Abstract
The dwarf pea (Pisum sativum) mutants lka and lkb are brassinosteroid (BR) insensitive and deficient, respectively. The dwarf phenotype of the lkb mutant was rescued to wild type by exogenous application of brassinolide and its biosynthetic precursors. Gas chromatography-mass spectrometry analysis of the endogenous sterols in this mutant revealed that it accumulates 24-methylenecholesterol and isofucosterol but is deficient in their hydrogenated products, campesterol and sitosterol. Feeding experiments using 2H-labeled 24-methylenecholesterol indicated that the lkb mutant is unable to isomerize and/or reduce the Δ24(28) double bond. Dwarfism of the lkb mutant is, therefore, due to BR deficiency caused by blocked synthesis of campesterol from 24-methylenecholesterol. The lkb mutation also disrupted sterol composition of the membranes, which, in contrast to those of the wild type, contained isofucosterol as the major sterol and lacked stigmasterol. The lka mutant was not BR deficient, because it accumulated castasterone. Like some gibberellin-insensitive dwarf mutants, overproduction of castasterone in the lka mutant may be ascribed to the lack of a feedback control mechanism due to impaired perception/signal transduction of BRs. The possibility that castasterone is a biologically active BR is discussed.
Steroid hormones play important roles in growth and development of various organisms. These include the sex hormones glucocorticoids and mineral corticoids in animals, the molting hormones ecdysteroids in insects and crustaceans, and an antheridiogen, antheridiol, in the microorganism Achlya bisexualis. Grove et al. (1979) reported the isolation of brassinolide as a plant growth-regulating steroid, and since then more than 40 analogs have been identified from various plant sources (Sakurai and Fujioka, 1993; Adam et al., 1996; Fujioka and Sakurai, 1997b). These steroids are known collectively as BRs (Mandava, 1988). Structurally, BRs are C27, C28, and C29 steroids with different substituents on the A- and B-rings and side chains (Fujioka and Sakurai, 1997b; Yokota, 1997). Brassinolide, the most biologically active BR, is a C28 steroid and, along with biosynthetically related compounds, it is distributed widely in the plant kingdom. Recently, brassinolide biosynthetic pathways have been elucidated by feeding 2H-labeled intermediates to suspension cultures of Catharanthus roseus (for reviews, see Fujioka and Sakurai, 1997a; Yokota, 1997; Fig. 1). It has long been established that BRs elicit a variety of effects on the growth of higher plants (Mandava, 1988; Sasse, 1997). However, it was the recent discovery of dwarf mutants of Arabidopsis and garden pea (Pisum sativum) with BR biosynthesis and sensitivity lesions that provided compelling evidence that BRs are a prerequisite for cell elongation and hence have a defined hormonal role in the regulation of higher plant growth and development (for reviews, see Clouse, 1996, 1997; Yokota, 1997; Clouse and Sasse, 1998).
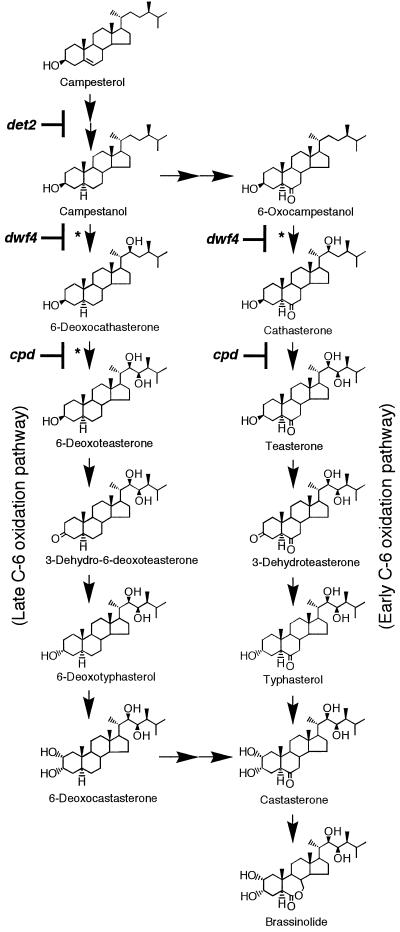
Figure 1.
Proposed biosynthesis pathways for brassinolide from campesterol. Conversions marked with asterisks are hypothetical. Lesions in the BR biosynthesis of Arabidopsis mutants det2, dwf4, and cpd are shown.
The roles of three genes in BR biosynthesis have been determined using dwarf mutants of Arabidopsis, det2 (d-etiolated 2) (Li et al., 1996, 1997), cpd (constitutive photomorphogenesis and dwarfism) (Szekeres et al., 1996), and dwf4 (dwarf4) (Azpiroz et al., 1998; Choe et al., 1998). It has been shown that the dwf6 mutant (Choe et al., 1998) is an allele of det2 and that cpd, cbb3 (cabbage3) (Kauschmann et al., 1996), and dwf3 (Choe et al., 1998) are all allelic. DET2 encodes a steroid 5α-reductase that hydrogenates the (24R)-24-methylcholest-4-en-3-one intermediate involved in the conversion of campesterol to campestanol (Fujioka et al., 1997). CPD encodes a Cyt P450 enzyme, designated CYP90A1, that catalyzes C-23 hydroxylation (Szekeres et al., 1996), whereas DWF4 encodes a Cyt P450 enzyme, CYP90B1, that catalyzes C-22 hydroxylation (Choe et al., 1998). In addition, there is evidence that the dim (diminuto) mutant of Arabidopsis (Takahashi et al., 1995; Klahre et al., 1998), which is an allele of cbb1 (Kauschmann et al., 1996) and dwf1 (Choe et al., 1998), and the dwarf mutant of tomato (Bishop et al., 1996, 1999), are involved in BR biosynthesis. Severe dwarfism is also observed in the Arabidopsis mutant bri1 (brassinosteroid-insensitive1), the root growth of which is not inhibited by exogenous BRs (Clouse et al., 1993, 1996). The gene BRI1 was found to encode a putative Leu-rich repeat receptor kinase probably involved in BR signal transduction (Li and Chory, 1997).
In the case of pea, the stunted phenotypes of the lka, lkb, lkc, and lk mutants are not fully reversed by treatment with traditional plant hormones such as GA and auxin (Reid and Ross, 1989; Reid et al., 1991; Lawrence et al., 1992; Reid and Davies, 1992; McKay et al., 1994; Yang et al., 1996). It was reported that the dwarfism of the lkb mutant is caused by BR deficiency, whereas the lka mutant, which has a similar dwarf phenotype, was predicted to be a BR-response mutant (Nomura et al., 1997). Preliminary studies indicate that the lk mutant is also BR deficient (Y. Kitasaka, T. Nomura, S. Takatsuto, J.B. Reid, and T. Yokota, unpublished data). In the present work we further analyzed the biochemical lesions in the lka and lkb mutants by GC-MS analysis of endogenous BRs and their probable precursor sterols, by feeding experiments with isotopically labeled intermediates, and by investigating the effects of different BRs on the growth of the two pea mutants.
MATERIALS AND METHODS
Plant Materials
The pure lines of garden pea (Pisum sativum) used in this study were cv Torsdag (WT, tall) and the two single-gene mutant lines derived from this cultivar mutagenized with ethyl methanesulfonate, NGB5865 (lka) and NGB5862 (lkb), which are held in the University of Tasmania collection at Hobart (Reid and Ross, 1989). Shoots (49 d old; Table I) used for analysis of the endogenous BRs and sterols were sown on October 27, 1997, and grown in a greenhouse under a natural photoperiod. Other plant seedlings used in this study were grown in a growth cabinet, as described by Nomura et al. (1997).
Table I.
