SUMMARY
Obstructive sleep apnoea syndrome is a disease characterized by a collapse of the pharyngeal airway resulting in repeated episodes of airflow cessation, oxygen desaturation, and sleep disruption. It is a common disorder affecting at least 2-4% of the adult population. The role of nasal resistance in the pathogenesis of sleep disordered breathing and sleep apnoea has not been completely clarified. Aim of the present study was to establish whether nasal resistance and nasal volumes, measured by means of Active Anterior Rhinomanometry and Acoustic Rhinometry together with Muco-Ciliary Transport time play a positive predictive role in the evaluation of Obstructive sleep apnoea syndrome patients before running a nocturnal polysomnographic recording. A retrospective study was performed analysing 223 patients referred for suspected Obstructive sleep apnoea syndrome. All patients were submitted to complete otorhinolaryngological evaluation and underwent nocturnal polysomnography. On the basis of polysomnographic data analysis, the apnoea-hypopnoea index and snoring index, patients were classified into two groups: Group 1 (110/223 patients) with a diagnosis of mild-moderate Obstructive sleep apnoea syndrome (apnoea-hypopnoea index < 30) and Group 2 (113/223 patients) affected by snoring without associated hypoxaemia/hypercapnia. A control group of 76 subjects, not complaining of sleep disorders and free from nasal symptoms was also selected. The results showed, in all the snoring and Obstructive sleep apnoea syndrome patients, total nasal resistance and increased Muco-Ciliary Transport time compared to standard values. Furthermore, the apnoea-hypopnoea index was significantly higher in patients with higher nasal resistence and significantly different between the groups. These results allow us to propose the simultaneous evaluation of nasal functions by Active Anterior Rhinomanometry, Acoustic Rhinometry, and Muco-Ciliary Transport time in the selection of patients undergoing polysomnography.
KEY WORDS: Sleep respiratory disorders, Obstructive sleep apnoea syndrome, Nasal functionality tests, Polysomnography
RIASSUNTO
La sindrome delle apnee ostruttive del sonno, patologia che affligge circa il 2-4% degli adulti, è una malattia caratterizzata dal collasso delle strutture faringee con conseguenti ripetuti episodi di desaturazione, pause respiratorie e risvegli notturni. Ipotizzando che lo studio della funzionalità nasale possa avere un ruolo predittivo in questi pazienti prima di effettuare un esame polisonnografico abbiamo retrospettivamente analizzato le cartelle di 223 pazienti afferenti all'ambulatorio di Rinologia che riferivano disturbi del sonno. Tutti i pazienti erano stati sottoposti a completa valutazione obbiettiva otorinolaringoiatrica, studio della funzionalità nasale mediante rinomanometria, rinometria acustica, valutazione del tempo di trasporto mucociliare e polisonnografia. I pazienti sono stati suddivisi in due gruppi in base ai valori di polisonnografia (Apnoea-Hypopnoea Index AHI/ Snoring index SI): Gruppo 1 (110/223 pazienti) affetti da OSAS (AHI < 30) lieve e moderata; Gruppo 2 (113/223 pazienti) con diagnosi di russamento semplice senza segni di ipossiemia ed ipercapnia. I risultati hanno evidenziato nei pazienti affetti da russamento semplice e OSAS, valori di resistenze nasali e di tempo di trasporto mucociliare più elevati rispetto al gruppo di controllo. All'analisi statistica lo AHI era significativamente elevato nei pazienti con elevate resistenze nasali totali. Alla luce dei nostri risultati riteniamo utile la simultanea valutazione della funzionalità nasale mediante rinomanometria, rinometria acustica e valutazione del tempo di trasporto mucociliare per indirizzare i pazienti verso un esame polisonnografico.
Introduction
Obstructive sleep apnoea syndrome (OSAS) is a pathological condition characterized by a collapse of the pharyngeal airway resulting in repeated episodes of airflow cessation, oxygen desaturation, and sleep disruption. It is a common disorder affecting at least 2-4% of the adult population and it is increasingly recognized by the public 1.
