Abstract
Objective
To evaluate the combination of cerebrospinal fluid biomarkers of Aβ42, tau, and phosphorylated tau (ptau181) with education and normalized whole brain volume (nWBV) to predict incident cognitive impairment and test the cognitive/brain reserve hypothesis.
Design
Longitudinal cohort study.
Setting
Charles F. and Joanne Knight Alzheimer’s Disease Research Center of Washington University, St. Louis, Missouri.
Participants
Convenience sample of 197 participants aged 50 years and above, with normal cognition (Clinical Dementia Rating [CDR] of 0) at baseline, followed for a mean of 3.3 years.
Main outcome measure
Time to cognitive impairment (CDR ≥ 0.5).
Results
Three-factor interactions between the baseline biomarker values, education, and nWBV were found for Cox proportional hazards models testing tau (p=.03) and ptau (p=.008). Among those with lower tau values, nWBV (hazard ratio [HR]=.54, 95% confidence interval [CI]=.31–.91; p=.02), but not education, was related to time to cognitive impairment. For participants with higher tau values, education interacted with nWBV to predict incident impairment (p=.01). For individuals with lower ptau values, there was no effect of education or nWBV. Education interacted with nWBV to predict incident cognitive impairment among those with higher ptau values (p=.02). In models testing Aβ42, larger nWBV was associated with a slower time to cognitive impairment (HR=.84, 95%CI=.71–.99, p=.0348), but there was no effect of Aβ42 or education.
Conclusions
Among individuals with higher levels of CSF tau and ptau, but normal cognition at baseline, time to incident cognitive impairment is moderated by education and brain volume as predicted by the cognitive/brain reserve hypothesis.
Lower educational attainment and smaller brain, or head, size have been frequently studied as risk factors for Alzheimer’s disease (AD).1–4 Educational attainment is a proxy measure of cognitive reserve: the efficient use of brain networks or the ability to recruit alternate brain networks or cognitive strategies.1,5 Brain size is thought to reflect brain reserve: the number and health of neurons.5–8 Greater amounts of both types of reserve are thought to provide resistance to brain damage due to AD, delaying the time to cognitive impairment.1,5–8
Cross-sectional studies suggest that educational attainment4,7,9–11 and brain size4,6,7 interact with AD pathology to determine current cognitive functioning, such that the impact of a given amount of AD pathology on cognition varies depending on one’s education and brain size. However, until the recent advent of biomarkers of AD pathology, it was not possible to test whether education and brain size modify the association between AD pathology in cognitively normal individuals with the later development of cognitive impairment. The cerebrospinal fluid (CSF) biomarkers of amyloid-beta42 (Aβ42), the primary component of amyloid plaques, are decreased among individuals with AD whereas levels of tau and phosphorylated tau (ptau181), the primary components of neurofibrillary tangles, are increased in AD.12 Abnormal levels of these biomarkers have also been found among cognitively normal individuals and are predictive of later cognitive impairment.13–15
We tested how the CSF biomarkers of Aβ42, tau, and ptau181 combine with education and brain volume to predict incident cognitive impairment in individuals with normal cognition at baseline.
Methods
Participants
Data were collected prospectively from participants enrolled in longitudinal studies at the Charles F. and Joanne Knight Alzheimer’s Disease Research Center at Washington University. Study protocols were approved by the Washington University Medical Center Human Subjects Committee, and written informed consent was obtained from all participants. Detailed information on recruitment and assessment procedures are available.16 In brief, participants in these studies are recruited through word-of-mouth, advertisements, and community events from the greater St. Louis, Missouri area for yearly assessment sessions. Individuals with health conditions, such as metastatic cancer, that may interfere with longitudinal follow-up are excluded from participation.
