Abstract
The concept that early-life experience influences the brain long-term has been extensively studied over the past 50 years, whereas genetic factors determine the sequence and levels of expression of specific neuronal genes, this genetic program can be modified enduringly as a result of experience taking place during critical developmental periods. This programming is of major importance because it appears to govern many behavioral and physiological phenotypes and promote susceptibility or resilience to disease. An established example of the consequences of early-life experience-induced programming includes the effects of maternal care, where patterns of augmented care result in decreased neuroendocrine stress responses, improved cognition and resilience to depression in the recipients of this care. Here, we discuss the nature and mechanisms of this programming phenomenon, focusing on work from our lab that was inspired by Seymour Levine and his fundamental contributions to the field.
Keywords: early-life experience, maternal care, handling, corticotropin releasing hormone, CRH, CRF, hypothalamo-pituitary-adrenal axis, programming, stress, resilience, HPA, epigenetics, depression, glucocorticoid receptors
IMPORTANCE OF EARLY-LIFE EXPERIENCE
Long-lasting influence of early-life experience on neuroendocrine and behavioral responses to threatening situations has been suspected in humans, and considered to involve changes in the hypothalamic-pituitary-adrenal (HPA) axis (reviewed in Heim, Plotsky, & Nemeroff, 2004). This influence has been directly demonstrated in experimental animals including rodents (Hess, 1969; Levine & Lewis, 1959; Meaney et al., 1996) and primates (Levine, 1993a; Heim, Owens, Plotsky, & Nemeroff, 1997). Indeed, modulation of the early-life experience of the neonatal rat by controlled experimental manipulations has been successfully used to permanently influence the HPA axis and the hormonal responses to stress during adulthood (Brunson, Avishai-Eliner, Hatalski, & Baram, 2001; Levine, 2000).
The set-point and magnitude of the responses to stress are under tight and intricate regulation (Joels & Baram, 2009; Walker & Dallman, 1993) and are influenced by both hippocampal glucocorticoid receptors (GR) and hypothalamic corticotropin releasing hormone (CRH). In both mature (Heinrichs, Menzaghi, Merlo, Britton, & Koob, 1995; Rivier & Vale, 1983) and developing (Yi & Baram, 1994) rats, CRH is released from the hypothalamic paraventricular nucleus (PVN) within seconds of stress onset, to influence pituitary ACTH secretion and release of adrenal glucocorticoids. These hormones interact with GRs in hippocampus, PVN, prefrontal cortex, and pituitary (Peiffer, Lapointe, & Barden, 1991; Spencer, Miller, Stein, & McEwen, 1991; Swanson & Simmons, 1989) to generate a negative feedback onto the hormonal stress response (see Fig. 1; Dallman et al., 1987).
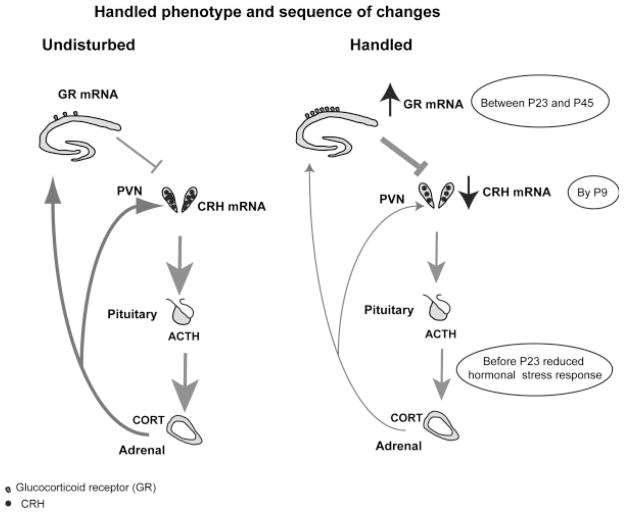
FIGURE 1.
The spectrum and sequence of molecular and hormonal changes induced by maternal care alterations via early-life handling. Corticotropin releasing hormone (CRH) expression in the hypothalamic paraventricular nucleus (PVN) of adult rats handled on postnatal (P) days P2–P9 is reduced, as are plasma ACTH and corticosterone (CORT) responses to stressors. Glucocorticoid receptor (GR) expression in the hippocampus is increased in these rats compared with controls. These changes occur in a sequential manner with reduced CRH present already by P9, followed by reduced ACTH response to stress by P23 and reduced hippocampal GR appearing between P23 and P45. The sequence of changes supports the concept of CRH modulation as an early and essential step in bridging handling-induced enhanced maternal care and the enduring changes in the HPA system.
Exposure of neonatal rats to age-appropriate physiological and psychological stressors such as cold (Yi & Baram, 1994) or prolonged or repeated maternal separation (for 3 hr or longer) results in short-term enhancement of HPA reactivity (Avishai-Eliner, Yi, Newth, & Baram, 1995; Dent, Smith, & Levine, 2000; Dent, Okimoto, Smith, & Levine, 2000; Plotsky & Meaney, 1993; Suchecki, Mozaffarian, Gross, Rosenfeld, & Levine, 1993). Interest in the long-term effects of early-life experience on the HPA system was driven by Levine’s pioneering observation that simply separating mother and pups daily for as little as 3 min during the first weeks of life may influence neuroendocrine and behavioral responses to stress long-term, with major consequences for cognitive and emotional health throughout life (Levine, 1957; Levine & Lewis, 1959; Levine, 1993a,b; Levine, 2000). This procedure, named handling, has been applied in countless studies since: The typical handling procedure involves brief (15 min) daily separation of rat pups from their mother followed by returning the pups to the home cage. This commences on postnatal day 2 for a minimum of 1 week (Avishai-Eliner, Eghbal-Ahmadi, Tabachnik, Brunson, & Baram, 2001; Fenoglio, Chen, & Baram, 2006; Weaver et al., 2001), or up to 3 weeks (Bhatnagar & Meaney, 1995; Hess, 1969; Levine & Lewis, 1959; Plotsky & Meaney, 1993).
