in protective immunity to mycobacteria. In: Kotb M, Calandra T, editors. Cytokines and Chemokines in Infectious Diseases Handbook. Totowa, NJ: Humana Press; 2003. p. 151. [Google Scholar]
- 34.Petrofsky M, Bermudez LE. Neutrophils from Mycobacterium avium-infected mice produce TNF-alpha, IL-12, and IL-1 beta and have a putative role in early host response. Clin. Immunol. 1999;91:354. doi: 10.1006/clim.1999.4709. [DOI] [PubMed] [Google Scholar]
- 35.Schneider BE, Korbel D, Hagens K, et al. A role for IL-18 in protective immunity against Mycobacterium tuberculosis. Eur. J. Immunol. 2010;40:396. doi: 10.1002/eji.200939583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winthrop KL, Chang E, Yamashita S, Iademarco MF, LoBue PA. Nontuberculous mycobacteria infections and anti-tumor necrosis factor-alpha therapy. Emerg. Infect. Dis. 2009;15:1556. doi: 10.3201/eid1510.090310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jung MY, Son MH, Kim SH, Cho D, Kim TS. IL-32gamma induced the maturation of dendritic cells with Th1- and Th17-polarizing ability through enhanced IL-12 and IL-6 production. J. Immunol. 2011;185:6848. doi: 10.4049/jimmunol.1003996. [DOI] [PubMed] [Google Scholar]
- 38.Nold MF, Nold-Petry CA, Pott GB, et al. Endogenous IL-32 controls cytokine and HIV-1 production. J. Immunol. 2008;181:557. doi: 10.4049/jimmunol.181.1.557. [DOI] [PubMed] [Google Scholar]
- 39.Li W, Liu Y, Mukhtar MM, et al. Activation of interleukin-32 pro-inflammatory pathway in response to influenza A virus infection. Plos One. 2008;3:e1985. doi: 10.1371/journal.pone.0001985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li W, Yang F, Liu Y, et al. Negative feedback regulation of IL-32 production by iNOS activation in response to dsRNA or influenza virus infection. Eur. J. Immunol. 2009;39:1. doi: 10.1002/eji.200838885. [DOI] [PubMed] [Google Scholar]
- 41.Li W, Sun W, Liu L, et al. IL-32: a host proinflammatory factor against influenza viral replication is upregulated by aberrant epigenetic modifications during influenza A virus infection. J. Immunol. 2010;185:5056. doi: 10.4049/jimmunol.0902667. [DOI] [PubMed] [Google Scholar]
- 42.Rasool ST, Tang H, Wu J, et al. Increased level of IL-32 during human immunodeficiency virus infection suppresses HIV replication. Immunol. Lett. 2008;117:161. doi: 10.1016/j.imlet.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Netea MG, Azam T, Ferwerda G, et al. IL-32 synergizes with nucleotide oligomerization domain (NOD) 1 and NOD2 ligands for IL-1beta and IL-6 production through a caspase 1-dependent mechanism. Proc. Natl Acad. Sci. USA. 2005;102:16309. doi: 10.1073/pnas.0508237102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joosten LA, Netea MG, Kim SH, et al. IL-32, a proinflammatory cytokine in rheumatoid arthritis. Proc. Natl Acad. Sci. USA. 2006;103:3298. doi: 10.1073/pnas.0511233103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dinarello CA, Kim S-H. IL-32, a novel cytokine with a possible role in disease. Ann. Rheum. Dis. 2006;65(Suppl. 3):61. doi: 10.1136/ard.2006.058511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calabrese F, Baraldo S, Bazzan E, et al. IL-32, a novel proinflammatory cytokine in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2008;178:894. doi: 10.1164/rccm.200804-646OC. [DOI] [PubMed] [Google Scholar]
- 47.Chang LY, Crapo JD. Quantitative evaluation of minimal injuries. In: Gil J, editor. Models of Lung Disease: Microscopy and Structural Models. New York, NY: Marcel Dekker, Inc; 1990. p. 597. [Google Scholar]
- 48.Theus SA, Cave MD, Eisenach KD. Activated THP-1 cells: an attractive model for the assessment of intracellular growth rates of Mycobacterium tuberculosis isolates. Infect. Immun. 2004;72:1169. doi: 10.1128/IAI.72.2.1169-1173.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stockes RW, Doxsee D. The receptor-mediated uptake, survival, replication, and drug sensitivity of Mycobacterium tuberculosis within the macrophage-like cell line THP-1: a comparison with human monocyte-derived macrophages. Cell. Immunol. 1999;197:1. doi: 10.1006/cimm.1999.1554. [DOI] [PubMed] [Google Scholar]
- 50.Riendeau CJ, Kornfeld H. THP-1 cell apoptosis in response to Mycobacterial infection. Infect. Immun. 2003;71:254. doi: 10.1128/IAI.71.1.254-259.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bai X, Wilson SE, Chmura K, Feldman NE, Chan ED. Morphometric analysis of Th(1) and Th(2) cytokine expression in human pulmonary tuberculosis. Tuberculosis. 2004;84:375. doi: 10.1016/j.tube.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 52.Wang T, Lafuse WP, Zwilling BS. NFkB and Sp1 elements are necessary for maximal transcription of Toll-like receptor 2 induced by Mycobacterium avium. J. Immunol. 2001;167:6924. doi: 10.4049/jimmunol.167.12.6924. [DOI] [PubMed] [Google Scholar]
