SUMMARY
The aim of the study was to evaluate the efficacy and potential pitfalls of selective neck dissection of levels II-IV in controlling occult neck disease in clinically negative neck (cN0) of patients with laryngeal squamous cell carcinoma. Charts of 96 consecutive cN0 laryngeal cancer patients undergoing 122 neck dissections at the University of Florence from January 2000 to December 2004 were reviewed. N0 neck was defined with contrast enhanced computed tomography scan. Occult neck disease rate was 12.5%, involvement per level was: 47.6% at level II, 38.1% at level III, 9.5% at level IV. Six patients developed neck recurrence (6.25%) after selective neck dissection of levels II-IV within the first two years after treatment. In conclusion, selective neck dissection of levels II-IV is effective in N0 laryngeal squamous cell carcinoma; posterior limits of surgical resection are missing therefore if post-operative radiation is required, the field should be extended beyond the dissected levels. The low incidence of occult neck disease indicates the need to refine treatment strategy, restricting elective neck dissection only to supraglottic T2 with epilaryngeal involvement, supraglottic T3-4 and glottic T4 tumours, and considering a "wait and see" protocol implemented with imaging techniques and cytological assessments for other lesions.
KEY WORDS: Neck dissection, Laryngeal carcinoma, Selective neck dissection, Modified radical neck dissection, Partial laryngectomy
RIASSUNTO
Abbiamo valutato le possibili problematiche e l'efficacia dello svuotamento latero-cervicale selettivo dei livelli II-IV nel controllo delle metastasi occulte in pazienti affetti da carcinomi squamocellulari della laringe senza evidenza clinica di adenopatie laterocervicali (cN0). Abbiamo rivalutato le cartelle cliniche di 96 pazienti affetti da cancro della laringe cN0 sottoposti a svuotamento latero-cervicale selettivo dei livelli II-IV presso l'Università di Firenze tra il gennaio 2000 e dicembre 2004. Clinicamente il paziente è stato considerato cN0 dopo essere stato sottoposto a TC con mezzo di contrasto. Metastasi occulte latero-cervicali sono state riscontrate nel 12,5% dei casi. L'interessamento metastatico registrato per ciascun livello è stato: 47,6% livello II, 38,1% livello III, 9,5% livello IV. Sei pazienti sottoposti a svuotamento laterocervicale selettivo dei livelli II-IV hanno sviluppato una recidiva sul collo (6,25%) entro due anni dal trattamento. Lo svuotamento laterocervicale selettivo dei livelli II-IV è una procedura oncologicamente adeguata nei pazienti con collo N0. Poiché non esistono limiti posteriori nell'esecuzione dello svuotamento, il campo di irraggiamento dell'eventuale radioterapia postoperatoria dovrebbe essere esteso a comprendere anche i livelli I e V. La bassa incidenza di metastasi occulte indica la necessità di rivedere le indicazioni chirurgiche riservando lo svuotamento elettivo solo ai tumori sopraglottici T2 con estensione epilaringea, sopraglottici T3-4 e ai glottici T4, sottoponendo gli altri pazienti a un attento follow-up strumentale e citologico.
Introduction
In patients with squamous cell carcinoma (SCC) of the larynx, the management of regional lymph nodes is a crucial component of the overall treatment plan. If metastases to the cervical lymph nodes are clinically evident at diagnosis, treatment of the neck is mandatory. The situation is more controversial when no clinical signs of neck disease are found (N0 neck). In this situation, the surgeon must decide whether to electively treat the neck or wait for metastases to develop and then treat the patient whenever they occur 1. The limitations in the identification of micrometastases and the negative impact of recurrences in the neck are still a challenge.
The lymphatic tumour spread to the neck follows predictable paths 2-6; this evidence justified the development of selective neck dissection (SND) for removing only those levels at risk. The SND of levels II-IV is, therefore, considered adequate in patients with cN0 laryngeal cancer 7-9. Furthermore, in order to minimize post-operative morbidity, it has recently been proposed by several Authors to carry out a super-selective dissection of levels IIA-III because sub-level IIB and level IV are seldom involved without involvement of sub-level IIA and level III 10-15.
