Abstract
Although mature T cells divide and differentiate when they receive strong TCR stimulation, most immature CD4+CD8+ thymocytes die. The molecular basis for this marked difference in response is not known. Observations that TCR-stimulated CD4+CD8+ thymocytes fail to polarize their microtubule-organizing center (MTOC), one of the first events that occurs upon antigen activation of mature T cells, suggests that TCR signaling routes in immature and mature T cells diverge early and upstream of MTOC polarization. To better understand the source of the divergence, we examined the molecular basis for the difference in TCR-mediated MTOC polarization. We show that unstable microtubules are a feature of immature murine CD4+CD8+ thymocytes, which also exhibit higher levels of glycogen synthase kinase 3 (GSK3) activity, a known inhibitor of microtubule stability. Importantly, CD4+CD8+ thymocytes gained the ability to polarize their MTOC in response to TCR signals when GSK3 activity was inhibited. GSK3 inhibition also abrogated TCR-mediated apoptosis of immature thymocytes. Together, our results suggest that a developmentally regulated difference in GSK3 activity has a major influence on immature CD4+CD8+ thymocyte versus mature T-cell responses to TCR stimulation.
Keywords: GSK3, MTOC, TCR, thymocyte
Introduction
The αβTCR is first expressed at the CD4+CD8+ [double-positive (DP)] stage, the major target of selection events that shape the T-cell repertoire (1). The fate of DP thymocytes, which browse MHC/peptide complexes expressed by thymic epithelial cells, depends on the strength and duration of TCR signals, as well as the costimulatory signals that accompany them (2, 3). Whereas immature thymocytes that experience intermediate affinity TCR signals mature to the single-positive (SP) stage of development, thymocytes that experience high-affinity TCR signals, in the presence of costimulatory signals such as those provided by CD28, die (4–6). However, when thymocytes mature fully, they interpret high-affinity TCR signals very differently. Rather than die in response to TCR stimulation, mature SP T cells survive and proliferate. The molecular basis for this difference in cell fate is still incompletely understood, although we and others have shown that several intracellular events differ between immature and mature T cells in response to TCR engagement (7). This report focuses on a developmental difference in microtubule reorganization that is apparent within minutes of TCR stimulation of mature versus immature T cells. Specifically, mature SP T cells but not immature DP thymocytes rapidly recruit the microtubule-organizing center (MTOC) to the point of contact with an antigen-presenting cells [the immunological synapse (IS) (8)] in response to TCR stimulation (9).
Reorientation of microtubules after T-cell activation regulates division and vesicular trafficking during both cytotoxic and helper T-cell responses (10–12). TCR-mediated MTOC polarization requires proximal TCR signaling components ZAP70, lck, fyn, LAT and SLP76 (13), is calcium dependent (10) and involves coordinator of actin organization, including VAV1, CDC42 and RAC1 (14–18).
MTOC recruitment is regulated by interactions between tubulin and molecular motors (e.g. dynein), which interact with TCR adaptor proteins at the IS (19). Critical to the ability of the MTOC to polarize to the synapse is the stability of these participating microtubules. Microtubules are very dynamic structures composed of tubulin dimers, which can rapidly assemble and disassemble in response to a variety of intracellular and molecular changes. Post-translational modifications of tubulin regulate microtubule interactions and polymerization and the tyrosination status of alpha tubulin has a particularly significant influence on the stability of microtubule polymers. Tyrosine is added to free alpha tubulin via tubulin tyrosine ligase and is removed via tubulin carboxypeptidase (20). Unstable microtubules include more tyrosinated tubulin. On the other hand, detyrosination is associated with tubulin stablity (21–23) and, in fact, may directly enhance motor protein attachment. Specifically, the motor protein kinesin-1 associates with and moves more readily along detyrosinated tubulin (24).
The balance between unstable and stable microtubules is also regulated by microtubule-associated proteins (MAPs) such as collapsing response mediator protein 2 (CRMP-2), adenomatous polyposis coli (AdPC), MAP2C and Tau, which bind to microtubules and enhance their stability (25, 26). MAPs, in turn, are regulated by kinases including glycogen synthase kinase 3 (GSK3), a serine/threonine kinase that influences multiple cellular processes (growth, differentiation and survival) (27). Active GSK3 specifically enhances microtubule instability by phosphorylating and inactivating Tau, AdPC and CRMP-2 (28) and by phosphorylating and activating MAP1b (25, 29).
Given that microtubule stability is likely to influence MTOC polarization, we decided to examine the possibility that differences in microtubule regulation might underlie differences in the ability of immature DP thymocytes and mature SP T cells to mobilize their MTOC in response to TCR signals.
Methods
Antibodies and reagents
Anti-TCR (H57-597), anti-CD2 (RM2-5) and anti-CD28 (37.51) were purchased from Biolegend (San Diego, CA, USA). Anti-tyrosinated tubulin (TUB-1A2) and SB415286 were obtained from Sigma–Aldrich (St Louis, MO, USA). Anti-Akt, cleaved caspase 3 (Asp175) and anti-phospho-GSK3 (Ser9, #9336) were purchased from Cell Signaling Technologies (Danvers, MA, USA). Anti-beta catenin (14) was obtained from BD Biosciences (San Jose, CA, USA). Alexa 488 goat anti-mouse IgG and Alexa 488 goat anti-rabbit were purchased from Invitrogen (Carlsbad, CA, USA). Lithium was purchased from Calbiochem (San Diego, CA, USA). Anti-actin was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Mice
Female C57BL/6 mice were obtained from Taconic Laboratories. Thymocytes and T cells were harvested from mice <6 months of age. Animal studies have been reviewed and approved by Haverford College Institutional Animal Care and Use Committee.