Growth data for 49-d-old pea shoots
Authentic BRs and Sterols
Brassinolide, castasterone, typhasterol, and teasterone were kindly supplied by Dr. M. Aburatani (Fuji Chemical Industries, Takaoka, Japan). We synthesized the remaining BRs. Campesterol was obtained from Tama Chemicals (Tokyo, Japan). Campestanol was obtained by catalytic hydrogenation (10% palladium on charcoal) of campesterol. 24-Methylenecholesterol was supplied by Dr. T. Akihisa (Nihon University, Japan). Isofucosterol was extracted from bean seeds (Kim et al., 1988). Sitosterol, sitostanol, stigmasterol, and cholesterol were obtained from Fluka, Sigma, Tokyo Kasei (Tokyo, Japan), and Kanto Chemical (Tokyo, Japan), respectively.
2H Standards
26,27-2H6 labeling of brassinolide, castasterone, typhasterol, and teasterone were reported by Takatsuto and Ikekawa (1986). [2H6]3-Dehydroteasterone was synthesized as reported by Yokota et al. (1994), [2H6]6-deoxocastasterone (melting point, 238°C), [2H6]6-deoxotyphasterol (melting point, 224°C), and [2H6]6-deoxoteasterone (melting point, 227°C) were synthesized from [2H6]castasterone, [2H6]typhasterol, and [2H6]teasterone, respectively, according to the method of Mori et al. (1984). [2H6]3-Dehydro-6-deoxoteasterone (melting point, 167°C) was synthesized from [2H6]6-deoxoteasterone as described by Yokota et al. (1994). [26,27-2H6]Campestanol was supplied by Dr. S. Fujioka (RIKEN, Saitama, Japan). [25,26,27-2H7]-24-Methylenecholesterol, [26,27-2H6]24-methyldesmosterol, and [26,27-2H6]campesterol were synthesized by the procedures of Takatsuto et al. (1998).
GC-SIM
GC-SIM, in the electron impact mode (70 eV), was carried out on a JMS AX 505 instrument (JEOL) fitted with either a DB-5 or DB-1 column (0.25 mm × 15 m; 0.25-mm film thickness; J & W Scientific, Folsom, CA). The carrier gas was He at a flow rate of 1 mL min−1, the injection port temperature was 260°C, and the samples were introduced by splitless injection. The column oven temperature was programmed to 170°C for 1.5 min, increased to 280°C at 37°C min−1, and then increased to 300°C at 1.5°C min−1.
Effects of Brassinolide, Its Precursors, and Sterols on Growth
Brassinolide precursors in 5 μL of ethanol containing 0.15% Tween 20 were applied to the fourth internode when the third leaf was almost fully expanded about 8 d after planting in a growth cabinet. Sterols were mixed with a fractionate lanolin (Mitchell and Livingston, 1968), and the lanolin paste was applied to the fourth internode. Control seedlings were treated with the solvent only. After 3 d the lengths of the fourth and fifth internodes were measured.
Effects of Brassinolide on Sterol Content
The procedure of brassinolide application was the same as described above, except that the surface of the expanding third leaf was treated with 100 ng of brassinolide dissolved in 10 μL of the solvent. After 2 d the third leaves were removed, and the apical portions (each with 20 shoots) were harvested by cutting at the third node and used for sterol analysis.
Metabolism of 2H-Sterols
Seeds of the lkb mutant were surface-sterilized with sodium hypochlorite solution (0.5% active chlorine) and were placed in 100-mL conical flasks (one seed per flask) containing 15 mL of Murashige and Skoog medium (JRH Biosciences, Lenexa, KS). The seeds were grown for 6 d at 25°C under a 16-h light and 8-h dark regime on a reciprocal shaker (60 rpm); then ethanol solutions (40 μL) of [2H7]24-methylenecholesterol (40 μg) or [2H6]24-methyldesmosterol (40 μg) were added to the conical flasks and incubated for an additional 3 d under the same conditions. Roots and shoots were separated and subjected to sterol analysis.
Preparation of Microsomal and Soluble Fractions
All operations were done at 4°C. Ten fresh segments (approximately 5 g) excised at the sixth node from 23-d-old seedlings were homogenized by a Polytron (Kinematica, Littau/Luzern) with 20 mL of 0.1 m Tris-HCl buffer, pH 7.8, containing 0.5 m Suc and 1 mm EDTA, and the homogenate was filtered through four layers of gauze. The filtrate was centrifuged at 6,000g for 15 min, and the supernatant was then centrifuged at 100,000g for 90 min according to the methods described by Gachotte et al. (1995). The pellet and supernatant obtained by 100,000g centrifugation, which represent microsomal membrane and soluble cellular fractions, respectively, were subjected to sterol analysis.
Extraction and Purification of BRs from 49-d-Old Seedlings
The methanol extracts of 49-d-old shoots excised at soil levels (Table I) were spiked with 2H6-labeled internal standards, 0.3 μg of [2H6]brassinolide, 0.5 μg each of [2H6]castasterone and [2H6]typhasterol, and 1 μg each of [2H6]3-dehydroteasterone, [2H6]6-deoxocastasterone, [2H6]6deoxotyphasterol, [2H6]3-dehydro-6-deoxoteasterone, and [2H6]6-deoxoteasterone before reduction to an aqueous residue. The aqueous residue was partitioned against chloroform. The chloroform phase was washed with 0.5 m K2HPO4 buffer, pH 9.0, evaporated to dryness, and partitioned between hexane and 80% methanol. The hexane and 80% methanol phases were used for analysis of sterols and BRs, respectively.
The 80% methanol fraction was evaporated to dryness and the residual solid was purified on a column of silica gel (7 g; Wakogel C-300, Wako Pure Chemicals, Osaka, Japan) eluted with chloroform containing 0%, 0.5%, 1%, 2.5%, 5%, 7%, 10%, 20%, and 50% methanol (first, chloroform was washed with water to remove ethanol used for stabilizer, dried, and distilled). Biological activity in the fractions was assayed by the rice lamina inclination test (Yokota et al., 1996). Eluates obtained with 1% to 7% methanol in chloroform were combined, dissolved in 60% methanol, and loaded onto a column of charcoal (chromatography grade, 5 g; Wako Pure Chemicals), then eluted with methanol:water (6:4 and 8:2, v/v), methanol, and methanol:chloroform (9:1, 5:5, 3:7, and 1:9, v/v). The methanol:chloroform (5:5 and 3:7, v/v) fractions were combined and chromatographed on a column of Sephadex LH-20 (bed volume, 500 mL; Pharmacia) using methanol:chloroform (4:1, v/v) as a mobile phase. Successive 10-mL fractions were collected. Fractions 33 to 39 were combined and purified on a column of diethylaminopropyl silica Bondesil (0.2 g; Varian, Palo Alto, CA) using methanol as the mobile phase. The eluate was subjected to reversed-phase HPLC on a Pak ODS-3251-D column (8 × 250 mm; Senshu Science, Tokyo, Japan) eluted with the following acetonitrile-water gradient: 0 to 20 min, 45% acetonitrile; 20 to 40 min, 45% to 100% acetonitrile; and 40 to 50 min, 100% acetonitrile, at a flow rate of 2.5 mL min−1. Fractions were collected every 1 min. The column oven temperature was maintained at 40°C. The following fractions were collected for analysis by GC-SIM: fractions 14 and 15 (brassinolide), 20 to 22 (castasterone), 30 to 33 (teasterone), 35 to 37 (typhasterol and 3-dehydroteasterone), 38 to 40 (6-deoxocastasterone), and 43 to 47 (6-deoxoteasterone, 3-dehydro-6-deoxoteasterone, and 6-deoxotyphasterol).
