Abstract
Purpose
To define the maximum tolerated dose (MTD), toxicities, and pharmacokinetics of 17-allylamino-17-demethoxygeldanamycin (17-AAG) when administered using continuous and intermittent dosing schedules.
Experimental Design
Patients with progressive solid tumor malignancies were treated with 17-AAG using an accelerated titration dose escalation schema. The starting dose and schedule were 5 mg/m2 daily for 5 days with cycles repeated every 21 days. Dosing modifications based on safety, pharmacodynamic modeling, and clinical outcomes led to the evaluation of the following schedules: daily × 3 repeated every 14 days; twice weekly (days 1, 4, 8, and 11) for 2 weeks every 3 weeks; and twice weekly (days 1 and 4) without interruption. During cycle 1, blood was collected for pharmacokinetic and pharmacodynamic studies.
Results
Fifty-four eligible patients were treated. The MTD was schedule dependent: 56 mg/m2 on the daily × 5 schedule; 112 mg/m2 on the daily × 3 schedule; and 220 mg/m2 on the days 1, 4, 8, and 11 every-21-day schedule. Continuous twice-weekly dosing was deemed too toxic because of delayed hepatotoxicity. Hepatic toxicity was also dose limiting with the daily × 5 schedule. Other common toxicities encountered were fatigue, myalgias, and nausea. This latter adverse effect may have been attributable, in part, to the DMSO-based formulation. Concentrations of 17-AAG above those required for activity in preclinical models could be safely achieved in plasma. Induction of a heat shock response and down-regulation of Akt and Raf-1 were observed in biomarker studies.
Conclusion
The MTD and toxicity profile of 17-AAG were schedule dependent. Intermittent dosing schedules were less toxic and are recommended for future phase II studies.
Heat shock protein 90 (Hsp90) is a molecular chaperone required for the stress-survival response, protein refolding, and the conformational maturation of a subset of signaling proteins (1–3). Several natural products that bind selectively to Hsp90 and inhibit its function have been used to determine its biological role (4–7). These natural products, which include geldanamycin and radicicol, induce the selective degradation of proteins whose activity and/or expression are regulated by Hsp90 (6–8). Sensitive Hsp90 clients include protein kinases [human epidermal growth factor receptor 2 (HER2), Raf-1, and Akt], steroid hormone receptors (androgen receptor and estrogen receptor), and mutant oncoproteins (mutant p53, bcr-abl, and B-Raf; refs. 9–15).
Geldanamycin, the lead compound of the class, proved too toxic for clinical use (16). However, 17-allylamino-17-demethoxygeldanamycin (17-AAG) has activity in human xenograft and genetic murine tumor models as a single agent (12, 17–20). Hsp90 inhibition also has additive and synergistic effects in combination with cytotoxics, biologics, radiation, and anti-angiogenics (17, 20–24). The objectives of the current trial were to determine the safety, pharmacokinetics, and pharmacodynamics of 17-AAG and to define a dose and schedule of administration that could be used in phase II studies.
Patients and Methods
Patient eligibility
Patients with histologically documented, progressive solid tumor malignancies that were refractory to standard therapy were considered. Progressive disease was defined as the development of new lesions or an increase in preexisting lesions on bone scintigraphy, computerized tomography, magnetic resonance imaging, or by physical examination. For prostate cancer patients, progression could also be based on an increasing prostate-specific antigen (PSA) level (>25%). Patients with other solid tumors were ineligible if their sole manifestation of progression was an increase in biochemical markers or an increase in symptoms.
Patients must have recovered from the acute toxicities of any prior therapy and should not have received chemotherapy, radiation therapy, or other investigational anticancer therapeutic drugs for at least 4 weeks. Patients were excluded if they had a history of an allergy to egg or egg products and, because of potential interactions with CYP3A, if they were taking coumadin, verapamil, miconazole, erythromycin, or ketoconazole.
Pre-enrollment testing included a complete history and laboratory studies including complete blood count, differential and platelet counts, prothrombin time, and partial thromboplastin times, and a comprehensive panel consisting of aspartate aminotransferase (AST), albumin, alkaline phosphatase, bilirubin, calcium, creatinine, glucose, total protein, blood urea nitrogen, sodium, potassium, chloride, lactate dehydrogenase, phosphorus, uric acid, bicarbonate, and magnesium. Depending on the disease, studies on markers such as PSA, prostatic acid phosphatase, carcinoembryonic antigen, cancer antigen 125, or carbohydrate antigen 15-3 were done. Entry required an age ≥18 years, KPS ≥70%, and adequate hematologic (WBC ≥3,500 cells/μL; platelets ≥100,000 cells/μL), renal (creatinine ≤1.5 mg/dL or CrCl >60 mL/min), hepatic [bilirubin ≤1.2 × the upper limit of normal; AST/alanine aminotransferase (ALT) <1.5 × upper limit of normal], and coagulation (prothrombin time < the upper limit of normal) function.
Additional diagnostic studies were required for patients who required diuretics for reasons other than hypertension, digoxin for reasons other than atrial fibrillation, and patients with a history of mild to moderate congestive heart failure and patients with the following electrocardiogram results: (a) significant Q waves (>3 mm or >1/3 the height of the QRS complex); (b) ST elevation or depressions of >2 mm that were not attributable to hypertension strain; (c) the absence of a regular sinus rhythm; or (d) the presence of a bundle block. Such patients could be treated if radionuclide angiocardiography showed an ejection fraction >45% and there was no evidence of ventricular aneurysm or other abnormal wall motion.
Other exclusions included myocardial infarction within the previous 6 months; active angina pectoris, heart disease of New York Heart Association class III or IV, severe debilitating valvular or pulmonary disease, uncontrolled hypertension or intermittent claudication; active central nervous system or epidural tumor; infection requiring i.v. antibiotic treatment; other severe comorbid medical problems that would increase the patient’s risk for toxicity; and grade ≥2 peripheral neuropathy. Women of child-bearing age not using reliable means of contraception and those pregnant or lactating were excluded.
Trial design and treatment plan
The study was conducted in two phases. The first was an accelerated phase during which one to two patients were enrolled per cohort, and the dose of 17-AAG was escalated 100% with each cohort until either a grade 3 toxicity or two incidences of grade 2 toxicity were encountered. This was followed by a standard phase in which three to six patients per dose level were treated with incremental dose escalations of 40% until a maximum tolerated dose (MTD) was defined. 17-AAG was provided by the Cancer Therapy Evaluation Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute (Bethesda, MD), in sterile vials, each of which contained 50 mg of 17-AAG in 2 mL of DMSO. EPL diluent (egg phospholipid diluent; NSC704057) was supplied in 50-mL vials that contained 48 mL of 2% egg phospholipids in 5% dextrose in water. On the day of treatment, aliquots of the 17-AAG/DMSO concentrate were thawed and added to 48 mL of EPL diluent to produce a 1 mg/mL solution, which was administered i.v. over a 1- to 1.5-h period.