Cell purification of CD4+CD8+ thymocytes and T cells
CD4+CD8+ thymocytes and T cells were purified by magnetic cell sorting (Miltenyi Biotec). A single-cell suspension of thymocytes was prepared and labeled with anti-mouse CD8α (Ly-2) microbeads (10 μl 10−7 cells) for positive selection of immature DP cells. Lymph nodes were harvested from cervical, axillary, inguinal and mesenteric areas and gently squashed with a 1 cc syringe plunger to release cells. Lymphocytes were labeled with anti-mouse CD90.2 (Thy1.2) microbeads (10 μl 10−7 cells). Labeled cells were positively selected by passage over Miltenyi Biotec LS columns in midiMACS separators according to manufacturer’s protocol. After magnetic separation, thymocyte preps were >90% CD4+CD8+ and lymphocytes were >95% CD4 SP and CD8 SP. Cells were suspended in media containing RPMI 1640, 10% fetal bovine serum, along with L-glutamine, non-essential amino acids, sodium pyruvate, 0.05 M 2-mercaptoethanol, penicillin and streptomycin and kept at 4°C or incubated at 37°C 5% CO2 as indicated.
Cell stimulation
Surfactant-free polystyrene beads were purchased from Interfacial Dynamics (Portland, OR, USA) or latex beads (sulfate latex, 4% w/v, 5 μM) from Invitrogen (Eugene, OR, USA). Beads were incubated with 1 μg ml−1 anti-TCR, 1 μg ml−1 CD2 and 5 μg ml−1 anti-CD28 in PBS overnight at 37°C and then washed three times with serum containing media. Control beads were prepared using uncoated beads or beads coated with 5 μg ml−1 of anti-CD28 alone. Note that because TCR signaling is not optimal in purified CD4+CD8+ thymocytes unless lck-associated molecules are aggregated with the TCR, we included anti-CD2 in our antibody mix (7, 30, 31). Beads were incubated with cells at 37°C at a ratio of 1:1 at a concentration of 20 million beads ml−1 for the indicated times.
For plate-bound stimulation, polystyrene plates were coated with 10 μg ml−1 anti-TCR, 10 μg ml−1 anti-CD2 and 50 μg ml−1 anti-CD28 in PBS overnight at 4°C. Plates were washed with serum containing media three times before use.
Immunofluorescence
Cells and beads were placed on poly-L-lysine- (Sigma–Aldrich) coated slides and fixed/permeabilized with ice-cold methanol. Wells were washed with PBS + 1% BSA, followed by blocking with PBS + 5% non-fat dry milk. For MTOC visualization, cells were stained with anti-tyrosinated tubulin overnight at room temperature. Slides were washed three times, incubated with goat anti-mouse Alexa 488 for 1 h at room temperature and followed by three washes including one with 300 nM 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI,Invitrogen) as a nuclear counterstain. Images were acquired on an immunofluorescence scope Nikon Eclipse 80i with SimplePCI software.
Western blot analysis
Cells were lysed at 2 × 108 cells ml−1 with SDS lysis buffer [0.5% w/v SDS, 0.05 M Tris (pH 8.0), 1 mM Na3VO4, 100 nM calyculin A, 1 mM dithiothreitol and protease inhibitor cocktail] for 10 min at 4°C. Lysates were sonicated and centrifuged at 14 000 r.p.m. at 4°C. Proteins were separated by SDS–PAGE and transferred to immobilon-P polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA). Blots were blocked with blotto (5% non-fat dry milk and 0.001% Tween 20 in PBS) for 30 min at room temperature; all washes were completed with PBS Tween (0.001% Tween 20 in PBS) and incubated rotating overnight at 4°C with primary antibody diluted in PBS Tween with 1% BSA. HRP-coupled secondary antibodies were incubated with the blot for 30 min at room temperature. Chemiluminesence was visualized with Supersignal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific, Rockford, IL, USA) and imaged with a FluorChem SP or a FluorChem HD2 digital imager (Alpha Innotech). Note that because immature DP thymocytes and mature SP T cells express different levels of actin per cell, we use Akt as a loading control for most experiments (7).
For quantification of western blot results, relative intensities of bands are indicated and represent the ratio of spot densitometry measurements of the bands indicated, with background subtracted (Alpha Innotech software). Note that the ratios will vary with the exposure selected for analysis but will not be influenced by any changes in brightness or contrast made after imaging for presentation purposes. The values are representative of at least two and typically three distinct experiments.
Flow cytometry
Cells were fixed with 4% PFA (Electron Microscopy Sciences) for 30 min at room temperature, washed twice with staining medium (1 × PBS containing 10% fetal bovine serum and 0.05% sodium azide), permeabilized with 0.1% Trition X-100 in PBS for exactly 4 min at room temperature and washed three times before overnight incubation at 4°C with anti-cleaved caspase 3 diluted 1:200 in staining media. Cells were incubated with goat anti-rabbit Alexa 488 diluted 1:200 for 30 min at room temperature, washed and analyzed on a FACSCalibur with CellQuest software (BD Biosciences).
Drug treatment
Cells were incubated in the presence or absence of 60 mM lithium chloride (Calbiochem) or 30 μM SB415286 (Sigma–Aldrich) for the duration of stimulation.