Handling has consistently been found to modulate the reactivity of the HPA system (Fig. 1). More specifically, concentrations of plasma corticosterone are lower in adult rats handled early in life compared to non-handled (NH) controls following exposure to novel stimuli (Levine, Haltmeyer, Karas, & Denenberg, 1967) or to subsequent handling (Ader, Stanford, Friedman, Grota, & Schaefer, 1968). In contrast, elevations in plasma corticosterone following electric shock are more rapid and initially higher in animals handled in infancy (Levine, 1962). However, in handled rats there is a more rapid return to basal levels after noxious stimulation (Haltemeyer, Denenberg, & Zarrow, 1967). Thus, rats handled in infancy seem to be endowed with improved differential response to varying intensities of stressful stimuli (but see Ader, 1970; Ader et al., 1968). They perceive and respond to mild challenging stimuli that are associated with improved cognitive function, yet recover more rapidly from strong stressors that might have adverse effects on neuronal function (Chen et al., 2010). In addition, handling leads to resilience to depressive-like behavior (Meaney et al., 1991) and improved hippocampus-dependent cognitive function (Fenoglio et al., 2005; Korosi & Baram, 2009; Liu, Diorio, Day, Francis, & Meaney, 2000) during adulthood.
More recently, the molecular basis for the altered reactivity of the HPA axis has been under study. For example, there is reduction of hypothalamic CRH in the hypothalamic PVN (Fig. 2) of handled rats, and this reduced expression is persistent (Fig. 2), and accompanied by augmented levels of hippocampal GR expression (e.g., Plotsky et al., 1993; Sanchez, Ladd, & Plotsky, 2001; Fenoglio et al., 2006). Together, these molecular changes are expected to reduce CRH, ACTH and hence corticoid release in response to stress, and augment a negative feedback that shuts-down the hormonal stress response. As mentioned above, the importance of these experimental manipulations and the related molecular changes derive from the fact that early-life experience (in combination with genetic factors) may similarly modulate the HPA axis in humans, influencing cognitive and emotional health (Nelson et al., 2007; Nemeroff & Vale, 2005; Wilson, 2007). For example, major depression is characterized by enhanced activation of the HPA axis, evident from increased cerebrospinal fluid and plasma levels of CRH and cortisol, respectively (Nemeroff, 1988). Further, it is generally believed that resilience to depression involves the ability of the HPA system to respond differently to stresses of different magnitudes and to be shut-off effectively (Bale & Vale, 2003). Because handling produces precisely these consequences in a controlled experimental model, this model enables mechanistic studies with potential therapeutic and social implications (Bredy, Humpartzoomian, Cain, & Meaney, 2003; Fenoglio et al., 2005; Korosi et al., 2010; Nelson et al., 2007). Put differently, understanding the neuro-biological basis of the enduring consequences of this programming is fundamental for promoting healthy human neurological function and preventing stress-related cognitive and affective disorders (Nestler et al., 2002). These mechanisms form the focus of this review.
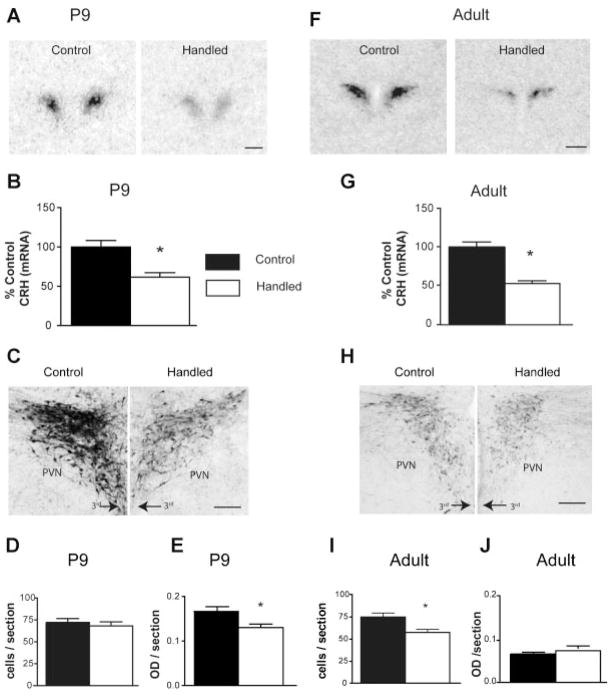
FIGURE 2.