The starting dose was 5 mg/m2/d for 5 consecutive days every 21 days. This dose was chosen because it represented 1/20 of the LD10 (100 mg/m2/d) in dogs. Adverse events were considered to be any event that occurred during the study or for up to 4 weeks posttreatment, regardless of causality, and were graded using the standard four-point scale of the National Cancer Institute Common Toxicity Criteria, version 2.0.
For patients treated on the daily × 5 and daily × 3 schedules, dose-limiting toxicity (DLT) was defined as grade ≥3 toxicity during the first cycle of treatment. For patients enrolled on the twice-weekly schedules, two treatment delays during the first 3 weeks of therapy because of grade 2 hepatic toxicity, thrombocytopenia, or leukopenia/neutropenia were also considered as DLTs. The MTD was defined as the highest dose level with an observed incidence of DLT in no more than one of six patients. All patients were assessed for toxicity and response if they received any treatment. In patients with measurable disease, standard WHO phase II response criteria were used, and radiographs underwent a blinded review by a radiologist.
In addition to the daily × 5 schedule, three additional dosing schedules were evaluated. These included daily × 3 (every 14 days); days 1, 4, 8, and 11 (every 21 days); and days 1 and 4, weekly without a break. These alternative schedules were chosen based on the results of preclinical studies and the preliminary results of this and other ongoing phase I trials of 17-AAG.
Pharmacokinetics
Plasma concentrations of 17-AAG and its active metabolite 17-amino-17-demethoxygeldanamycin (17-AG) were quantitated by high-performance liquid chromatography in samples collected before and 0.25, 0.5, 1, 2, 4, 6 to 8, 24, and 48 h after the start of the 17-AAG infusion. The lower limit of quantitation of the assay was 0.1 μmol/L for both 17-AAG and 17-AG, and the assay was linear between 0.1 and 25.6 μmol/L. The time courses of 17-AAG and 17-AG in plasma were analyzed noncompartmentally. The area under the plasma concentration versus time curve (AUC) from zero to infinity and the terminal half-life (t1/2) were estimated using the LaGrange function (25), as implemented by the LAGRAN computer program (26). Total body clearance for 17-AAG was calculated as dose per AUC.
Analysis of target protein expression in peripheral blood mononuclear cells
Peripheral blood mononuclear cells (PBMC) were assessed for changes in Hsp70, Akt, and Raf-1 following 17-AAG treatment. p85 phosphatidylinositol 3-kinase, a protein whose expression is unaffected by 17-AAG, was used as a control. Blood samples were drawn into heparin-containing tubes (Vacutainer CPT, Becton Dickinson, Franklin Lakes, NJ) and PBMCs were isolated by centrifugation. Up to six samples were drawn during cycle 1 only. Cells were lysed in NP40 lysis buffer [50 mmol/L Tris (pH 7.4), 1% NP40, 150 mmol/L NaCl, 40 mmol/L NaF, 1 mmol/L Na3VO4, 1 mmol/L phenylmethylsulfonylfluoride, and 10 μg/mL each of leupeptin, aprotinin, and soybean trypsin inhibitor] for 30 min on ice. Lysates were centrifuged at ~13,000 × g for 10 min and the protein concentration of the supernatant was determined by bicinchoninic acid assay (Pierce, Rockford, IL). Equal amounts of total protein were resolved by SDS-PAGE and transferred onto nitrocellulose membranes. Blots were probed overnight at 4°C with the primary antibody. After incubation with horseradish peroxidase–conjugated secondary antibodies, proteins were detected by chemiluminescence. The following primary antibodies were used: Akt (Cell Signaling, Beverly, MA), Hsp70 (StressGene, Victoria, British Columbia, Canada), p85 subunit of phosphatidylinositol 3-kinase (Upstate Biotechnology, Lake Placid, NY), and Raf-1 (Santa Cruz Biotechnology, Santa Cruz, CA).
Results
Patient demographics
Fifty-four patients were treated on the study (Table 1). The male/female ratio was 35:19 and the median age was 58 years (range, 22–83 years). The most common tumor types were breast and prostate.
Table 1.
Patient demographic characteristics
| Patients treated | 54 |
| Age, y | |
| Median | 58 |
| Range | 22–83 |
| Sex | |
| Male | 35 |
| Female | 19 |
| Race | |
| White non-Hispanic | 47 |
| White Hispanic | 1 |
| Black non-Hispanic | 5 |
| Asia/Pacific Islander | 1 |
| KPS | |
| Median | 90 |
| Range | 70–90 |
| No. prior chemotherapy regimens | |
| Median | 2 |
| Range | 0–8 |
| Tumor type | |
| Prostate | 18 |
| Breast | 8 |
| Renal | 7 |
| Lung | 6 |
| Bladder | 5 |
| Melanoma | 4 |
| Head and neck | 3 |
| Other | 3 |
Dose escalation and toxicity
Daily × 5 (every-21-day) schedule
The treatment cohorts are outlined in Table 2. The starting dose and schedule were 5 mg/m2 daily × 5 every 21 days. The accelerated phase ended when the first patient enrolled at 80 mg/m2 developed grade 3 abdominal pain and grade 2 diarrhea and AST elevation on day 4 of cycle 1 (Table 3). This patient underwent cholecystectomy for cholecystitis, but as the cholecystitis was not clearly drug related, he was rechallenged at the same dose level and tolerated five additional cycles without further serious liver or gastrointestinal toxicity. However, two additional DLTs were observed at the expanded 80 mg/m2 dose level. One patient developed grade 3 thrombocytopenia and the second developed grade 3 diarrhea and AST elevation and grade 2 ALT elevation. Two additional patients treated at the 80 mg/m2 dose level also developed delayed grade ≥3 liver toxicity. One did so during cycle 2, and the second did so during cycle 3. In summary, five patients treated with 17-AAG at the 80 mg/m2 daily × 5 dose level developed grade ≥3 toxicities. In all cases, the transaminitis was reversible following discontinuation of 17-AAG. Additional patients were then treated at 40 mg/m2 and 56 mg/m2 daily × 5. These patients all tolerated the treatment well. However, at that point, based on preclinical data suggesting that a daily × 3 schedule was superior to the daily ×5 schedule, the protocol was amended.