Results
Immature CD4+CD8+ thymocytes fail to polarize their MTOC in response to TCR stimulation
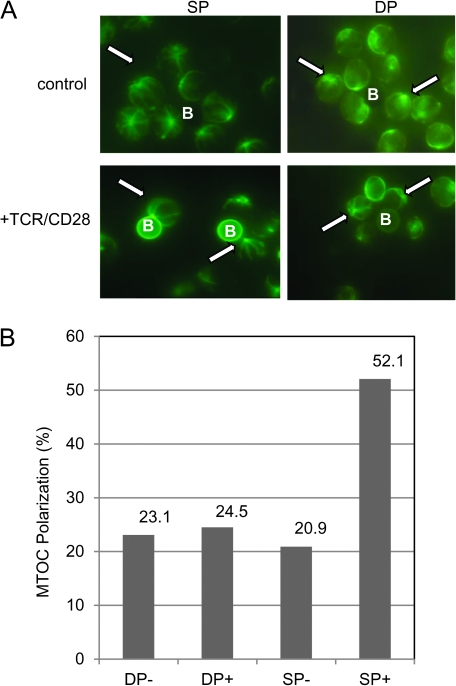
In agreement with previous work by Hailman and Allen (9), we find that TCR stimulation results in polarization of the MTOC in mature SP T cells but not immature CD4+CD8+ thymocytes (Fig. 1). Using an in vitro assay where antigen-presenting cell (APC) interactions are mimicked by beads coated with anti-TCR/anti-CD28/anti-CD2 antibodies (APC surrogates), we assessed MTOC polarization by staining for tubulin and observing bead–cell conjugates by fluorescent microscopy (Fig. 1A). After 20 min of incubation with antibody-coated beads, 52.1% of mature T cells, but only 24.5% of immature T cells, polarized their MTOC to the point of contact. Under non-stimulatory conditions (in the presence of beads that were not coated with antibody), only 20.9 and 23.1% of mature and immature T cells, respectively, were scored positive for MTOC polarization (Fig. 1B). Stimulation via anti-TCR/anti-CD2-coated beads also results in MTOC polarization by mature SP T cells but not immature CD4+CD8+ thymocytes, indicating that CD28 co-engagement is not absolutely necessary for this response (data not shown).
Fig. 1.
Immature CD4+CD8+ thymocytes do not polarize their MTOC in response to TCR stimulation. Purified mature CD4+ and CD8+ (SP) and immature CD4+CD8+ (DP) cells were stimulated with uncoated beads [control (−)] or beads coated with anti-TCR, anti-CD2 and anti-CD28 (+) for 20 min. (A) Cells were fixed and stained for tyrosinated tubulin and visualized using immunofluoresence microscopy. Images were scored for polarization without observer knowledge of stimulation condition or cell type. Cells were counted as polarized if the most intense staining point (MTOC, see arrows) was in complete contact with the beads (B). (B) The percent of positively scored cells (MTOC polarized) seen in each condition is shown on the bar graph. These results are representative of at least 10 experiments.
We were interested in understanding the molecular basis for the differences in the ability of CD4+CD8+ thymocytes and mature T cells to polarize their MTOC in response to acute TCR stimulation. We qualitatively, yet consistently, observed that microtubules originating from the MTOC of mature T cells were well defined, extending across the diameter of the cell. In contrast, microtubules within immature thymocytes were less defined and tubulin staining appeared more diffuse, raising the possibility that a larger proportion of tubulin molecules were not incorporated into the microtubules (see Fig. 1A and data not shown). These preliminary observations led us to speculate that microtubules in immature CD4+CD8+ thymocytes might be less stable, leading to inefficient MTOC polarization.
CD4+CD8+ thymocytes contain more tyrosinated tubulin than mature T cells
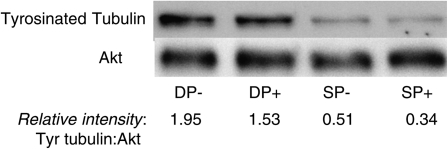
Tubulin dimers are tyrosinated at the C-terminus when synthesized but typically lose these terminal tyrosine residues after incorporation into microtubule. Recent data suggest that detyrosination by a carboxypeptidase enhances microtubule stability by inhibiting disassembly (32). To pursue our hypothesis that microtubule stability differs between immature CD4+CD8+ thymocytes and mature T cells, we compared levels of tyrosinated tubulin by western blot (Fig. 2). Our data show that CD4+CD8+ thymocytes express markedly higher levels of tyrosinated tubulin, both before and after TCR stimulation, indicating that their microtubules are less stable.
Fig. 2.
CD4+CD8+ thymocytes express more tyrosinated tubulin than mature SP T cells. Purified immature DP and mature SP T cells were cultured for 2 h in the presence (+) or absence (−) of plate-bound anti-TCR/CD2/CD28 antibodies. Protein lysates were separated by SDS–PAGE (8%) and probed with antibodies to tyrosinated tubulin and Akt (loading control). The results of the western blot are shown and relative band intensities (tyrosinated tubulin/Akt) are indicated.
Activity of GSK3 differs between CD4+CD8+ cells and their mature descendants
Given that tubulin is present in its destabilizing form in CD4+CD8+ thymocytes, we focused attention on a key upstream regulator, GSK3, which phosphorylates specific MAPs, reducing their ability to bind to and stabilize microtubules (26, 33, 34). Increased GSK3 activity is associated with decreased microtubule stability; therefore, we hypothesized that GSK3 activity is higher in immature CD4+CD8+ thymocytes than mature T cells.
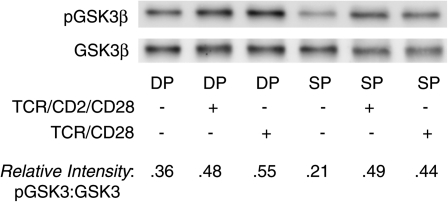
Activity of GSK3 can be inferred from its phosphorylation status. Specifically, phosphorylation of GSK3 at an N-terminal serine residue (Ser 9 for GSK3β, Serine 21 for GSK3α) is an indirect marker of decreased GSK3 activity. Therefore, we first predicted that more GSK3 would be phosphorylated and inactivated in mature T cells versus immature thymocytes. However, we saw no consistent differences in Ser9/21 phosphorylation between unstimulated immature and mature T cells (Fig. 3): both cell subpopulations exhibited a basal level of phosphorylation of GSK3 and increased its phosphorylation in response to TCR stimulation (both TCR/CD28 and TCR/CD2/CD28 co-engagement). In some (but not all) experiments, freshly isolated immature DP thymocytes even exhibited higher levels of GSK3 phosphorylation than freshly isolated mature SP T cells. However, we were reluctant to draw definitive conclusions from this indirect measure of inactivity, particularly given recent data suggesting that other phosphorylation sites that are not as readily assessed, particularly in the C-terminus (35), are also critical determinants of activity.