The present report refers to a series of 96 consecutive cN0 laryngeal cancer patients treated at the Clinic of Otolaryngology- Head & Neck Surgery of the University of Florence, between 2000 and 2004 with a critical analysis of the results and potential pitfalls in SND II-IV.
Material and methods
Inclusion criteria to define N0 neck, in laryngeal cancer patients in this series, were based upon characteristics of detectable lymph nodes at contrast enhanced CT scan: lesser diameter < 10 mm, absence of central necrosis, absence of contrast enhancement of lymph node capsule. The medical records of 96 consecutive patients with SCC of the larynx matched these criteria and represent the source data for this study. None of these patients had ever received radiotherapy or chemotherapy and all were submitted to surgery at the Otolaryngology-Head & Neck Surgery Clinic of the University of Florence, between January 2000 to December 2004 and then observed at strict follow-up. The UICC-AJCC TNM 6th edition staging system 15 was used for the staging of patients. The population study (Table I) comprised 80 males and 16 females, with a mean age of 63 years (median 67, range 38-82). Of these, 57 presented a supraglottic localization of laryngeal SCC, staged as follows: 11 T1, 24 T2, 10 T3, and 12 T4a; 39 presented a glottic localization staged as follows: 20 T2, 17 T3, and 2 T4a. Of these, 14 had a total laryngectomy (all T4a cases with massive extra-laryngeal extension), in the remaining 82 cases a conservative approach was performed according to tumour site and stage: 31 transoral CO2 laser resections, 16 supraglottic horizontal laryngectomies; 33 supracricoid partial laryngectomies (21 crico-hyoid-pexis: CH P and 12 crico-hyoid-epiglotto-pexis: CHE P) and 2 hemilaryngectomies. A total of 122 elective neck dissections were performed: in 23 cases, a modified radical neck dissection (mRND) and in 64 cases a SND II-IV; in 35 patients, a contralateral neck dissection was also performed: 4 mRND and 31 SND II-IV. All mRNDs were performed preserving the internal jugular vein, sternocleidomastoid muscle and spinal accessory nerve (this type of neck dissection was performed in the early period of the series before institutional consolidation of SND II-IV).
Table I.
Patient overview.
| No. patients | 96 |
| Sex | |
| Male | 80 |
| Female | 16 |
| Site of T | |
| Supraglottic (57) | |
| T1 | 11 |
| T2 | 24 |
| T3 | 10 |
| T4a | 12 |
| Glottic (39) | |
| T2 | 20 |
| T3 | 17 |
| T4a | 2 |
| Type of Surgery | |
| Total Laryngectomy | 14 |
| Hemilaryngectomy | 2 |
| CHP | 21 |
| CHEP | 12 |
| SPL | 16 |
| CO2 laser | 31 |
| Neck procedures (122) | |
| Homolateral mRND | 23 |
| Homolateral SND II-IV | 64 |
| Contralateral mRND | 4 |
| Contralateral SND II-IV | 31 |
CHP: crico-hyoid-pexy; CHEP: crico-hyoid-epiglotto-pexy; SPL: Supraglottic horizontal Partial Laryngectomy; mRND: modified Radical Neck Dissection; SND: Selective Node Dissection.
Each neck specimen was divided into levels by the surgeon and sent for pathological analysis, it was then sectioned in a routine manner and studied by the pathologist. Lymph nodes were mainly studied on a single section (double section only in lymph nodes with larger diameter > 10/15 mm).
Of the 96 patients, 19 (19.8%) received post-operative radiotherapy with standard fractioning of 200 cGy daily for 5 days a week. The average dose of irradiation was 56.8 G y (50-65 G y). Indications for post-operative radiotherapy included advanced stage of the primary tumour, positive margin, presence of 2 or more occult positive nodes and lymph node metastases with extra capsular tumour spread (ECS). The radiation was delivered to the primary and both neck sides. In the case of positive margins and ECS, chemotherapy was added to post-operative radiation. Follow-up was for a minimum of 5 years or until death in all patients (mean 90 ± 19 months, min. 63 and max. 133).