Table 2.
Patient cohorts and DLTs
| Dose level | Schedule | 17-AAG dose (mg/m2) | Cycle duration (d) | Patients | DLTs | Description of DLTs |
|---|---|---|---|---|---|---|
| 1 | Daily × 5 | 5 | 21 | 1 | 0 | |
| 2 | Daily × 5 | 10 | 21 | 1 | 0 | |
| 3 | Daily × 5 | 20 | 21 | 2 | 0 | |
| 4 | Daily × 5 | 40 | 21 | 3 | 0 | |
| 5 | Daily × 5 | 80 | 21 | 7 | 2 | Thrombocytopenia (1 pt), diarrhea/hepatitis (1 pt) |
| 6 | Daily × 5 | 56 | 21 | 3 | 0 | |
| 7 | Daily × 3 | 80 | 14 | 3 | 0 | |
| 8 | Daily × 3 | 112 | 14 | 8 | 1 | Emesis (1 pt) |
| 9 | Daily × 3 | 157 | 14 | 4 | 2 | Nausea (1 pt), dyspnea (1 pt) |
| 10 | Days 1, 4, 8, and 11 | 112 | 21 | 3 | 0 | |
| 11 | Days 1, 4, 8, and 11 | 157 | 21 | 4 | 0 | |
| 12 | Days 1, 4, 8, and 11 | 220 | 21 | 6 | 1 | Hepatitis (1 pt) |
| 13 | Days 1, 4, 8, and 11 | 307 | 21 | 3 | 2 | Seizure (1 pt), abdominal pain/nausea/fever (1 pt) |
| 14 | Days 1 and 4 | 150 | 21 | 3 | 0 | |
| 15 | Days 1 and 4 | 210 | 21 | 3 | 0 |
Table 3.
Adverse events (all cycles)
| Daily × 5
|
Daily × 3
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| No. patients | 17 | 15 | ||||||
| No. courses | 50 | 55 | ||||||
| Adverse events | ||||||||
| Nausea | 9 (53%) | 1 (6%) | — | — | 6 (40%) | 1 (7%) | 1 (7%) | — |
| Emesis | 5 (29%) | 1 (6%) | — | — | 2 (13%) | 1 (7%) | 1 (7%) | — |
| Diarrhea | 3 (18%) | 1 (6%) | 1 (6%) | — | 5 (33%) | 3 (20%) | — | — |
| AST | 6 (35%) | 2 (12%) | 1 (6%) | 1 (6%) | 6 (40%) | 4 (27%) | — | — |
| ALT | 5 (29%) | 1 (6%) | 1 (6%) | 1 (6%) | 6 (40%) | 2 (13%) | 1 (7%)* | — |
| Platelets | 3 (18%) | — | 1 (6%) | — | 4 (27%) | 1 (7%) | — | — |
| Days 1, 4, 8, and 11
|
Days 1 and 4 weekly
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| No. patients | 16 | 6 | ||||||
| No. courses | 56 | 33 | ||||||
| Adverse events | ||||||||
| Nausea | 6 (38%) | 4 (25%) | — | — | 1 (17%) | 2 (33%) | — | — |
| Emesis | 4 (25%) | 2 (13%) | — | — | — | 1 (17%) | — | — |
| Diarrhea | 5 (31%) | 3 (19%) | 1 (6%) | — | 3 (50%) | 1 (17%) | — | — |
| AST | 4 (25%) | 4 (25%) | — | — | 1 (17%) | 1 (17%) | 2 (33%) | 1 (17%) |
| ALT | 4 (25%) | 1 (6%) | — | — | — | 1 (17%) | 4 (67%) | — |
| Platelets | 2 (13%) | — | — | — | — | — | 1 (17%) | — |
Abnormal lab value in this patient likely attributable to antibiotic therapy.
Daily × 3 (every-14-day) schedule
The starting dose for the daily × 3 schedule was 80 mg/m2 with cycles repeated every 2 weeks. One patient in the 112 mg/m2 cohort was hospitalized on day 4 of cycle 1 for grade 3 vomiting, grade 2 AST/ALT elevations, grade 2 thrombocytopenia, and grade 2 diarrhea; therefore, this cohort was expanded. At the 157 mg/m2 dose level, two DLTs were observed in the first four patients enrolled. One patient developed grade 3 nausea, grade 2 AST elevation, grade 1 ALT elevation, and acute abdominal pain after 2 days of treatment. The second, a 71-year-old male with stage IV non–small-cell lung cancer, developed grade 3 dyspnea, grade 2 AST, and grade 1 ALT elevations and abdominal pain after 2 days of treatment. This latter patient had a history of coronary artery disease, and his dyspnea was attributed to unstable angina. Because of this event, the protocol was modified. This was the second DLT at this dose level. Therefore, the MTD for the daily × 3 schedule was defined as 112 mg/m2.
Twice-weekly, two-out-of-three week schedule
The starting dose level for the twice-weekly (days 1, 4, 8, and 11 every 21 days) schedule was set at 112 mg/m2. At the 307 mg/m2 dose level, two of three patients experienced DLTs. One patient had a suspected seizure. The second DLT at 307 mg/m2 occurred in a patient with prostate cancer who was hospitalized with grade 3 abdominal pain, nausea, and fever following the second treatment dose of cycle 1. This pain flare was accompanied by an acute 79% increase in his PSA. However, his PSA then declined to 41% of his pretreatment level over the next 2 months despite no further therapy. Given the PSA decline following treatment, he was rechallenged at 220 mg/m2 and received three additional cycles, which he tolerated well. However, he did not respond to the lower dose.
Three additional patients were then added to the 220 mg/m2 dose level, and one of them experienced a DLT. Therefore, 220 mg/m2 was defined as the MTD for the twice-weekly, two-out-of-three week schedule. The one DLT at this dose level was grade 2 hepatitis that required treatment to be held on days 8 and 11. A second patient at the 220 mg/m2 dose level had delayed grade 3 diarrhea associated with grade 2 AST elevation during cycle 3.