- 53.Tse HM, Josephy SI, Chan ED, Fouts D, Cooper AM. Activation of the mitogen-activated protein kinase signaling pathway is instrumental in determining the ability of Mycobacterium avium to grow in murine macrophages. J. Immunol. 2002;168:825. doi: 10.4049/jimmunol.168.2.825. [DOI] [PubMed] [Google Scholar]
- 54.Bermudez LE, Young LS. Factors affecting invasion of HT-29 and HEp-2 epithelial cells by organisms of the Mycobacterium avium complex. Infect. Immun. 1994;62:2021. doi: 10.1128/iai.62.5.2021-2026.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bermudez LE, Goodman J. Mycobacterium tuberculosis invades and replicates within type II alveolar cells. Infect. Immun. 1996;64:1400. doi: 10.1128/iai.64.4.1400-1406.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reddy VM, Kumar B. Interaction of Mycobacterium avium complex with human respiratory epithelial cells. J. Infect. Dis. 2000;181:1189. doi: 10.1086/315327. [DOI] [PubMed] [Google Scholar]
- 57.García-Pérez BE, Mondragón-Flores R, Luna-Herrera J. Internalization of Mycobacterium tuberculosis by macropinocytosis in non-phagocytic cells. Microb. Pathog. 2003;35:49. doi: 10.1016/s0882-4010(03)00089-5. [DOI] [PubMed] [Google Scholar]
- 58.Choi JD, Bae SY, Hong JW, et al. Identification of the most active interleukin-32 isoform. Immunology. 2008;126:535. doi: 10.1111/j.1365-2567.2008.02917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keane J, Balcewicz-Sablinska MK, Remold HG, et al. Infection by Mycobacterium tuberculosis promotes human alveolar macrophage apoptosis. Infect. Immun. 1997;65:298. doi: 10.1128/iai.65.1.298-304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keane J, Remold HG, Kornfeld H. Virulent Mycobacterium tuberculosis strains evade apoptosis of infected alveolar macrophages. J. Immunol. 2000;15:2016. doi: 10.4049/jimmunol.164.4.2016. [DOI] [PubMed] [Google Scholar]
- 61.Molloy A, Laochumroonvorapong P, Kaplan G. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette-Guerin. J. Exp. Med. 1994;180:1499. doi: 10.1084/jem.180.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee J, Hartman M, Kornfeld H. Macrophage apoptosis in tuberculosis. Yonsei Med. J. 2009;50:1. doi: 10.3349/ymj.2009.50.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shioya M, Nishida A, Yagi Y, et al. Epithelial overexpression of interleukin-32alpha in inflammatory bowel disease. Clin. Exp. Immunol. 2007;149:480. doi: 10.1111/j.1365-2249.2007.03439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith D, Hänsch H, Bancroft G, Ehlers S. T-cell-independent granuloma formation in response to Mycobacterium avium: role of tumour necrosis factor-alpha and interferon-gamma. Immunology. 1997;92:413. doi: 10.1046/j.1365-2567.1997.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ulrichs T, Kaufmann SH. New insights into the function of granulomas in human tuberculosis. J. Pathol. 2006;208:261. doi: 10.1002/path.1906. [DOI] [PubMed] [Google Scholar]
- 66.Winston BW, Chan ED, Riches DWH. Activation of p38mapk, MKK3, and MKK4 by tumor necrosis factor-alpha in mouse bone marrow-derived macrophages. J. Immunol. 1997;159:4491. [PubMed] [Google Scholar]
- 67.Ko NY, Mun SH, Lee SH, et al. Interleukin-32a production is regulated by MyD88-dependent and independent pathways in Il-1b-stimulated human alveolar epithelial cells. Immunobiology. 2011;216:32. doi: 10.1016/j.imbio.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 68.Zepp JA, Nold-Petry CA, Dinarello CA, Nold MF. Protection from RNA and DNA viruses by IL-32. J. Immunol. 2011;186:4110. doi: 10.4049/jimmunol.1000081. [DOI] [PubMed] [Google Scholar]
- 69.Bals R, Hiemstra PS. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur. Respir. J. 2004;23:327. doi: 10.1183/09031936.03.00098803. [DOI] [PubMed] [Google Scholar]
- 70.Kato A, Schleimer RP. Beyond inflammation: airway epithelial cells are at the interface of innate and adaptive immunity. Curr. Opin. Immunol. 2007;19:711. doi: 10.1016/j.coi.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 2005;73:1908. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kang JW, Choi SC, Cho MC, et al. A proinflammatory cytokine interleukin-32beta promotes the production of an anti-inflammatory cytokine interleukin-10. Immunology. 2009;128(1 Suppl.):e532. doi: 10.1111/j.1365-2567.2008.03025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Choi J, Bae S, Hong J, et al. Paradoxical effects of constitutive human IL-32{gamma} in transgenic mice during experimental colitis. Proc. Natl Acad. Sci. USA. 2010;107:21082. doi: 10.1073/pnas.1015418107. [DOI] [PMC free article] [PubMed] [Google Scholar]