SUMMARY
Minimally-invasive autologous fat injection of the head and neck region can be considered a valid alternative to major invasive surgical procedures both for aesthetic and functional purposes. The favourable outcomes of autologous fat injection in otolaryngological practice are due to the filling of soft tissue and, mainly, to the potential regenerative effect of adipose-derived mesenchymal stem cells. Herewith, some important biological preliminary remarks are described underlying the potential of autologous fat injection in regenerative medicine, and personal experience in using it for both consolidated clinical applications, such as fat grafting to the face and vocal fold augmentation in the treatment of glottic incompetence, and more recent applications including the treatment of post-parotidectomy Frey syndrome and velopharyngeal insufficiency.
KEY WORDS: Head and neck, Autologous fat injection, Fat grafting, Adipose-derived stem cells, Adipocytes
RIASSUNTO
L'iniezione di grasso autologo del distretto testa e collo costituisce un trattamento mini-invasivo che può essere considerato una valida alternativa ad alcune procedure chirurgiche maggiori, sia per finalità estetiche che funzionali. L'efficacia dell'iniezione di grasso autologo nel trattamento di alcune patologie d'interesse otorinolaringoiatrico è dovuto, principalmente, al potenziale effetto rigenerativo intrinseco, ascrivibile alle cellule staminali adiposo-derivate. Nel presente lavoro verranno descritte le importanti premesse biologiche che sono alla base del potenziale impatto dell'iniezione di grasso autologo nella medicina rigenerativa e la nostra esperienza in questo ambito. Verranno in particolar modo discusse sia le applicazioni cliniche più consolidate, tra cui l'iniezione di grasso autologo nel distretto cutaneo cervico-facciale ed il trattamento dell'insufficienza glottica mediante iniezione di grasso autologo intra-cordale, sia quelle di più recente introduzione, quali l'iniezione di grasso autologo per il trattamento della sindrome di Frey post-parotidectomia e dell'insufficienza velofaringea.
Introduction
The use of fat to improve contour irregularities and correct depressions goes back to the end of the Nineteenth Century when Gustav Neuber (1850-1932) described the first transplantation of parcels of arm adipose tissue to the lower margin of the orbit as a means of improving adherent scars due to osteomyelitis1. Thereafter, many Authors 2 3 described the advantages to be obtained from fat grafting for various clinical applications in functional/ reconstructive and cosmetic surgery.
However, the technique fell out of favour because the tendency of the grafts to resorb, form cysts and be almost completely replaced by fibrous tissue, made the results unpredictable, especially in the field of facial aesthetics. The advent of liposuction, in the early 1980s, renewed interest in autologous fat re-injection 4 but, despite numerous attempts, the injected lipoaspirate continued to disappear almost completely. The problems of reabsorption were finally overcome in the 1990s, when Sidney Coleman developed a new atraumatic technique for fat harvesting and placement 5 that preserved the fragile adipocytes. In his opinion, the keys for success were: 1) harvesting with low negative pressure; 2) purifying the lipoaspirate by centrifugation; and 3) placing minimal amounts of adipocytes in multiple tunnels in order to maximise contact with the surrounding tissues and increase the survival rate 6. He formalised the steps of the procedure, christened it Lipostructure® 7 and, since then, the outcomes of various applications, such as restoring fullness, correcting asymmetries and scars, etc., have improved considerably and there has been a dramatic decrease in the reabsorption rate.
Recent studies on the extracellular matrix have shown that fatty tissue not only contains adipocytes, but also pre-adipocytes, endothelial cells, fibroblasts and adipose-derived adult mesenchymal stem cells (ADSCs) that are capable of differentiating into many lineages, thus indicating that fat can provide a basis for soft tissue regeneration 8 9. Although only recent, it already seems that the idea of use of ADSCs for reconstructive and functional purposes is likely to affect various fields of head and neck surgery 10-14.
The aims of this report are: 1) to outline the basic principles underlying autologous fat injections (AFIs) and their implications for regenerative medicine; 2) to describe the technical details of fat harvesting, purification and placement; and 3) to provide otolaryngologists with an overview of the various functional and aesthetic uses of AFIs in the face and neck.
Basic principles of fat grafting
Adipose-derived stem cells
The most promising research, in the field of regenerative medicine, involves the use of totipotent, pluripotent and multi-potent stem cells: i.e., undifferentiated cells with significant self-renewal capacities. Totipotent stem cells, which can be obtained starting from the blastocyst stage, are capable of triggering various human cell lineages, including human embryonic stem cells (hESCs); pluripotent stem cells can differentiate into any of the: endoderm, mesoderm or ectoderm germ layers (i.e., induced pluripotent stem cells,); and multi-potent stem cells can produce daughter cells of a limited number of lineages, including haematopoietic progenitor cells that can develop into different types of blood cells, but not into cell types of other origin. There are still a number of major limitations concerning the therapeutic use of hESCs and iPS, including ethical concerns, cell regulation and gene defects 15, but a number of clinical trials using adult stem cells have shown that they can have beneficial clinical effects 16.
Friedenstein et al. 17 were the first to discover that bone marrow contains cells that can differentiate into other mesenchymal cells as well as into fibroblasts. A few hours after placing whole bone marrow in plastic culture dishes, they removed the non-adherent cells (and, therefore, most of the haematopoietic cells) and found that, although the remaining cells were heterogeneous in appearance, the most tightly adherent cells were spindle-shaped. They also found that the cells could differentiate into colonies that resembled small deposits of bone or cartilage. These observations were extended by other Authors, throughout the 1980s 18, who established that cells isolated ususing Friedenstein's method 17 were multi-potent and could differentiate into osteoblasts, chondrocytes, adipocytes and even myoblasts. These adherent cells are currently referred to as mesenchymal stem cells (MSCs), because of their ability to differentiate into mesenchymal-type cells, or marrow stromal cells, because they appear to arise from the complex array of supporting structures found in bone marrow.
Bone marrow has long since been the main source of MSCs, but these cells account for only a small percentage of the cells found in bone marrow. The other most common source of MSCs is adipose tissue, which not only offers an abundant and easily accessible source of ADSCs (Fig. 1), but can also be harvested by means of a minimally- invasive procedure and be processed for clinical applications in accordance with current Good Manufacturing Practice guidelines.
Fig. 1.
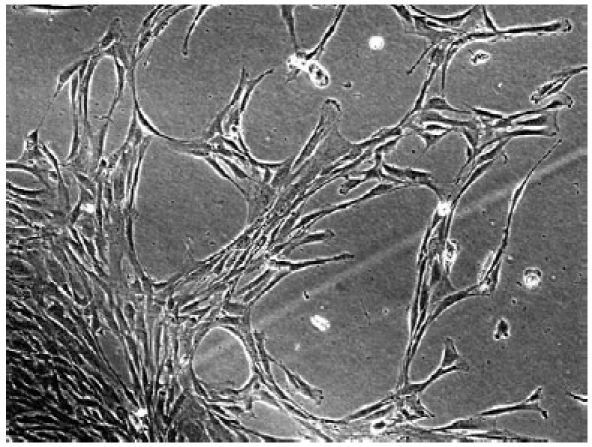
Multipotent adipose-derived mesenchymal stem cells.