Clinical assessment, CSF, and brain volume measurement
At the initial and each annual assessment thereafter, participants take part in neurological and physical examinations, and are accompanied by a collateral source (CS) who knows the participant well. Experienced clinicians obtain health and medication histories and conduct semi-structured interviews with the participant and CS separately. The clinicians use the information obtained from the participant and CS interviews to generate a Clinical Dementia Rating (CDR),17–19 reflecting the presence or absence of dementia. The global CDR is based on a standard scoring algorithm which integrates functioning in six individual domains: memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care. The CDR Sum of Boxes (CDR-SB) is obtained by summing the scores from the six domains.18 The CDR has established reliability.20,21 Global CDR scores of 0=normal cognition; 1=mild dementia, 2=moderate dementia, and 3=severe dementia. CDR 0.5 designates “uncertain dementia” if the etiology of the cognitive impairment cannot be determined or very mild dementia if on clinical grounds an etiologic diagnosis can be made.
Our participants with cognitive impairment at the CDR 0.5 stage can be diagnosed with very mild dementia of the Alzheimer type (DAT) when there is a history of the gradual onset and progression of cognitive problems that represent a decline from that individual’s prior level of cognitive function and interfere to at least some degree with usual activities at home and in the community. We have demonstrated that our CDR 0.5/DAT participants have progressive cognitive deterioration typical for DAT and of those coming to autopsy, AD is confirmed in 92%.22 Moreover, it is well recognized that some individuals rated as CDR 0.5 can merit a DAT diagnosis.23
To obtain CSF from participants, trained neurologists use a 22-gauge Sprotte spinal needle to draw 20–30 mL of CSF at 8:00 AM following an overnight fast. CSF samples are gently inverted and centrifuged at low speed to avoid possible gradient effects and then frozen at −84°C24 after aliquoting into polypropylene tubes. CSF samples for Aβ42, tau, and ptau181 are analyzed using enzyme-linked immunosorbant assay (INNOTEST; Innogenetics, Ghent, Belgium).
Normalized whole brain volume (nWBV), reflecting the percentage of the intracranial cavity occupied by brain, was obtained using previously established methods.25 Briefly, the MP-RAGE data were intensity normalized.26 A validated segmentation tool was then used to classify brain tissue as cerebral spinal fluid (CSF), gray, or white matter.27,28 Correction of intensity inhomogeneity was accomplished by an automated procedure to minimize intensity variation within contiguous regions. Based on intensity limits and contour (intensity gradient) detection, contiguous region boundaries were identified (without brain masking). The bias field was modeled as a general, second-order polynomial in three dimensions (10 free parameters).26 Segmentation began with an initial estimation step to obtain and classify tissue parameters. Using a three-step, expectation-maximization algorithm, class labels and tissue parameters were then updated to iterate toward the maximum likelihood estimates of a hidden Markov random field model. This model used spatial proximity to constrain the probability with which voxels of a given intensity are assigned to each tissue class. Finally, the brain volume estimate was taken as the sum of white and gray-matter voxels within the atlas-based brain mask and expressed as the percentage of the mask.
Inclusion criteria
Archival data from participants who (1) donated CSF between June 18, 1998 and May 18, 2009; (2) were aged 50 years or older at the time of donation; (3) had normal cognition (CDR 0) at the closest clinical assessment within one-year prior, or one month following, donation; (4) had magnetic resonance imaging (MRI) with measurement of brain volume within one year of donation; and (5) had at least one subsequent clinical assessment were used in this study.
Statistical analyses
Cox proportional hazards models were used to test the three-factor interaction of each of the biomarker variables (Aβ42, tau, ptau) with education in years and nWBV in determining time from the baseline assessment to cognitive impairment (i.e., CDR > 0). All predictor variables were treated as continuous.
For models where the three factor interaction was significant, Cox proportional hazards models were conducted separately for individuals with biomarker values above, and below, the median; and the two-factor interaction between education and nWBV tested in these models.
For models where the three-factor interaction was not significant, the models were repeated testing two-factor interactions among the biomarker, education, and nWBV variables. If no two-factor interactions were significant, the final model was comprised of the main effects of each variable. All models included terms adjusting for, and simultaneously testing the effects of, gender, age, race, the presence of an APOE ε4 allele, and the MRI scanner used.