Statistical analysis
The statistical analysis was performed with an IBM computer using STATA (Stata Corporation, College Station, TX, USA). Fisher's exact test was used for categorical variables and Student's t-test for continuous variables to compare the outcomes in SND II-IV treated patients versus those who underwent mRND. The actuarial survival time was defined as the interval between the date of surgery and either the date of the last consultation for censored observations or the date of death for uncensored observations. The disease-free interval was measured on the basis of the dates of surgery and the diagnosis of first recurrence. Actuarial survival and loco-regional control was studied for the SND II-IV group and mRND group and for pN0 and pN+ groups with the Kaplan-Meier method, differences between groups were studied by log rank test. Statistical significance was set at p < 0.05.
Results
a). Histopathological findings
Occult neck disease was documented in 12 out of 96 patients, 12.5% (Table II). In the 122 elective neck dissections (27 mRND and 95 SNDII-IV), occult neck metastases were found in 12 specimens (9.8%, 7 SND II-IV and 5 mRND), the pathological staging was pN1 in 9 cases, pN2b in 2 cases and pN2c in one case (Table II). The incidence of occult neck disease was 15.7% for supraglottic tumours (9 of 57: 4T2 16.6%, 3T3 30%, 2T4a 16.6%) and 10.3% for glottic tumours (3 of 39: 1T3 5.8%, 2T4a 100%).
Table II.
Occult neck metastases overview.
| By type of neck dissections (N = 122) | |
| SNDI-IV | 7/95 (7.36%) |
| mRND | 5/27 (18.51%) |
| By T size and site | |
| Supraglottic (N = 9) | 9/57 (15.7%) |
| T2 | 4/24 (16.6%) |
| T3 | 3/10 (30%) |
| T4a | 2/12 (16.6%) |
| Glottic (N = 3) | 3/39 (10.3%) |
| T3 | 1/17 (5.8%) |
| T4a | 2/2 (100%) |
| By positive nodes (N = 21) for neck level | |
| Level I | 1/21 (5.3%) |
| Level II | 10/21 (47.6%) |
| Level III | 8/21 (38.1%) |
| Level IV | 2/21 (9.5%) |
| Level V | 0/21 (0%) |
mRND: modified Radical Neck Dissection; SND: Selective Node Dissection.
The average number of lymph nodes examined by pathologists was 31 per neck (range 25-42). A histopathological node negative neck was documented in 110 neck dissection specimens. ECS in occult metastasis was documented in two nodes from one pN2b neck after mRND. The total number of occult positive nodes was 21: 10 located at level II (47.6%), 8 at level III (38.1%), 2 at level IV (9.5%), none at level V and only 1 (5.3%) was found in level I in a patient with positive nodes at level II and III who received a mRND.
b). Neck relapse
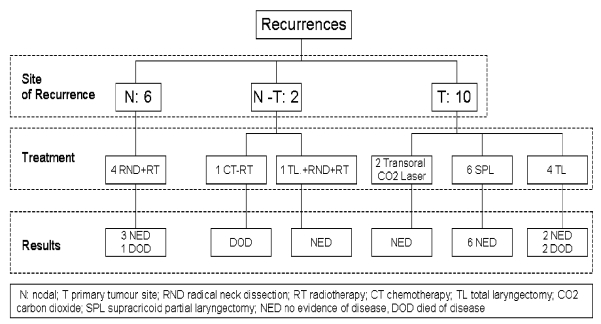
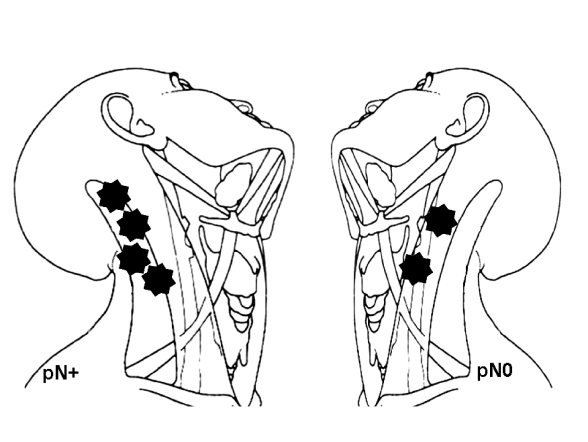
Six patients developed a neck recurrence (6.25%) in a previously dissected neck within the first 2 years after treatment (Fig. 1). Among these patients, 4 developed regional recurrence without concomitant local failure (at 6, 11, 14 and 17 months after surgery) while in the remaining 2 cases the neck recurrence was documented in association with a local failure, in one case after a supraglottic laryngectomy for pT2N0 and in another after total laryngectomy with post-operative radiotherapy for a pT4aN2c, at the 10th and 8th month, respectively. All recurrences affected necks previously treated with SND II-IV (6 of 95 vs 0 of 27 for the mRND group), however, this difference is not statistically significant (p = 0.336). The 4 neck relapses without concomitant local failure were recorded in 3 clinically and histologically negative necks (one supraglottic T1, one glottic T2 and one supraglottic T3) and one in a patient who had undergone total laryngectomy and post-operative radiotherapy on account of a pT4aN1 glottic cancer. Looking at the sites of recurrence (Fig. 2), it was found that for the pN0 