Augmented early-life experience leads to early-onset and persistent reduction of CRH expression in parvocellular PVN at both mRNA and protein levels. (A) Representative bright-field photomicrographs of coronal sections at the level of the PVN from undisturbed controls and handled rats. The sections were subject to in situ hybridization for CRH mRNA. (B) Quantitative analysis of CRH mRNA expression in the two groups: CRH mRNA expression was reduced by 52% in postnatal day (P) 9, handled rats compared with undisturbed controls. (C) Bright-field photomicrographs, taken under similar viewing parameters, showing CRH immunohistochemistry in PVN of control and handled rats. (D) Quantitative analysis of the numbers of CRH immunoreactive (ir) neurons and (E) intensity of the immunoreactivity. The changes observed for mRNA expression were translated to protein levels as apparent from the ~20% reduction in the intensity of CRH expression in handled rats. (F,G) Representative autoradiographs after CRH mRNA in situ hybridization, and quantification of CRH mRNA signal in adult control and handled rat PVN. CRH expression in handled adult rats was 50% lower compared to controls, indicating that repressed CRH expression, found on P9, was long-lasting. (H,I,J) Bright-field photomicrographs and quantitative analysis of CRH immunohistochemistry in adult PVN. The enduring suppression of CRH expression observed at the mRNA level was translated to the protein level as evident from the ~21% reduction of CRH-ir cells in the handled rats. 3rd = third ventricle. Scale bars in A,F: 500 μm, in C,H: 200 μm. *p <.05. (Figure modified from Korosi et al., 2010.)
MOTHER–PUP INTERACTION IS A KEY REGULATOR OF THE PROGRAMMING OF THE HPA AXIS
The mechanisms underlying the effects of early-life handling as first proposed by Levine, Chevalier, and Korchin (1956) were thought to result, at least in part, from the direct physical effects of the procedure, for example, cooling of the pups and/or stress imposed on the pups while separated. However, others proposed that handling might act indirectly on the pups via its effects on the nature of mother–infant interaction (Barnett & Burn, 1967; Denenberg, Taylor, & Zarrow, 1969; Bell, Nitschke, Bell, & Zachman, 1974; Smotherman, Brown, & Levine, 1977). Indeed, handling has been shown to enhance mother–pup interaction by provoking bursts of maternal sensory stimulation of pups immediately after their return to the home cage (Brown, Smotherman, & Levine, 1977; Fenoglio et al., 2006; Korosi et al., 2010; and see Fig. 3). In this manner, handling likely mimics conditions that occur in nature, where short separations of the dam from the pups are common, and are associated with bouts of maternal care upon the return of the dam to the nest. In nature, it is likely that some mothers provide more care/stimulation to pups than others. This hypothesis was systematically tested in the late 1990s (Caldji et al., 1998; Francis, Diorio, Liu, & Meaney, 1999; Liu et al., 1997). Individual differences in quantity and quality of active maternal care (licking and nursing) of rats were found to be associated with differences in stress responses of the pups when they became adults. The hormonal stress response of adults reared by dams exhibiting high levels of licking and arched back nursing was reduced. This was accompanied by reduced CRH expression in the hypothalamus and increased hippocampal GR expression when compared with pups from mothers with lower levels of caring activities (Meaney, 2001; Plotsky & Meaney, 1993). In addition, the importance of sensory stimulation as a regulator of expression levels of molecules involved in the stress response such as CRH and CRH receptors has also been demonstrated (Eghbal-Ahmadi, Avishai-Eliner, Hatalski, & Baram, 1999; Fenoglio et al., 2006). Together, these studies indicate that, by recapitulating the natural variation of maternal care and creating groups that segregate more clearly (handling vs. controls), the handling procedure programs the molecules and processes that comprise the stress response.
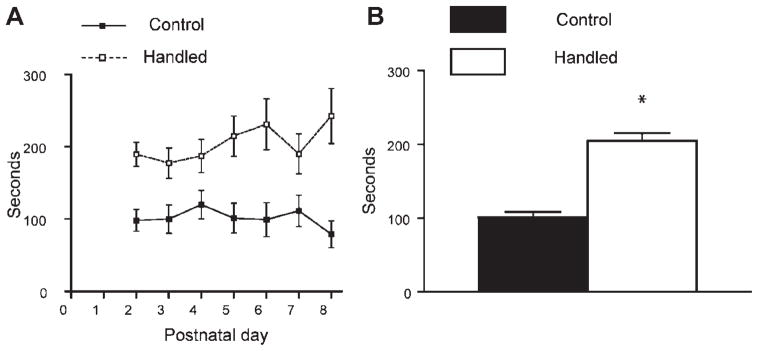
FIGURE 3.
Sensory stimulation of pups by the dam is enhanced daily after brief separation of pups from their mother. (A) Maternal stimulation of the pups, and specifically licking was observed and quantified daily from P2 to P8 during the 30 min following the return of separated pups and dams to home cages (handling procedure starting at 08:30 AM; light on at 07:00 AM.). Duration of the sensory stimulation of the pups was significantly higher in litters that were briefly separated (handled) compared to control litters on each day (n = 16 dams per group; repeated measure ANOVA F1,31 = 39.94, p < .0001). (B) Collapsed for the whole week, duration of nurturing activity of the dams was twofold higher in handled litters compared with controls. *p <.0001.
There has been some debate in the literature about the appropriate control group when studying the effects of early-life experiences, including handling on expression of stress-related molecules such as GR and CRH, as well as on the functional outcome. One of the most widely used reference groups is one left completely undisturbed (NH). NH and handled conditions differ in several aspects (e.g., cage opening, picking up of the dam and pups and their transfer to new cages, placing them back). Because the majority of the neuroscience and behavioral literature that employs rodents is derived from studies on animal facility reared rats, the possibility has been raised that pups raised under routine animal facility care (AFR) should be the correct controls (Plotsky et al., 2005). AFR consists of exposing rats to cage changes (typically twice a week): Dams and pups undergo repeated cage cleaning involving transferring of the mother and pups to a clean, novel cage, and this procedure may therefore be considered a variant of the handling procedure. Indeed, hypothalamic CRH levels of adults exposed to either handling or AFR rearing conditions are comparable (Plotsky et al., 1993; Viau, Sharma, Plotsky, & Meaney, 1993) and both AFR and the handling protocol produce similar neurobehavioral outcomes (Caldji, Diorio, & Meaney, 2000; Pryce, Bettschen, & Feldon, 2001; Pryce & Feldon, 2003). These data suggest that even relatively limited handling might suffice to program the HPA axis. Indeed, whereas a single handling episode does not alter hypothalamic CRH levels (Fenoglio et al., 2006), as few as five daily procedures suffice (Fenoglio et al., 2005). Therefore, we believe that a suitable control group for the handling procedure is the NH rearing condition. In the undisturbed group—in contrast to the handled group—dams are never stimulated to provide bursts of sensory stimulation to the pups by brief experimentally induced separations that take place during cage changes.