To graphically display significant interaction effects, the biomarker, education, and nWBV variables were each dichotomized, reflecting lower and higher values on the variable, using a median split and Kaplan-Meier survival curves were generated for each combination of these variables.
We also explored whether there were differences in the slope of scores across the follow-up period as a function of these 8 possible combinations of higher and lower values of the biomarker, education, and nWBV variables. In these analyses, mixed linear models tested whether the slope of scores on the CDR Sum of Boxes, Mini-Mental State Examination29 (MMSE), and Short Blessed Test30 differed as a function of the combination variable while adjusting for gender, age, race, and APOE4.
Results
One-hundred ninety-seven participants, followed for a mean of 3.3 (SD=2.0) years, met inclusion criteria (Table 1). Of these, N=26 developed cognitive impairment a mean of 3.01 (SD=1.93) years following baseline. Table 2 shows the clinical diagnoses assigned at the time of first CDR>0. We consider those individuals who received a DAT diagnosis to meet “formal” criteria for very mild dementia, although we acknowledge that as the boundaries for MCI and dementia overlap, others may classify these individuals as MCI. At the time of first CDR=0.5, individuals with a DAT diagnosis had greater impairment than those with an Uncertain diagnosis as reflected in worse mean performance on an autobiographical memory test31 (1.25 vs. 1.68, p=.0432) and in higher mean CDR Sum of Boxes (1.94 vs. 0.85, p=.0193).
Table 1.
Baseline demographics (N=197).
| Characteristics | |
|---|---|
| Age, mean (SD), y | 68.6 (9.0) |
| Women, No. (%) | 128 (65.0) |
| Minority race, No. (%) | 16 (8.1) |
| Education, mean (SD), y | 15.7 (2.9) |
| APOE genotype, No. (%) | |
| 22 | 2 (1.0) |
| 23 | 26 (13.2) |
| 24 | 9 (4.6) |
| 33 | 98 (49.8) |
| 34 | 55 (27.9) |
| 44 | 7 (3.55) |
| nWBV, mean (SD), % of intracranial volume | 77.7 (3.4) |
| MMSE, mean (SD) | 29.0 (1.3) |
| Aβ42, mean (SD), pg/mL | 616.8 (251.3) |
| tau, mean (SD), pg/mL | 304.1 (161.4) |
| ptau, mean (SD), pg/mL | 55.9 (24.7) |
| Follow-up time, mean (SD), y | 3.3 (2.0) |
Abbreviations: nWBV=normalized whole brain volume; MMSE=Mini-Mental State Examination; SD=standard deviation.
Table 2.
Clinical diagnoses at the time of first CDR>0 for participants who progressed.
| Participant | Dx 1 | Dx 2 | Dx 3 | Dx 4 | Dx 5 |
|---|---|---|---|---|---|
| 1 | DAT | ||||
| 2 | DAT | ||||
| 3 | DAT | ||||
| 4 | DAT | ||||
| 5 | DAT | ||||
| 6 | DAT | ||||
| 7 | DAT | ||||
| 8 | DAT Uncertain |
||||
| 9 | dementia Uncertain |
||||
| 10 | dementia Uncertain |
||||
| 11 | dementia Uncertain |
||||
| 12 | dementia Uncertain |
||||
| 13 | dementia Uncertain |
||||
| 14 | dementia Uncertain |
||||
| 15 | dementia Uncertain |
||||
| 16 | dementia Uncertain |
||||
| 17 | dementia Uncertain |
||||
| 18 | dementia Uncertain |
Mood disorder | |||
| 19 | dementia Uncertain |
Mood disorder | |||
| 20 | dementia Uncertain |
Mood disorder | |||
| 21 | dementia Uncertain |
Mood disorder | |||
| 22 | dementia | Mood disorder | |||
| Uncertain | Global cerebral | ||||
| 23 | dementia Uncertain |
hypoperfusion | |||
| 24 | dementia | ADHD | |||
| Uncertain | Anxiety | Sleep | |||
| 25 | dementia | Alcoholism | ADHD | disorder | disorder |
| Vascular | Cerebrovascular | ||||
| 26 | dementia | disease |
Abbreviations: Dx = diagnosis; CDR = Clinical Dementia Rating; DAT = Dementia of the Alzheimer type; ADHD = Attention deficit hyperactivity disorder.