Twice-weekly continuous schedule
No DLTs occurred during cycle 1 in patients treated with 17-AAG twice weekly at doses of 150 and 210 mg/m2. However, five of the six patients (all three treated at 150 mg/m2 and two of three at 210 mg/m2) developed delayed hepatotoxicity. The only patient on the continuous twice-weekly schedule who did not develop delayed liver toxicity had rapid disease progression and was taken off therapy after 5 weeks. At 150 mg/m2, all three patients had delayed grade 3 ALT elevations, which occurred during cycles 2, 5, and 8, respectively. At 210 mg/m2, one patient developed grade 2 ALT elevation during cycle 3 and then again during cycle 4, which prompted dose reduction (Fig. 1). This patient with prostate cancer had a 25% decline in PSA and a minor response in measurable disease; however, following dose reduction, his PSA began to increase. A second patient treated at 210 mg/m2 had one dose of 17-AAG held for grade 2 ALT/AST elevation during cycle 1. He then developed streptococcal sepsis during cycle 2, which was associated with grade 3 ALT and grade 4 AST elevations. The patient died 4 days after his last dose of 17-AAG. Following this event, further enrollment on the continuous twice-weekly cohort was suspended because this schedule was deemed too toxic.
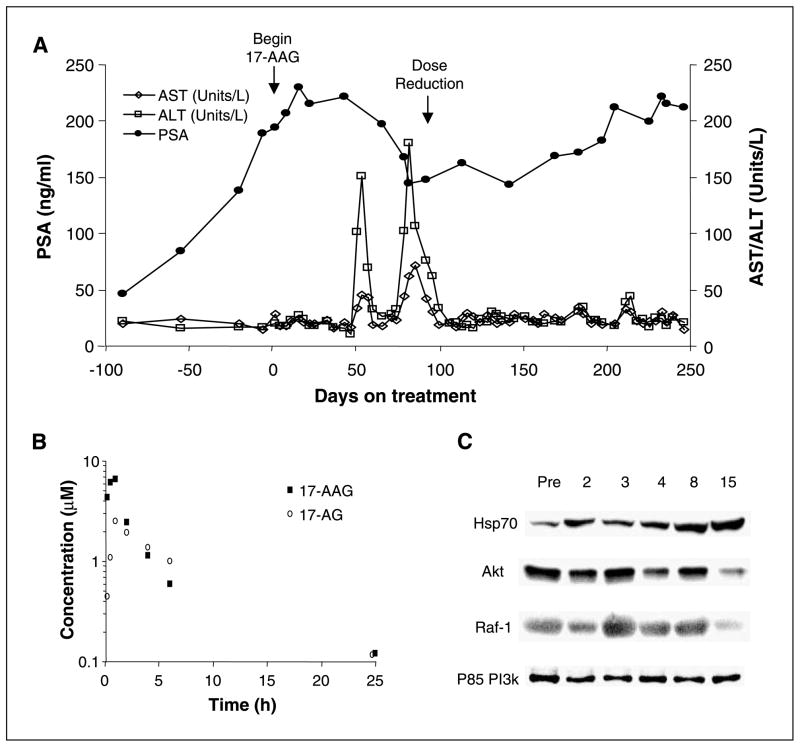
Fig. 1.
A, time course of PSA change in a patient with prostate cancer treated with 210 mg/m2 17-AAG on the continuous twice-weekly schedule (dose level 15). This patient had a 25% decline in PSA after beginning 17-AAG. Because of delayed grade 2 transaminitis, treatment was held on cycle 3, week 2 (day 53), and again during cycle 4, week 3 (day 81). Following the second treatment delay, 17-AAG was resumed but at the next lower dose level (150 mg/m2) on day 92. Following dose reduction, the patient’s PSA began to increase. B and C, serum levels of 17-AAG and 17-AG (B) and PBMC studies (C) from this patient. Pharmacodynamic studies of PBMCs show a heat shock response with induction of Hsp70 and down-regulation of Akt and Raf-1 by day 15.
Other toxicities
The most frequent grade 1/2 toxicities seen with all schedules were fatigue (91%), constipation (52%), myalgia (57%), and anemia (76%). Based on preclinical studies suggesting that Hsp90 plays a role in the maturation of the hERG pump (27), all patients treated with the twice-weekly schedules had pretreatment and posttreatment electrocardiograms. No patients had QTc prolongation after treatment with 17-AAG. Two patients developed asymptomatic second-degree atrioventricular block that resolved without intervention.
Clinical efficacy
There were no partial or complete responses observed. Patients with prolonged stable disease included those treated daily × 3 at 112 mg/m2 (renal and breast); days 1, 4, 8, and 11 at doses of 157 mg/m2 (lung and thyroid) and 220 mg/m2 (renal); and days 1 and 4 continuously at doses of 150 mg/m2 (renal) and 210 mg/m2 (prostate cancer). As an example, the latter patient with prostate cancer was treated with 11 cycles of 17-AAG and had a 25% decline in PSA (Fig. 1) and a minor objective response.
Pharmacokinetics
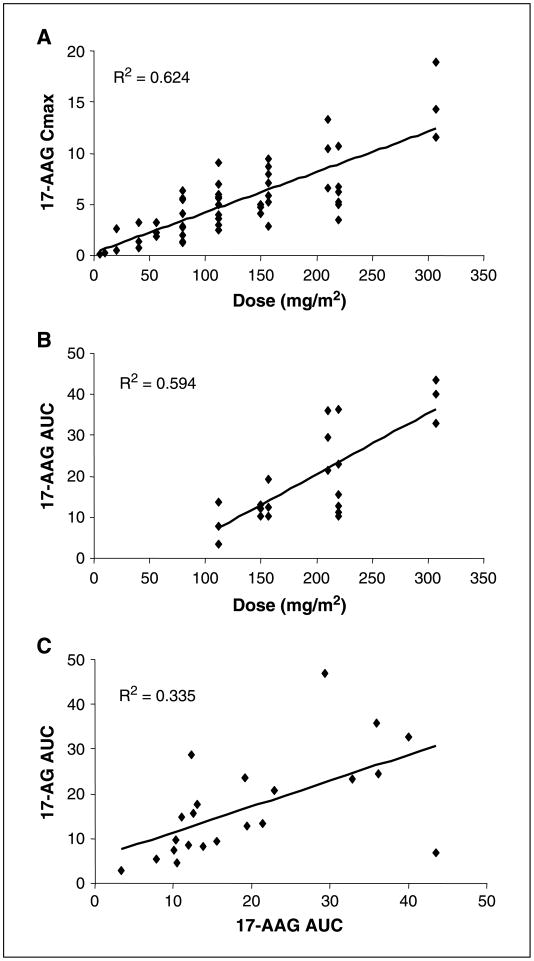
The pharmacokinetics of 17-AAG and 17-AG were studied during cycle 1 (Table 4). Peak plasma concentrations of both 17-AAG and its active metabolite 17-AG were greater than those required for antitumor effects in preclinical models. At the 220 mg/m2 dose level (n = 6), which is recommended for phase II studies using schedule 3 (days 1, 4, 8, and 11 every 21 days), the mean Cmax was 6.21 μmol/L and the t1/2 and clearance values for 17-AAG were 3.4 ± 1.64 h and 25.28 ± 10.38 L/h/m2, respectively. A linear increase in 17-AAG Cmax was observed with increasing dose (Fig. 2). The active metabolite, 17-AG, was detected at all dose levels. At the 220 mg/m2 dose level, mean peak 17-AG concentrations of 2.4 ± 0.7 μmol/L were observed and the mean t1/2 was 6.2 ± 4.7 h.