HANDLING EVOKED CHANGES ARISE SEQUENTIALLY
An important step in our understanding of the molecular and behavioral phenotype induced by the handling procedure is defining how it arises and the sequential steps that are involved. Persistently altered expression levels of CRH in hypothalamic neurons and of GR in the hippocampus have been established as key elements of the experience-dependent programming (Plotsky & Meaney, 1993; Fig. 1). Specifically, CRH mRNA levels in the hypothalamus are reduced, whereas GR expression levels in the hippocampus are elevated. Discovering which of these fundamental changes occurs first should help in identifying the location and nature of the initial programming steps triggered by the early-life experience. What is then the precise timing and sequence of the handling-induced alterations? Increased GR expression has been proposed as an early and critical effect of the enriched sensory input (Meaney et al., 1996; Liu et al., 1997; Francis & Meaney, 1999). The increased GR levels would then transmit negative glucocorticoid feedback more efficiently to the HPA axis, downregulating hypothalamic CRH and responses to subsequent stress. However programming of Crh gene expression in PVN neurons to lower levels takes place already by the end of the daily week-long handling period [postnatal day (P) 9; Avishai-Eliner et al., 2001; Fenoglio et al., 2005). This reduction is followed sequentially by attenuated hormonal responses to stress and then by enhancement of hippocampal GR expression, which take place by P23 and between P23 and P45, respectively (Fig. 1). Further support for the importance of the early reduction of CRH expression in the programming that culminates in the “handled phenotype” is apparent from the fact that reducing the activation of the CRH receptor type 1 by its endogenous ligand via a week-long administration of a selective blocker in NH rats during P10–P17, was sufficient to upregulate hippocampal GR persistently and to confer the behavioral phenotype of improved cognitive functions seen in adult handled rats in both Morris Water Maze and object-recognition tests (Fenoglio et al., 2005; Korosi & Baram, 2009). These findings establish that the modulation of CRH expression precedes the increased GR expression, indicating that programming of the levels of CRH gene expression is an early and essential step in the molecular cascade bridging maternal care and the enduring changes of the HPA system. The precise mechanism for the sequence and the timing of the onset of the reduced hormonal stress–response and augmented GR expression alterations has not yet been fully understood and requires further study.
HOW IS THE SENSORY INPUT FROM THE MOTHER CONVEYED TO CRH-EXPRESSING CELLS WITHIN PUPS’ PVN?
If the initial consequence of the augmented maternal-derived sensory input (handling) experience is to change CRH expression levels in the PVN, then we need to understand how maternal signals reach this brain region, and more specifically, the parvocellular CRH-expressing neurons in the PVN. Using the immediate-early gene Fos to visualize neurons activated by maternal-derived sensory signals, a pathway regulating the hypothalamic PVN emerged. After a single day of handling Fos expression was induced in the bed nucleus of the stria terminalis (BnST) and the central nucleus of the amygdala (ACe; Fenoglio et al., 2006) and both these regions generally augment CRH expression in PVN (Akana & Dallman, 1997; Choi et al., 2007, 2008; Feldman, Conforti, Itzik, & Weidenfeld, 1994). In contrast, recurrent handling induced Fos expression also in the thalamic paraventricular nucleus (PVT), a region with major inhibitory output onto ACe (Bhatnagar & Dallman, 1998; Spencer, Fox, & Day, 2004). The activation of PVT neurons after recurrent handling likely altered their activity (firing rate, neurotransmitter release), changing activity of BnSTand ACe, and thus the sum and pattern of afferent information arriving at CRH-expressing neurons in PVN. Indeed, PVT has been proposed previously as a region involved in processing memories/experiences related to the stress–response system (Bhatnagar & Dallman, 1998; Bell, Bhatnagar, Akana, Choi, & Dallman, 2000).
In summary, recurrent daily handling was required for both the involvement of PVT and for reduced hypothalamic CRH expression (Fenoglio et al., 2006), suggesting that a repeated, consistent pattern of maternal care is the signal that programs CRH expression at lower levels (Fenoglio et al., 2006; Korosi & Baram, 2008).
Comprehensive characterization of the anatomic and chemical identities of the neuronal pathways conducting these signals to the hypothalamus requires further work. However, the data presented above brings to focus crucial new questions about the fundamental mechanisms of the experience-dependent programming induced by maternal sensory input. These important questions include the nature of the modified neuronal-signaling received by the CRH neuron in the PVN and the nature of the mechanisms that translate this information into persistent repression of Crh gene expression.
WHAT HAPPENS IN THE HYPOTHALAMIC CRH-NEURON ONCE THE SIGNAL IS RECEIVED?