In the survival models testing Aβ42, there were no interactions with education or nWBV. The final model indicated that larger nWBV was associated with a slower time to cognitive impairment (Hazard ratio [HR]=.81, 95% confidence interval [CI]=.68–.97, p=.02), but there was no effect of Aβ42 (p=.24) or education (p=.07).
Three-factor interactions between the biomarker values, education, and nWBV were found for models testing tau (p=.02) and ptau (p=.008).
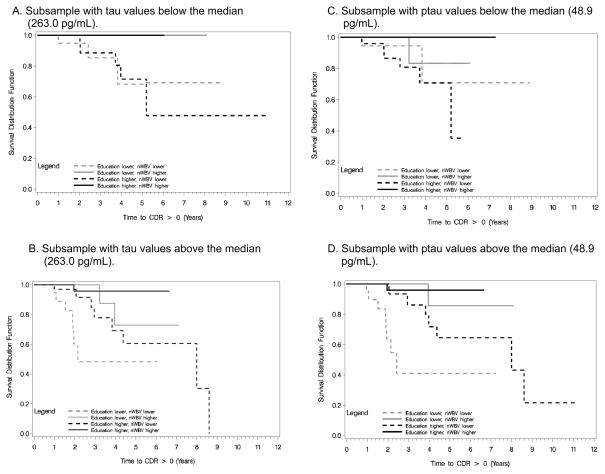
Among those with baseline tau values below the median, nWBV (HR=.54, 95%CI=.31–.91; p=.02), but not education (p=.996), was related to time to cognitive impairment, and there was no interaction between these variables (p=.39; Figure 1A). Of the 8 individuals with lower tau values who developed cognitive impairment (all of whom had nWBV values below the median nWBV) only 2 (25%) received a subsequent diagnosis of DAT at some time during the follow-up period. The remaining 6 had diagnoses of uncertain dementia (N=5; 3 of these with a secondary diagnosis of mood disorder) or vascular dementia with a secondary diagnosis of Parkinson’s disease (N=1). By contrast, 8 of 15 (53.3%) participants with smaller nWBV but with higher tau values received DAT diagnoses at some point over follow-up. For those with tau values above the median, education interacted with nWBV to predict incident impairment (p=.01; Figure 1B).
Figure 1.
Kaplan-Meier curves illustrating the 3-factor interactions among education, normalized whole brain volume (nWBV), and the cerebrospinal biomarkers of tau and ptau.
For individuals with lower ptau values, there was no effect of education (p=.89) or nWBV (p=.14), and no interaction between them (p=.9373; Figure 1C). However, education and nWBV interacted to predict incident cognitive impairment among those with higher ptau values (p=.02; Figure 1D).
Other variables that independently predicted time to impaired cognition were minority race, which was associated with a faster time to impairment in each of the biomarker models (p<.007), and male gender (p=.04) which was associated with more rapid cognitive impairment in the model including tau. There was no relationship between age, APOE4, or scanner type and incident impairment after adjustment for other variables in the model.