Clinically, OSAS is defined by the occurrence of daytime sleepiness, loud snoring, witnessed breathing interruptions, or awakenings due to gasping or choking in the presence of at least 5 obstructive respiratory events (apnoeas, hypopnoeas or respiratory effort-related arousals) per hour of sleep 1 2. The presence of 15 or more obstructive respiratory events per hour of sleep, in the absence of sleep-related symptoms, is also sufficient for the diagnosis of OSAS due to the greater association of this severity of obstruction with important consequences such as increased cardiovascular disease risk 3.
The relationship between obstructed nasal passages and sleep-disordered breathing has been studied for over 30 years 3. It has been speculated that increased nasal resistance (NR) may be associated with increases in snoring activity and apnoic events during sleep 4-6. Some studies have demonstrated a weak association between NR and the severity of obstructive sleep apnoea 7-10. On the contrary, others did not show any correlation between the degree of nasal obstruction and the severity of snoring or sleep apnoea 11 12.
In the present study, by focusing on nasal functions, measured by Active Anterior Rhinomanometry (AAR), Acoustic Rhinometry (AR) and the evaluation of nasal Muco-Ciliary Transport time (MCTt), a group of patients was examined suffering from OSAS and a group of patient suffering from Primary Snoring (PS) i.e., snoring without associated hypoxaemia, hypercapnia, sleep disruption or daytime symptoms and these data were then compared with those in a control group without sleep disorders.
The aim was to establish whether the status of the nasal passages has a significant predictive positive role in the evaluation of these patients before running a nocturnal polysomnographic recording.
Materials and methods
Patients
Inclusion criteria were age > 18 years, body mass index (BMI) < 33, tonsil size Grades 1 and 2, elongated uvula, all Mallampati grades, minimal collapse of the tongue base (< 25%) as seen on the modified Müller manoeuvre 13, Fujita (retro-palatal obstruction) Grade I, simple snorers (apnoea-hypopnoea index AHI < 5), and patients with mild-moderate OSA (AHI < 30).
All patients had had a previous complete ENT evaluation, including clinical examination, fiberoptic nasopharyngoscopy with modified Müller manoeuvre; then all underwent nasal functionality tests and more precisely: AAR, AR and nasal MCTt determination. Nocturnal polysomnography was, thereafter, performed on all the patients.
Fiberoptic nasopharyngoscopy
The clinical evaluation included a complete traditional ENT examination of the upper airways and an endoscopic examination, with a flexible fibroscope (Olympus LF-DP, Tokyo, Japan), of the nasal, nasopharyngeal, and hypopharyngeal cavities. During this latter examination, a modified Müller manoeuvre was performed in the oropharyngeal areas (retropalate region) 1 3 14 15.
Otorhinolaryngologic findings of the upper airway were graded as follows:
-
Tonsil size
Grade 1: tonsils are in tonsillar fossa, barely seen behind the anterior pillars.
Grade 2: tonsils are visible behind the anterior pillars.
Grade 3: tonsils are extended three quarters of the way to the mid-line.
-
Modified Mallampati grade (MMP)
Grade 1: tonsils, pillars and soft palate are clearly visible.
Grade 2: uvula, pillars and upper pole are visible.
Grade 3: soft palate is partly visible; while tonsils, pillars and the uvula base are all invisible.
Grade 4: hard palate only is visible.
-
Fujita scale
Type 1: collapse of palato-pharyngeal arch only.
Type 2: collapse of palato-pharyngeal arch and retroglossal space.
Type 3: collapse of retroglossal space only.
-
Retroglossal space
Grade 1: the retroglossal space is widely patent allowing visualization of the oropharynx.
Grade 2: the retroglossal space hardly allows visualization of the oropharynx but the opposite walls are not in contact.
Grade 3: the retroglossal space is very narrow with the barely contacting opposite walls.
Grade 4: the retroglossal space is in constant contact with the opposite walls.