Because bursts of maternal sensory stimulation (in nature or evoked by handling) elicit a signal that reaches the CRH neuron in the PVN, it is reasonable to ask what the cellular consequences of the signal are, and how they reduce CRH levels in a persistent manner. Altered activation of transcription factors involved in the regulation of CRH expression is an attractive possibility. Phosphorylation of cAMP response element-binding protein (CREB) and extracellular signal-regulated kinase (ERK) influence the initial activation of the critical cAMP-response element (CRE) domain on the Crh gene promoter (Seasholtz, Thompson, & Douglass, 1988). In addition, phosphorylation of the transcription factor ERK (pERK) is crucial for maintaining CREB phosphorylated beyond the first seconds after synaptic activation, contributing to plasticity at a longer timescale (West, Griffith, & Greenberg, 2002). CREB and ERK are ubiquitously phosphorylated in the PVN of undisturbed P9 rats and can therefore be candidates for deactivation by reduction of excitatory input onto these neurons (or by augmented inhibitory inputs). Indeed, recent work has demonstrated a drastic reduction in the number and function of excitatory synapses on CRH expressing neurons of handled pups (Korosi et al., 2010). These findings demonstrate a novel structural basis for the remarkable plasticity of the HPA system in response to early-life experience. In essence, these data finally answer Seymour Levine’s lifelong query of how early-life experience might alter the brain.
The reduced excitatory input to CRH neurons in hypothalami of handled pups was translated into a strong decrease in the number of pERK immunoreactive cells in the PVN (Fenoglio et al., 2006). Accordingly, transcription of the Crh gene (measured by CRH mRNA) in response to separation stress decreased in the PVN of recurrently handled pups (Fenoglio et al., 2006). This reduced transcription of the Crh gene should result in reduced steady-state CRH mRNA levels, as is indeed found in handled rats throughout their lives.
SUMMARY
Much progress has already been made in delineating the nature of experience-induced programming of the molecular and behavioral responses to stress, that is, the question raised by Seymour Levine over 50 years ago. The neuronal populations in which initial plasticity takes place appear to reside in the hypothalamus and the cellular changes seem to include repression of the Crh gene early and persistently. However, much remains unclear about the mechanisms by which experience-dependent programming takes place and future studies will examine both the initiation and maintenance of this programmed gene expression. Whereas the reduced excitatory input to CRH neurons probably initiates this programming (Korosi et al., 2010), epigenetic processes may lead to the maintenance of the molecular changes including reduction of CRH expression. These may include DNA methylation and/or neuronal restrictive silencing factor-driven histone deacetylation (Korosi et al., 2010; Meaney & Szyf, 2005; Thatcher & LaSalle, 2006).
Such future studies are necessary because this phenomenon, described originally by Seymour Levine and his peers, is of major clinical significance. Indeed, employing the principles learned from the salubrious consequences of augmented maternal care would greatly improve human health. One potential candidate for promoting resilience to affective disorders that emerges from the work described above is the CRH receptor antagonist. As mentioned above partially blocking the CRH receptor type 1 (and thus presumably reducing the consequences of activation of this receptor by CRH) in immature rats that did not receive augmented maternal care was sufficient to upregulate hippocampal GR persistently and to confer the behavioral phenotype induced by augmented maternal care (Fenoglio et al., 2005). In line with the concept of CRH receptor as a molecular target for influencing emotional and cognitive function by early-life experience, the presence of specific combinations of single nucleotide polymorphisms in the Crhr1 gene is protective against depressive symptoms in individuals maltreated in childhood (Bradley et al., 2008; Tyrka et al., 2009). Therefore, in addition to obvious social and behavioral intervention, pharmacological interventions targeting CRH-CRH receptor signaling may enable enhanced resilience to human stress-related disorders associated with early-life experience.
Acknowledgments
Contract grant sponsor: NIH
Contract grant numbers: NS28912, MH73136
References
- Ader R. The effects of early experience on adreno-cortical response to different magnitudes of stimulation. Physiology and Behavior. 1970;5:837–839. doi: 10.1016/0031-9384(70)90168-x. [DOI] [PubMed] [Google Scholar]
- Ader R, Friedman SB, Grota LJ, Schaefer A. Attenuation of the plasma corticosterone response to handling and electric shock stimulation in the infant rat. Physiology and Behavior. 1968;3:327–331. [Google Scholar]
- Akana SF, Dallman MF. Chronic cold in adrenalectomized, corticosterone (B)-treated rats: Facilitated corticotropin responses to acute restraint emerge as B increases. Endocrinology. 1997;138(8):3249–3258. doi: 10.1210/endo.138.8.5291. [DOI] [PubMed] [Google Scholar]