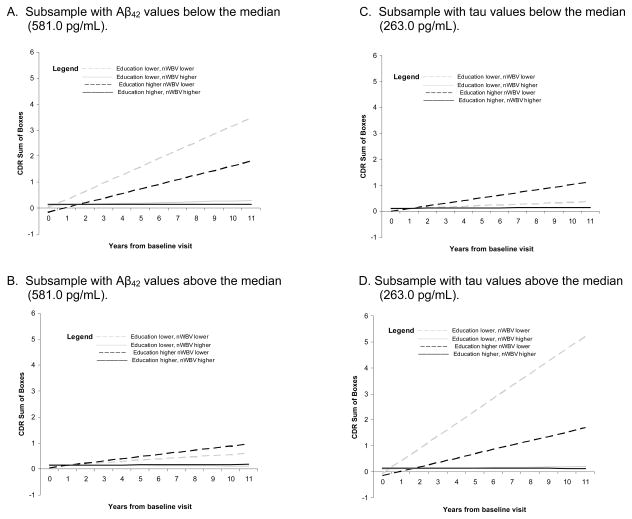
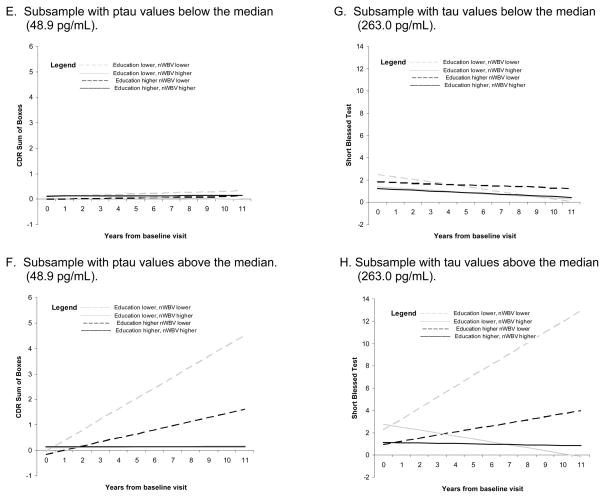
In the mixed model analyses testing the 8 possible combinations of higher and lower values of the biomarker, education, and nWBV variables, the slope of scores on the CDR-SB differed as a function of the “combination” variable, for analyses testing Aβ42 (p=.0007), tau (p<.0001), and ptau (<.0001); and on the SBT for the analysis testing tau (p=.0173). As shown in Figure 2, the significant results generally confirm those found using CDR>0 as the endpoint. The slope of scores on the MMSE did not differ across the combination variable levels.
Figure 2.
Mean slope of global scale scores for combinations of higher and lower values of the biomarker, education, and nWBV variables for significant mixed-model analyses.
Comment
Accumulating evidence suggests that the presence of AD biomarkers in cognitively normal persons is a harbinger of eventual cognitive impairment,13–15 and much current effort is devoted to developing therapies that can halt the disease process. When these therapies are ready for use, it is thought that they may be most effective if administered at the time that biomarkers show abnormal values, but before dementia symptoms occur.15 However, since biomarker levels may become abnormal a decade or more before clinical symptoms appear,32 it is vital to understand the time course between abnormal biomarker values, the onset of cognitive impairment, and characteristics that influence that time course, to avoid exposing healthy individuals to medications, and their potential side effects, many years before they are needed.
Our results indicate that among individuals with higher levels of CSF tau and ptau, but normal cognition at baseline, the time to incident cognitive impairment is moderated by education and brain volume. More education and larger nWBV appear to slow the rate of impairment onset in the presence of tau-related pathology, whereas individuals with both lower levels of education and smaller nWBV have the most rapid onset. As theorized by others, greater education may provide resistance to dementia in the presence of brain damage because more education may be associated with the use of particular cognitive processing approaches or enlistment of compensatory processes, or, may serve as a proxy for another factor, such as innate intelligence.5 Individuals with larger nWBVs may have sufficient neuronal resources to continue normal functioning in the presence of AD pathology for a longer time,33 and/or, these individuals may have experienced less neuronal neurodegeneration despite having similar abnormal biomarker levels as other individuals. Education and nWBV do not interact to predict future cognitive impairment when lower levels of brain tau and ptau are present.