Sleep Study
Standard overnight polysomnography (Alice 3, Healthdyne, Technologies, Ohio, USA) was performed in a conventional manner to record sleep parameters and architecture in every patient. The electro-encephalogram (EEG ), electrooculogram (EOG ), electromyogram (EMG ) and electrocardiogram (ECG) were recorded continuously, and respiration was monitored with oro-nasal thermistors and thoraco-abdominal piezo sensors. The parameters used in this study were AHI, snoring index (SI) and minimal oxygen saturation (MSAT). AHI was defined as the total number of apnea and hypopnea episodes per hour of sleep. An apnea episode was defined as cessation of airflow lasting longer than 10 seconds, whereas a hypopnea episode was defined as a reduction of 50% or greater in combined oral and nasal flow lasting longer than 10 seconds. SI was defined as the number of spikes in sound intensity exceeding 50 dB per hour of sleep. MSAT was defined as the minimal O2 saturation detected during the polysomnographic test period. The single technician who scored the polysomnographic studies was blind to NR measurements 15-17.
Nasal Airflow study
Rhinomanometry
Nasal resistance was measured by AAR (Ryno Zig Rhinomanometer by Menfis Biomedica srl, BO, Italy) in the daytime. Active rhinomanometry is a quick test that requires subjects to generate airflow through the nose by their own effort 18 19. In accordance with the International Committee on standardization of rhinomanometry, the nasal airflow resistance was measured at standard pressure (150 Pa), and the total nasal resistance was calculated from the unilateral rhinomanometry recordings 20 21. The measurements were not taken during the symptomatic period for acute common cold or seasonal allergic rhinitis, and patients in whom nasal resistance was not measurable due to severe nasal obstruction were excluded from this study. In contrast to other studies 15, we measured total nasal resistance only in the sitting position which allowed us to determine a stable situation of the upper airways (not influenced by wakefulness/sleep or sitting/supine position) and its eventual influence on snoring or sleep apnoea.
Acoustic rhinometry
Acoustic rhinometry (Rhinoklack-RK1000, Stimotron Co., Wendelstein, Germany) was conducted while the patient was breathing quietly after inserting a nocepiece into the nostril. This technique evaluates nasal patency by analysing reflections of a sound pulse introduced by the nostril. By analysing the amplitude of sound reflected from the nasal cavity, an estimate of cavity geometry can be produced by PC-software as a plot of the cross sectional area against the distance from the nostril. In this graph, narrowing is seen as a peak and widening as a dip. Minimal nasal cross-sectional areas, cross-sectional areas, at a defined distance from the nostril and volumes of defined regions of nasal cavities, can be derived by further manipulation of the data 21-23. We added these values to our analysis because, on the basis of our previous studies, acoustic rhinometry is more specific and more sensitive than rhinomanometry in diagnosing rhinopathies in patients with structural anomalies 24.
In this research, it was found that symptom scores, as rated by patients on a visual analogue scale frequently did not correlate with objective measurements, as patients often overestimated the severity of their obstruction. On the contrary, for a few patients, a correlation between symptom scores and MCTt could be observed. This is why we performed also this test in all our patients.
Evaluation of Nasal Muco-Ciliary Transport time
All subjects underwent nasal MCTt determination using a mixture of charcoal powder and 3% saccharin. Nasal MCTt is calculated as being the time elapsing between the moment in which the charcoal powder is placed on the head of the inferior turbinate and the moment in which a blackish colouring appears in the oro-pharynx, as shown by direct pharyngoscopy; saccharin clearance, on the other hand, is calculated at the moment in which the subject being tested notices a sensation of sweetness 25.
Statistical analysis
All statistical analyses were made using the SPSS for Windows. Continuous variables (age, height, body weight, BMI) were compared with the independent t test.
The clinical data and PSG variables are expressed as mean values ± SD. The correlation between nasal resistance and the parameters monitored during PSG were evaluated using the Mann-Whitney test.
Assuming nasal resistance as a reference value for the severity of OSAS, the specificity, sensitivity, and positive and negative predictive values of nasal function test were calculated using the standard technique to obtain the Receiver Operator Characteristics (RO C) curve. A p value < 0.05 was considered statistically significant.