cases, neck failure was located at the posterior limit of the resection at levels II and III, while the sites of failure in the 2 cases cN0/pN+ were located outside the dissected field (levels I and V, respectively). A salvage radical neck dissection (RND) followed by post-operative radiotherapy was performed in 3 neck relapses without primary failure, resulting in 3 patients free of disease at 25, 28 and 31 months. A RND was performed for the pN+ previously submitted to total laryngectomy and post-operative radiotherapy, the patient died following a new recurrence in the mastoid area. Of the remaining 2 patients with loco-regional recurrence, one underwent palliative treatment with chemo-radiation (the patient had undergone a total laryngectomy with post-operative radiation for a pT4aN2c) and died with peristomal recurrence, the other one was saved by a total laryngectomy (glottic recurrence after supraglottic laryngectomy) and RND plus radiotherapy and is free of disease at 63 months. The 5-year neck recurrence rate as estimated by the Kaplan- Meyer method, for all patients (n = 96), was 4.7%. No significant difference in the rate of 5-year neck recurrence was documented between node positive (pN+) and node negative (pN0) groups (p = 0.893) between surgery only and combined therapy groups (p = 0.490), as well as between SND II-IV and mRND type III groups (p = 0.425). No cases of neck failure affecting a nondissected neck side was recorded in this series.
Fig. 1.
Site of recurrence, treatment and results of T and neck failures.
Fig. 2.
Analysis of the pattern of neck failure.
c). Local relapse
A total of 14 patients (14.6%) developed local recurrence in absence of regional relapse, all of whom were initially pN0 (Fig. 2). A total of 12 patients received salvage surgery: 2 transoral CO2 laser resections and 1 supra-cricoid partial laryngectomy on account of failure after previous laser surgery; 9 total laryngectomy after previous supraglottic and supracricoid partial laryngectomy (4 and 5, respectively). During total laryngectomy, we also performed a contralateral SND II-IV in 5 patients previously pN0 who had received a SNDII-IV initially, no occult neck disease was found. Two patients presented with recurrence after total laryngectomy and post-operative radiotherapy (one peristomal and the other at the neopharynx), these patients received palliative chemotherapy but died from the condition.
Discussion
Ultrasound (US) images and positron emission tomography (PET) are, today, used in clinical practice to stage patients with head and neck tumours and might be useful in the case of lymph nodes with uncertain characteristics. Sensitivity and specificity of diagnostic images to stage neck lymph nodes could, therefore, improve and the incidence of clinically negative lymph node could be reduced. Nevertheless, subcentimetric metastasis are still a challenge, however recently encouraging data are emerging from computed tomography perfusion (CTP) scan that has been shown to depict small, subcentimetric nodal metastases even less than 1 cm in size. However, this technique needs to be further evaluated since, to date, the role of CTP imaging in the discrimination of malignant from benign lymph nodes has been investigated in a limited number of studies 16-18.
In this series, an incidence of occult neck metastases of 12.5% was found, these data reflect an increased accuracy for the pre-operative assessment of cN0 neck compared to 18% for occult neck disease documented in a series treated during the 1990s in our Institute 19. This more accurate estimate in clinical staging is certainly related to the policy of considering cN0 only patients without any suggestive sign of lymph node metastasis at contrast enhanced CT scan.