Quantitative Analysis of BRs
Random aliquots of extracts derived from pooled plant materials were analyzed by GC-SIM in duplicates. BRs were converted to either MBs or BMBs with pyridine that contains methaneboronic acid (2 mg mL−1) at 70°C for 30 min. Typhasterol, teasterone, 6-deoxotyphasterol, and 6-deoxoteasterone were further trimethylsilylated to yield MB-TMSi derivatives. The 2H0/2H6 ions monitored were m/z 528/534 (M+), 374/374 and 155/161 for brassinolide BMB, m/z 512/518 (M+), 358/358 and 155/161 for castasterone BMB, m/z 544/550 (M+), 529/535 and 515/521 for typhasterol MB-TMSi and teasterone MB-TMSi, m/z 470/476 (M+), 316/316 and 155/161 for 3-dehydroteasterone MB, m/z 498/504 (M+), 273/273 and 155/161 for 6-deoxocastasterone BMB, m/z 530/536 (M+), 440/446 and 215/215 for 6-deoxotyphasterol MB-TMSi and 6-deoxoteasterone MB-TMSi, and m/z 456/462 (M+), 231/231, and 155/161 for 3-dehydro-6-deoxoteasterone MB. The contents of BRs were calculated from the peak area ratios of 2H0 and 2H6 M+ ions.
Analysis of Sterols
Random aliquots of extracts derived from pooled plant materials were analyzed by GC-SIM in duplicates. Plant tissues were extracted with methanol:chloroform (4:1, v/v), whereas the microsomal membrane pellet was extracted with methanol:dichloromethane (1:2, v/v). In the case of 49-d-old seedlings, the hexane-soluble phase was obtained as described above for sterol analysis. The extracts were partitioned between ethyl acetate and 0.5 m K2HPO4 buffer, pH 9.0, and the organic phases were used for sterol analysis. Sterol extracts equivalent to 100 mg fresh weight of tissue were spiked with 1 μg of [2H6]campestanol as an internal standard and saponified with 1 n sodium hydroxide in methanol at 80°C for 1.5 h. The hydrolysate was partitioned between chloroform and water. The chloroform phase was evaporated to dryness, redissolved in chloroform, and then passed through a short silica gel (Wakogel C-300) column. The eluate was trimethylsilylated at room temperature and subjected to GC-SIM. The levels of sterols were determined using calibration curves constructed from the ratios of the M+ peak area of [2H6]campestanol TMSi (m/z 480) to those of cholesterol TMSi (m/z 458), 24-methylenecholesterol TMSi (m/z 470), campesterol/24-epicampesterol TMSi (m/z 472), campestanol/24-epicampestanol TMSi (m/z 474), stigmasterol TMSi (m/z 484), sitosterol TMSi (m/z 486), sitostanol TMSi (m/z 488), and isofucosterol TMSi (m/z 484). Campesterol and 24-epicampesterol (22-dihydrobrassicasterol), as well as campestanol/24-epicampestanol, were analyzed as a mixture because they were not resolved by GC.
RESULTS
Levels of BRs in lkb Plants Are Reduced, Whereas Those in lka Plants Are Not
Nomura et al. (1997) demonstrated that 36-d-old lkb plants contain significantly lower levels of brassinolide, castasterone, and 6-deoxocastasterone than WT plants. To locate the defective biosynthetic step, the levels of the endogenous BRs situated in the early sections of the biosynthetic pathway were analyzed in extracts from shoots of 49-d-old lkb plants by GC-SIM. BRs (Table II) were rigorously identified because the relative intensities of the M+ ion and two daughter ions for each molecule (see Methods) were consistent with those of the corresponding 2H internal standard (data not shown). The contents of BRs, which were calculated from the peak area ratios of 2H0 and 2H6 M+ ions, are shown in Table II. In the case of 6-oxo-BRs, the levels of castasterone and typhasterol were 23% and 15%, respectively, of the WT levels. The castasterone level was significantly greater than that for the 36-d-old plants, according to previously published results (Nomura et al., 1997). In keeping with this, the size of 49-d-old plants relative to the WT (Table I) was greater than that previously described for 36-d-old plants. It seems that environmental and/or age differences modified the levels of BRs, thereby affecting plant growth. There was no substantial difference in the levels of the earlier precursors, 3-dehydroteasterone and teasterone. Brassinolide was not present at the detectable levels in any genotype. As for 6-deoxo-BRs, the levels of 6-deoxocastasterone and 6-deoxotyphasterol were 7% and 29%, respectively, of the WT levels, whereas no large reduction was observed in the levels of the earlier precursors, 3-dehydro-6-deoxoteasterone and 6-deoxoteasterone (Table II).
Table II.
Endogenous levels of BRs in 49-d-old shoots of WT, lka, and lkb of pea
| BR | BR Level
|
||
|---|---|---|---|
| WT | lka | lkb | |
| ng kg−1 fresh wt | |||
| Brassinolide | NDa | ND | ND |
| Castasterone | 491 ± 0.3 | 3005 ± 2.5 | 112 ± 0.2 |
| Typhasterol | 26 ± 0.1 | 39 ± 1.5 | 4 ± 0.0 |
| 3-Dehydroteasterone | 20 ± 1.4 | 21 ± 1.9 | 15 ± 0.4 |
| Teasterone | 5 ± 0.1 | 5 ± 0.5 | 4 ± 0.2 |
| 6-Deoxocastasterone | 2937 ± 22 | 4053 ± 73 | 208 ± 7.8 |
| 6-Deoxotyphasterol | 835 ± 2.1 | 983 ± 17 | 245 ± 13 |
| 3-Dehydro-6-deoxoteasterone | 182 ± 1.9 | 339 ± 4.2 | 101 ± 4.1 |
| 6-Deoxoteasterone | 180 ± 2.8 | 197 ± 1.1 | 176 ± 2.9 |
The levels of BRs were determined by GC-SIM using 2H internal standards. Data are means ± se of duplicate determinations.
ND, Not detected.
In keeping with the data of Nomura et al. (1997), the BR content of lka plants was not altered greatly compared with WT seedlings, except for an accumulation of castasterone (Table II).
Brassinolide and Its Biosynthetic Precursor BRs Rescue the Dwarf Phenotype of lkb Plants
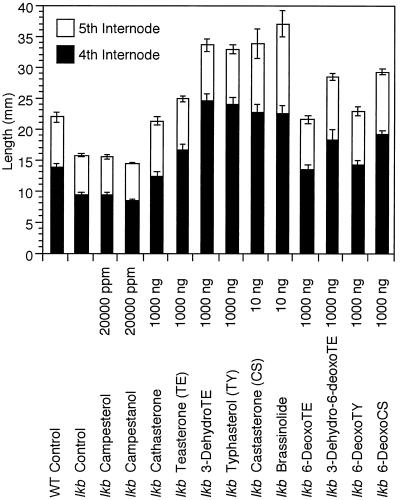
Previously, Nomura et al. (1997) applied 6-oxo-BRs, including brassinolide, castasterone, typhasterol, 3dehydroteasterone, and teasterone to the third leaf of lkb plants, and this resulted in increased elongation of the fourth and fifth internodes. 6-Deoxocastasterone, however, was inactive. In the current study, the fourth internodes of 8-d-old seedlings were treated with BRs to minimize transportation effects, and under these conditions all of the 6-oxo-BRs and 6-deoxo-BRs exhibited activity when applied in doses of 1 μg and less (Fig. 2). Overall, it is apparent that the biological activities of the BRs tend to increase with their proximity to brassinolide in the biosynthetic pathway, as reported by Yokota and Mori (1992) and Fujioka et al. (1995). Campesterol and campestanol failed to promote the internode elongation when applied at high doses in a lanolin paste (Fig. 2). This may reflect low-efficiency conversion to brassinolide and/or poor uptake by the plant tissues.