Fig. 3.
Phosphorylation of GSK3 at Ser9/21 does not differ between immature CD4+CD8+ thymocytes and mature SP T cells. Purified DP thymocytes and SP T cells were incubated at 37°C with anti-TCR/CD2 or anti-TCR/CD2/CD28-coated plates for 1 h, after which they were lysed and prepared for western blotting with anti-phospho-GSK3 (Ser9/21) and anti-total GSK3. Phospho-GSK3β shows up at ∼46 kD. (Phospho-GSK3α shows up as a much fainter band at ∼52 kD and is not always evident on western blots.)
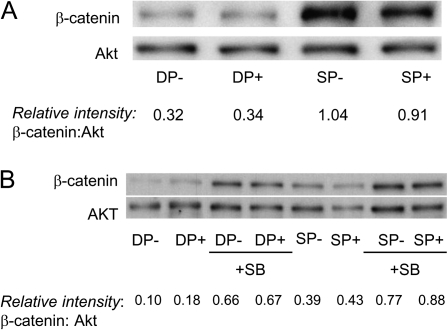
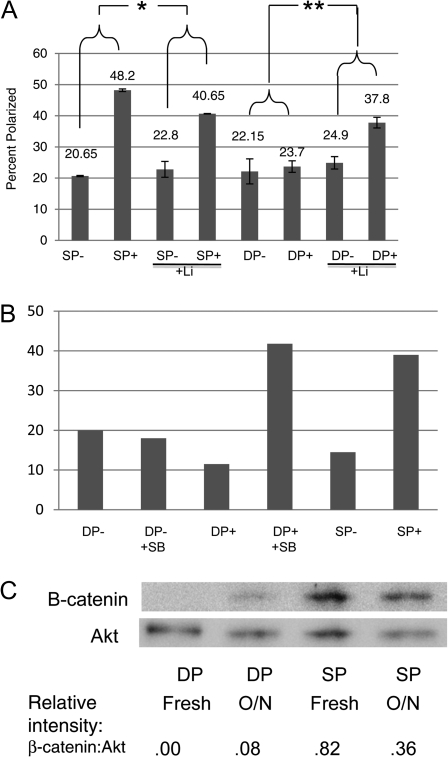
Given that GSK3 activity is incompletely predicted by its phosphorylation status, we turned to another indirect but arguably more reliable assay for activity (36–38): changes in levels of β-catenin protein, which is directly targeted for degradation when phosphorylated by GSK3. We compared levels of β-catenin in immature CD4+CD8+ thymocytes and mature T cells by western blot and found, in agreement with others (39), that freshly isolated CD4+CD8+ thymocytes express markedly lower β-catenin levels than mature T cells (Fig. 4). A pharmacological inhibitor of GSK3, SB415286, confirmed that β-catenin levels are regulated by GSK3 (Fig. 4B); in its presence, β-catenin levels increased in CD4+CD8+ thymocytes. These data suggest that GSK3 is, indeed, more active in CD4+CD8+ thymocytes than mature T cells and raise the possibility that it may regulate microtubule stability and MTOC polarization of immature versus mature T cells.
Fig. 4.
GSK3 is more active in immature CD4+CD8+ thymocytes than mature T cells. (A) Purified immature CD4+CD8+ thymocytes (DP) and mature T cells (SP) were incubated for 2 h in the presence (+) or absence (−) of plate-bound anti-TCR/CD2/CD28 antibodies. Expression of β-catenin (which increases with decreased GSK3 activity) and Akt (loading control) was assessed by western blot. Relative intensities of western bands (β-catenin/Akt) are indicated. (B) DP and SP thymocytes were stimulated for 2 h with plate-bound anti-TCR/CD2/CD28 antibodies (+) for 2 h in the presence or absence of the GSK3 inhibitor, SB415286 (SB). Relative intensities of western bands (β-catenin/Akt) are indicated.
CD4+CD8+ thymocytes acquire the ability to polarize the MTOC when stimulated in the presence of GSK3 inhibitors
To directly address our hypothesis that GSK3 activity inhibits TCR-mediated MTOC polarization in CD4+CD8+ thymocytes, we examined the effects of known pharmacological inhibitors of GSK3, lithium (Li) and SB415285, on immature and mature T-cell responses. Immature CD4+CD8+ thymocytes gained the capacity to polarize MTOC in response to TCR stimulation when either GSK3 inhibitor was present (Fig. 5A and B). In the absence of lithium, an average of 23.7% CD4+CD8+ thymocytes polarized their MTOC in response to TCR stimulation, while in its presence, an average of 37.8% polarized their MTOC. In the presence of SB415286, the frequency of DP thymocytes that polarized their MTOC in response to TCR stimulation increased by >3-fold. GSK3 inhibitors did not enhance the ability of mature T cells to polarize their MTOCs. Together, these results show that GSK3 activity abrogates TCR-mediated MTOC polarization in CD4+CD8+ T cells. They also suggest that immature thymocytes actually have the potential to respond to TCR stimulation like their mature T-cell descendants; however, they appear to be actively inhibited from doing so.
Fig. 5.