Human MSCs are typically isolated retrospectively from the mononuclear cell layer of bone marrow after separation by means of density gradient centrifugation, but they can be isolated from the stromal vascular fraction of adipose tissue by digesting the whole tissue with collagenase. Fat tissue can contain even more stem cells per gram than bone marrow (5000 vs. 100-1000 cells) 19. Some in vivo and in vitro studies have shown that ADSCs come from vessel-associated pericytes, which are in contact with the intimal surfaces of small vessels within the tissue and, if sorted and cultured over the long term, give rise to adherent multi-lineage progenitor cells with the features of MSCs.
MSCs are phenotypically heterogeneous in terms of morphology, physiology and surface antigen expression. No specific marker has yet been identified that exclusively characterises MSCs, which express a large number of adhesion molecules, extracellular matrix proteins, cytokines and growth factor receptors. As stem cells are characterized by their ability to self-renewal and differentiate towards multiple cell lineages, a further way of identifying possible MSC populations is by inducing them to differentiate into bone, fat and cartilage in vitro. MSCs from different sources have been successfully differentiated into osteoblasts, chondrocytes, adipocytes, fibroblasts, myoblasts and cardiomyocytes, hepatocytes, tenocytes, and even neurons.
Some MSCs are already used for orthopaedic, cardiac and neuro-repair, while others are being investigated. It is known that MSCs home in on injured or pathological tissues by means of still unknown mechanisms but that possibly involve chemokines and their receptors, as well as adhesion molecules.
Stem cell niche therapy using adipose tissue
A niche refers to the complex relationships that ADSCs establish with all of the physiological or ectopic factors contributing to determine their identity and fate. A cell niche not only applies to interactions with neighbouring cells or the surrounding extracellular matrix, but also to long-distance interactions through circulating blood, the lymphatic system and nerve pathways. The phenotypical identity of any cell and its reaction to a given stimulus are, therefore, not only determined by the genetic or epigenetic equipment of the cell, but also depend on the specific niche context in which it resides. A change in niche parameters or the transplantation of cells into other niches can have a considerable effect on cell physiology and alter its properties.
The concept of a niche as a specialised micro-environment housing stem cells was first proposed by Schofield 20 almost 30 years ago with reference to mammalian haematology. Although this field of stem cell biology is still young and very unclear, a number of aspects have been clarified for certain stem cell populations and provides reference points for defining a common stem cell niche paradigm. The best-known stem cell niche is that of haematopoietic stem cells (HSCs) 21 and, although their role is still not completely understood, it is known that the signals transduced by members of the Wingless (Wnt) family are involved in HSCs homeostasis and fate 22.
Research has shown that the signals controlling the embryonic origin of ADSCs and their differentiation in adult adipose tissue include the same pathways as those involved in the homeostasis of other adult and embryonic stem cell populations 23, and their complex orchestration can have different effects depending on the concentration, stage of differentiation and extrinsic niche factors, such as cell-matrix and cell-cell interactions, the presence of vasculature, and the level and type of innervation. Despite extensive research into the intracellular signalling involved in ADSC homeostasis and differentiation, little is known about the role of cell-cell and cell-matrix interactions. Zannettino et al. 24 have suggested that ADSCs reside in perivascular niches, which prompts the speculation that perivascular structures (cells and extracellular matrix) may provide signals that balance the maintenance of ADSCs in an undifferentiated state and their commitment to differentiation.
It has recently been shown that in vitro culture and expansion significantly alter the transcriptional phenotype of ADSCs. Freshly isolated stromal vascular fractions (SVF) express haematopoietic markers (CD34) that are lost within a few days of culturing 25. In line with previously published findings 26 27, we have found the increased expression of mesenchymal stem cell-associated markers in cultures of both in human and murine ADSCs, whereas the longer term loss of markers of undifferentiated status, such as undifferentiated transcription factor (UTF-1), indicated a shift towards differentiation. Prolonged culturing also significantly down-regulated various isoforms of pro-collagen, matrix metallopeptidases and inflammatory cytokines, thus indicating adaptation to the artificial environment. Under extreme conditions, it has even been shown that prolonged in vitro culturing can induce the neoplastic transformation of ADSCs 28, although it is still unclear whether these changes are reversible or how they may affect the therapeutic potential of the cell. However, ADSCs can be used in many clinical applications (particularly in the fields of plastic and reconstructive surgery) by means of transplantation without removing them from their fat niche.
In addition to molecular and cell biology, the dynamic and regenerative nature of fat grafts has been established in various areas of plastic surgery, and clinical practice has shown that the long-term outcomes of fat grafting can include rejuvenation of skin texture 29-32. The ‘regenerative protocols' used in the pioneering work of Coleman included centrifuging fat at 3000x g before transplantation with the aim of reducing fat volume and removing as much debris, oil, blood and water as possible without significantly damaging the tissue to be transplanted 33.
Ultrastructural studies of centrifuged fat have revealed that mature adipocytes show interruptions in their cytoplasmic membrane and various degrees of degeneration including cell necrosis, but the SVF appears to be well preserved 34. Rigotti et al. 34 compared the ultrastructure of mammary radio-lesions before, and at different times after, fat grafting, and found clear signs of ongoing regeneration, with evidence of new adipocyte formation and a large number of precursors at different stages of differentiation toward the adipocyte phenotype. Before transplantation, almost all of the adipocytes were seriously damaged by centrifugation and, in the radio-damaged recipient tissue, there were neither mature adipocytes nor differentiating pre-adipocytes. Reasonable interpretations of these findings are that the differentiating pre-adipocytes observed after treatment were either adipocyte-committed ADSCs originally embedded within the transplanted fat, or locally present endogenous adipocyte-committed ADSCs activated by the ectopically transplanted fat. The massive survival of ADSCs in fat transplants, despite liposuction and centrifugation procedures, strongly supports the first hypothesis, but the second may be equally valid as it assumes that transplanted fat enriched with ADSCs by centrifugation can behave as an atypical ectopic niche that orchestrates tissue regeneration by modulating endogenous tissue resources.
The term ‘atypical ectopic niche' could be used as a common paradigm of stem cell-based therapies. It is based on the idea of a ‘bystander' mechanism in which the stem cells ectopically transplanted into a generic lesion (radiolesion, myocardial infarction, cerebral stroke, etc.) do not replace tissue-specific cells by direct differentiation, but locally form an atypical stem cell niche that suppresses inflammation, promotes neo-angiogenesis, and favours the activation of endogenous stem cell precursors by releasing trophic factors, such as cytokines, pro-angiogenic molecules and growth factors.
Clinical applications of fat grafting
Fat harvesting, purification, placement and complications
Fat harvesting
Usually 50 cc of lipoaspirate are sufficient to solve face and neck problems, but 10-12 cc are enough in many cases involving vocal folds, scars or fistulae. However, it may sometimes be difficult to obtain enough material as reconstructive surgeons often have to treat thin patients. The choice of the site of harvesting is, therefore, extremely important. The primary source is the lower abdomen, followed by the inner thigh and the inner knee (we generally prefer to avoid the lateral thigh or Bichat fat pad because of possible post-operative asymmetries or deformities).