Previously, cross-sectional autopsy studies including individuals with dementia as well as those with normal cognition prior to death have suggested that education and brain volume interact with AD pathology to predict concurrent cognitive performance.34,35 In these studies, education was found to interact with amyloid plaque, but not tangle, pathology. 34,35 In the present study, conducted only with individuals who were cognitively normal at baseline, we found a modifying effect of education and nWBV on incident cognitive impairment for tau-based, but not amyloid-based, pathology. In fact, the main effect of Aβ42 itself was not significant in the primary multivariate analyses. This is consistent with our previous finding, using a smaller subsample of these individuals, of only a marginally significant effect (p=.09) of Aβ42 on incident AD when education and nWBV were included in the same model.36 However, Aβ42 combined with education and nWBV to predict the slope of CDR-SB scores across the follow-up period, suggesting that Aβ42 interacts with education and nWBV in a similar manner to that exhibited by tau and ptau, although as shown in Figure 2, the effect is less dramatic. With a longer follow-up period, or larger sample size, it is possible that a significant 3-way interaction effect among Aβ42, education, and nWBV would be found using the endpoint of CDR>0. The categorical variable reflecting combined levels of the biomarkers, education, and nWBV was unrelated to the slope of scores on the MMSE. As pointed out by others,37 the MMSE may be less sensitive to cognitive decline compared to global dementia severity measures such as the CDR-SB and SBT.
Interestingly, nWBV was found to be associated with incident cognitive impairment even among individuals with tau levels below the baseline median. Brain volume decline, in addition to occurring as a consequence of neuron loss in AD, also occurs as a function of normal aging.38 Although based on small sample numbers, individuals with smaller nWBV and lower tau levels who developed cognitive impairment were less likely to receive DAT diagnoses as an explanation of their cognitive problems, compared to individuals with smaller nWBV and higher tau values. This suggests that individuals with smaller nWBV may be more vulnerable to cognitive impairment due to reasons other than underlying AD. However, this interpretation should be viewed with caution, since the effect of nWBV was not significant when examined in the presence of low ptau.
Relatedly, we found no effect of age on incident impairment in the multivariate models. As previously noted, age and nWBV are tightly correlated among our participants.36 Thus, when one variable is present in the model, the other adds little additional predictive power.
There were no significant effects of APOE4 status when considered together with the CSF biomarkers in predicting incident cognitive impairment. This result is similar to our previous finding that APOE4 did not increase the predictive accuracy of CSF biomarker models for development of incident AD.36 That study also demonstrated that APOE4 was helpful in distinguishing prevalent AD from normal cognition.36 It is possible that APOE genotype, when tested together with CSF biomarkers, might show independent effects on incident cognitive impairment in studies using a larger sample size or longer follow-up period.
Limitations of the study include the use of a convenience sample as well as a relatively short average follow-up time of 3.3 years. Given these limitations, our results provide strong support for the brain and cognitive reserve hypotheses,1,5–8 and suggest that education and nWBV are influential in mediating the time to cognitive impairment when tau-based pathology is present.
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke [grant number P30 NS057105]; National Institute on Aging [grant numbers P50 AG005681, P01 AG003991, and P01 AG026276]; the National Center for Research Resources [grant numbers KL2RR024994 and 1UL1RR024992]; and the Charles F. and Joanne Knight Alzheimer’s Research Initiative of the Washington University Alzheimer’s Disease Research Center. The authors thank the participants, investigators, and staff of the Alzheimer’s Disease Research Center Clinical (participant assessments) and Genetics Cores (genotyping) and the investigators and staff of the Biomarker Core for the Adult Children Study (P01 AG026276) for cerebrospinal fluid analytes. Mark Mintun is currently employed by Avid Radiopharmaceuticals.
Footnotes
Disclosures: Dr. David Holtzman is on the scientific advisory boards of Satori, En Vivo, and C2N Diagnostics and has consulted for Pfizer, Bristol-Myers Squibb, and Innogenetics. Dr. Morris has participated or is currently participating in clinical trials of antidementia drugs sponsored Elan, Eli Lilly and Company, and Wyeth; and he has served as a consultant for, or has received speaking honoraria, from AstraZeneca, Bristol-Myers Squibb, Eisai, Elan/Janssen Alzheimer Immunotherapy Program, Genentech, Lilly, Merck, Novartis, Otsuka Pharmaceuticals, Pfizer/Wyeth, Schering Plough.