Figure 2.
Effects of brassinolide and its biosynthetic precursors on the internode length of lkb plants of pea. The lengths of the fourth and fifth internodes were measured 3 d after the fourth internodes were treated. Results are means ± se (n = 5). Campesterol and campestanol were applied in lanolin paste.
Sterol Content of the Shoots and Seeds Is Drastically Altered in lkb, but Not in lka
The levels of sterols in the shoots of 49-d-old plants used for BR analysis and mature seeds of WT, lka, and lkb were analyzed quantitatively by GC-MS, and the data obtained are shown in Table III. All of the sterols listed were rigorously identified by full-scan MS.
Table III.
Endogenous levels of sterols in 49-d-old shoots and mature seeds of WT, lka, and lkb of pea
| Sterol | Sterol Level
|
|||||
|---|---|---|---|---|---|---|
| WT
|
lka
|
lkb
|
||||
| Shoot | Seed | Shoot | Seed | Shoot | Seed | |
| μg g−1 fresh wt | ||||||
| 24-Methylenecholesterol | 0.7 ± 0.0 | NDa | 0.5 ± 0.0 | ND | 17.5 ± 0.2 | 14.5 ± 0.4 |
| Campesterol/24-epicampesterol | 21.1 ± 0.1 | 85.8 ± 1.0 | 25.0 ± 0.4 | 88.2 ± 2.0 | 2.3 ± 0.0 | 6.0 ± 0.0 |
| Campestanol/24-epicampestanol | 1.3 ± 0.0 | 5.3 ± 0.1 | 1.4 ± 0.0 | 5.7 ± 0.0 | 0.1 ± 0.0 | 0.5 ± 0.0 |
| Isofucosterol | 20.3 ± 0.2 | 6.8 ± 0.1 | 16.5 ± 0.3 | 6.1 ± 0.1 | 252.5 ± 2.0 | 1514.5 ± 20 |
| Sitosterol | 122.4 ± 0.8 | 872.0 ± 17 | 143.5 ± 0.8 | 943.2 ± 39 | 11.7 ± 0.1 | 21.7 ± 0.2 |
| Stigmasterol | 46.9 ± 0.2 | 42.0 ± 1.1 | 57.5 ± 0.4 | 60.4 ± 2.1 | 0.6 ± 0.0 | 4.4 ± 0.0 |
| Sitostanol | 5.8 ± 0.0 | 15.9 ± 0.3 | 6.3 ± 0.0 | 15.7 ± 0.1 | ND | ND |
| Cholesterol | 1.4 ± 0.0 | 1.4 ± 0.0 | 1.2 ± 0.0 | 2.2 ± 0.0 | 2.0 ± 0.0 | 1.9 ± 0.0 |
| Total sterols | 219.9 | 1029.2 | 251.9 | 1121.5 | 286.7 | 1563.5 |
| End-pathway sterolsb | 191.8 | 1001.2 | 227.2 | 1094.0 | 16.6 | 34.0 |
The levels of sterols were determined by GC-SIM using [2H6]campestanol as an internal standard. Data are means ± se of duplicate determinations.
ND, Not detected.
Campesterol plus 24-epicampesterol plus sitosterol plus stigmasterol plus cholesterol.
In shoots of WT plants the end-pathway sterols, sitosterol, stigmasterol, campesterol/24-epicampesterol, and cholesterol, account for 87% of the total sterol content, as is the case in most plant species (Nes, 1977). Sitosterol, stigmasterol, and campesterol/24-epicampesterol were the major sterols present (Table III). The levels of 24-methylenecholesterol and isofucosterol were much lower than those of their hydrogenated products, campesterol and sitosterol, respectively. Campestanol/24-epicampestanol and sitostanol were also detected in low levels. The WT and lka seedlings had very similar sterol contents. In contrast to the WT and lka mutant, the lkb plants contained isofucosterol and 24-methylenecholesterol as the major sterols, whereas the levels of the end-pathway sterols, campestanol/24-epicampestanol and sitostanol, were extremely low. It is interesting that the level of cholesterol in the lkb shoots was not altered.
The total sterol content of mature seeds of the WT, lka, and lkb were 4- to 5-fold higher than the levels in the corresponding shoots, although the spectrum of sterols present in the seeds was very similar to that found in shoots (Table III). Thus, in seeds of the WT and lka mutant end-pathway sterols occupied about 97% of the total sterol pool, whereas in lkb seeds isofucosterol and 24-methylenecholesterol accounted for 98% of the total sterol content.
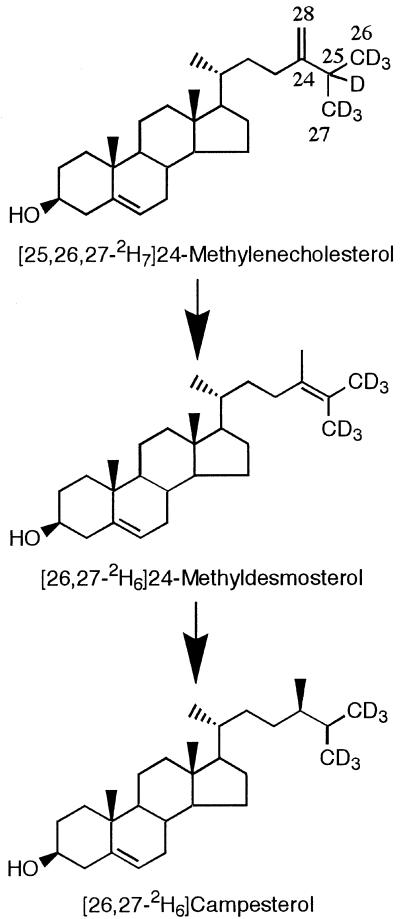
Conversion of [25,26,27-2H7]24-Methylenecholesterol to [26,27-2H6]Campesterol Is Blocked in the lkb Mutant
Since campesterol has been reported to be synthesized from 24-methylenecholesterol via 24-methyldesmosterol (Fig. 3; Goodwin, 1985; Benveniste, 1986), [25,26,27-2H7]24-methylenecholesterol was added to the media in which 6-d-old WT and lkb seedlings had been aseptically grown. After 3 d data were obtained by analyzing roots but not by analyzing shoots: It appears that sterols absorbed by the roots that were submerged in the medium did not move to the shoots. The roots of the WT plants were found to contain similar levels (0.5 μg g−1 fresh weight) of [25,26,27-2H7]24-methylenecholesterol and [26,27-2H6]campesterol, but no [26,27-2H6]24-methyldesmosterol was present (Fig. 4). In contrast, the roots of the lkb plants contained [25,26,27-2H7]24-methylenecholesterol (0.5 μg g−1 fresh weight), but [26,27-2H6]campesterol and [26,27-2H6]24-methyldesmosterol were not detected. Unexpectedly, after feeding [26,27-2H6]24-methyldesmosterol to the WT and lkb tissues, neither this compound nor its expected metabolite, [26,27-2H6]campesterol, was present in root extracts. One of the possible reasons for this result is that [26,27--2H6]24-methyldesmosterol might be rapidly metabolized to other products before reaching the proper reaction site.
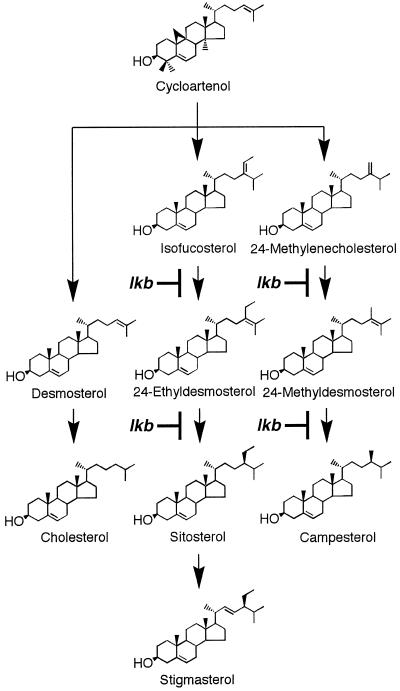
Figure 3.