CD4+CD8+ thymocytes acquire the ability to polarize MTOC in response to TCR stimulation when GSK3 is inhibited. (A) Purified immature CD4+CD8+ thymocytes (DP) and mature T cells (SP) were incubated for 4 h in the presence (+) or absence (−) of plate-bound anti-TCR/CD2/CD28 antibodies and in the presence or absence of 60 mM lithium (Li). The percentage of cells that scored positive for MTOC polarization in three experiments was calculated (as described under Fig. 1). Analysis of variance statistics were applied to the results and the asterixes identify the groups that displayed statistically significant differences (*P < 0.05, **P < 0.01). (B) Purified immature CD4+CD8+ thymocytes (DP) and mature T cells (SP) were incubated for 1 h in the presence (+) or absence (−) of plate-bound anti-TCR/CD2/CD28 antibodies and in the presence or absence of the GSK3 inhibitor, SB415286 (SB). Percent cells that scored positive for MTOC polarization are shown (and are representative of two experiments). (C) CD4+CD8+ thymocytes and mature T cells were lysed immediately after purification or incubated in RPMI/10% FCS at 37°C overnight. Expression of β-catenin (which increases with decreased GSK3β activity) and Akt (loading control) was assessed by western blot. Relative intensities of bands (β-catenin/Akt) are indicated.
Interestingly, Hailman and Allen (9) showed that MTOC polarization was significantly enhanced when CD4+CD8+ thymocytes were cultured from 4 to 20 h in single-cell suspensions prior TCR stimulation. Our data raise the possibility that this phenomenon is related to GSK3 activity and specifically predict that GSK3 activity is lower in cultured versus freshly isolated thymocytes. Consistent with this prediction, β-catenin levels increase in CD4+CD8+ thymocytes after overnight incubation (Fig. 5C). We speculate that growth factors present in the serum of most culture mediums (as well as in some serum free media) activate signaling pathways that inhibit GSK3 activity.
GSK3 inhibition influences thymocyte survival
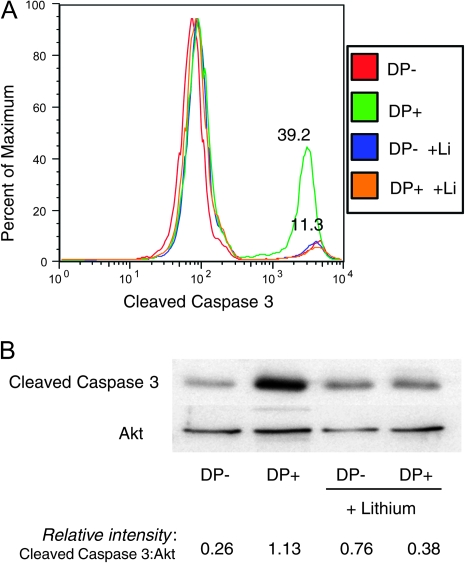
TCR/CD28 stimulation induces cell death in CD4+CD8+ thymocytes but not mature T cells (4–6). The molecular basis for this marked difference in response is still unclear, but the data presented here raise the possibility that GSK3 activity may play a role in the susceptibility of CD4+CD8+ thymocytes to TCR-mediated apoptosis. Consistent with this possibility, lithium addition profoundly reduced TCR-mediated increases in caspase 3 cleavage among CD4+CD8+ thymocytes (Fig. 6A and B).
Fig. 6.
GSK3 inhibition enhances survival of CD4+CD8+ thymocytes in response to negative selecting TCR signals. (A) Purified DP thymocytes were cultured in the presence or absence of plate-bound anti-TCR/CD2/CD28 antibodies and the presence or absence of 60 mM lithium for 4 h. Cells were fixed, permeabilized, stained for cleaved caspase 3 and analyzed by flow cytometry. Results from the four conditions are shown as an overlay histogram. TCR-stimulated DP thymocytes were the only group that showed an increase in the frequency of cleaved caspase 3 ± cells. All three other groups (unstimulated DP in the presence or absence of lithium and stimulated DP in the presence of lithium) showed essentially the same frequency of cleaved caspase 3+ cells (∼11%). These results are representative of at least three experiments. (B) In a different experiment, purified DP and SP thymocytes were subject to the same treatment as in (a), lysed and analyzed for cleaved caspase 3 by western blot. Relative intensities of bands (cleaved caspase 3/Akt) are indicated.
Discussion
In this report, we examine the molecular basis for the failure of immature CD4+CD8+ thymocytes to polarize their MTOC in response TCR stimulation (9) and provide evidence that GSK3-mediated instability of tubulin polymers plays a key role. We show that CD4+CD8+ thymocytes contain an abundance of tyrosinated tubulin, an indicator of microtubule instability, as well as a relatively high level of GSK3 activity, which regulates microtubule stability. Most importantly, immature CD4+CD8+ thymocytes acquire the ability to polarize their MTOC to the site of TCR stimulation in the presence of GSK3 inhibitors. Interestingly, GSK3 inhibition also provides CD4+CD8+ thymocytes with a survival advantage in response to pro-apoptotic TCR signals.
Our observations also imply that GSK3 activity differs between immature CD4+CD8+ and mature thymocytes—even prior to their receipt of TCR signals. This difference is likely to reflect differences in the receptor–ligand interactions they are experiencing in situ. Our data suggest that mature T cells constitutively experience signals that abrogate GSK3 activity. There are several possible candidate signaling pathways (40). Growth factor receptors (including the insulin receptor) and antigen receptors activate kinases, including Akt, p70-S6K, p90-rsk and PKA that phosphorylate GSK3 at serine 9/21 and inhibit its activity (41–43). However, our observation that GSK3 phosphorylation at Ser9/21 does not differ between freshly isolated immature and mature T cells suggest that other influences play a role in establishing GSK3 activity levels in developing T cells. GSK3 can be inhibited by phosphorylation at a distinct site by p38 (35), which can also be activated by growth factors. However, p38 is also activated by stress and appears to play more of a role in apoptosis than survival of thymocytes (44, 45).