Author contributions:
Study concept and design: Roe, Holtzman
Acquisition of data: Morris, Fagan, Grant, Marcus, Benzinger, Mintun
Analysis and interpretation of data: Roe,
Drafting of the manuscript: Roe. Marcus
Critical revision of the manuscript for important intellectual content: Roe, Morris, Fagan, Holtzman, Grant, Benzinger, Mintun
Statistical analysis: Roe,
Obtained funding: Morris,
Administrative, technical, or material support: Morris, Fagan, Holtzman, Grant, Marcus, Benzinger, Mintun
Study supervision: Morris, Benzinger
References
- 1.Stern Y, Gurland B, Tatemichi TK, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA. 1994;271(13):1004–1010. [PubMed] [Google Scholar]
- 2.Mori E, Hirono N, Yamashita H, et al. Premorbid brain size as a determinant of reserve capacity against intellectual decline in Alzheimer’s disease. Am J Psychiatry. 1997;154(1):18–24. doi: 10.1176/ajp.154.1.18. [DOI] [PubMed] [Google Scholar]
- 3.Graves AB, Mortimer JA, Bowen JD, et al. Head circumference and incident Alzheimer’s disease. Neurology. 2001;57(9):1453–1460. doi: 10.1212/wnl.57.8.1453. [DOI] [PubMed] [Google Scholar]
- 4.Mortimer JA, Snowdon DA, Markesbery WR. Head circumference, education, and risk of dementia: findings from the Nun Study. J Clin Exp Neruopsychol. 2003;25(5):671–679. doi: 10.1076/jcen.25.5.671.14584. [DOI] [PubMed] [Google Scholar]
- 5.Stern Y. Cognitive reserve and Alzheimer disease. Alz Dis Assoc Disord. 2006;20(2):112–117. doi: 10.1097/01.wad.0000213815.20177.19. [DOI] [PubMed] [Google Scholar]
- 6.Katzman R, Terry R, DeTeresa R, et al. Clinical, pathological, and neurochemical changes in dementia: A subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol. 1988;23(2):138–144. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- 7.Mortimer JA, Borenstein AR, Gosche KM, Snowdon DA. Very early detection of Alzheimer neuropathology and the role of brain reserve in modifying its clinical expression. J Geriatr Psychiatry Neurol. 2005;18(4):218–223. doi: 10.1177/0891988705281869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valenzuela MJ. Brain reserve and the prevention of dementia. Curr Opin Psychiatry. 2008;21(3):296–302. doi: 10.1097/YCO.0b013e3282f97b1f. [DOI] [PubMed] [Google Scholar]
- 9.Bennett DA, Wilson RS, Schneider JA, et al. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology. 2003;60(12):1909–1915. doi: 10.1212/01.wnl.0000069923.64550.9f. [DOI] [PubMed] [Google Scholar]
- 10.Roe CM, Mintun MA, D’Angelo G, et al. Alzheimer disease and cognitive reserve: variation of education effect varies with carbon 11-labeled Pittsburgh Compound B uptake. Arch Neurol. 2008;65:1467–1471. doi: 10.1001/archneur.65.11.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kemppainen NM, Aalto S, Karrasch M, et al. Cognitive reserve hypothesis: Pittsburgh Compound B and fluorodeoxyglucose positron emission tomography in relation to education in mild Alzheimer’s disease. Ann Neurol. 2008;63(1):112–118. doi: 10.1002/ana.21212. [DOI] [PubMed] [Google Scholar]
- 12.Fagan AM, Holtzman DM. Cerebrospinal fluid biomarkers of Alzheimer’s disease. Biomark Med. 2010;4(1):51–63. doi: 10.2217/BMM.09.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fagan AM, Roe CM, Xiong C, Morris JC, Holtzman DM. Cerebrospinal fluid tau/β-amyloid42 ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64(3):343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 14.Li G, Sokal I, Quinn JF, et al. CSF tau/Abeta42 ratio for increased risk of mild cognitive impairment: a follow-up study. Neurology. 2007;69(7):631–639. doi: 10.1212/01.wnl.0000267428.62582.aa. [DOI] [PubMed] [Google Scholar]