The sterol biosynthesis pathways. Lesions in the BR biosynthesis of the lkb mutant of pea are shown.
Figure 4.
Proposed synthetic pathway from [25,26,27-2H7]24-methylenecholesterol to [26,27-2H6] campesterol.
Brassinolide-Treated lkb Plants Had Lower Levels of Endogenous Sterols
The effect of exogenous brassinolide on the levels of endogenous sterols was investigated because it was suspected that the abnormal sterol content of the lkb plants might be normalized by exogenous brassinolide. WT seedlings (8 d old) were treated with 100 ng of brassinolide. After 2 d elongation was enhanced, and this was accompanied by an increase in the fresh weight of the shoot. The brassinolide treatment also increased the total sterol content per shoot in proportion to the growth increase, although when expressed on a fresh-weight basis this effect was not evident (Table IV). A similar result was obtained in lka plants (Table IV).
Table IV.
Effects of brassinolide on the growth and sterol levels of WT, lka, and lkb shoots of pea
| Growth and Level | WT
|
lka
|
lkb
|
|||
|---|---|---|---|---|---|---|
| Control | Brassinolide (100 ng) | Control | Brassinolide (100 ng) | Control | Brassinolide (100 ng) | |
| Profile | ||||||
| Fourth internode length (mm ± se) | 11.2 ± 0.4 | 17.2 ± 0.6 | 7.7 ± 0.2 | 11.5 ± 0.4 | 8.7 ± 0.2 | 21.4 ± 0.7 |
| Averaged weight per apical segment (g) | 0.18 | 0.22 | 0.19 | 0.22 | 0.19 | 0.27 |
| Sterol (μg g−1 fresh wt) | ||||||
| 24-Methylenecholesterol | 1.2 ± 0.0 | 1.4 ± 0.0 | 1.5 ± 0.0 | 1.9 ± 0.1 | 43.7 ± 0.4 | 32.1 ± 0.3 |
| Campesterol/24-epicampesterol | 38.3 ± 0.2 | 39.0 ± 0.1 | 49.9 ± 0.1 | 46.0 ± 0.3 | 2.0 ± 0.1 | 1.3 ± 0.0 |
| Campestanol/24-epicampestanol | 0.9 ± 0.0 | 0.8 ± 0.0 | 1.3 ± 0.0 | 1.4 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| Isofucosterol | 32.2 ± 0.3 | 31.1 ± 0.1 | 21.4 ± 0.0 | 26.8 ± 0.3 | 311.8 ± 8.0 | 161.1 ± 2.5 |
| Sitosterol | 100.5 ± 0.4 | 100.2 ± 2.2 | 131.7 ± 1.4 | 121.1 ± 0.3 | 9.5 ± 0.0 | 6.5 ± 0.1 |
| Stigmasterol | 132.0 ± 0.0 | 132.5 ± 1.3 | 144.9 ± 0.3 | 137.8 ± 1.1 | 0.8 ± 0.0 | 0.5 ± 0.0 |
| Sitostanol | 3.3 ± 0.0 | 3.2 ± 0.0 | 4.7 ± 0.2 | 4.4 ± 0.0 | NDa | ND |
| Cholesterol | 7.2 ± 0.1 | 8.4 ± 0.1 | 16.9 ± 0.2 | 13.9 ± 0.1 | 7.7 ± 0.0 | 3.4 ± 0.0 |
| Total sterols (μg g−1 fresh wt) | 315.6 | 316.6 | 372.3 | 353.3 | 375.6 | 205.0 |
| Total sterols (μg per segment) | 56.9 | 69.6 | 70.8 | 77.7 | 71.4 | 55.5 |
Third leaves of 8-d-old seedlings (n = 20) were treated with brassinolide. After 2 d growth data and sterol levels were determined. The levels of sterols were determined by GC-SIM using [2H6]campestanol as an internal standard. Sterol levels are expressed as means ± se of duplicate determinations.
ND, Not detected.
Treatment of 8-d-old lkb seedlings with 100 ng of brassinolide induced an elevated rate of internode elongation that was accompanied by an increase in the fresh weight of the shoot. The endogenous sterol levels of lkb, however, tended to decrease (Table IV), with the sterol content on a fresh-weight basis decreasing to 55% and the total sterol content per shoot segment decreasing to 78%.
The lkb Mutant Has an Altered Sterol Composition in the Membrane
A microsomal membrane fraction and a soluble fraction were obtained from 23-d-old WT, lka, and lkb shoots by centrifugation at 100,000g. The microsomal fractions sedimented at 100,000g are composed of a mixture of vesicles originating from various membrane types (Hartmann and Benveniste, 1987). Sterol compositions in these fractions and whole shoots are shown in Table V. The microsomal membranes of WT contained sitosterol and stigmasterol as the major sterols, whereas those of the lkb mutant contained isofucosterol and sitosterol as the principal components. In the WT membranes the level of sitosterol was increased 1.4-fold compared with that of the whole shoots. In the lkb membrane the levels of sitosterol and campesterol/24-epicampesterol were elevated 25- and 4-fold, respectively, compared with those of the whole shoots. The levels of cholesterol in the microsomal fraction, soluble fraction, and whole shoots of the lkb mutant were nearly comparable to those of the WT. Surprisingly, the sterol compositions of the WT and lkb soluble fractions were indistinguishable. Sterol compositions in all samples obtained from lka seedlings closely resembled those of the WT.
Table V.
Subcellular distribution of sterols in 23-d-old WT, lka, and lkb seedlings of pea
| Sterol | Sterol Composition
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Microsomal fraction
|
Soluble
fraction
|
Whole shoot
|
|||||||
| WT | lka | lkb | WT | lka | lkb | WT | lka | lkb | |
| % wt of total sterols | |||||||||
| 24-Methylenecholesterol | NDa | ND | 3 | ND | ND | ND | ND | ND | 9 |
| Campesterol/24-epicampesterol | 9 | 10 | 4 | 11 | 11 | 11 | 11 | 12 | 1 |
| Campestanol/24-epicampestanol | 1 | 1 | 0.5 | 1 | 5 | 2 | 0.5 | 0.5 | 0.1 |
| Isofucosterol | 8 | 7 | 64 | ND | ND | 2 | 14 | 10 | 88 |
| Sitosterol | 52 | 52 | 25 | 73 | 74 | 73 | 38 | 38 | 1 |
| Stigmasterol | 24 | 25 | 0.5 | 1 | 1 | 2 | 34 | 37 | 0.4 |
| Sitostanol | 3 | 3 | 1 | 1 | 1 | 1 | 2 | 2 | ND |
| Cholesterol | 3 | 2 | 2 | 13 | 8 | 9 | 0.5 | 0.5 | 0.5 |
Sterols were quantified by GC-SIM using [2H6]campestanol as an internal standard. Data determined in duplicates were averaged and used to calculate sterol compositions.
ND, Not detected.