Results
This retrospective study was performed analyzing 223 patients (77 female, 145 male; age range 25 to 77 years, mean age 53 ± 14 SD) referred to the E.N.T. Clinic of Siena for suspected OSAS, from January 2002 to December 2005. On the basis of polysomnographic data (AHI and SI), patients were divided into two groups as follows: Group 1 (110/223 patients) with a diagnosis of mild-moderate OSAS (AHI < 30) and Group 2 (113/223 patients) affected by snoring without associated hypoxaemia, hyper-capnia, sleep disruption or daytime symptoms (AHI < 5). These two patient groups were compared with a control group (Group 3), comprising 76 adults (27 female, 49 male, age range 27 - 75 years, mean age 57 ± 16 SD) free from nasal symptoms or upper respiratory infections for at least 2 weeks, and without sleep or snoring complaints.
No significant correlation between nasal resistance and other anthropometric parameters (patient age, height, body weight, BMI) was observed (P > 0.05).
The results of AAR, AR and MCTt in the mild-moderate OSAS group, in snoring group and in a control group are shown in Table I.
Table I.
Study population data.
| Study Groups Mean ± SD |
Control Group Mean ± SD |
|
|---|---|---|
| No. | 223 (110 Group 1) (113 Group 2) |
76 |
| Age (yrs) | 53 ± 14 | 57 ± 16 |
| Body Mass Index | 31.3 ± 0.7 (Group 1) 27.4 ± 0.5 (Group 2) |
20.4 ± 0.3 |
| AHI | 25.3 ± 2.7 (Group 1) 4.78 ± 0.5 (Group 2) |
< 1 |
AHI: apnoea-hypopnoea index
Total Nasal Resistance correlated significantly, and in the expected direction, with AHI (p = 0.006) (Fig. 1). The values of nasal MCTt were also significantly correlated with AHI (p = 0.004) (Fig. 2). There was no significant correlation between rhinomanometric parameters and polysomnography. As far as concerns the rhinometric parameters, a decrease of the minimal cross-sectional areas was observed in all the patients, but no statistically significant correlation with AHI was observed.
Fig. 1.
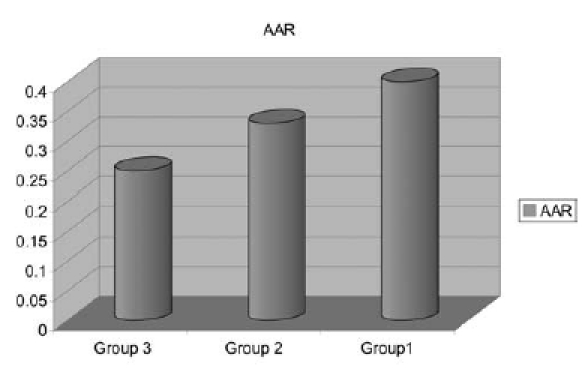
Nasal resistance value. AAR: Active Anterior Rhinomanometry
Fig. 2.
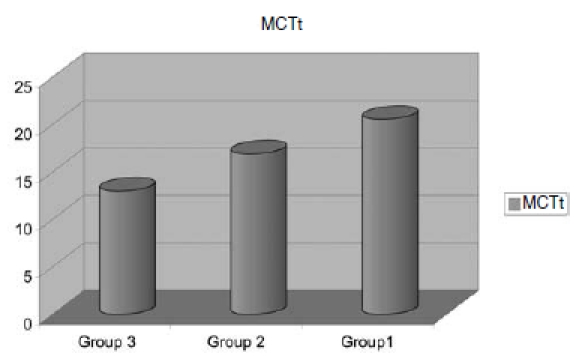
Muco-Ciliary Transport time (MCTt).
Total NR also correlated with SI (p = 0.02): inter-group comparison revealed a significant difference in SI with the higher NR group showing higher SI.
Stepwise multiple regression models revealed that high NR was predictive of AHI (p = 0.006).
The RO C curve of nasal resistance is shown in Figure 3, without a specific cut-off point; the possibility to identify patients with mild-moderate OSAS is greatly affected by varying the threshold value along the curve.
Fig. 3.