Table 4.
Summary of pharmacokinetic parameters of 17-AAG and 17-AG
| Dose (mg/m2) | n | 17-AAG Cmax (μmol/L)
|
17-AAG AUC (μmol/L × h)
|
17-AAG t1/2 (h)
|
17-AAG clearance (L/h/m2)
|
|---|---|---|---|---|---|
| Mean (SD) | |||||
| 112 | 3 | 4.96 (0.99) | 8.33 (5.22) | 1.57 (0.32) | 31.57 (21.99) |
| 150–157 | 7 | 6.05 (1.95) | 13.81 (3.88) | 2.11 (0.57) | 20.16 (4.75) |
| 210–220 | 9 | 7.52 (3.25) | 21.69 (10.24) | 3.78 (2.15) | 21.19 (10.38) |
| 307 | 3 | 14.90 (3.75) | 38.77 (5.46) | 2.80 (0.92) | 13.73 (2.03) |
| Dose (mg/m2) | n | 17-AG Cmax (μmol/L)
|
17-AG Tmax (h)
|
17-AG AUC (μmol/L × h)
|
17-AG t1/2 (h)
|
|---|---|---|---|---|---|
| Mean (SD) | |||||
| 112 | 3 | 1.6 (0.4) | 1.0 (0.9) | 5.4 (2.7) | 5.0 (3.8) |
| 150–157 | 7 | 2.3 (1.1) | 1.4 (0.5) | 15.1 (8.7) | 7.5 (5.7) |
| 210–220 | 9 | 3.0 (1.2) | 1.7 (0.5) | 21.0 (13.0) | 6.9 (4.3) |
| 307 | 3 | 2.8 (1.4) | 1.7 (0.6) | 21.0 (13.2) | 9.7 (11.1) |
Abbreviations: Cmax, maximum plasma concentration; AUC, area under the curve, t1/2, elimination half-life; Tmax, time to maximum plasma concentration.
Fig. 2.
A and B, relationship between 17-AAG dose and 17-AAG Cmax (A) and 17-AAG AUC (B). C, relationship between 17-AAG AUC and 17-AG AUC.
Pharmacodynamic studies
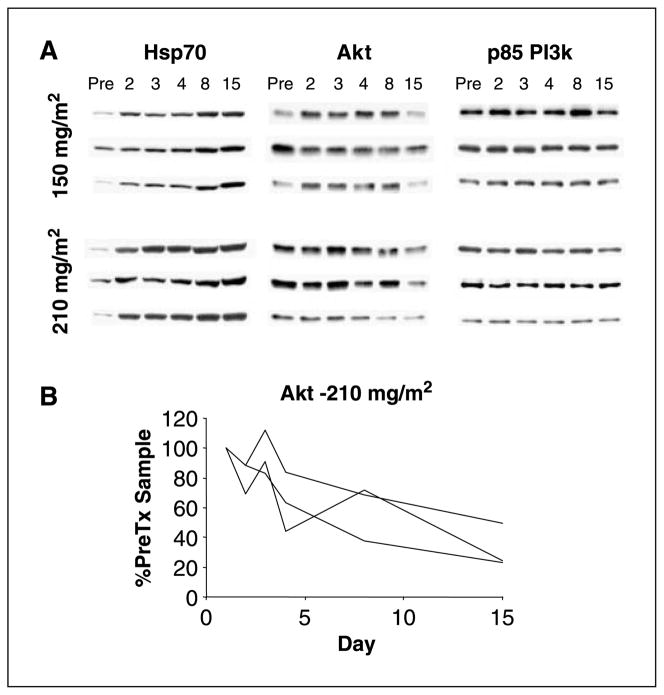
Beginning with patients enrolled at the 112 mg/m2 daily × 3 dose level, PBMCs were collected to assess for changes in the expression of Hsp70 and Hsp90 client proteins following treatment with 17-AAG (Figs. 1 and 3 and data not shown). Protein levels were quantitated by Western blot. p85 phosphatidylinositol 3-kinase was used as a control because this protein is unaffected by Hsp90 inhibition. We observed evidence of a heat shock response following treatment with 17-AAG with an increase posttreatment in Hsp70 expression in PBMCs that began 6 h after treatment (Fig. 3 and data not shown). This increase in Hsp70 was durable with levels remaining elevated in the pretreatment PBMCs collected on day 4. Changes in Raf-1 expression were variable, but decreased Akt protein expression was observed in some patients posttreatment (Fig. 3).
Fig. 3.
Induction of Hsp70 and down-regulation of Akt expression in PBMCs collected from patients treated with 17-AAG. A, Western blot analysis of PBMCs of patients treated with 17-AAG on the continuous twice-weekly dosing schedule. PBMCs were collected on days 1, 2, 3, 4, 8, and 15 of cycle 1. Samples on days 1, 4, 8, and 15 were collected pretreatment. p85 phosphatidylinositol 3-kinase (PI3k) was used as a loading control because this protein is unaffected by Hsp90 inhibition. B, quantitation of the Akt Western blot results for patients treated at the 210 mg/m2 dose level. Akt expression levels were normalized to each patient’s day 1 pretreatment sample.
Discussion
Hsp90 is a molecular chaperone required for protein refolding and the conformational maturation of a subset of signaling proteins (1). Interest in Hsp90 as a therapeutic target can be traced to its identification as the target for geldanamycin, a natural product isolated in 1970 from a broth culture of S. hygroscopicus (5, 28). Geldanamycin proved too toxic for human use, but 17-AAG, a 17-substituted derivative, retains activity against Hsp90 and has a more favorable toxicity profile (16, 29).