- 15.Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6(3):131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 16.Berg L, McKeel DW, Jr, Miller JP, et al. Clinicopathologic studies in cognitively healthy aging and Alzheimer disease: Relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol. 1998;55(3):326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- 17.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 18.Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 19.Williams MM, Roe CM, Morris JC. Stability of the Clinical Dementia Rating: 1979–2007. Arch Neurol. 2009;66(6):773–777. doi: 10.1001/archneurol.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burke WJ, Miller JP, Rubin EH, et al. Reliability of the Washington University Clinical Dementia Rating. Arch Neurol. 1988;45(1):31–32. doi: 10.1001/archneur.1988.00520250037015. [DOI] [PubMed] [Google Scholar]
- 21.Morris JC, Ernesto C, Schaefer K, et al. Clinical dementia rating (CDR) training and reliability protocol: The Alzheimer Disease Cooperative Study experience. Neurology. 1997;48(6):1508–1510. doi: 10.1212/wnl.48.6.1508. [DOI] [PubMed] [Google Scholar]
- 22.Storandt M, Grant EA, Miller JP, Morris JC. Longitudinal course and neuropathological outcomes in original versus revised MCI and in PreMCI. Neurology. 2006;67(3):467–473. doi: 10.1212/01.wnl.0000228231.26111.6e. [DOI] [PubMed] [Google Scholar]
- 23.Petersen RC, Morris JC. Clinical Features. In: Peterson RC, editor. Mild Cognitive Impairment: Aging to Alzheimer’s Disease. New York, New York: Oxford University Press; 2003. pp. 15–39. [Google Scholar]
- 24.Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Aβ42 in humans. Ann Neurol. 2006;59(3):512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 25.Buckner RL, Head D, Parker J, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. NeuroImage. 2004;23(2):724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 26.Styner M, Brechbuhler C, Szekely G, Gerig G. Parametric estimate of intensity inhomogeneities applied to MRI. IEEE Trans Med Imag. 2000;19(3):153–165. doi: 10.1109/42.845174. [DOI] [PubMed] [Google Scholar]
- 27.Smith SM, Zhang Y, Jenkinson M, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17(1):479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algoithm. IEEE Trans Med Imaging. 2001;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 29.Folstein MF, Folstein SE, McHugh PR. Mini-mental State: A practical method for grading the cognitive state of patients for the clinicians. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 30.Katzman R, Brown T, Fuld P, et al. Validation of a short orientation-memory-concentration test of cognitive impairment. Am J Psychiatry. 1983;140(6):734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 31.Dreyfus DM, Roe CM, Morris JC. Autobiographical memory task in assessing dementia. Arch Neurol. 2010;67(7):862–866. doi: 10.1001/archneurol.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fagan AM, Head D, Shah AR, et al. Decreased cerebrospinal fluid Aβ42 correlates with brain atrophy in cognitively normal elderly. Ann Neurol. 2009;65(2):176–183. doi: 10.1002/ana.21559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stern Y. Cognitive reserve. Neuropsychologia. 2009;47(10):2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Education modifies the association of amyloid but not tangles with cognitive function. Neurology. 2005;65(6):953–955. doi: 10.1212/01.wnl.0000176286.17192.69. [DOI] [PubMed] [Google Scholar]
- 35.Roe CM, Xiong C, Miller JP, Cairns NJ, Morris JC. Interaction of neuritic plaques and education predicts dementia. Alz Dis Assoc Disord. 2008;22(2):188–193. doi: 10.1097/WAD.0b013e3181610fff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roe CM, Fagan AM, Williams MM, et al. Improving CSF biomarker accuracy in predicting prevalent and incident Alzheimer’s disease. Neurology. 2011;76(6):501–510. doi: 10.1212/WNL.0b013e31820af900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reisberg B. Global measures: utility in defining and measuring treatment response in dementia. Int Psychogeriatr. 2007;19(3):421–456. doi: 10.1017/S1041610207005261. [DOI] [PubMed] [Google Scholar]
- 38.Fotenos AF, Snyder AZ, Girton LE, Morris JC, Buckner RL. Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology. 2005;64(6):1032–1039. doi: 10.1212/01.WNL.0000154530.72969.11. [DOI] [PubMed] [Google Scholar]