The basic harvesting equipment includes a 3 mm diameter, 15 cm long, 2-hole distal opening blunt-tipped cannula (Byron Medical, Tucson, AZ, USA), a 10 cc Luer-Lock syringe, and a backhouse towel forceps. The selected area is infiltrated with 10-15 cc of a solution of 2.0% carbocaine with 1:200,000 epinephrine. A 2 mm stab incision, large enough to allow the insertion of the cannula connected to the syringe, is made in the inferior pole of the umbilicus using a No. 11 blade. When inserting the cannula for fat harvesting, it is essential to pinch the skin firmly between the thumb and fingers in order to avoid penetrating the abdominal cavity or the saphenous vein (if the inner thigh is used). The plunger of the syringe is gently retracted to provide negative pressure, throughout the suction manoeuvre using a backhouse towel forceps (Fig. 2). The procedure should be the least traumatic possible in order to avoid damaging the fragile adipocytes. Once the necessary amount of fat is obtained, the stab incision is closed using one or two 5/0 Ethilon stitches. It is advisable to use an elastic garment to reduce the risk of haematoma in the donor area.
Fig. 2.
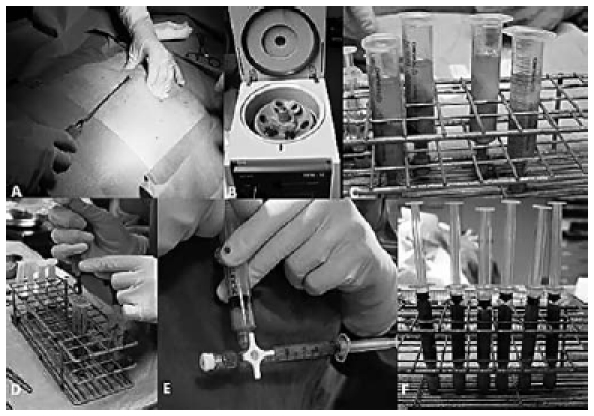
Fat harvesting from the abdomen (A); fat purification by means of centrifugation (B); lipoaspirate after centrifugation: note the three different layers (C);removal of oily and aqueous components from the fatty layer (D); the refined fatty layer is then transferred to a 3.0 cc Luer-lock syringe by means of a three-way tap (E); lipoaspirate ready to be injected (F).
Purification
Separating the different lipoaspirate components is essential. The purification equipment includes a centrifuge with a sterilisable central rotor, metal sleeves to hold the 10 cc Luer-lock syringes, plastic plugs for locking them, a rack for holding them, Codman neuropads, and a threeway tap. The plugged syringes are placed in the centrifuge, protected by sterile metal sleeves. The lipoaspirate is centrifuged at 1,200 j for 3' to separate three components: the upper layer mainly consists of oil from ruptured adipocytes; the middle layer includes viable adipocytes; and the lower, mainly aqueous, layer contains blood, carbocaine, etc. The upper layer is discarded using the Codman neuropads, and the aqueous component is released by removing the plug from the syringe. A three-way tap is then used to transfer the refined fatty layer a 3.0 cc Luerlock syringe for placement.
Placement
The placement equipment includes not only a 3.0 cc Luer- Lock syringe, but also various types of 18 gauge blunt, straight or curved, 9 cm long style I and II Coleman cannulas, and an 18 gauge V-shaped dissector Coleman cannula (Byron Medical, Tucson, AZ, USA). Correct placement is crucial, and pre-surgical markings are made in a sitting position to highlight the areas in which the fat is to be inserted. Fat grafting is usually performed under local anaesthesia with sedation, great care being taken to ensure sterility at all times since bacterial contamination may cause infection and the complete loss of the transplanted fat. In our protocol, a prophylactic antibiotic is routinely administered intravenously during the operation. To maximize contact between the graft and the surrounding tissue, it is essential to create multiple tunnels running in different directions so that a minimum number of fat parcels can be slowly positioned upon cannula withdrawal. A bolus should be avoided because the insufficiently vascularised graft core will inevitably die, leading to liquefaction and necrosis, as well as unsightly irregularities or cysts. Choosing the appropriate cannula is important: an 18 gauge blunt style 1 or 2 Coleman cannula with only one hole at the distal end allows the least traumatic creation of the tunnels and also minimizes the risk of damaging vessels or nerves. However, in the presence of scars, contractures or fibrous tissue, a sharp V-shaped dissector cannula is necessary as it disrupts adhesions between the skin and the underlying structures while creating a cavity. An alternative means of releasing the skin from the dermis is to make continuous clockwise and counter-clockwise movements with a sharp 16-18 gauge bevelled needle to sever fibrotic adherences and to create a plane in which the adipocytes can be deposited.
After following the basic principles for correct placement, it is necessary to establish the appropriate depth of the graft as the level of infiltration varies in each anatomical area: an immediate subcutaneous plane along the cheek; which should be very superficial around the chin and jawline to avoid damaging the marginal branch of the facial nerve; deeper within the vocalis muscle in the case of vocal fold augmentation; and very superficially for filling depressions or correcting scar contractures or radiodermatitis. The amount injected should be recorded.
Patients should be told that a second, and sometimes a third, procedure at 3-4 month intervals may be necessary to achieve the ideal volume.
Complications
Fat grafting can be performed as an outpatient procedure with little morbidity and few complications. However, as with any surgical technique, problems can arise. Care must be taken to avoid potentially life-threatening gut perforation during harvesting (especially in thin patients) by pinching the skin before inserting the cannula. An intravascular fat injection can lead to pulmonary embolism, stroke or blindness, but this can be avoided by using small blunt cannulas for soft tissue dissection and fat placement 35. Meticulous asepsis is essential to prevent infections. Finally, in order to avoid adipocyte rupture, leading to unpleasant oily cysts, minimal amounts of fat should be homogeneously placed in multiple tunnels 35.
AFI to the face and neck
Congenital malformations
AFI may be useful to treat some congenital malformations (including facial asymmetry in patients with hemifacial microsomia) and the post-surgical sequelae of cleft lips.
In patients with hemifacial microsomia, the traditional treatment programme includes distraction osteogenesis of the hypoplastic mandible at an early age, and LeFort1 osteotomy with sagittal splitting of the mandible and a contralateral costochondral graft and sliding genioplasty at completing of growth. In either case, the residual facial asymmetry, due to soft tissue deficit, can be corrected by repeated AFIs (at least 3 sessions at 4-month intervals, for a total of 60 cc), which lead to nearly normal symmetry and more predictable results than dermal fat grafts or use of implants.
In the case of cleft lips, AFI (generally two sessions for a total of 7-8 cc) can reduce scarring and increase upper lip volume.
Acquired deformities
Sequelae of tracheostomy
The frequent aesthetic and sometimes functional sequelae of tracheostomy can create concern.
Management traditionally requires the surgical transposition of surrounding tissue to fill the depression, and the use of local flaps to close the soft tissue defect once the scars have been excised. On the basis of our experience, AFI can be used to improve the sequelae due to fibrotic bands between the skin and underlying tissue and correct hypertrophic scars (Fig. 3). This simple and repeatable procedure (usually three sessions) can be performed under local anaesthesia obtained by infiltrating 2.0% carbocaine with a 1:200,000 epinephrine solution. The fibrotic bands between the skin and tissue can be interrupted by inserting an 18 gauge sharp needle subcutaneously and moving the tip clockwise and counter- clockwise, taking care not to leave an excessively large dead space that would reduce contact between the adipocytes and surrounding tissue. Later, using a sharp V-shaped dissector cannula, AFI is performed by means of multiple radial infiltrations between the skin and subcutaneous tissue. The first session usually only corrects the depression (total amount 5 cc) and, if the tissue is still recessed, a further 3-4 cc can be delivered after 4 months to improve scarring retraction, increase tissue volume and restore eutrophic skin. After a further 4 months, the residual scar is excised to obtain a better aesthetic result.