Hsp90 is required for maintaining the malignant phenotype of cancer cells, and Hsp90 inhibitors, including 17-AAG, are selectively toxic to tumor cells (30, 31). 17-AAG has activity in xenograft and transgenic models both as a single agent and in combination with cytotoxics, biologics, radiation, and anti-angiogenics (12, 17, 18, 20, 24). In preclinical studies, 17-AAG, at nontoxic doses, was effective in down-regulating the expression of Hsp90 client proteins such as HER2 for up to 48 h (12, 17, 18). In mice, multiple doses of 17-AAG each week were superior to more intermittent dosing schedules; however, intermittent treatment was sufficient to sensitize tumors to cytotoxic agents such as paclitaxel (17).
The initial clinical trials of 17-AAG explored one of two dosing schedules: weekly and daily × 5 repeated every 3 weeks. Consistent with the results of the two other trials using the daily × 5 dosing schedule, we found that hepatotoxicity was dose limiting at concentrations significantly below those achievable using a weekly dosing schedule (32–35). Therefore, we do not recommend further exploration of the daily × 5 schedule.
Concurrent with the conduct of the clinical trial, ongoing preclinical studies in our laboratory suggested that daily × 3 and twice-weekly schedules of 17-AAG retained activity in xenograft model systems (17). Therefore, we amended our study to examine a daily × 3 schedule (with cycles repeated every 2 versus 3 weeks) and later twice-weekly dosing. With the daily × 3 schedule, less hepatotoxicity was observed, which allowed an increase in the MTD from 56 to 112 mg/m2. A days 1, 4, 8, and 11 every-3-week schedule was also well tolerated. However, twice-weekly continuous dosing was unacceptably toxic because all five patients who received more than 5 weeks of treatment developed delayed liver toxicity. These data suggest that, in patients treated twice-weekly with 17-AAG, treatment breaks will be required to minimize hepatotoxicity. Because of its limited oral bioavailability, 17-AAG has been administered only by i.v. infusion. 17-Dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG) is a second-generation, 17-carbon–substituted geldanamycin derivative now in phase I testing. 17-DMAG has greater oral bioavailability than 17-AAG, and trials of orally administered 17-DMAG are planned (36). As some of the toxicities observed with 17-AAG may be attributable, in part, to the DMSO formulation, 17-DMAG, which is water soluble, may be less toxic than 17-AAG when administered i.v. However, as preclinical studies of 17-DMAG also suggest that hepatotoxicity will be dose limiting, intermittent treatment breaks or weekly dosing may still be required with 17-DMAG despite its oral formulation.
One obstacle to the clinical development of Hsp90 inhibitors has been the difficulty of quantitatively assessing the effect of 17-AAG on Hsp90 function in patients. A direct assay of Hsp90 chaperone function has not been developed, and the “gold standard” has been to obtain pretreatment and posttreatment tumor tissues for analysis of treatment-induced changes in the expression of relevant Hsp90 client proteins. Such biopsies were successfully obtained in a trial reported by Banerji et al. (34) in nine patients treated weekly at the 375 and 450 mg/m2 dose levels. In that study, a posttreatment induction of Hsp70 and depletion of cyclin-dependent kinase 4 were observed in eight of the nine posttreatment tumor biopsies (34). Raf-1 down-regulation was also shown in four of the six patients with detectable Raf-1 in their baseline sample (34).
Although these data suggest that 17-AAG can induce a heat shock response and down-regulate the expression of at least some Hsp90 clients, we believe that a reliance on such methods is insufficient for several reasons. First, in our experience, tumor tissue is accessible in only a small fraction of the solid-tumor patients considered for enrollment on a phase I study. Second, even when available, typically only a single posttreatment time point can feasibly be assessed, and, even then, sufficient material can often only be collected for immunohistochemical analysis. However, immunohistochemistry is not quantitative and lacks sufficient sensitivity for detecting potentially meaningful partial loss of Hsp90 client protein expression. Finally, our preclinical studies suggest that the most sensitive Hsp90 clients (HER2 and steroid receptors) are not found in abundance in most tumors and that significant variability is observed in the doses of 17-AAG required to induce the degradation of individual clients in xenograft tumors. For this reason, we opted, as the trial progressed, to pursue pretreatment and posttreatment tumor biopsies as part of disease-specific phase II trials. This would allow for uniformity in the target proteins to be assessed (e.g., B-Raf and Raf-1 in the case of our melanoma phase II study). It would also speed accrual and thus the identification of the MTD. This approach, however, fails to identify the minimum dose of 17-AAG necessary to induce the degradation of a specific Hsp90 client.
We did examine the effect of 17-AAG on Hsp90 function using a normal tissue (PBMCs). While we were successful in collecting PBMCs, preclinical studies suggest that 17-AAG accumulates in tumor versus normal tissues and that the affinity of 17-AAG for Hsp90 (and thus the concentration required for its inhibition) may differ significantly in tumor and normal tissues (30). For these reasons, changes in the expression of Hsp90 client proteins in normal tissues may not reflect those occurring in the tumor. Further, although some Hsp90 client proteins are expressed in PBMCs (Raf-1, cyclin-dependent kinase 4, and Akt), the clients we find to be most sensitive to Hsp90 inhibitors in animal models (HER2 and steroid receptors) are not expressed at sufficient levels to be assessed by Western blot. Therefore, normal tissues cannot be used to evaluate the effect of 17-AAG on these most sensitive clients. As a result, we chose to define the recommended phase II dose of each schedule as the MTD although biomarker changes were observed in PBMCs at doses below the MTD.
As an alternative to tumor biopsies, we have also developed a method for the noninvasive quantification of changes in HER2 protein expression in tumors (37, 38). 17-AAG induces the rapid degradation of HER2 and loss of its expression on the membrane (17, 39). We took advantage of this unique property of Hsp90 inhibitors to develop a positron emission tomography imaging probe that could noninvasively quantitate changes in HER2 expression as a marker of Hsp90 inhibition. To accomplish this goal, we generated and labeled an F(ab′)2 fragment of trastuzumab with 68Ga, a short half-life positron emitter, which allows for the sequential noninvasive quantitation of HER2 expression using positron emission tomography imaging (37). The rapid elimination of this radiotracer from the blood allows for serial determinations of HER2 expression in xenograft models pretreatment and posttreatment with 17-AAG, and we hope to use this technology in future trials of novel Hsp90 inhibitors.