DISCUSSION
BR Biosynthesis Is Retarded in the lkb Mutant
In the biosynthesis of brassinolide, campesterol is first converted to campestanol, which is metabolized to castasterone via either the early or late C-6 oxidation pathway, after which castasterone is converted to brassinolide (Fig. 1). The analysis of endogenous BRs in the present study indicated the presence of components of both the early and late C-6 oxidation pathways in pea (Choi et al., 1997; Table II). Low levels of castasterone, typhasterol, 6-deoxocastasterone, and 6-deoxotyphasterol in shoots of 49-d-old lkb plants indicate that both pathways are affected. However, the lkb plants had levels of 3-dehydroteasterone and teasterone in the early C-6 oxidation pathway, as well as of 3-dehydro-6-deoxoteasterone and 6-deoxoteasterone in the late C-6 oxidation pathway, comparable to those of the WT. It is possible to speculate that the reduction of the 3-oxo group to 3β-hydroxyl function may be blocked in the lkb mutant. However, this possibility is unlikely because both 3-dehydroteasterone and 3-dehydro-6-deoxoteasterone are biologically active (Fig. 2), probably via the conversion to typhasterol and 6-deoxotyphasterol, respectively (Fig. 1). All of the BRs examined could counteract the dwarfism of the lkb mutant (Fig. 2), suggesting a blockage at a very early step in BR biosynthesis, possibly even as far back as the sterol biosynthesis pathway.
BR Deficiency in the lkb Mutant Is Due to Impaired Synthesis of Campesterol from 24-Methylenecholesterol
Sterol compositions of 10-, 23-, and 49-d-old lkb shoots, which are summarized in Tables IV, V, and III, respectively, as well as those of mature seeds (Table III), were almost comparable. Such sterol profiles indicate lesions in the reductive conversion of 24-methylenecholesterol to campesterol and of isofucosterol to sitosterol in shoots and seeds of the lkb mutant. The inability to metabolize [25,26,27-2H7]24-methylenecholesterol to [26,27-2H6]campesterol was confirmed using 6-d-old lkb seedlings. This conversion occurred in WT tissues with a loss of the 2H at C-25, indicating that the Δ24(28) double bond in 24-methylenecholesterol is first isomerized to a Δ24(25) double bond, yielding 24-methyldesmosterol, which, in turn, is hydrogenated to campesterol (Fig. 4), as was postulated by Goodwin (1985) and Benveniste (1986). Thus, it is possible that the LKB protein has a dual role in that it catalyzes both isomerization and reduction of the Δ24(28) double bond in the hydrogenation of 24-methylenecholesterol and isofucosterol. However, we do not exclude the possibility that the LKB gene product may act as a regulator influencing gene expression or enzyme activity of one or more sterol isomerase(s)/reductase(s) or that the LKB gene may encode either an isomerase or a reductase that act together in a tightly coupled fashion. Nonetheless, we conclude that the dwarfism of the lkb mutant is due to BR deficiency caused by blocked conversion of 24-methylenecholesterol to campesterol. Recently, Klahre et al. (1998) found that the dwarf dim mutant of Arabidopsis has the same defect as the lkb mutant.
[26,27-2H6]24-Methyldesmosterol could not be detected after feeding [25,26,27-2H7]24-methylenecholesterol to WT and lkb tissues. Furthermore, 24-methyldesmosterol, as well as 24-ethyldesmosterol, which is an intermediate between isofucosterol and sitosterol, could not be detected as endogenous components in either intact shoots or seeds of WT and lkb. This suggests that 24-methyldesmosterol, as well as 24-ethyldesmosterol, is synthesized and further metabolized without being released from the surface of the enzyme(s).
24-Methylenecholesterol has been regarded as a putative substrate for BRs having a 24-methylene group, e.g. in seeds of Lablab purpurea (L.) Sweet (formerly Dolichos lablab L.; Yokota et al., 1984; Yokota, 1997). However, the stunted phenotype of the lkb and dim mutants indicates that such a pathway is not operative in either pea or Arabidopsis.
Hydrogenation of the Δ24(25) double bond has been suggested to occur in the reductive synthesis of cholesterol from desmosterol (Fig. 3) in plants (Grunwald, 1975; Goodwin, 1985) and animals (Rilling and Chayet, 1985). The LKB gene may not be involved in this conversion because the lkb mutant and WT contained similar levels of cholesterol in their shoots, seeds, and microsomal membranes (Tables III and V). Some plant species, such as tomato (Yokota et al., 1997) and Ornithopus sativus (Spengler et al., 1995), contain, in addition to C28 BRs derived from campesterol, C27 BRs having no alkyl substituent at C-24, which are probably synthesized from cholesterol (Yokota, 1997). Thus, such plants may not show a clear dwarf phenotype, even when the gene orthologous to LKB is impaired, because they can probably synthesize C27 BRs, which may compensate for the loss of campesterol-derived BRs.
Abnormal Sterol Compositions in the lkb Mutant May Damage Functions of Membrane
In an attempt to examine the effect of brassinolide on the sterol composition, we found that sterol synthesis was retarded in 10-d-old lkb seedlings treated with brassinolide, despite the dwarf phenotype being counteracted with internode elongation being promoted strongly (Table IV). The suppression of sterol synthesis suggests that membranes of the lkb mutant may have an unusual sterol composition, which may, in turn, affect adversely the activity of membrane-bound enzymes (Nes, 1977; Benveniste, 1986; Hartmann, 1998), including those involved in sterol biosynthesis. The membranes of 23-d-old lkb shoots were most unusual in that they contain isofucosterol as the major sterol (64% in weight), in contrast to the WT and lka membranes in which sitosterol is the major component (52% in weight). Surprisingly, the sitosterol content in the lkb membranes increased to 25%, although sitosterol in the whole shoot accounts for only 1% of the total sterols (Table V). A similar increase was also observed in the campesterol/24-epicampesterol content. It is well known that sterols affect membrane fluidity (Hartmann, 1998). Sitosterol and campesterol/24-epicampesterol have been found to be the most efficient sterols for restricting the mobility of fatty acyl chains in soybean phosphatidylcholine bilayers and appear to be very active in reducing the water permeability of the soybean membranes (Schuler et al., 1991). Thus, the partial recovery in the sitosterol and campesterol/24-epicampesterol level in the lkb membranes could be due to homeostatic control restoring sterol composition and hence membrane functions. The finding that the soluble fraction of the lkb mutant has the same composition as WT also may be due to homeostatic control, although the role of sterols in the soluble fraction seems to be undefined (Gachotte et al., 1995).
A further defect of the lkb membranes is the loss of stigmasterol (Table V). In celery cells growth inhibition caused by a sterol biosynthetis inhibitor was restored by either stigmasterol or mixtures of cholesterol and a low concentration of stigmasterol (Goad, 1990). Recently, it was found that stigmasterol and cholesterol stimulate proton pumping in H+-ATPase of maize, whereas sitosterol and 24-methylcholesterol act as inhibitors (Grandmougin-Ferjani et al., 1997). These findings indicate that stigmasterol has important functions in the metabolism and regulation of plants.
Thus, whereas circumstantial evidence suggests that the changes in sterol composition of the lkb membrane may slow biochemical reactions of certain membrane-bound enzymes (Table IV), the phenotypic similarity of the lka and lkb mutants suggests that the phenotype of both plants is controlled only by their similarly perceived BR levels rather than endogenous sterol levels.
The Level of Castasterone May Be Controlled by a Feedback Mechanism
Although the lka mutant has a phenotype similar to the lkb mutant, it is not BR deficient (Table II) and its dwarfism is not counteracted by exogenous brassinolide (Nomura et al., 1997). Furthermore, the sterol compositions of the lka mutant are almost identical with those of the WT (Tables III–V). Such evidence suggests that the lka mutant has a defect in the perception or signal transduction pathway of BRs.