Fig. 3.
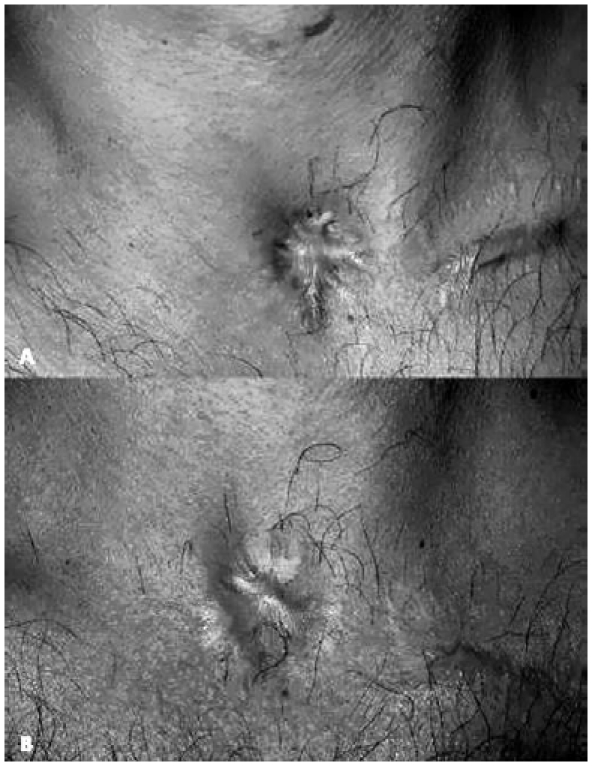
Sequela of tracheostomy (A); the same patient after autologous fat injection (two procedures for a total amount of 10 cc) (B).
Pharyngo-cutaneous fistulae
Pharyngo-cutaneous fistulae are quite common sequelae of the surgical treatment or radiotherapy of head and neck cancer. Large fistulae or pharyngostomes should be closed using local or distant flaps (direct suturing inevitably fails and may also increase the size of the fistula) but small fistulae can be treated with AFI (Fig. 4). The procedure is simple and does not require any special pre- or post-operative care. Under local anaesthesia, 2-2.5 cc of adipocytes are placed between the skin and mucosa, at a distance of 1 cm all around the fistula, using an 18 gauge blunt style I cannula. After 6 weeks, there is an improvement in skin texture and quality, with an increase in the volume of the dystrophic tissue. The residual fistula is closed by directly closing its margins in a double layer.
Fig. 4.
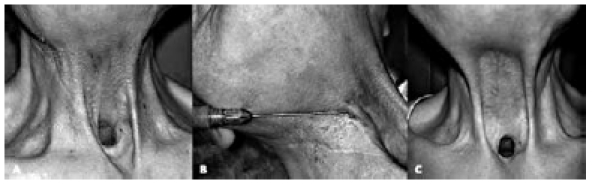
Pharyngo-cutaneous fistula after total laryngectomy (A); autologous fat injection (total amount 2.5 cc) (B); complete closure of pharyngo-cutaneous fistula four months after the procedure (C).
Sequelae of radiotherapy
The well-known dramatic consequences of radiotherapy on the head and neck lead to soft tissue deterioration and skin atrophy, particularly in children. Instead of invasive and often unpredictable major surgery, repeated AFI (generally 2-3 sessions) can provide sufficient bulk to the affected side of the face. It is also useful in making the previously stiff and atrophied skin softer and more pliable, and improving its texture and colour match.
Sequelae of rhinoplasty
Fat grafting to the nose is a challenging means of improving rhinoplasty results. The most obvious indications are to correct the contours of the dorsum in the case of saddle nose deformity, camouflage cartilage or bone irregularities, or provide better skin texture in the case of atrophy or scarring. It may also improve airway obstruction when placed at the level of the nasal valve 36.
Between 0.5 and 2.5 cc of fat should be injected into multiple intradermal or subcutaneous tunnels depending on the type of defect. The wounds are dressed using steristrips for 10-15 days in order to avoid fat displacement.
Sequelae of burns
It is always difficult to improve the facial scarring caused by burns both functionally and aesthetically, but repeated AFI (usually 2-3 sessions at 4-month intervals) can lead to better skin texture, scar quality and skin colour, and also increase volume in retracted areas and reestablish gliding tissue, thus improving the contractures and limited mobility caused by fibrotic tissue and skin graft adhesions. The use of a sharp V-shape dissector cannula is recommended to reduce the fibrosis and interrupt subcutaneous adhesions, and only small amounts of fat should be placed under the skin graft to avoid the risk of fat necrosis.
Vocal fold augmentation
Fat auto-grafting in the vocal folds, to correct defective closure, was first described in the early 1990s by Mikaelian et al. 37 and Brandenburg et al. 38, who reported the successful treatment of unilateral laryngeal paralysis. Subsequently, various authors used fat grafting to treat glottic incompetence but, as most of them found that reabsorption caused long-term failure 39-41, vocal fold fat augmentation was considered to be only a temporary solution and was abandoned in favour of prosthetic implants 42 or injectable alloplastics. Both absorbable materials such as collagen 43 or hyaluran derivatives 44, and permanent fillers have been used but it has been shown that non-resorbable implants such as Teflon and silicon have a tendency to migrate and cause foreign body reactions 45, and that "biomaterials" such as collagen can cause delayed hypersensitivity reactions 46 and vocal fold stiffness due to fibrosis 47. As an alternative to injections, vocal folds can be successfully increased by deeply burying a typically silicon prosthetic implant after fenestrating the thyroid cartilage 42. However, this "thyroplasty" requires an external approach that causes an anterior cervical scar, and may also be followed by implant extrusion 48.
The advent of liposuction made autologous fat easily available, and aroused new interest in using AFI to augment paretic or defective vocal folds. Furthermore, recent basic research has shown that fat is a vital organ and that its stromal vascular fraction contains cells displaying the typical features of MSCs 49 capable of self-renewal and differentiation into multiple cell lineages 13 50 51. The main indications for vocal fold AFI are shown in Table I. After harvesting and purification (as described above), fat parcels are introduced into the barrel of a pistol (Medtronic, Micro-France, Jacksonville, FL MCL-55) using a 1 mm diameter bayonet needle, with air exposure being minimised to avoid cytoplasmic lysis 7. In the case of vocal fold paralysis, the fat is injected deeply into the paralysed vocalis muscle, usually starting at the posterior third under direct microlaryngoscopy and general anaesthesia. The injection is made while retracting the needle in order to allow the fat to diffuse in layers. In patients with scarring or congenital soft tissue defects, the injections are made in the vocalis muscle and a number of sites in the superficial layer of the lamina propria (Reinke's space) depending on the aetiology and severity of the glottic incompetence. In cases of sequelae of cordectomy, AFI might also be placed in the paraglottic space in order to enhance medialization. The aim of injecting the superficial layer is to free adherent tissue in the cover and restore the gliding layer in which the mucosal wave flows to produce sound. Injecting areas of scar tissue also undermines the epithelium while softening the scar itself (Fig. 5). It is difficult to quantify the amount of grafted fat exactly as some of it oozes out while removing the needle, but we estimate that 1-3 cc are injected for each vocal fold treated. Paralytic vocal folds are overcorrected until their volume approximately doubles (if both vocal folds are treated, only one is over-inflated).
Table I.