In conclusion, the current study highlights the schedule-dependent toxicity of the Hsp90 inhibitor 17-AAG. We show that a biologically effective dose can be administered safely using an intermittent dosing schedule. As we have previously shown that intermittent dosing with 17-AAG is effective in enhancing the activity of cytotoxic agents, the current formulation of 17-AAG may be most useful in combination with such agents. Phase I trials of 17-AAG in combination with docetaxel, irinotecan, and other cytotoxics are currently ongoing. The delayed hepatotoxicity observed with the twice-weekly continuous dosing schedule also supports ongoing efforts to develop non-ansamycin Hsp90 inhibitors with a more favorable toxicity profile.
Acknowledgments
Grant support: National Cancer Institute grants P50-CA92629, R21 85506-01, and U01-CA69856 and the Prostate Cancer Foundation.
We thank Qing Ye, Ayana Sawai, and Christine Pratilas, M.D., for technical assistance with the PBMC studies.
References
- 1.Schneider C, Sepp-Lorenzino L, Nimmesgern E, et al. U Pharmacologic shifting of a balance between protein refolding and degradation mediated by Hsp90. Proc Natl Acad Sci U S A. 1996;93:14536–41. doi: 10.1073/pnas.93.25.14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Obermann WM, Sondermann H, Russo AA, Pavletich NP, Hartl FU. In vivo function of Hsp90 is dependent on ATP binding and ATP hydrolysis. J Cell Biol. 1998;143:901–10. doi: 10.1083/jcb.143.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panaretou B, Prodromou C, Roe SM, et al. ATP binding and hydrolysis are essential to the function of the Hsp90 molecular chaperone in vivo. EMBO J. 1998;17:4829–36. doi: 10.1093/emboj/17.16.4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uehara Y, Hori M, Takeuchi T, Umezawa H. Phenotypic change from transformed to normal induced by benzoquinonoid ansamycins accompanies inactivation of p60src in rat kidney cells infected with Rous sarcoma virus. Mol Cell Biol. 1986;6:2198–206. doi: 10.1128/mcb.6.6.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein HSP90-60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci U S A. 1994;91:8324–8. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mimnaugh EG, Chavany C, Neckers L. Polyubiquitination and proteasomal degradation of the p185c-erbB-2 receptor protein-tyrosine kinase induced by geldanamycin. J Biol Chem. 1996;271:22796–801. doi: 10.1074/jbc.271.37.22796. [DOI] [PubMed] [Google Scholar]
- 7.Sepp-Lorenzino L, Ma Z, Lebwohl DE, Vinitsky A, Rosen N. Herbimycin A induces the 20S proteasome-and ubiquitin-dependent degradation of receptor tyrosine kinases. J Biol Chem. 1995;270:16580–7. doi: 10.1074/jbc.270.28.16580. [DOI] [PubMed] [Google Scholar]
- 8.Schulte TW, Blagosklonny MV, Ingui C, Neckers L. Disruption of the Raf-1-Hsp90 molecular complex results in destabilization of Raf-1 and loss of Raf-1-Ras association. J Biol Chem. 1995;270:24585–8. doi: 10.1074/jbc.270.41.24585. [DOI] [PubMed] [Google Scholar]
- 9.Citri A, Alroy I, Lavi S, et al. Drug-induced ubiquitylation and degradation of ErbB receptor tyrosine kinases: implications for cancer therapy. EMBO J. 2002;21:2407–17. doi: 10.1093/emboj/21.10.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulte TW, Blagosklonny MV, Romanova L, et al. Destabilization of Raf-1 by geldanamycin leads to disruption of the Raf-1-MEK-mitogen-activated protein kinase signalling pathway. Mol Cell Biol. 1996;16:5839–45. doi: 10.1128/mcb.16.10.5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basso AD, Solit DB, Chiosis G, Giri B, Tsichlis P, Rosen N. Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J Biol Chem. 2002;277:39858–66. doi: 10.1074/jbc.M206322200. [DOI] [PubMed] [Google Scholar]
- 12.Solit DB, Zheng FF, Drobnjak M, et al. 17-Allylamino-17-demethoxygeldanamycin induces the degradation of androgen receptor and HER-2/neu and inhibits the growth of prostate cancer xenografts. Clin Cancer Res. 2002;8:986–93. [PubMed] [Google Scholar]
- 13.Blagosklonny MV, Toretsky J, Bohen S, Neckers L. Mutant conformation of p53 translated in vitro or in vivo requires functional HSP90. Proc Natl Acad Sci U S A. 1996;93:8379–83. doi: 10.1073/pnas.93.16.8379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorre ME, Ellwood-Yen K, Chiosis G, Rosen N, Sawyers CL. BCR-ABL point mutants isolated from patients with imatinib mesylate-resistant chronic myeloid leukemia remain sensitive to inhibitors of the BCR-ABL chaperone heat shock protein 90. Blood. 2002;100:3041–4. doi: 10.1182/blood-2002-05-1361. [DOI] [PubMed] [Google Scholar]
- 15.Grbovic OM, Basso AD, Sawai A, et al. V600EB-Raf requires the Hsp90 chaperone for stability and is degraded in response to Hsp90 inhibitors. Proc Natl Acad Sci U S A. 2006;103:57–62. doi: 10.1073/pnas.0609973103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Supko JG, Hickman RL, Grever MR, Malspeis L. Preclinical pharmacologic evaluation of geldanamycin as an antitumor agent. Cancer Chemother Pharmacol. 1995;36:305–15. doi: 10.1007/BF00689048. [DOI] [PubMed] [Google Scholar]
- 17.Solit DB, Basso AD, Olshen AB, Scher HI, Rosen N. Inhibition of heat shock protein 90 function down-regulates akt kinase and sensitizes tumors to Taxol. Cancer Res. 2003;63:2139–44. [PubMed] [Google Scholar]
- 18.Banerji U, Walton M, Raynaud F, et al. Pharmacokinetic-pharmacodynamic relationships for the heat shock protein 90 molecular chaperone inhibitor 17-allylamino, 17-demethoxygeldanamycin in human ovarian cancer xenograft models. Clin Cancer Res. 2005;11:7023–32. doi: 10.1158/1078-0432.CCR-05-0518. [DOI] [PubMed] [Google Scholar]