Nevertheless, does this low rate of occult disease justify an elective procedure which is beneficial only in one tenth of the treated population? This aspect is probably the most important pitfall arising from our series, clearly indicating the need to refine treatment strategies aimed at better characterization of high risk patients for occult neck disease.
For supraglottic T2 tumours, an incidence of occult neck disease of 16.6% (4 of 24) was found, however in 14 cases with tumour involving the epilaryngeal portion of the supraglottis (suprahyoid epiglottis, aryepiglottic folds, arytenoids), the incidence was 21.4% (3 of 14) while in the 10 T2 cases, without epilaryngeal involvement, the incidence was 10% (1 of 10). Therefore, our data indicate that it would be appropriate to reserve elective neck dissection only for cases of supraglottic T2 with epilaryngeal involvement, supraglottic T3-4 and glottic T4 tumours and considering a "wait-and-see" protocol implemented with imaging techniques and cytological assessments to be more appropriate for "other lesions" in order to avoid unnecessary overtreatment.
At present, to decrease postoperative morbidity as much as possible, super-selective neck dissection at levels IIa- III has been advocated by several Authors 11-13 20-22. Data emerging from results of the present study might sustain this approach since no isolated metastases at level IV were found, in this series; unfortunately, we are unable to provide data on sublevel IIb.
As far as concerns the effectiveness of staging procedure, SND II-IV revealed 7 of 10 necks with occult metastases (not revealing 3 neck recurrences on pN0 that developed during follow-up without primary site recurrence); mRND revealed 5 of 5 cases of occult neck disease and no relapses were documented in the group. Nevertheless, only one case showed a metastasis at level I, but positive lymph nodes at level II and level III were found, and these would have been detected by SND II-IV.
Serial sections for pathologic lymph-node examination and other immunohistochemical or bio-molecular assessments allow detection of micrometastases and/or isolated tumour cells that could be otherwise missed with standard sectioning 23 24. In fact, this could have been the case for the 3 before-mentioned pN0 necks for which it cannot be excluded that standard lymph node sectioning did not detect minimal neck disease. Nevertheless, these techniques are not routinely used in clinical practice because of the high costs. Moreover, the utility of these extended techniques have the most impact on clinically N0 patients for whom the sentinel lymph node biopsy technique may eventually become the standard of care 25.
From our data, we could make comment about post-operative radiotherapy: in cases of documented occult neck disease, post-operative radiotherapy should include all neck levels, since the finding of metastasis at levels other than II-IV is not infrequent in this situation. We have observed two neck relapses, respectively, at level I and level V, in two pN+ patients that received post-operative radiation. The analysis of the post-operative radiation field revealed that it was limited to the dissected levels, this non-surgical pitfall should be avoided. The results, in terms of regional control, indicate that no failure was recorded in the mRND group while 6 neck relapses were seen in the SND II-IV group. These data however, must be further analyzed because, for 3 patients, the regional failure is hardly imputable to the possible surgical inadequacy of SND II-IV: 2 patients were pathologically staged pN+ after SND II-IV and also received post-operative radiotherapy as initial treatment, and in one patient recurrence was detected in the neck with local supraglottic failure. The analysis of the pattern of neck failure is interesting (Fig. 2): in patients cN0/pN0, we found that all recurrences involved the upper posterior or mid-posterior limit of the dissection at levels II.
This site of failure seems to suggest that SND II-IV was less than complete, from the surgical point of view, highlighting a technical question regarding the absence of a clear anatomic separation between the posterior limit of levels II-III-IV and the anterior limit of level V. During surgery, we set this limit at the posterior edge of the sternocleidomastoid muscle, when encountering the emergency of the branches of the cervical plexus (that are usually preserved), however an exact anatomic boundary is lacking. Nevertheless, all necks presenting recurrence initially staged pN0 were successfully salvaged with RND and post-operative radiotherapy. The present study, not unlike previous reports from our group 26 27, indicates that SND II-IV is oncologically adequate for the elective surgical treatment of N0 laryngeal patients, but a better codification of the high risk population must be further studied to avoid useless overtreatment.