In the dwarf but GA-insensitive mutants Rht3 (wheat), D8 (maize), and gai (Arabidopsis), the levels of GA1 are elevated substantially. It was suggested that GA action results in the production of a transcriptional repressor that limits the expression of GA biosynthetic enzymes. Mutants with an impaired response to GA would lack this repressor and have an elevated rate of GA production (Hedden and Kamiya, 1997). In 49-d-old shoots of the lka mutant a large accumulation of castasterone was observed. However, no quantitative information was obtained for brassinolide because its levels in both the lka mutant and WT plants were below detectable limits. However, it has been demonstrated that the level of brassinolide in the 36-d-old seedlings of the lka mutant is not elevated (Nomura et al., 1997). These findings indicate that, at least in pea shoots, synthesis of castasterone rather than brassinolide may be controlled by a feedback mechanism similar to that proposed for GA1. Recently, it was demonstrated that transcription of the Arabidopsis CPD gene is controlled in this manner by BR (Mathur et al., 1998). Brassinolide is the most biologically active BR, and hence, the conversion of castasterone to brassinolide is deemed an activation step. However, such conversion has not been observed in bioassay systems in which castasterone is biologically active (Yokota, 1997). All of the evidence currently available indicates that castasterone, in addition to brassinolide, may be a biologically active BR.
ACKNOWLEDGMENTS
We thank Akihiko Saito for the bioassay of BRs. We also thank Dr. Alan Crozier (Institute of Biochemical and Life Sciences, University of Glasgow, UK) and Dr. Gerard Bishop (The Institute of Physics and Chemistry, Wako, Japan) for providing helpful comments to improve this manuscript.
Abbreviations:
- BMB
bismethaneboronate
- BR
brassinosteroid
- MB
monomethaneboronate
- SIM
selected ion monitoring
- TMSi
trimethylsilyl ether
- WT
wild type
Footnotes
This work was supported by a Grant-in-Aid for Cooperative Research from the Ministry of Education, Science and Culture of Japan (grant no. 06305010). T.N. was supported by Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists.
LITERATURE CITED
- Adam G, Porzel A, Schmidt J, Schneider B, Voigt B (1996) New developments in brassinosteroid research. In ST Atta-ur-Rahman, eds, Studies in Natural Products Chemistry, Vol 18. Elsevier, New York, pp 495–549
- Azpiroz R, Wu Y, LoCascio JC, Feldmann KA. An Arabidopsis brassinosteroid-dependent mutant is blocked in cell elongation. Plant Cell. 1998;10:219–230. doi: 10.1105/tpc.10.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste P. Sterol biosynthesis. Annu Rev Plant Physiol. 1986;37:275–308. [Google Scholar]
- Bishop GJ, Harrison K, Jones JDG. The tomato Dwarf gene isolated by heterologous transposon tagging encodes the first member of a new cytochrome P450 family. Plant Cell. 1996;8:959–969. doi: 10.1105/tpc.8.6.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop GJ, Nomura T, Yokota T, Harrison K, Noguchi T, Fujioka S, Takatsuto S, Jones JDG, Kamiya Y. The tomato DWARF enzyme catalyses C-6 oxidation in brassinosteroid biosynthesis. Proc Natl Acad Sci USA. 1999;96:1761–1766. doi: 10.1073/pnas.96.4.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe S, Dilkes BP, Fujioka S, Takatsuto S, Sakurai A, Feldmann KA. The DWF4 gene of Arabidopsis encodes a cytochrome P450 that mediates multiple 22α-hydroxylation steps in brassinosteroid biosynthesis. Plant Cell. 1998;10:231–243. doi: 10.1105/tpc.10.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YH, Fujioka S, Nomura T, Harada A, Yokota T, Takatsuto S, Sakurai A. An alternative brassinolide biosynthetic pathway via late C-6 oxidation. Phytochemistry. 1997;44:609–613. [Google Scholar]
- Clouse SD. Molecular genetic studies confirm the role of brassinosteroids in plant growth and development. Plant J. 1996;10:1–8. doi: 10.1046/j.1365-313x.1996.10010001.x. [DOI] [PubMed] [Google Scholar]
- Clouse SD. Molecular genetic analysis of brassinosteroid action. Physiol Plant. 1997;100:702–709. [Google Scholar]
- Clouse SD, Hall AF, Langford M, McMorris TC, Baker ME. Physiological and molecular effects of brassinosteroids on Arabidopsis thaliana. J Plant Growth Regul. 1993;12:61–66. [Google Scholar]
- Clouse SD, Langford M, McMorris TC. A brassinosteroid-insensitive mutant in Arabidopsis thaliana exhibits multiple defects in growth and development. Plant Physiol. 1996;111:671–678. doi: 10.1104/pp.111.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse SD, Sasse MD. Brassinosteroids: essential regulators of plant growth and development. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:427–451. doi: 10.1146/annurev.arplant.49.1.427. [DOI] [PubMed] [Google Scholar]
- Fujioka S, Inoue T, Takatsuto S, Yanagisawa T, Yokota T, Sakurai A. Biological activities of biosynthetically-related congeners of brassinolide. Biosci Biotechnol Biochem. 1995;69:1973–1975. [Google Scholar]
- Fujioka S, Li J, Choi YH, Seto H, Takatsuto S, Noguchi T, Watanabe T, Kuriyama H, Yokota T, Chory J and others. The Arabidopsis deetiolated2 mutant is blocked early in brassinosteroid biosynthesis. Plant Cell. 1997;9:1951–1962. doi: 10.1105/tpc.9.11.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka S, Sakurai A. Biosynthesis and metabolism of brassinosteroids. Physiol Plant. 1997a;100:710–715. [Google Scholar]
- Fujioka S, Sakurai A. Brassinosteroids. Nat Prod Rep. 1997b;14:1–10. doi: 10.1039/np9971400001. [DOI] [PubMed] [Google Scholar]
- Gachotte D, Meens R, Benveniste P. An Arabidopsis mutant deficient in sterol biosynthesis: heterologous complementation by ERG 3 encoding a Δ7-sterol-C-5-desaturase from yeast. Plant J. 1995;8:407–416. doi: 10.1046/j.1365-313x.1995.08030407.x. [DOI] [PubMed] [Google Scholar]
- Goad LJ. Application of sterol synthesis inhibitor to investigate the sterol requirements of protozoa and plants. Biochem Soc Trans. 1990;18:63–65. doi: 10.1042/bst0180063. [DOI] [PubMed] [Google Scholar]
- Goodwin TW (1985) Biosynthesis of plant sterols. In H Danielsson, J Sjövall, eds, Sterol and Bile Acids. Elsevier Science, New York, pp 175–198
- Grandmougin-Ferjani A, Schuler-Muller I, Hartmann M-A. Sterol modulation of the plasma membrane H+-ATPase activity from corn roots reconstituted into soybean lipids. Plant Physiol. 1997;113:163–174. doi: 10.1104/pp.113.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove MD, Spencer GF, Rohwedder WK, Mandava N, Worley JF, Warthen JD, Steffens GL, Flippen-Anderson J, Cook JC., Jr Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature. 1979;281:216–217. [Google Scholar]
- Grunwald C. Plant sterols. Annu Rev Plant Physiol. 1975;26:209–236. [Google Scholar]
- Hartmann M-A. Plant sterols and the membrane environment. Trends Plant Sci. 1998;3:170–175. [Google Scholar]
- Hartmann M-A, Benveniste P. Plant membrane sterols: isolation, identification and biosynthesis. Methods Enzymol. 1987;148:632–650. [Google Scholar]