- Avishai-Eliner S, Eghbal-Ahmadi M, Tabachnik E, Brunson KL, Baram TZ. Down-regulation of hypothalamic corticotropin-releasing hormone messenger ribonucleic acid (mRNA) precedes early-life experience-induced changes in hippocampal glucocorticoid receptor mRNA. Endocrinology. 2001;142(1):89–97. doi: 10.1210/endo.142.1.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avishai-Eliner S, Yi SJ, Newth CJ, Baram TZ. Effects of maternal and sibling deprivation on basal and stress induced hypothalamic-pituitary-adrenal components in the infant rat. Neuroscience Letters. 1995;192(1):49–52. doi: 10.1016/0304-3940(95)11606-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Vale WW. Increased depression-like behaviors in corticotropin-releasing factor receptor-2-deficient mice: Sexually dichotomous responses. Journal of Neuroscience. 2003;23:5295–5301. doi: 10.1523/JNEUROSCI.23-12-05295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett SA, Burn J. Early stimulation and maternal behaviour. Nature. 1967;213(5072):150–152. doi: 10.1038/213150a0. [DOI] [PubMed] [Google Scholar]
- Bell ME, Bhatnagar S, Akana SF, Choi S, Dallman MF. Disruption of arcuate/paraventricular nucleus connections changes body energy balance and response to acute stress. Journal of Neuroscience. 2000;20(17):6707–6713. doi: 10.1523/JNEUROSCI.20-17-06707.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RW, Nitschke W, Bell NJ, Zachman TA. Early experience, ultrasonic vocalizations and maternal responsiveness in rats. Developmental Psychobiology. 1974;7(3):235–242. doi: 10.1002/dev.420070307. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84(4):1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Meaney MJ. Hypothalamic-pituitary-adrenal function in chronic intermittently cold-stressed neonatally handled and non handled rats. Journal of Neuroendocrinology. 1995;7(2):97–108. doi: 10.1111/j.1365-2826.1995.tb00672.x. [DOI] [PubMed] [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, et al. Influence of child abuse on adult depression: Moderation by the corticotropin-releasing hormone receptor gene. Archives of General Psychiatry. 2008;65:1336–1337. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredy TW, Humpartzoomian RA, Cain DP, Meaney MJ. Partial reversal of the effect of maternal care on cognitive function through environmental enrichment. Neuroscience. 2003;118(2):571–576. doi: 10.1016/s0306-4522(02)00918-1. [DOI] [PubMed] [Google Scholar]
- Brown CP, Smotherman WP, Levine S. Interaction-induced reduction in differential maternal responsiveness: An effect of cue-reduction or behavior? Developmental Psychobiology. 1977;10(3):273–280. doi: 10.1002/dev.420100311. [DOI] [PubMed] [Google Scholar]
- Brunson KL, Avishai-Eliner S, Hatalski CG, Baram TZ. Neurobiology of the stress response early in life: Evolution of a concept and the role of corticotropin releasing hormone. Molecular Psychiatry. 2001;6(6):647–656. doi: 10.1038/sj.mp.4000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Meaney MJ. Variations in maternal care in infancy regulate the development of stress reactivity. Biological Psychiatry. 2000;48:1164–1174. doi: 10.1016/s0006-3223(00)01084-2. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proceedings of the National Academy of Science of the United States of America. 1998;95(9):5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Rex CS, Rice CJ, Dubé CM, Gall CM, Lynch G, et al. Correlated memory defects and hippocampal dendritic spine loss after acute stress involve corticotropin-releasing hormone signaling. Proceedings of the National Academy of Science of the United States of America. 2010;107(29):13123–13128. doi: 10.1073/pnas.1003825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DC, Evanson NK, Furay AR, Ulrich-Lai YM, Ostrander MM, Herman JP. The anteroventral bed nucleus of the stria terminalis differentially regulates hypothalamic-pituitary-adrenocortical axis responses to acute and chronic stress. Endocrinology. 2008;149(2):818–826. doi: 10.1210/en.2007-0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. Bed nucleus of the stria terminalis subregions differentially regulate hypothala-mic-pituitary-adrenal axis activity: Implications for the integration of limbic inputs. Journal of Neuroscience. 2007;27(8):2025–2034. doi: 10.1523/JNEUROSCI.4301-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Jacobson L, Levin N, Cascio CS, Shinsako J. Characterization of corticosterone feedback regulation of ACTH secretion. Annals of the New York Academy of Science. 1987;512:402–414. doi: 10.1111/j.1749-6632.1987.tb24976.x. [DOI] [PubMed] [Google Scholar]
- Denenberg VH, Taylor RE, Zarrow MX. Maternal behavior in the rat: An investigation and quantification of nest building. Behaviour. 1969;34(1):1–16. doi: 10.1163/156853969x00369. [DOI] [PubMed] [Google Scholar]
- Dent GW, Okimoto DK, Smith MA, Levine S. Stress-induced alterations in corticotropin-releasing hormone and vasopressin gene expression in the paraventricular nucleus during ontogeny. Neuroendocrinology. 2000;71:333–342. doi: 10.1159/000054554. [DOI] [PubMed] [Google Scholar]
- Dent GW, Smith MA, Levine S. Rapid induction of corticotropin-releasing hormone gene transcription in the paraventricular nucleus of the developing rat. Endocrinology. 2000;141(6):1593–1598. doi: 10.1210/endo.141.5.7455. [DOI] [PubMed] [Google Scholar]
- Eghbal-Ahmadi M, Avishai-Eliner S, Hatalski CG, Baram TZ. Differential regulation of the expression of corticotropin-releasing factor receptor type 2 (CRF2) in hypothalamus and amygdala of the immature rat by sensory input and food intake. Journal of Neuroscience. 1999;19(10):3982–3991. doi: 10.1523/JNEUROSCI.19-10-03982.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman S, Conforti N, Itzik A, Weidenfeld J. Differential effect of amygdaloid lesions on CRF-41, ACTH and corticosterone responses following neural stimuli. Brain Research. 1994;658(1–2):21–26. doi: 10.1016/s0006-8993(09)90005-1. [DOI] [PubMed] [Google Scholar]