Conclusions
These results suggest that even if SND II-IV is effective in controlling occult neck disease, the indication for elective neck treatment might be less than compelling in most cN0 laryngeal cancer patients, therefore it would be appropriate to reserve elective neck dissection only for supraglottic T2 with epi-laryngeal involvement, supraglottic T3-4 and glottic T4 tumours. Our data also suggest that if radiotherapy is indicated for a pN+ neck treated with SND II-IV, then the radiation field should be extended beyond the surgical limits of the dissection, encompassing also levels I and V.
References
- 1.Yuen AP, Wei WI, Wong SH. Critical appraisal of watchful waiting policy in the management of N0 neck of advanced laryngeal carcinoma. Arch Otolaryngol Head Neck Surg. 1996;122:742–745. doi: 10.1001/archotol.1996.01890190038010. [DOI] [PubMed] [Google Scholar]
- 2.Redaelli de Zinis LO, Nicolai P, Tomenzoli D, et al. The distribution of lymph node metastases in supraglottic squamous cell carcinoma: therapeutic implications. Head Neck. 2002;24:913–920. doi: 10.1002/hed.10152. [DOI] [PubMed] [Google Scholar]
- 3.Gallo O, Fini-Storchi I, Napolitano L. Treatment of the contralateral negative neck in supraglottic cancer patients with unilateral node metastases (N1-3) Head Neck. 2000;22:386–392. doi: 10.1002/1097-0347(200007)22:4<386::aid-hed12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 4.Shah JP, Medina JE, Shaha AR, et al. Cervical lymph node metastasis. Curr Probl Surg. 1993;30:1–335. doi: 10.1016/0011-3840(93)90012-6. [DOI] [PubMed] [Google Scholar]
- 5.Shah JP. Patterns of cervical lymph node metastasis from squamous carcinomas of the upper aerodigestive tract. Am J Surg. 1990;160:405–409. doi: 10.1016/s0002-9610(05)80554-9. [DOI] [PubMed] [Google Scholar]
- 6.Candela FC, Shah J, Jaques DP, et al. Patterns of cervical node metastases from squamous carcinoma of the larynx. Arch Otolaryngol Head Neck Surg. 1990;116:432–435. doi: 10.1001/archotol.1990.01870040054013. [DOI] [PubMed] [Google Scholar]
- 7.Spiro RH, Gallo O, Shah JP. Selective jugular node dissection in patients with squamous carcinoma of the larynx or pharynx. Am J Surg. 1993;166:399–402. doi: 10.1016/s0002-9610(05)80341-1. [DOI] [PubMed] [Google Scholar]
- 8.Zhang B, Xu ZG, Tang PZ. Elective lateral neck dissection for laryngeal cancer in the clinically negative neck. J Surg Oncol. 2006;93:464–467. doi: 10.1002/jso.20478. [DOI] [PubMed] [Google Scholar]
- 9.Brazilian Head and Neck Cancer Study Group, author. End results of a prospective trial on elective lateral neck dissection vs type III modified radical neck dissection in the management of supraglottic and transglottic carcinomas. Head Neck. 1999;21:694–702. doi: 10.1002/(sici)1097-0347(199912)21:8<694::aid-hed3>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 10.Brazilian Head and Neck Cancer Study Group, author. Results of a prospective trial on elective modified radical classical versus supraomohyoid neck dissection in the management of oral squamous carcinoma. Am J Surg. 1998;176:422–427. doi: 10.1016/s0002-9610(98)00230-x. [DOI] [PubMed] [Google Scholar]
- 11.Celik B, Coskun H, Kumas FF, et al. Accessory nerve function after level 2b-preserving selective neck dissection. Head Neck. 2009;31:1496–1501. doi: 10.1002/hed.21112. [DOI] [PubMed] [Google Scholar]
- 12.Selcuk A, Selcuk B, Bahar S, et al. Shoulder function in various types of neck dissection. Role of spinal accessory nerve and cervical plexus preservation. Tumori. 2008;94:36–39. doi: 10.1177/030089160809400108. [DOI] [PubMed] [Google Scholar]
- 13.Santoro R, Franchi A, Gallo O, et al. Nodal metastases at level IIb during neck dissection for head and neck cancer: clinical and pathologic evaluation. Head Neck. 2008;30:1483–1487. doi: 10.1002/hed.20907. [DOI] [PubMed] [Google Scholar]