Indications for autologous fat injection of vocal folds.
| Indications for autologous fat injection of vocal folds |
|---|
|
Unilateral laryngeal paralysis or paresis Malformations of the vocal fold structure (sulcus glottidis or vergeture) * Scarring due to previous surgery (for benign or malignant lesions of the vocal folds) Scarring due to prolonged intubation or laryngeal fracture/contusion Vocal fold atrophy due to previous radiotherapy Secondary procedure following failure of previous augmentation by means of injection or prosthetic implant (with possible inflammatory reaction and secondary tissue stiffness). |
Congenital adherence of the epithelium to the ligament with lack of gliding tissue in Reinke's space.
Fig. 5.
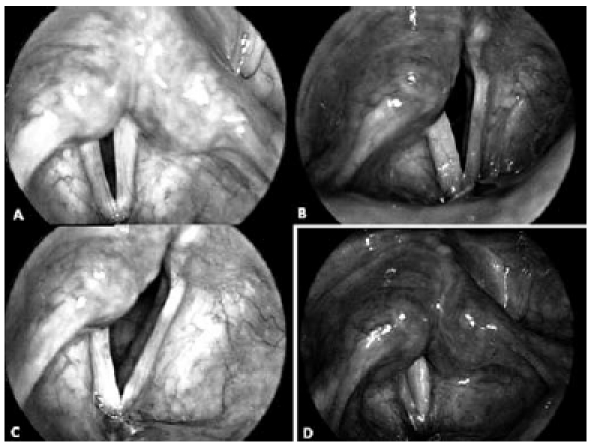
Videolaryngoscopic images in a 37-year-old female, with a 10- month history of post-thyroidectomy right vocal fold paralysis. Pre-operative view of vocal folds on inspiration (A) and on phonation (B), N.B. The wide gap of glottic closure due to hypotrophy and flaccidity of the right paralyzed fold. Result 3 months' post-operatively: the right fold still has a yellowish colour (C), and complete glottic closure is achieved during phonation (D).
On the basis of our experience, AFI is simple and effective, and leads to good and stable voice improvement without the risk of a foreign body reaction inducing vocal fold stiffness, and without impairing inspiratory and expiratory flows 52. The fat obtained by liposuction is soft and easily diffuses into the vocal fold layers, and therefore it does not alter the elasticity of the glottic vibrator even if injected into Reinke's space because the viscosity is similar to that of Reinke's space 53. However, despite these favourable characteristics, the role of vocal fold fat injection is still debated mainly because of its unpredictable reabsorption. Nevertheless, in our opinion maximizing contact between the fat parcels and host tissue, by injecting the fat into several layers, favours fat cell nutrition, oxygenation and integration, and leads to stable results 54.
Furthermore, it has been shown that AFI may relieve swallowing impairment due to defective sphincteric closure of the larynx following previous surgery or paralytic incompetence 10 55 56.
Frey syndrome
Frey syndrome consists of profuse sweating and cutaneous flushing in the area innervated by the auriculotemporal nerve. It frequently occurs after parotidectomy probably because of misdirected resprouting of the post-ganglionic parasympathetic fibres feeding the parotid gland to the cutaneous sweat glands 57. The incidence of the subjective clinical occurrence of post-parotidectomy Frey syndrome (PPFS) is 10-40% 58 depending on the case series, but most patients show a positive objective reaction to the diagnostic Minor's iodine starch test 58 59. Many forms of treatment have been proposed, including the local application of anti-cholinergic ointments 58 or multiple intra-cutaneous botulinun toxin A injections 60 61 and traditional and more invasive surgical procedures such as timpanic neurectomy 62 or the interposition of an autologous graft (dermal, the temporoparietal fascia) 63 to create a barrier between the skin and the residual parotid gland. However, as the former are only partially and transiently effective in most cases, and the latter involve the risk of possible facial nerve injury 58, there is still no treatment of choice 58. On the basis of the encouraging results obtained in other fields, such as aesthetic surgery, and the experience of Curry et al. 64 who have used autologous fat graft reconstruction with superficial musculo-aponeurotic system elevation as prophylaxis for Frey syndrome, autologous AFI in the parotid area may be a minimally-invasive option as the fat would create a barrier between the skin and the residual parotid gland, and may prevent abnormal nerve neo-anastomoses to the sweat glands. In addition to being useful for treating the unpleasant salivary sweating and flushing typical of PPFS, AFI could also have a positive aesthetic impact as it may fill the gap left by the excision of the parotid. It can be performed in a one-day-surgery setting with the patients under local anaesthesia and sedation, but needs to be preceded by carefully marking of the whole of the affected area identified by Minor's iodine starch test 59 (Fig. 6). This consists of applying an iodine solution (iodine 1.5 g, castor oil 10 g, and 95% ethanol 125 ml) to the parotid skin surface, and then sprinkling it with white starch powder; when the patient starts to eat, sweating in the skin areas affected by PP FS turn the white solution to dark purple. We recommend marking the affected area with a 1 cm grid drawn on the patient's skin, and labelling the more intensely coloured areas with a "+". Some standardised clinical scores have been suggested to assess the severity of the syndrome, including that of Luna-Ortiz et al. 65, which evaluates the objective clinical extent of the affected area (determined on the basis of Minor's iodine starch test) and the subjective impact of PPFS on the patients' quality of life. Once the pre-operative marking has been completed, fat is harvested as described above. The dark purple part of the parotid area is then carefully infiltrated with a 1:200,000 carbocaine:epinephrine mixture, and a 2 mm stab incision is made 3 mm in front of the ear lobe (carefully avoiding the facial nerve) and a 19 gauge Coleman V-shaped dissector is carefully and gently inserted to dissect into a sub-dermal plane (once again taking care not to damage the underlying facial nerve). The adipocytes transferred into a 19 gauge Coleman style II cannula are then medially placed by means of a gentle dissection into the immediate sub-dermal plane. Using Coleman's technique 14, the adipocytes are deposited in multiple tunnels created by advancing the cannula to the most distal site and retracting it to the proximal site in more than one direction, with the fat being continuously injected while retracting the cannula. More fat is injected where the staining is darkest (the squares labelled "+"). The procedure also includes fat graft deposition to fill the post-auricular area by means of a 1-2 mm stab incision. Minimally-invasive AFI of the parotid area can be considered safe and effective in PPFS, and also has an aesthetic impact as it can fill the gap left by the parotid gland excision (Fig. 7). However, patients should be informed that the procedure may need to be repeated to achieve a definitive result, although this limitation may be overcome by scrupulously respecting the pre-operative grid and using multiple tunnels for placement. Furthermore, when indicated, subsequent procedures can be performed in a one-day setting under local anaesthesia with minimal patient discomfort.
Fig. 6.