- 19.Burger AM, Fiebig HH, Stinson SF, Sausville EA. 17-(Allylamino)-17-demethoxygeldanamycin activity in human melanoma models. Anticancer Drugs. 2004;15:377–87. doi: 10.1097/00001813-200404000-00011. [DOI] [PubMed] [Google Scholar]
- 20.de Candia P, Solit DB, Giri D, et al. Angiogenesis impairment in Id-deficient mice cooperates with an Hsp90 inhibitor to completely suppress HER2/neu-dependent breast tumors. Proc Natl Acad Sci U S A. 2003;100:12337–42. doi: 10.1073/pnas.2031337100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munster PN, Basso A, Solit D, Norton L, Rosen N. Modulation of Hsp90 function by ansamycins sensitizes breast cancer cells to chemotherapy-induced apoptosis in an RB- and schedule-dependent manner. Clin Cancer Res. 2001;7:2228–36. [PubMed] [Google Scholar]
- 22.Nguyen DM, Lorang D, Chen GA, Stewart JH, 4th, Tabibi E, Schrump DS. Enhancement of paclitaxel-mediated cytotoxicity in lung cancer cells by 17-allylamino geldanamycin: in vitro and in vivo analysis. Ann Thorac Surg. 2001;72:371–8. doi: 10.1016/s0003-4975(01)02787-4. discussion 378–9. [DOI] [PubMed] [Google Scholar]
- 23.Sain N, Krishnan B, Ormerod MG, et al. Potentiation of paclitaxel activity by the HSP90 inhibitor 17-allylamino-17-demethoxygeldanamycin in human ovarian carcinoma cell lines with high levels of activated AKT. Mol Cancer Ther. 2006;5:1197–208. doi: 10.1158/1535-7163.MCT-05-0445. [DOI] [PubMed] [Google Scholar]
- 24.Enmon R, Yang WH, Ballangrud AM, et al. Combination treatment with 17-N-allylamino-17-demethoxy geldanamycin and acute irradiation produces supra-additive growth suppression in human prostate carcinoma spheroids. Cancer Res. 2003;63:8393– 9. [PubMed] [Google Scholar]
- 25.Yeh KC, Kwan KC. A comparison of numerical integrating algorithms by trapezoidal, Lagrange, and spline approximation. J Pharmacokinet Biopharm. 1978;6:79–98. doi: 10.1007/BF01066064. [DOI] [PubMed] [Google Scholar]
- 26.Rocci ML, Jr, Jusko WJ. LAGRAN program for area and moments in pharmacokinetic analysis. Comput Programs Biomed. 1983;16:203–16. doi: 10.1016/0010-468x(83)90082-x. [DOI] [PubMed] [Google Scholar]
- 27.Ficker E, Dennis AT, Wang L, Brown AM. Role of the cytosolic chaperones Hsp70 and Hsp90 in maturation of the cardiac potassium channel HERG. Circ Res. 2003;92:e87–100. doi: 10.1161/01.RES.0000079028.31393.15. [DOI] [PubMed] [Google Scholar]
- 28.DeBoer C, Meulman PA, Wnuk RJ, Peterson DH. Geldanamycin, a new antibiotic. J Antibiot (Tokyo) 1970;23:442–7. doi: 10.7164/antibiotics.23.442. [DOI] [PubMed] [Google Scholar]
- 29.Schulte TW, Neckers LM. The benzoquinone ansamycin 17-allylamino-17-demethoxygeldanamycin binds to HSP90 and shares important biologic activities with geldanamycin. Cancer Chemother Pharmacol. 1998;42:273–9. doi: 10.1007/s002800050817. [DOI] [PubMed] [Google Scholar]
- 30.Kamal A, Thao L, Sensintaffar J, et al. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature. 2003;425:407–10. doi: 10.1038/nature01913. [DOI] [PubMed] [Google Scholar]
- 31.Vilenchik M, Solit D, Basso A, et al. Targeting wide-range oncogenic transformation via PU24FCl, a specific inhibitor of tumor Hsp90. Chem Biol. 2004;11:787–97. doi: 10.1016/j.chembiol.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 32.Grem JL, Morrison G, Guo XD, et al. Phase I and pharmacologic study of 17-(allylamino)-17-demethoxygeldanamycin in adult patients with solid tumors. J Clin Oncol. 2005;23:1885–93. doi: 10.1200/JCO.2005.12.085. [DOI] [PubMed] [Google Scholar]
- 33.Goetz MP, Toft D, Reid J, et al. Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer. J Clin Oncol. 2005;23:1078–87. doi: 10.1200/JCO.2005.09.119. [DOI] [PubMed] [Google Scholar]
- 34.Banerji U, O’Donnell A, Scurr M, et al. Phase I pharmacokinetic and pharmacodynamic study of 17-allylamino, 17-demethoxygeldanamycin in patients with advanced malignancies. J Clin Oncol. 2005;23:4152–61. doi: 10.1200/JCO.2005.00.612. [DOI] [PubMed] [Google Scholar]
- 35.Ramanathan RK, Trump DL, Eiseman JL, et al. Phase I pharmacokinetic-pharmacodynamic study of 17-(allylamino)-17-demethoxygeldanamycin (17AAG, NSC 330507), a novel inhibitor of heat shock protein 90, in patients with refractory advanced cancers. Clin Cancer Res. 2005;11:3385–91. doi: 10.1158/1078-0432.CCR-04-2322. [DOI] [PubMed] [Google Scholar]
- 36.Hollingshead M, Alley M, Burger AM, et al. A.In vivo antitumor efficacy of 17-DMAG (17-dimethylaminoethylamino-17-demethoxygeldanamycin hydrochloride), a water-soluble geldanamycin derivative. Cancer Chemother Pharmacol. 2005;56:115– 25. doi: 10.1007/s00280-004-0939-2. [DOI] [PubMed] [Google Scholar]
- 37.Smith-Jones PM, Solit DB, Akhurst T, Afroze F, Rosen N, Larson SM. Imaging the pharmacodynamics of HER2 degradation in response to Hsp90 inhibitors. Nat Biotechnol. 2004;22:701–6. doi: 10.1038/nbt968. [DOI] [PubMed] [Google Scholar]
- 38.Smith-Jones PM, Solit DB. Generation of DOTA-conjugated antibody fragments for radioimmunoimaging. Methods Enzymol. 2004;386:262–75. doi: 10.1016/S0076-6879(04)86012-9. [DOI] [PubMed] [Google Scholar]
- 39.Basso A, Solit D, Munster P, Rosen N. Ansamycin antibiotics inhibit Akt activation and tumor growth in human breast cancers that overexpress HER2. Oncogene. 2002;21:1159–66. doi: 10.1038/sj.onc.1205184. [DOI] [PMC free article] [PubMed] [Google Scholar]