Perhaps, then, the strongest candidate for influencing the developmental differences in GSK3 activity between immature and mature T cells is the Wnt signaling pathway. Wnt/Frizzled interactions inhibit GSK3 activity by promoting its dissociation from the ‘destruction’ complex (which includes Axin, APC and β-catenin). Wnt signaling plays a key role in multiple stages of T-cell development, including the final maturation steps. Thymic epithelial cells produce Wnt ligands and both epithelial cells and thymocytes express Frizzled receptors (39, 46). Importantly, the expression pattern of Wnt ligands and Frizzled receptors vary by microenvironment (46). For instance, different Wnt ligands dominate in the thymic cortex versus the thymic medulla, suggesting that immature and mature thymocytes experience different levels and/or quality of Wnt signaling (46, 47). Indeed, mature T cells exhibit evidence of more robust Wnt signaling, which appears to regulate survival of immature CD4+CD8+ thymocytes during positive selection (39, 47, 48). Although the studies that generated these observations focused primarily on the influence of TCF/LEF, transcription factors downstream of GSK3 and β-catenin, they suggest a basis for the differences in GSK3 activity responsible for TCR regulation of MTOC polarization that we describe in this report.
Our observation that GSK3 inhibition also abrogates TCR-mediated apoptosis could be related to GSK3’s more downstream effects on TCF/LEF or other transcription factors, including NFAT (49). However, it is tempting to speculate that its more acute effects on TCR-stimulated MTOC polarization play a role. MTOC polarization is known to facilitate not only the directional delivery of cytokine and/or granzyme containing vesicles to the cell surface but also the delivery of influential signaling molecules to the site of interaction (10, 11, 15, 50). In fact, T lymphocytes fail to form an organized IS when MTOC polarization is abrogated in part because vesicles containing signaling regulators never reach the site of T-cell activation (8). It is therefore possible that the inability of DP thymocytes to polarize their MTOC has a direct impact on their ability to interpret signals—quantitatively (because of a reduction in delivery of additional signaling molecules) and/or qualitatively (because of reduction in delivery of specific anti-apoptotic signaling molecules, perhaps via distinct vesicles).
Finally, it is important to recognize that GSK3 activity is assessed, in this and other studies of primary cells, by examining the behavior of its substrates. β-catenin stabilization is considered one of the more reliable indicators (36) but remains an indirect measure of GSK3 activity. One can also infer GSK3 activity in T cells from the phosphorylation and subcellular localization of another of its substrates, the transcription factor, NFAT (49): phosphorylated NFAT is retained in the cytoplasm. Our analysis of the subcellular localization of NFAT in primary cells indicates that NFAT is strictly cytoplasmic in freshly isolated immature DP thymocytes but is found in multiple subcellular compartments (cytoplasmic, organellar, cytoskeletal and to a small extent nuclear) in freshly isolated SP T cells (data not shown). These data provide independent support for the indications from our β-catenin analysis that GSK3 is more active in DP than SP T cells. However, we recognize that the regulation of NFAT localization is complex. For instance, it can be phosphorylated by other kinases present in T lymphocytes, including p38 (51), so this measure is also indirect, at best. Ultimately, genetic analysis will need to be done to confirm the albeit strong indications of this study that GSK3 activity differs between immature and mature T cells and directly regulates MTOC stabilization.
In summary, these results suggest that GSK3 can exert a proximal acute effect on TCR signals by influencing microtubule function (40). They also raise the provocative possibility that immature CD4+CD8+ thymocytes are not incapable of responding to TCR signals like their mature descendants but instead may be actively inhibited from doing so.
Funding
National Science Foundation (MCB-0744570 to J.A.P.); Howard Hughes Medical Institute; Beckman Foundation; Haverford College Provost Office research funds.
Acknowledgments
Many thanks to all former students who have inspired and contributed to the work we describe, in particular Mary Chang and Emily Nietzreba.
References
- 1.Hogquist K, Baldwin T, Jameson S. Central tolerance: learning self-control in the thymus. Nat. Rev. Immunol. 2005;5:772. doi: 10.1038/nri1707. [DOI] [PubMed] [Google Scholar]
- 2.Alam S, Davies G, Lin C, et al. Qualitative and quantitative differences in T cell receptor binding of agonist and antagonist ligands. Immunity. 1999;10:227. doi: 10.1016/s1074-7613(00)80023-0. [DOI] [PubMed] [Google Scholar]
- 3.Starr T, Jameson S, Hogquist K. Positive and negative selection of T cells. Annu. Rev. Immunol. 2003;21:139. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 4.Punt J, Osborne B, Takahama Y, Sharrow S, Singer A. Negative selection of CD4+CD8+ thymocytes by T cell receptor-induced apoptosis requires a costimulatory signal that can be provided by CD28. J. Exp. Med. 1994;179:709. doi: 10.1084/jem.179.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li R, Page D. Requirement for a complex array of costimulators in the negative selection of autoreactive thymocytes in vivo. J. Immunol. 2001;166:6050. doi: 10.4049/jimmunol.166.10.6050. [DOI] [PubMed] [Google Scholar]