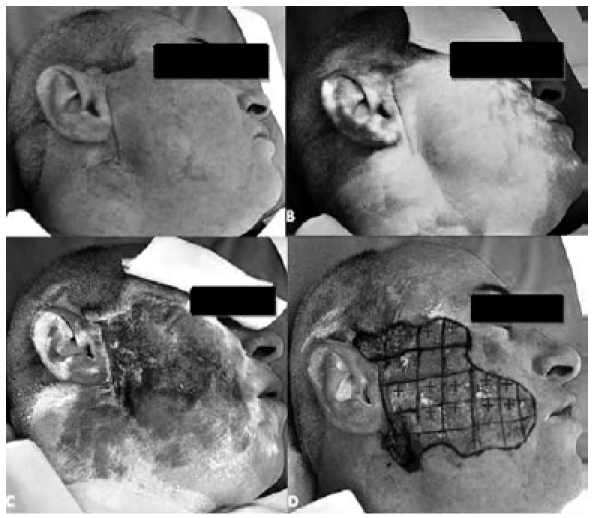
Pre-operative Minor's iodine starch test in a patient with PPFS: an iodine solution is applied to the parotid skin surface (A), and the skin is then sprinkled with white starch powder (B). When the patient starts to eat, the white iodine solution on the skin areas affected by PPFS becomes dark purple as a result of sweating (C). The affected area is marked with a 1 cm grid drawn on the patient's skin, and the most intensely coloured areas are labelled with a "+" (D).
Fig. 7.
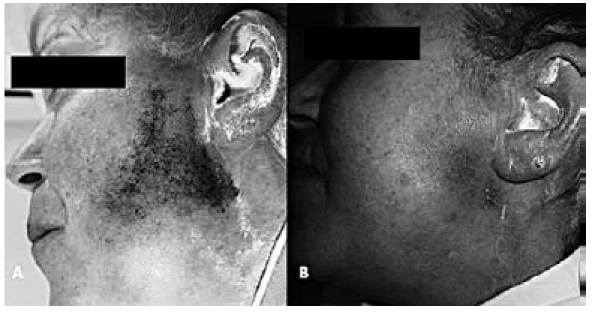
Pre-operative Minor's iodine starch test in a patient with PPFS (A). The same patient 2 months after AFI of the parotid area (total amount 24 cc): the area affected by Minor's iodine starch test is markedly smaller and the gap left by the parotid excision has been filled (B).
Velo-pharyngeal insufficiency
Velo-pharyngeal insufficiency (VPI) is most frequently due to congenital soft palate abnormalities (mainly a sequela of cleft palate repair), but may also be due to neurological causes (paralysis or paresis) or acquired conditions. Surgical treatment is traditionally based on velopharyngoplasties, including local flaps, sphincter reconstruction or the advancement of the posterior pharyngeal wall, which are adopted to narrow the passage between the naso- and oropharynx 66. Velopharyngoplasties lead to excellent results in terms of reducing hypernasality and improving voice resonance and speech articulation, but may also cause post-operative pain and bleeding 67. Post-operative snoring is also frequent, and obstructive sleep apnoea can be a transitory or persistent sequela 68 69. The use of implants to increase the posterior pharyngeal wall has been proposed as a less invasive alternative to major surgical procedures, but nonreabsorbable materials, such as hydroxyapatite, Goretex or silicone, can be associated with migration and extrusion, and re-absorbable materials only lead to a temporary improvement 70. The transplantation of autologous tissue can avoid these drawbacks 71. Fat grafting in the posterior pharyngeal wall, using an external approach, was originally suggested by Gaza in 1926 72, but was subsequently abandoned. The advent of liposuction led to autologous fat grafts being proposed as a means of increasing the posterior naso-pharyngeal wall in patients with moderate VPI 71 73 74
AFI is performed under general anaesthesia with oral endo-tracheal intubation. A Digman mouth gag is used to expose the naso-pharynx, and the soft palate is retracted by means of two rubber catheters passing through the nasal fossae to the mouth with their ends tied. A 70° Storz 4 mm nasal endoscope connected to a videocamera is inserted through the mouth to visualise the nasopharynx, and 3-5 stab incisions are made in the posterior oropharyngeal wall using an 11 blade. A 7 cm long, 1.5 mm diameter, malleable blunt cannula (Pouret Médical, Clichy, France) is inserted and gently advanced to create multiple tunnels upwards towards the atlas prominence. After harvesting and purification, as described above, the fatty tissue grafts are placed under endoscopic guidance while withdrawing the cannula in the submucosal and the intramuscular tissue of the superior constrictor muscle, taking care to remain above the pre-vertebral fascia. The injections are made in multiple layers as often as possible in order to maximize surface contact with the host tissue and improve survival 14 75. A stab incision is then made in the midline (cephalad to uvula) of the nasal surface of the velum, and the fat is injected (particularly distally and on the midline scarred tissue of cleft palate patients). Between 3.5 and 8 cc of fat is used for each patient.
On the basis of our preliminary experience, AFI is a straightforward and minimally-invasive means of treating VPI that significantly reduces hypernasality and improves overall speech quality in paediatric and adult patients. However, a longer follow-up is needed to confirm these findings, and patients should be informed that multiple procedures might be needed to optimise the results; in the case of incompleteness, subsequent velo-pharyngoplasties can be performed as AFI does not affect them.
Conclusions
On the basis of the presence of multi-potent ADSCs and our experience using AFI in the head and neck region, we believe that it is a safe and minimally-invasive procedure both for functional and aesthetic purposes, and a valid alternative to major surgery. The clinical applications of AFI now include fat grafting to the face, vocal fold augmentation for glottic incompetence, the treatment of post-parotidectomy Frey syndrome, and velo-pharyngeal insufficiency. Vocal fold augmentation for glottic incompetence has been standardized and used in our Clinic for several years, whereas using AFI to treat pharyngocutaneous fistulae after head and neck cancer surgery, post-parotidectomy Frey syndrome and velo-pharyngeal insufficiency are more recent proposals that have led to positive outcomes.
Hopefully the present report will encourage further studies on the effectiveness of AFI in other fields.
Acknowledgements
Elisa Montelatici, MD . Cell Factory, Centre for Transfusion Medicine, Cell Therapy and Cryobiology, Department of Regenerative Medicine, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milan, Italy; Mirco Galiè, MD . Human Anatomy and Histology Section, University of Verona, Verona, Italy; Gino Rigotti, MD . Plastic Surgery Unit, Azienda Ospedaliera di Verona, Verona, Italy; Pietro Panettiere, MD . Surgical and Anaesthesiological Sciences Department, University of Bologna, Bologna, Italy; Mario Mantovani, MD . Department of Specialist Surgical Sciences, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, University of Milan, Milan, Italy; Giovanna Baracca, MD . Department of Specialist Surgical Sciences, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, University of Milan, Milan, Italy.