- Fenoglio KA, Brunson KL, Avishai-Eliner S, Stone BA, Kapadia BJ, Baram TZ. Enduring, handling-evoked enhancement of hippocampal memory function and glucocorticoid receptor expression involves activation of the corticotropin-releasing factor type 1 receptor. Endocrinology. 2005;146(9):4090–4096. doi: 10.1210/en.2004-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio KA, Chen Y, Baram TZ. Neuro-plasticity of the hypothalamic-pituitary-adrenal axis early in life requires recurrent recruitment of stress-regulating brain regions. Journal of Neuroscience. 2006;26(9):2434–2442. doi: 10.1523/JNEUROSCI.4080-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286(5442):1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Francis D, Meaney MJ. Maternal care and the development of stress responses. Current Opinion in Neurobiology. 1999;9(1):128–134. doi: 10.1016/s0959-4388(99)80016-6. [DOI] [PubMed] [Google Scholar]
- Haltemeyer GC, Deenenberg VH, Zarrow MX. Modification of the plasma corticosterone response as a function of infantile stimulation and electric shock parameters. Physiology and Behavior. 1967;2:61–63. [Google Scholar]
- Heim C, Owens MJ, Plotsky PM, Nemeroff CB. The role of early adverse life events in the etiology of depression and posttraumatic stress disorder. Focus on corticotropin-releasing factor. Annals of the New York Academy of Science. 1997;821:194–207. doi: 10.1111/j.1749-6632.1997.tb48279.x. [DOI] [PubMed] [Google Scholar]
- Heim C, Plotsky PM, Nemeroff CB. Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychophar-macology. 2004;29(4):641–648. doi: 10.1038/sj.npp.1300397. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Menzaghi F, Merlo PE, Britton KT, Koob GF. The role of CRF in behavioral aspects of stress. Annals of the New York Academy of Science. 1995;771:92–104. doi: 10.1111/j.1749-6632.1995.tb44673.x. [DOI] [PubMed] [Google Scholar]
- Hess G. Perception of nursing role in a developing mental health center. Journal of Psychiatric and Mental Health Nursing. 1969;7(2):77–81. [PubMed] [Google Scholar]
- Joels M, Baram TZ. The neuro-symphony of stress. Nature Reviews Neuroscience. 2009;10(6):459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korosi A, Baram TZ. The central corticotropin releasing factor system during development and adulthood. European Journal of Pharmacology. 2008;583(2–3):204–214. doi: 10.1016/j.ejphar.2007.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korosi A, Baram TZ. The pathways from mother’s love to baby’s future. Frontiers Behavioral Neuroscience. 2009;3:27. doi: 10.3389/neuro.08.027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korosi A, Shanabrough M, McClelland S, Liu ZW, Borok E, Gao XB, et al. Early-life experience reduces excitation to stress-responsive hypothalamic neurons and reprograms the expression of corticotropin-releasing hormone. Journal of Neuroscience. 2010;30:703–713. doi: 10.1523/JNEUROSCI.4214-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S. Infantile experience and resistance to physiological stress. Science. 1957;126(3270):405. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- Levine S. Plasma free corticosteroid response to electric shock in rats stimulated in infancy. Science. 1962;135:795–796. doi: 10.1126/science.135.3506.795-a. [DOI] [PubMed] [Google Scholar]
- Levine S. The psychoendocrinology of stress. Annals of the New York Academy of Science. 1993a;697:61–69. doi: 10.1111/j.1749-6632.1993.tb49923.x. [DOI] [PubMed] [Google Scholar]
- Levine S. The influence of social factors on the response to stress. Psychotherapy and Psychosomatics. 1993b;60(1):33–38. doi: 10.1159/000288677. [DOI] [PubMed] [Google Scholar]
- Levine S. Influence of psychological variables on the activity of the hypothalamic-pituitary-adrenal axis. European Journal of Pharmacology. 2000;405(1–3):149–160. doi: 10.1016/s0014-2999(00)00548-3. [DOI] [PubMed] [Google Scholar]
- Levine S, Chevalier JA, Korchin SJ. The effects of early shock and handling on later avoidance learning. Journal of Personality. 1956;24(4):475–493. doi: 10.1111/j.1467-6494.1956.tb01283.x. [DOI] [PubMed] [Google Scholar]
- Levine S, Haltmeyer GC, Karas GG, Denenberg VH. Physiological and behavioral effects of infantile stimulation. Physiology and Behavior. 1967;2:55–59. [Google Scholar]
- Levine S, Lewis GW. The relative importance of experimenter contact in an effect produced by extra-stimulation in infancy. Journal of Comparative and Physiology Psychology. 1959;52(3):368–369. doi: 10.1037/h0044637. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nature Neuroscience. 2000;3(8):799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, et al. Maternal care, hippocampal glucocorticoid receptors and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277(5332):1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C, et al. Early environmental regulation of forebrain glucocorticoid receptor gene expression: Implications for adrenocortical responses to stress. Developmental Neuroscience. 1996;18(1–2):49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: Life at the interface between a dynamic environment and a fixed genome. Dialogues in Clinical Neurosciences. 2005;7(2):103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Viau V, Bhatnagar S, Betito K, Iny LJ, O’Donnell D, et al. Cellular mechanisms underlying the development and expression of individual differences in the hypothalamic-pituitary-adrenal stress response. The Journal of Steroid Biochemistry and Molecular Biology. 1991;39(2):265–274. doi: 10.1016/0960-0760(91)90072-d. [DOI] [PubMed] [Google Scholar]