- 14.Bolzoni Villaret A, Piazza C, Peretti G, et al. Multicentric prospective study on the prevalence of sublevel IIB metastases in head and neck cancer. Arch Otolaryngol Head Neck Surg. 2007;133:897–903. doi: 10.1001/archotol.133.9.897. [DOI] [PubMed] [Google Scholar]
- 15.Sobin LH, Wittekind Ch, editors. International Union Against Cancer. 6th ed. New York: Wiley-Liss; 2002. TNM classification of malignant tumors. [Google Scholar]
- 16.Liu Y, Bellomi M, Gatti G, et al. Accuracy of computed tomography perfusion in assessing metastatic involvement of enlarged axillary lymph nodes in patients with breast cancer. Breast Cancer Res. 2007;9:R40–R40. doi: 10.1186/bcr1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bisdas S, Baghi M, Smolarz A, et al. Quantitative measurements of perfusion and permeability of oropharyngeal and oral cavity cancer, recurrent disease, and associated lymph nodes using first-pass contrast-enhanced computed tomography studies. Invest Radiol. 2007;42:172–179. doi: 10.1097/01.rli.0000252496.74242.0b. [DOI] [PubMed] [Google Scholar]
- 18.Trojanowska A, Trojanowski P, Bisdas S, et al. Squamous cell cancer of hypopharynx and larynx. Evaluation of metastatic nodal disease based on computed tomography perfusion studies. Eur J Radiol. 2011 Feb 14; doi: 10.1016/j.ejrad.2011.01.084. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Gallo O, Boddi V, Bottai GV, et al. Treatment of the clinically negative neck in laryngeal cancer patients. Head Neck. 1996;18:566–572. doi: 10.1002/(SICI)1097-0347(199611/12)18:6<566::AID-HED12>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 20.Elsheick MN, Mahfouz ME, Salim EI, et al. Molecular assessment of neck dissections supports preserving level IIb lymph nodes in selective neck dissection for laryngeal squamous cell carcinoma with clinically negative neck. OR L J Otorhinolaryngol Relat Spec. 2006;68:177–184. doi: 10.1159/000091396. [DOI] [PubMed] [Google Scholar]
- 21.Lim YC, Choi EC, Lee JS, et al. Is dissection of level IV absolutely necessary in elective neck dissection for clinically N0 laryngeal carcinoma? Oral Oncol. 2006;42:102–107. doi: 10.1016/j.oraloncology.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 22.Ferlito A, Silver CE, Suarez C, et al. Preliminary multi-institutional prospective pathologic and molecular studies support preservation of sublevel IIb and level IV for laryngeal squamous carcinoma with clinically negative neck. Eur Arch Otorhinolaryngol. 2007;264:111–114. doi: 10.1007/s00405-006-0209-5. [DOI] [PubMed] [Google Scholar]
- 23.Barrera JE, Miller ME, Said S, et al. Detection of occult cervical micrometastases in patients with head and neck squamous cell cancer. Laryngoscope. 2003;113:892–896. doi: 10.1097/00005537-200305000-00022. [DOI] [PubMed] [Google Scholar]
- 24.Shores CG, Yin X, Funkhouser W, et al. Clinical evaluation of a new molecular method for detection of micrometastases in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2004;130:937–942. doi: 10.1001/archotol.130.8.937. [DOI] [PubMed] [Google Scholar]
- 25.Seethala RR. Current state of neck dissection in the United States. Head Neck Pathol. 2009;3:238–245. doi: 10.1007/s12105-009-0129-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallo O, Deganello A, Scala J, et al. Evolution of elective neck dissection in N0 laryngeal cancer. Acta Otorhinolaryngol Ital. 2006;26:335–344. [PMC free article] [PubMed] [Google Scholar]
- 27.Sarno A, Bocciolini C, Deganello A, et al. Does unnecessary elective neck treatment affect the prognosis of N0 laryngeal cancer patients? Acta Otolaryngol. 2004;124:980–985. doi: 10.1080/00016480410017341. [DOI] [PubMed] [Google Scholar]