References
- 1.Neuber G. Über die Wiederanheilung vollständig vom Körper getrennter, die ganze Fettschicht enthaltender Hautstücke. Zbl F Chirurgie. 1893;30:16–16. [Google Scholar]
- 2.Lexer E. Fettgewebsverplanzung. In: Lexer E, editor. Die freien Transplantationen. I. Teil. Stuttgart: Enke; 1919. pp. 219–547. [Google Scholar]
- 3.Holländer E. Über einen Fall von fortschreitenden Schwund des Fettgewebes und seinen kosmetischen Ersatz durch Menschenfett. Münch Med Wochenschr. 1910;57:1794–1795. [Google Scholar]
- 4.Illouz YG. The fat cell "graft". A new technique to fill depressions. Plast Reconstr Surg. 1986;78:122–123. [PubMed] [Google Scholar]
- 5.Coleman SR. The technique of periorbital lipoinfiltration. Oper Tech Plast Surg. 1997;1:120–126. [Google Scholar]
- 6.Coleman SR. Long-term survival of fat transplants: controlled demonstrations. Aesthetic Plast Surg. 1995;19:421–425. doi: 10.1007/BF00453875. [DOI] [PubMed] [Google Scholar]
- 7.Coleman SR. Facial recontouring with Lipostructure. Clin Plast Surg. 1997;24:347–367. [PubMed] [Google Scholar]
- 8.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 9.Zuk Zuk, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cantarella G, Mazzola RF, Domenichini E, et al. Vocal fold augmentation by autologous fat injection with lipostructure procedure. Otolaryngol Head Neck Surg. 2005;132:239–243. doi: 10.1016/j.otohns.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 11.Mazzola RF. Fat Injection, expanding opportunities. Panel. 17th Annual Meeting of the European Association of Plastic Surgeons; Istanbul. 2006. [Google Scholar]
- 12.Rigotti G, Marchi A, Galiè M, et al. Treatment of radiotherapy tissue damage by lipoaspirate transplant. A healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg J. 2007;119:1409–1422. doi: 10.1097/01.prs.0000256047.47909.71. [DOI] [PubMed] [Google Scholar]
- 13.Cicero V, Montelatici E, Cantarella G, et al. Do mesenchymal stem cells play a role in vocal fold graft survival? Cell Prolif. 2008;41:460–473. doi: 10.1111/j.1365-2184.2008.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coleman SR, Mazzola RF. Fat Injection: from Filling to Regeneration. St. Louis: Quality Medical Publishing; 2009. [Google Scholar]
- 15.Kang L, Kou Z, Zhang Y, Gao S, et al. Induced pluripotent stem cells (iPSCs) – a new era of reprogramming. J Genet Genomics. 2010;37:415–421. doi: 10.1016/S1673-8527(09)60060-6. [DOI] [PubMed] [Google Scholar]
- 16.Malgieri A, Kantzari E, Patrizi MP, et al. Bone marrow and umbilical cord blood human mesenchymal stem cells: state of the art. Int J Clin Exp Med. 2010;3:248–269. [PMC free article] [PubMed] [Google Scholar]
- 17.Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267–274. [PubMed] [Google Scholar]
- 18.Castro-Malaspina H, Gay RE, Resnick G, et al. Characterization of human bone marrow fibroblast colony-forming cells (CFU-F) and their progeny. Blood. 1980;56:289–301. [PubMed] [Google Scholar]
- 19.Strem BM, Hicok KC, Zhu M, et al. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54:132–141. doi: 10.2302/kjm.54.132. [DOI] [PubMed] [Google Scholar]
- 20.Schofield R. The relationship between the spleen colonyforming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 21.Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 22.Haegel H, Larue L, Ohsugi M, et al. Lack of beta-catenin affects mouse development at gastrulation. Development. 1995;121:3529–3537. doi: 10.1242/dev.121.11.3529. [DOI] [PubMed] [Google Scholar]
- 23.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Zannettino AC, Paton S, Arthur A, et al. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol. 2008;214:413–421. doi: 10.1002/jcp.21210. [DOI] [PubMed] [Google Scholar]
- 25.Miranville A, Heeschen C, Sengenes C, et al. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349–355. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell JB, McIntosh K, Zvonic S, et al. The immunophenotype of human adipose derived cells: Temporal changes in stromaland stem cell-associated markers. Stem Cells. 2005;24:376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 27.Yoshimura K, Shigeura T, Matsumoto D, et al. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol. 2006;208:64–76. doi: 10.1002/jcp.20636. [DOI] [PubMed] [Google Scholar]
- 28.Rubio D, Garcia-Castro J, Martin MC, et al. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65:3035–3039. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- 29.Tzikas TL. Lipografting: autologous fat grafting for total facial rejuvenation. Facial Plast Surg. 2004;20:135–143. doi: 10.1055/s-2004-861754. [DOI] [PubMed] [Google Scholar]
- 30.Trepsat F. Periorbital rejuvenation combining fat grafting and blepharoplasties. Aesthetic Plast Surg. 2003;27:243–253. doi: 10.1007/s00266-003-2126-y. [DOI] [PubMed] [Google Scholar]
- 31.Berman M. Rejuvenation of the upper eyelid complex with autologous fat transplantation. Dermatol Surg. 2000;26:1113–1116. [PubMed] [Google Scholar]
- 32.Coleman SR. Hand rejuvenation with structural fat grafting. Plast Reconstr Surg. 2002;110:1731–1744. doi: 10.1097/01.PRS.0000033936.43357.08. [DOI] [PubMed] [Google Scholar]
- 33.Boschert MT, Beckert BW, Puckett CL, et al. Analysis of lipocyte viability after liposuction. Plast Reconstr Surg. 2001;109:761–765. doi: 10.1097/00006534-200202000-00054. [DOI] [PubMed] [Google Scholar]
- 34.Rigotti G, Marchi A, Galie M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119:1409–1422. doi: 10.1097/01.prs.0000256047.47909.71. [DOI] [PubMed] [Google Scholar]
- 35.Coleman SR. Overview of Structural Fat Grafting. In: Coleman SR, Mazzola RF, editors. Fat Injection: from Filling to Regeneration. St. Louis: Quality Medical Publishing; 2009. pp. 95–110. [Google Scholar]
- 36.Coleman SR. Fat Grafting to the Nose. In: Coleman SR, Mazzola RF, editors. Fat Injection: from Filling to Regeneration. St. Louis: Quality Medical Publishing; 2009. pp. 425–446. [Google Scholar]
- 37.Mikaelian DO, Lowry LD, Sataloff RT. Lipo-injection for unilateral vocal cord paralysis. Laryngoscope. 1991;101:465–468. doi: 10.1288/00005537-199105000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Brandenburg JH, Kirkham W, Koschkee D. Vocal cord augmentation with autogenous fat. Laryngoscope. 1992;102:863–869. doi: 10.1288/00005537-199205000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Shindo ML, Zaretsky LS, Rice DH. Autologous fat injection for unilateral vocal fold paralysis. Ann Otol Rhinol Laryngol. 1995;104:1–4. doi: 10.1177/000348949510400101. [DOI] [PubMed] [Google Scholar]
- 40.McCulloch TM, Andrews BT, Hoffman HT, et al. Long-term follow-up of autologous fat injection laryngoplasty for unilateral vocal cord paralysis. Laryngoscope. 2002;112:1235–1238. doi: 10.1097/00005537-200207000-00017. [DOI] [PubMed] [Google Scholar]
- 41.Laccourreye O, Papon JF, Kania R, et al. Intracordal injection of autologous fat in patients with unilateral laryngeal nerve paralysis: long term results from the patient's perspective. Laryngoscope. 2003;113:541–545. doi: 10.1097/00005537-200303000-00027. [DOI] [PubMed] [Google Scholar]