- Hedden P, Kamiya Y. Gibberellin biosynthesis: enzymes, gene and their regulation. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:431–460. doi: 10.1146/annurev.arplant.48.1.431. [DOI] [PubMed] [Google Scholar]
- Kauschmann A, Jessop A, Koncz C, Szekeres M, Willmitzer L, Altmann T. Genetic evidence for an essential role of brassinosteroids in plant development. Plant J. 1996;9:701–713. [Google Scholar]
- Kim SK, Akihisa T, Tamura T, Matsumoto T, Yokota T, Takahashi N. 24-Methylene-25-methylcholesterol in Phaseolus vulgaris seed: structural relation to brassinosteroids. Phytochemistry. 1988;27:629–631. [Google Scholar]
- Klahre U, Noguchi T, Fujioka S, Takatsuto S, Yokota T, Nomura T, Yosida S, Chua NH. The Arabidopsis DIMINUTO/DWARF1 gene encodes a protein involved in steroid synthesis. Plant Cell. 1998;10:1677–1690. doi: 10.1105/tpc.10.10.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence NL, Ross JJ, Mander LN, Reid JB. Internode length in Pisum. Mutants lk, lka and lkb do not accumulate gibberellins. J Plant Growth Regul. 1992;11:35–37. [Google Scholar]
- Li J, Biswas MG, Chao A, Ruessel DW, Chory J. Conservation of function between mammalian and plant steroid 5α-reductases. Proc Natl Acad Sci USA. 1997;94:3554–3559. doi: 10.1073/pnas.94.8.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Chory J. A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell. 1997;90:929–938. doi: 10.1016/s0092-8674(00)80357-8. [DOI] [PubMed] [Google Scholar]
- Li J, Nagpal P, Vitart V, McMorris TC, Chory J. A role for brassinosteroids in light-dependent development of Arabidopsis. Science. 1996;272:398–401. doi: 10.1126/science.272.5260.398. [DOI] [PubMed] [Google Scholar]
- Mandava NB. Plant growth-promoting brassinosteroids. Annu Rev Plant Physiol Plant Mol Biol. 1988;39:23–52. [Google Scholar]
- Mathur J, Molnár G, Fujioka S, Takatsuto S, Sakurai A, Yokota T, Adam G, Voigt B, Nagy F, Maas C and others. Transcription of the Arabidopsis CPD gene, encoding a stereoidogenic cytochrome P450, is negatively controlled by brassinosteroids. Plant J. 1998;14:593–602. doi: 10.1046/j.1365-313x.1998.00158.x. [DOI] [PubMed] [Google Scholar]
- Mckay MJ, Ross JJ, Lawrence NL, Cramp RE, Beveridge CA, Reid JB. Control of internode length in Pisum sativum. Further evidence for the involvement of indole-3-acetic acid. Plant Physiol. 1994;106:1521–1526. doi: 10.1104/pp.106.4.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JW, Livingston GA (1968) Methods of studying plant hormones and growth-regulating substances. Agriculture Handbook No. 336. Agricultural Research Service, U.S. Department of Agriculture, Washington, DC, pp 26–28
- Mori H, Sakakibara M, Okada K. Synthesis of naturally occurring brassinosteroids employing cleavage of 23,24-epoxides as key reactions: synthesis of brassinolide, castasterone, dolicholide, dolichosterone, homodolicholide, homodolichosterone, 6-deoxocastasterone and 6-deoxodolichosterone. Tetrahedron. 1984;40:1767–1781. [Google Scholar]
- Nes WR. The biochemistry of plant sterols. Adv Lipid Res. 1977;15:233–324. [Google Scholar]
- Nomura T, Nakayama M, Reid JB, Takeuchi Y, Yokota T. Blockage of brassinosteroid biosynthesis and sensitivity causes dwarfism in Pisum sativum. Plant Physiol. 1997;113:31–37. doi: 10.1104/pp.113.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid JB, Davies PJ. The genetics and physiology of gibberellin sensitivity mutants in peas. In: Karssen CM, Van Loon LC, Vreugdenhil D, editors. Progress in Plant Growth Regulation. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992. pp. 214–225. [Google Scholar]
- Reid JB, Ross JJ. Internode length in Pisum: two further gibberellin insensitivity genes, lka and lkb. Physiol Plant. 1989;75:81–88. [Google Scholar]
- Reid JB, Ross JJ, Hasa O. Internode length in Pisum: gene lkc. J Plant Growth Regul. 1991;10:11–16. [Google Scholar]
- Rilling HC, Chayet LT (1985) Biosynthesis of cholesterol. In H Danielsson, J Sjövall, eds, Sterol and Bile Acids. Elsevier Science, New York, pp 1–39
- Sakurai A, Fujioka S. The current status of physiology and biochemistry of brassinosteroids. Plant Growth Regul. 1993;13:147–159. [Google Scholar]
- Sasse JM. Recent progress in brassinosteroid research. Physiol Plant. 1997;100:1–6. [Google Scholar]
- Schuler I, Milon A, Nakatanni Y, Ourisson G, Albrecht A-M, Benveniste P, Hartmann M-A. Differential effects of plant sterols on water permeability and on acyl chain ordering of soybean phosphatidylcholine bilayers. Proc Natl Acad Sci USA. 1991;88:6926–6930. doi: 10.1073/pnas.88.16.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler B, Schmidt J, Voigt B, Adam G. 6-Deoxo-28-norcastasterone and 6-deoxo-24-epicastasterone: two new brassinosteroids from Ornithopus sativus. Phytochemistry. 1995;40:907–910. [Google Scholar]
- Szekeres M, Nemeth K, Kalman ZK, Mathur J, Kauschmann A, Altmann T, Redei GP, Nagy F, Schell J, Koncz C. Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell. 1996;85:171–182. doi: 10.1016/s0092-8674(00)81094-6. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Gasch A, Nishizawa N, Chua NH. The DIMINUTO gene of Arabidopsis is involved in regulating cell elongation. Gene Dev. 1995;9:97–107. doi: 10.1101/gad.9.1.97. [DOI] [PubMed] [Google Scholar]
- Takatsuto S, Goto C, Noguchi T, Nomura T, Fujioka S, Yokota T. Synthesis of deuterio-labelled 24-methylenecholesterol and related steroids. J Chem Res. 1998;207:206–207. [Google Scholar]
- Takatsuto S, Ikekawa N. Synthesis of deuterio-labelled brassinosteroids, [26,28-2H6]brassinolide, [26,28-2H6]castasterone, [26,28-2H6]typhasterol, and [26,28-2H6]teasterone. Chem Pharm Bull. 1986;34:4045–4049. [Google Scholar]
- Yang T, Davies PJ, Reid JB. Genetic dissection of the relative roles of auxin and gibberellin in the regulation of stem elongation in intact light-grown peas. Plant Physiol. 1996;110:1029–1034. doi: 10.1104/pp.110.3.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T. The structure, biosynthesis and function of brassinosteroids. Trends Plant Sci. 1997;2:137–143. [Google Scholar]
- Yokota T, Baba J, Koba S, Takahashi N. Purification and separation of eight steroidal plant-growth regulators from Dolichoslablab seed. Agric Biol Chem. 1984;48:2529–2534. [Google Scholar]
- Yokota T, Matsuoka T, Koarai T, Nakayama M. 2-Deoxybrassinolide, a brassinosteroid from Pisum sativum seed. Phytochemistry. 1996;42:509–511. [Google Scholar]
- Yokota T, Mori K. Molecular structure and biological activity of brassinolide and related brassinosteroids. In: Duax WL, Bohl M, editors. Molecular Structure and Biological Activity of Steroids. Boca Raton, FL: CRC Press; 1992. pp. 317–340. [Google Scholar]
- Yokota T, Nakayama M, Wakisaka T, Schmidt J, Adam G. 3-Dehydroteasterone, a 3,6-diketobrassinosteroid as a possible biosynthetic intermediate of brassinolide from wheat grain. Biosci Biotechnol Biochem. 1994;58:1183–1185. [Google Scholar]
- Yokota T, Nomura T, Nakayama M. Identification of brassinosteroids that appear to be derived from campesterol and cholesterol in tomato shoots. Plant Cell Physiol. 1997;38:1291–1294. [Google Scholar]