- 6.Page D, Kane L, Allison J, Hedrick S. Two signals are required for negative selection of CD4+CD8+ thymocytes. J Immunol. 1993;151:1868. [PubMed] [Google Scholar]
- 7.Cunningham N, Artim S, Fornadel C, et al. Immature CD4+CD8+ thymocytes and mature T cells regulate Nur77 distinctly in response to TCR stimulation. J. Immunol. 2006;177:6660. doi: 10.4049/jimmunol.177.10.6660. [DOI] [PubMed] [Google Scholar]
- 8.Martín-Cófreces N, Robles-Valero J, Cabrero J, et al. MTOC translocation modulates IS formation and controls sustained T cell signaling. J. Cell. Biol. 2008;182:951. doi: 10.1083/jcb.200801014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hailman E, Allen P. Inefficient cell spreading and cytoskeletal polarization by CD4+CD8+ thymocytes: regulation by the thymic environment. J. Immunol. 2005;175:4847. doi: 10.4049/jimmunol.175.8.4847. [DOI] [PubMed] [Google Scholar]
- 10.Kupfer A, Swain S, Singer S. The specific direct interaction of helper T cells and antigen-presenting B cells. II. Reorientation of the microtubule organizing center and reorganization of the membrane-associated cytoskeleton inside the bound helper T cells. J. Exp. Med. 1987;165:1565. doi: 10.1084/jem.165.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kupfer A, Dennert G, Singer S. The reorientation of the Golgi apparatus and the microtubule-organizing center in the cytotoxic effector cell is a prerequisite in the lysis of bound target cells. J. Mol. Cell. Immunol. 1985;2:37. [PubMed] [Google Scholar]
- 12.Westermann S, Weber K. Post-translational modifications regulate microtubule function. Nat. Rev. Mol. Cell. Biol. 2003;4:938. doi: 10.1038/nrm1260. [DOI] [PubMed] [Google Scholar]
- 13.Kuhné M, Lin J, Yablonski D, et al. Linker for activation of T cells, zeta-associated protein-70, and Src homology 2 domain-containing leukocyte protein-76 are required for TCR-induced microtubule-organizing center polarization. J. Immunol. 2003;171:860. doi: 10.4049/jimmunol.171.2.860. [DOI] [PubMed] [Google Scholar]
- 14.Ardouin L, Bracke M, Mathiot A, et al. Vav1 transduces TCR signals required for LFA-1 function and cell polarization at the immunological synapse. Eur. J. Immunol. 2003;33:790. doi: 10.1002/eji.200323858. [DOI] [PubMed] [Google Scholar]
- 15.Lowin-Kropf B, Shapiro V, Weiss A. Cytoskeletal polarization of T cells is regulated by an immunoreceptor tyrosine-based activation motif-dependent mechanism. J. Cell. Biol. 1998;140:861. doi: 10.1083/jcb.140.4.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martín-Cófreces N, Sancho D, Fernández E, et al. Role of Fyn in the rearrangement of tubulin cytoskeleton induced through TCR. J. Immunol. 2006;176:4201. doi: 10.4049/jimmunol.176.7.4201. [DOI] [PubMed] [Google Scholar]
- 17.Stowers L, Yelon D, Berg L, Chant J. Regulation of the polarization of T cells toward antigen-presenting cells by Ras-related GTPase CDC42. Proc. Natl Acad. Sci. USA. 1995;92:5027. doi: 10.1073/pnas.92.11.5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez T, Kumar K, Medeiros R, Shimizu Y, Leibson P, Billadeau D. Formins regulate the actin-related protein 2/3 complex-independent polarization of the centrosome to the immunological synapse. Immunity. 2007;26:177. doi: 10.1016/j.immuni.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Combs J, Kim S, Tan S, et al. Recruitment of dynein to the Jurkat immunological synapse. Proc. Natl Acad. Sci. USA. 2006;103:14883. doi: 10.1073/pnas.0600914103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kreitzer G, Liao G, Gundersen G. Detyrosination of tubulin regulates the interaction of intermediate filaments with microtubules in vivo via a kinesin-dependent mechanism. Mol. Biol. Cell. 1999;10:1105. doi: 10.1091/mbc.10.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baas P, Ahmad F, Pienkowski T, Brown A, Black M. Sites of microtubule stabilization for the axon. J. Neurosci. 1993;13:2177. doi: 10.1523/JNEUROSCI.13-05-02177.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown A, Slaughter T, Black M. Newly assembled microtubules are concentrated in the proximal and distal regions of growing axons. J. Cell. Biol. 1992;119:867. doi: 10.1083/jcb.119.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Black M. Microtubule assembly and turnover in growing axons. J. Neurosci. 1996;16:531. doi: 10.1523/JNEUROSCI.16-02-00531.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunn S, Morrison E, Liverpool T, et al. Differential trafficking of Kif5c on tyrosinated and detyrosinated microtubules in live cells. J. Cell. Sci. 2008;121:1085. doi: 10.1242/jcs.026492. [DOI] [PubMed] [Google Scholar]
- 25.Goold R, Owen R, Gordon-Weeks P. Glycogen synthase kinase 3beta phosphorylation of microtubule-associated protein 1B regulates the stability of microtubules in growth cones. J. Cell. Sci. 1999;112:3373. doi: 10.1242/jcs.112.19.3373. [DOI] [PubMed] [Google Scholar]
- 26.Zumbrunn J, Kinoshita K, Hyman A, Näthke I. Binding of the adenomatous polyposis coli protein to microtubules increases microtubule stability and is regulated by GSK3 beta phosphorylation. Curr. Biol. 2001;11:44. doi: 10.1016/s0960-9822(01)00002-1. [DOI] [PubMed] [Google Scholar]