- 42.Isshiki N. Progress in laryngeal framework surgery. Acta Otolaryngol. 2000;120:120–127. doi: 10.1080/000164800750000748. [DOI] [PubMed] [Google Scholar]
- 43.Remacle M, Lawson G. Results with collagen injection into the vocal folds for medialization. Curr Opin Otolaryngol Head Neck Surg. 2001;15:148–152. doi: 10.1097/MOO.0b013e3281084e74. [DOI] [PubMed] [Google Scholar]
- 44.Hertegård S, Hallén L, Laurent C, et al. Cross-linked hyaluronan used as augmentation substance for treatment of glottal insufficiency: safety aspects and vocal fold function. Laryngoscope. 2002;112:2211–2219. doi: 10.1097/00005537-200212000-00016. [DOI] [PubMed] [Google Scholar]
- 45.Kasperbauer JL, Slavit DH, Maragos NE. Teflon granulomas and overinjection of Teflon: a therapeutic challenge for the otorhinolaryngologist. Ann Otol Rhinol Laryngol. 1993;102:748–751. doi: 10.1177/000348949310201002. [DOI] [PubMed] [Google Scholar]
- 46.Takayama E, Ikeda M, Tsuru S, et al. Is injectable collagen truly safe? J Laryngol Otol. 1992;106:704–708. doi: 10.1017/s0022215100120638. [DOI] [PubMed] [Google Scholar]
- 47.Spiegel JR, Sataloff RT, Gould JW. The treatment of vocal fold paralysis with injectable collagen: clinical concerns. J Voice. 1987;1:119–121. [Google Scholar]
- 48.Koufman JA, Isaacson G. Laryngoplastic phonosurgery. Otolaryngol Clin North Am. 1991;24:1151–1177. [PubMed] [Google Scholar]
- 49.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tholpady SS, Llull R, Ogle RC, et al. Adipose tissue: Stem cells and beyond. Clin Plast Surg. 2006;33:55–62. doi: 10.1016/j.cps.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 51.Rehman J, Traktuev D, Li J, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 52.Cantarella G, Fasano V, Maraschi B, et al. Airway resistance and airflow dynamics after autologous fat injection into vocal folds. Ann Otol Rhinol Laryngol. 2005;115:816–823. doi: 10.1177/000348940611501103. [DOI] [PubMed] [Google Scholar]
- 53.Chan RW, Titze IR. Viscosities of implantable biomaterials in vocal fold augmentation surgery. Laryngoscope. 1998;108:725–731. doi: 10.1097/00005537-199805000-00019. [DOI] [PubMed] [Google Scholar]
- 54.Cantarella G, Baracca G, Forti S, et al. Proceedings AAO-HNSF Annual Meeting. Boston, MA: 2010. Etiology-related outcome of vocal fold lipoinjection for glottal insufficiency; pp. 81–81. [Google Scholar]
- 55.Ricci Maccarini A, Stacchini M, Salsi D, et al. Surgical rehabilitation of dysphagia after partial laryngectomy. Acta Otorhinolaryngol Ital. 2007;27:294–298. [PMC free article] [PubMed] [Google Scholar]
- 56.Navach V, Calabrese LS, Zurlo V, et al. Functional base of tongue fat injection in a patient with severe postradiation dysphagia. Dysphagia. 2010 Aug 01; doi: 10.1007/s00455-010-9293-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 57.Thomas A. Le double réflexe vaso-dilatateur et sudoral de la face consecutive aux blessures de la loge parotidienne. Rev Neurol (Paris) 1927;1:447–460. [Google Scholar]
- 58.Bree R, Waal I, Leemans CR. Management of Frey syndrome. Head Neck. 2007;29:773–778. doi: 10.1002/hed.20568. [DOI] [PubMed] [Google Scholar]
- 59.Minor V. Eines neues Verfahren zu der klinsichen Untersugung der Schweissabsonderung. Dtsch Z Nervenh. 1928;101:258–261. [Google Scholar]
- 60.Beerens AJ, Snow GB. Botulinum toxin A in the treatment of patients with Frey syndrome. Br J Surg. 2002;89:116–119. doi: 10.1046/j.0007-1323.2001.01982.x. [DOI] [PubMed] [Google Scholar]
- 61.Capaccio P, Torretta S, Osio M, et al. Botulinum toxin therapy: a tempting tool in the management of salivary secretory disorders. Am J Otolaryngol. 2008;29:333–338. doi: 10.1016/j.amjoto.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 62.Hays LL, Novack AJ, Worsham JC. The Frey syndrome: a simple, effective treatment. Otolaryngol Head Neck Surg. 1982;90:419–425. doi: 10.1177/019459988209000410. [DOI] [PubMed] [Google Scholar]
- 63.MacKinnon C, Lovie M. An alternative treatment for Frey syndrome. Plast Reconstr Surg. 1999;103:745–746. doi: 10.1097/00006534-199902000-00076. [DOI] [PubMed] [Google Scholar]
- 64.Curry JM, Fisher KW, Heffelfinger RN, et al. Superficial musculoaponeurotic system elevation and fat graft reconstruction after superficial parotidectomy. Laryngoscope. 2008;118:210–215. doi: 10.1097/MLG.0b013e3181581f94. [DOI] [PubMed] [Google Scholar]
- 65.Luna-Ortiz K, Sansón-RíoFrío JA, Mosqueda-Taylor A. Frey syndrome. A proposal for evaluating severity. Oral Oncol. 2004;40:501–505. doi: 10.1016/j.oraloncology.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 66.Bardach J. Pharyngoplasty. In: Salyer K, Bardach J, editors. Atlas of craniofacial and cleft surgery. Vol. II. Cleft lip and palate surgery. Philadelphia, PA: Lippincott Raven Pub; 1999. pp. 783–807. [Google Scholar]
- 67.Valnicek SM, Zuker RM, Halpern LM, et al. Perioperative complications of superior pharyngeal flap surgery in children. Plast Reconstr Surg. 1994;93:954–958. doi: 10.1097/00006534-199404001-00009. [DOI] [PubMed] [Google Scholar]
- 68.Ysunza A, Garcia-Velasco M, Garcia-Garcia M, et al. Obstructive sleep apnea secondary to surgery for velopharyngeal insufficiency. Cleft Palate Craniofac J. 1993;30:387–390. doi: 10.1597/1545-1569_1993_030_0387_osasts_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 69.Lesavoy MA, Borud LJ, Thorson T, et al. Upper airway obstruction after pharyngeal flap surgery. Ann Plast Surg. 1996;36:26–30. doi: 10.1097/00000637-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 70.Lypka M, Bidros R, Rizvi M, et al. Posterior pharyngeal augmentation in the treatment of velopharyngeal insufficiency: a 40-year experience. Ann Plast Surg. 2010;65:48–51. doi: 10.1097/SAP.0b013e3181c1fec6. [DOI] [PubMed] [Google Scholar]
- 71.Leuchter I, Schweizer V, Hohlfeld J, et al. Treatment of velopharyngeal insufficiency by autologous AFT. Eur Arch Otorhinolaryngol. 2010;267:977–983. doi: 10.1007/s00405-009-1157-7. [DOI] [PubMed] [Google Scholar]
- 72.Gaza WV. Ueber freie Fettgewebstransplantation in den retropharyngealen Raum bei Gaumenspalte. Arch Klin Chir. 1926;142:590–599. [Google Scholar]
- 73.Dejonckere PH, Wijngaarden HA. Retropharyngeal autologous fat transplantation for congenital short palate: a nasometric assessment of functional results. Ann Otol Rhinol Laryngol. 2001;110:168–172. doi: 10.1177/000348940111000213. [DOI] [PubMed] [Google Scholar]
- 74.Bardot J, Niddam J. Velopharyngeal incompetence in cleft palate sequelae. In: Coleman SR, Mazzola RF, editors. Fat Injection: from Filling to Regeneration. St. Louis, MO: Quality Medical Publishing; 2009. pp. 717–734. [Google Scholar]
- 75.Cantarella G, Mazzola RF. Vocal fold augmentation by autologous Fat Injection. In: Coleman SR, Mazzola RF, editors. Fat Injection: from Filling to Regeneration. St. Louis, MO: Quality Medical Publishing; 2009. pp. 717–734. [Google Scholar]