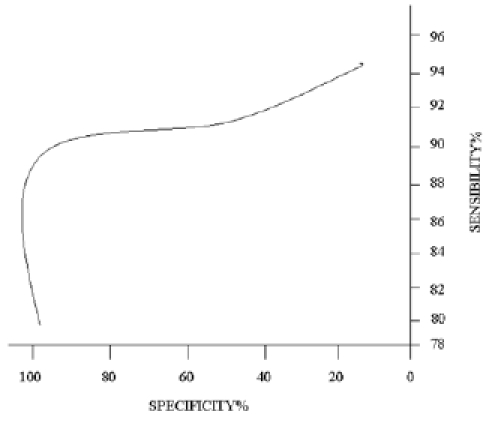
ROC curve of nasal resistance in detecting patients with mild-moderate OSAS.
Discussion
OSAS affects, at least, 2-4% of the adult population and is increasingly recognized by the public 1. The pathophysiology of OSAS involves the development of pharyngeal airway narrowing. The pharyngeal airway may behave like a Starling resistor due to decreased upper airway muscle tone and phasic inspiratory activity during sleep.
The collapsibility of the compliant wall is further enhanced by the increased resistive load caused by obesity which alters the physical characteristics of the wall.
The pharynx, connecting the nose to the larynx and acting as a Starling resistor, is more vulnerable to changes in airflow resistance. On the other hand, the nose has a rigid framework to maintain constant resistance both in waking and sleeping states 26-29. Thus, the role of NR in OSAS remains controversial.
A large number of adult surveys have been conducted in order to establish the presence or absence of OSAS by means of parameters such as age, sex, snoring, witnessed apnoea and daytime sleepiness, and giving a score for each clinical feature suggestive of sleep apnoea29,30.
Our results showed that in snoring and mild-moderate OSAS patients the values of nasal resistance and MCTt are increased with respect to standard values whereas minimal cross-sectional areas are decreased.
Specifically, a nasal resistance of 0.40 ± 0.06 Pa/cc3/sec, a minimal cross sectional area of 0.66 cm2 and a MCTt of 17.55 min could be predictors of AHI and SI (Table II).
Table II.
Results of nasal functionality tests in the three groups of patients.
| Group 3 | Group 2 | Group 1 | |
|---|---|---|---|
| AAR | 0.25 ± 0.03 Pa/cc3/sec |
0.33 ± 0.02 Pa/cc3/sec |
0.40 ± 0.06 Pa/cc3/sec |
| AR | 1.1 Cm2 | 0.83 Cm2 | 0.66 Cm2 |
| MCTt | 13+/- 2 Min. | 16.9 +/- 2 Min. | 17.55 +/- 2 Min. |
In this study, SI was significantly higher in patients with higher NR and significantly different in the three groups (p = 0.02).
Furthermore, there was a significant correlation between total NR and the severity of OSAS.
In this study, MSAT did not correlate significantly with any parameter from rhinomanometry and acoustic rhinometry. These results were consistent with previous data, and reinforced the hypothesis that nasal function may not contribute to severe sleep apnoea with hypoxaemia.
The low correlation level between rhinomanometric and some polysomnographic data, such as MSAT, makes it necessary to perform a careful evaluation of the entire upper respiratory tract during awakening and with specific manoeuvres or tests (Muller manoeuvre, somnendoscopy) feigning sleeping condition 31.
The RO C curve (Fig. 3) indicates that nasal resistance value of 0.40 Pa/cm3/s has a sensitivity of 91% and a specificity of 96%; these values show that AAR offers supplementary diagnostic opportunities and represents a simple and objective system to select patients saving the technician's time.
Conclusions
This study provides supporting evidence that the status of the nasal fossae has a significant positive predictive role in patients with simple snoring and mild-moderate OSAS.
Following the results obtained, we propose the simultaneous evaluation of nasal function by AAR, AR and MCTt in patients undergoing PSG recording.
However, as OSAS is a multi-factorial, multi-level disease, where nasal functions play a role, but together with other alterations at different levels of the pharynx (naso, oro, and hypopharynx) our results do not allow us to exclude a "systematic diagnostic and phased protocol" including fiberoptic endoscopy with the evaluation of tonsil size, Mallampati grading, Fujita grading with retro-glossal space evaluation, as stated and again stressed recently in the literature 32. These statements are even more important when considering any surgical approach in patients with severe OSAS 33.