- Nelson CA, III, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, Guthrie D. Cognitive recovery in socially deprived young children: The Bucharest Early Intervention Project. Science. 2007;318(5858):1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. The role of corticotropin-releasing factor in the pathogenesis of major depression. Pharmacop-sychiatry. 1988;21(2):76–82. doi: 10.1055/s-2007-1014652. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Vale WW. The neurobiology of depression: Inroads to treatment and new drug discovery. Journal of Clinical Psychiatry. 2005;66(7):5–13. [PubMed] [Google Scholar]
- Nestler EJ, Gould E, Manji H, Buncan M, Duman RS, Greshenfeld HK, et al. Preclinical models: Status of basic research in depression. Biological Psychiatry. 2002;52(6):503–528. doi: 10.1016/s0006-3223(02)01405-1. [DOI] [PubMed] [Google Scholar]
- Peiffer A, Lapointe B, Barden N. Hormonal regulation of type II glucocorticoid receptor messenger ribonucleic acid in rat brain. Endocrinology. 1991;129(4):2166–2174. doi: 10.1210/endo-129-4-2166. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Research Molecular Brain Research. 1993;18(3):195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsycho-pharmacology. 2005;30(12):2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Bettschen D, Feldon J. Comparison of the effects of early handling and early deprivation on maternal care in the rat. Developmental Psychobiology. 2001;38(4):239–251. doi: 10.1002/dev.1018. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Feldon J. Long-term neurobehavioral impact on the postnatal environment in rats: Manipulations, effect and mediating mechanisms. Neuroscience and Bio-behavior. 2003;27:57–71. doi: 10.1016/s0149-7634(03)00009-5. [DOI] [PubMed] [Google Scholar]
- Rivier C, Vale WW. Influence of the frequency of ovine corticotropin-releasing factor administration on adrenocorticotropin and corticosterone secretion in the rat. Endocrinology. 1983;113(4):1422–1426. doi: 10.1210/endo-113-4-1422. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: Evidence from rodent and primate models. Developmental Psychopathology. 2001;13(3):419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Seasholtz AF, Thompson RC, Douglass JO. Identification of a cyclic adenosine monophosphate-responsive element in the rat corticotropin-releasing hormone gene. Molecular Endocrinology. 1988;2(12):1311–1319. doi: 10.1210/mend-2-12-1311. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Brown CP, Levine S. Maternal responsiveness following differential pup treatment and mother–pup interactions. Hormones and Behavior. 1977;8(2):242–253. doi: 10.1016/0018-506x(77)90041-1. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Fox JC, Day TA. Thalamic paraventricular nucleus lesions facilitate central amygdala neuronal responses to acute psychological stress. Brain Research. 2004;997(2):234–237. doi: 10.1016/j.brainres.2003.10.054. [DOI] [PubMed] [Google Scholar]
- Spencer RL, Miller AH, Stein M, McEwen BS. Corticosterone regulation of type I and type II adrenal steroid receptors in brain, pituitary and immune tissue. Brain Research. 1991;549(2):236–246. doi: 10.1016/0006-8993(91)90463-6. [DOI] [PubMed] [Google Scholar]
- Suchecki D, Mozaffarian D, Gross G, Rosenfeld P, Levine S. Effects of maternal deprivation on the ACTH stress response in the infant rat. Neuroendocrinology. 1993;57(2):204–212. doi: 10.1159/000126361. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Simmons DM. Differential steroid hormone and neural influences on peptide mRNA levels in CRH cells of the paraventricular nucleus: A hybridization histochemical study in the rat. Journal of Comparative Neurology. 1989;285(4):413–435. doi: 10.1002/cne.902850402. [DOI] [PubMed] [Google Scholar]
- Thatcher KN, LaSalle JM. Dynamic changes in Histone H3 lysine 9 acetylation localization patterns during neuronal maturation require MeCP2. Epigenetics. 2006;1(1):24–31. doi: 10.4161/epi.1.1.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Gelernter J, Schepker C, Anderson GM, Carpenter LL. Interaction of childhood maltreatment with the corticotropin-releasing hormone receptor gene: Effects on hypothalamic-pituitary-adrenal axis reactivity. Biological Psychiatry. 2009;66:681–685. doi: 10.1016/j.biopsych.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau V, Sharma S, Plotsky PM, Meaney MJ. Increased plasma ACTH response to stress in nonhandled compared with handled rats require basal levels of corticosterone and are associated with increased levels of ACTH secretagogues in the median eminence. Journal of Neuroscience. 1993;13:1097–1105. doi: 10.1523/JNEUROSCI.13-03-01097.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CD, Dallman MF. Neonatal facilitation of stress-induced adrenocorticotropin secretion by prior stress: Evidence for increased central drive to the pituitary. Endocrinology. 1993;132(3):1101–1107. doi: 10.1210/endo.132.3.8382596. [DOI] [PubMed] [Google Scholar]
- Weaver IC, La Plante P, Weaver S, Parent A, Sharma S, Diorio J, et al. Early environmental regulation of hippocampal glucocorticoid receptor gene expression: Characterization of intracellular mediators and potential genomic target sites. Molecular and Cellular Endocrinology. 2001;185 (1–2):205–218. doi: 10.1016/s0303-7207(01)00635-9. [DOI] [PubMed] [Google Scholar]
- West AE, Griffith EC, Greenberg ME. Regulation of transcription factors by neuronal activity. Nature Reviews Neuroscience. 2002;3(12):921–931. doi: 10.1038/nrn987. [DOI] [PubMed] [Google Scholar]
- Wilson P. Recognising childhood neuropsychiatric disorders. Practitioner. 2007;251(1697):26–30. [PubMed] [Google Scholar]
- Yi SJ, Baram TZ. Corticotropin-releasing hormone mediates the response to cold stress in the neonatal rat without compensatory enhancement of the peptide’s gene expression. Endocrinology. 1994;135(6):2364–2368. doi: 10.1210/endo.135.6.7988418. [DOI] [PMC free article] [PubMed] [Google Scholar]