- 27.Forde J, Dale T. Glycogen synthase kinase 3: a key regulator of cellular fate. Cell. Mol. Life Sci. 2007;64:1930. doi: 10.1007/s00018-007-7045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshimura T, Kawano Y, Arimura N, Kawabata S, Kikuchi A, Kaibuchi K. GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell. 2005;120:137. doi: 10.1016/j.cell.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 29.Goold R, Gordon-Weeks P. Microtubule-associated protein 1B phosphorylation by glycogen synthase kinase 3beta is induced during PC12 cell differentiation. J. Cell. Sci. 2001;114:4273. doi: 10.1242/jcs.114.23.4273. [DOI] [PubMed] [Google Scholar]
- 30.McCarthy S, Kruisbeek A, Uppenkamp I, Sharrow S, Singer A. Engagement of the CD4 molecule influences cell surface expression of the T-cell receptor on thymocytes. Nature. 1988;336:76. doi: 10.1038/336076a0. [DOI] [PubMed] [Google Scholar]
- 31.Cibotti R, Punt J, Dash K, Sharrow S, Singer A. Surface molecules that drive T cell development in vitro in the absence of thymic epithelium and in the absence of lineage-specific signals. Immunity. 1997;6:245. doi: 10.1016/s1074-7613(00)80327-1. [DOI] [PubMed] [Google Scholar]
- 32.Peris L, Wagenbach M, Lafanechère L, et al. Motor-dependent microtubule disassembly driven by tubulin tyrosination. J. Cell. Biol. 2009;185:1159. doi: 10.1083/jcb.200902142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sánchez C, Pérez M, Avila J. GSK3beta-mediated phosphorylation of the microtubule-associated protein 2C (MAP2C) prevents microtubule bundling. Eur. J. Cell Biol. 2000;79:252. doi: 10.1078/s0171-9335(04)70028-x. [DOI] [PubMed] [Google Scholar]
- 34.Lovestone S, Hartley C, Pearce J, Anderton B. Phosphorylation of tau by glycogen synthase kinase-3 beta in intact mammalian cells: the effects on the organization and stability of microtubules. Neuroscience. 1996;73:1145. doi: 10.1016/0306-4522(96)00126-1. [DOI] [PubMed] [Google Scholar]
- 35.Thornton TM, Pedraza-Alva G, Deng B, et al. Phosphorylation by p.38 MAPK as an alternative pathway for GSK3beta inactivation. Science. 2008;320:667. doi: 10.1126/science.1156037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang F, Phiel C, Spece L, Gurvich N, Klein P. Inhibitory phosphorylation of glycogen synthase kinase-3 (GSK-3) in response to lithium. Evidence for autoregulation of GSK-3. J. Biol. Chem. 2003;278:33067. doi: 10.1074/jbc.M212635200. [DOI] [PubMed] [Google Scholar]
- 37.Yost C, Farr G, Pierce S, Ferkey D, Chen M, Kimelman D. GBP, an inhibitor of GSK-3, is implicated in Xenopus development and oncogenesis. Cell. 1998;93:1031. doi: 10.1016/s0092-8674(00)81208-8. [DOI] [PubMed] [Google Scholar]
- 38.Hedgepeth C, Conrad L, Zhang J, Huang H, Lee V, Klein P. Activation of the Wnt signaling pathway: a molecular mechanism for lithium action. Dev. Biol. 1997;185:82. doi: 10.1006/dbio.1997.8552. [DOI] [PubMed] [Google Scholar]
- 39.Yu Q, Sharma A, Sen JM. TCF1 and beta-catenin regulate T cell development and function. Immunol. Res. 2010;47:45. doi: 10.1007/s12026-009-8137-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dominguez I, Green J. Missing links in GSK3 regulation. Dev. Biol. 2001;235:303. doi: 10.1006/dbio.2001.0317. [DOI] [PubMed] [Google Scholar]
- 41.Clodfelder-Miller B, De Sarno P, Zmijewska A, Song L, Jope R. Physiological and pathological changes in glucose regulate brain Akt and glycogen synthase kinase-3. J. Biol. Chem. 2005;280:39723. doi: 10.1074/jbc.M508824200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fang X, Yu S, Lu Y, Bast RJ, Woodgett J, Mills G. Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc. Natl Acad. Sci. USA. 2000;97:11960. doi: 10.1073/pnas.220413597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sutherland C, Leighton I, Cohen P. Inactivation of glycogen synthase kinase-3 beta by phosphorylation: new kinase connections in insulin and growth-factor signalling. Biochem. J. 1993;296:15. doi: 10.1042/bj2960015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sohn SJ, Thompson J, Winoto A. Apoptosis during negative selection of autoreactive1 thymocytes. Curr. Opin. Immunol. 2007;19:510. doi: 10.1016/j.coi.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 45.Sugawara T, Moriguchi T, Nishida E, Takahama Y. Differential roles of ERK and p.38 MAP kinase pathways in positive and negative selection of T lymphocytes. Immunity. 1998;9:565. doi: 10.1016/s1074-7613(00)80639-1. [DOI] [PubMed] [Google Scholar]
- 46.Pongracz J, Hare K, Harman B, Anderson G, Jenkinson E. Thymic epithelial cells provide WNT signals to developing thymocytes. Eur. J. Immunol. 2003;33:1949. doi: 10.1002/eji.200323564. [DOI] [PubMed] [Google Scholar]
- 47.Staal F, Luis T, Tiemessen M. WNT signalling in the immune system: WNT is spreading its wings. Nat. Rev. Immunol. 2008;8:581. doi: 10.1038/nri2360. [DOI] [PubMed] [Google Scholar]
- 48.Mulroy T, Xu Y, Sen J. beta-Catenin expression enhances generation of mature thymocytes. Int. Immunol. 2003;15:1485. doi: 10.1093/intimm/dxg146. [DOI] [PubMed] [Google Scholar]
- 49.Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109(Suppl):S67. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- 50.Kuhn J, Poenie M. Dynamic polarization of the microtubule cytoskeleton during CTL-mediated killing. Immunity. 2002;16:111. doi: 10.1016/s1074-7613(02)00262-5. [DOI] [PubMed] [Google Scholar]
- 51.Macian F. NFAT proteins: key regulators of T-cell development and function. Nat. Rev. Immunol. 2005;5:472. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]