References
- 1.Epstein LJ, Kristo D, Strollo PJ, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2009;5:263–276. [PMC free article] [PubMed] [Google Scholar]
- 2.Aubert-Tulkens G, Hamoir M, Eeckault J. Failure of tonsil and nose surgery in adults with long standing severe pulmonary sleep apnea syndrome. Arch Intern Med. 1989;149:2118–2121. [PubMed] [Google Scholar]
- 3.Olsen KD, Kern EB, Westbrook PR. Sleep and breathing disturbance secondary to nasal obstruction. Otolaryngol Head Neck Surg. 1981;89:804–810. doi: 10.1177/019459988108900522. [DOI] [PubMed] [Google Scholar]
- 4.Lenders H, Schaefer J, Pirsig W. Turbinate hypertrophy in habitual snorers and patients with obstructive sleep apnea: findings of acoustic rhinometry. Laryngoscope. 1991;101(6 Pt 1):614–618. doi: 10.1288/00005537-199106000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Zeng B, Ng AT, Qian J, et al. Influence of nasal resistance on oral appliance treatment outcome in obstructive sleep apnea. Sleep. 2008;31:543–547. doi: 10.1093/sleep/31.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kemppainen T, Ruoppi P, Seppä J, et al. Effect of weight reduction on rhinometric measurements in overweight patients with obstructive sleep apnea. Am J Rhinol. 2008;22:410–415. doi: 10.2500/ajr.2008.22.3203. [DOI] [PubMed] [Google Scholar]
- 7.Sériès F, St. Pierre S, Carrier G. Effects of surgical correction of nasal obstruction in the treatment of obstructive sleep apnea. Am Rev Respir Dis. 1992;146:1261–1265. doi: 10.1164/ajrccm/146.5_Pt_1.1261. [DOI] [PubMed] [Google Scholar]
- 8.Rombaux P, Liistro G, Hamoir M. Nasal obstruction and its impact on sleep-related breathing disorders. Rhinology. 2005;43:242–250. [PubMed] [Google Scholar]
- 9.Hoffstein V, Chaban R, Cole P. Snoring and upper airway properties. Chest. 1988;94:87–89. doi: 10.1378/chest.94.1.87. [DOI] [PubMed] [Google Scholar]
- 10.Mates A, Ohki M, Cole P. Snoring, apnea and nasal resistance in men and women. J Otolaryngol. 1991;20:57–61. [PubMed] [Google Scholar]
- 11.Atkins M, Taskar V, Clayton N, et al. Nasal resistance in obstructive sleep apnea. Chest. 1994;105:1133–1135. doi: 10.1378/chest.105.4.1133. [DOI] [PubMed] [Google Scholar]
- 12.Miljeteig H, Hoffstein V, Cole P. The effect of unilateral and bilateral nasal obstruction on snoring and sleep apnea. Laryngoscope. 1992;102:1150–1152. doi: 10.1288/00005537-199210000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Sher AE, Thorpy MJ, Spielman AJ, et al. Predictive value of Mueller's manoeuver in selection of patients for uvulopalatopharyngoplasty. Laryngoscope. 1985;95:1483–1487. doi: 10.1288/00005537-198512000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Yagi H, Nakata S, Tsuge H, et al. Morphological examination of upper airway in obstructive sleep apnea. Auris Nasus Larynx. 2009;36:444–449. doi: 10.1016/j.anl.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Vito A, Berrettini S, Carabelli A, et al. The importance of nasal resistance in obstructive sleep apnea syndrome: a study with positional rhinomanometry. Sleep Breath. 2001;5:3–11. doi: 10.1007/s11325-001-0003-y. [DOI] [PubMed] [Google Scholar]
- 16.Mehta A, Qian J, Petocz P, et al. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163:1457–1461. doi: 10.1164/ajrccm.163.6.2004213. [DOI] [PubMed] [Google Scholar]
- 17.Gotsopoulos H, Chen C, Qian J, et al. Oral appliance therapy improves symptoms in obstructive sleep apnea: a randomized, controlled trial. Am J Respir Crit Care Med. 2002;166:743–748. doi: 10.1164/rccm.200203-208OC. [DOI] [PubMed] [Google Scholar]
- 18.Desfonds P, Planès C, Fuhrman C, et al. Nasal resistance in snorers with or without sleep apnea: effect of posture and nasal ventilation with continuous positive airway pressure. Sleep. 1998;21:625–632. doi: 10.1093/sleep/21.6.625. [DOI] [PubMed] [Google Scholar]
- 19.Li HY, Wang PC, Hsu CY, et al. Nasal resistance in patients with obstructive sleep apnea. ORL J Otorhinolaryngol Relat Spec. 2005;67:70–74. doi: 10.1159/000084337. [DOI] [PubMed] [Google Scholar]
- 20.Clement PAR, Godts F. Consensus report on acoustic rhinometry and rhinomanometry. Rhinology. 2005;43:169–179. [PubMed] [Google Scholar]
- 21.Dunagan D, Georgitis J, Kemp S, et al. Intranasal disease and provocation: diagnostic testing of allergic disease. Vol. 3539. New York: Marcel Dekker; 2000. pp. 151–173. [Google Scholar]
- 22.Clement PAR. Committee report on standardization of rhinomanometry. Rhinology. 1984;22:151–155. [PubMed] [Google Scholar]
- 23.Chung Seop K, Byeong Kweon M, Dong Hak J, et al. Correlation between nasal obstruction symptoms and objective parameters of acoustic rhinometry and rhinomanometry. Auris Nasus Larynx. 1998;25:45–48. doi: 10.1016/s0385-8146(97)10011-6. [DOI] [PubMed] [Google Scholar]
- 24.Passàli D, Mezzedimi C, Passàli GC, et al. The role of rhinomanometry, acoustic rhinometry, and mucociliary transport time in the assessment of nasal patency. Ear Nose Throat J. 2000;79:397–400. [PubMed] [Google Scholar]
- 25.Passàli D, Bellussi L, Seta E. Experiences in the determination of nasal mucociliary transport time. Acta Oto-Laryngologica. 1984;97:319–323. doi: 10.3109/00016488409130995. [DOI] [PubMed] [Google Scholar]
- 26.Zhong G, Kong W, Yue J, et al. The study of the relationship between obstructive sleep apnea syndrome and positional nasal resistance. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2003;17:351–353. (Chinese) [PubMed] [Google Scholar]
- 27.Lee NR. Evaluation of the obstructive sleep apnea patient and management of snoring. Oral Maxillofac Surg Clin North Am. 2009;21:377–387. doi: 10.1016/j.coms.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Madani M, Madani F. Epidemiology, pathophysiology, and clinical features of obstructive sleep apnea. Oral Maxillofac Surg Clin North Am. 2009;21:369–375. doi: 10.1016/j.coms.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Berger G, Berger R, Oksenberg A. Progression of snoring and obstructive sleep apnoea: the role of increasing weight and time. Eur Respir J. 2009;33:338–45. doi: 10.1183/09031936.00075408. [DOI] [PubMed] [Google Scholar]
- 30.Sugiura T, Noda A, Nakata S, et al. Influence of nasal resistance on initial acceptance of continuous positive airway pressure in treatment for obstructive sleep apnea syndrome. Respiration. 2007;74:56–60. doi: 10.1159/000089836. [DOI] [PubMed] [Google Scholar]
- 31.Campanini A, Canzi P, Vito A, et al. Awake versus sleep endoscopy: personal experience in 250 OSAHS patients. Acta Otorhinolaryngol Ital. 2010;30:73–77. [PMC free article] [PubMed] [Google Scholar]
- 32.Powell NB. Contemporary surgery for obstructive sleep apnea syndrome. Clin Exp Otorhinolaryngol. 2009;2:107–114. doi: 10.3342/ceo.2009.2.3.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campanini A, Vito S, Frassinetti S, et al. Temporary tracheotomy in the surgical treatment of obstructive sleep apnea syndrome: personal experience. Acta Otorhinolaryngol Ital. 2003;23:474–478. [PubMed] [Google Scholar]


