SUMMARY
In order to analyze the diagnostic efficiency of saccadic and pursuit eye movements compared to findings from brain magnetic resonance imaging in patients with central vertigo, 108 patients were selected from 580 dizzy patients as cases of suspected central origin; the saccadic and pursuit eye movements were evaluated by electronystagmography and findings were compared to information from magnetic resonance imaging. The study of oculomotor movements in patients suspected of having a central lesion revealed a 83.3% sensitivity and 21.2% specificity. Restricting consideration to severe alterations in eye movements as indicative of a central origin, this test gives a 71.4% sensitivity and 50.0% specificity. In conclusion, the study of alterations in oculomotor movements, in patients with suspected central lesions, proved to be a test with good sensitivity also compared with results of magnetic resonance imaging.
KEY WORDS: Vertigo, Dizziness, Saccadic movements, Smooth pursuit, Magnetic resonance imaging
RIASSUNTO
Allo scopo di analizzare l'efficacia diagnostica dei movimenti oculari saccadici e di inseguimento lento nei pazienti con una vertigine di origine centrale mettendoli a confronto con i riscontri ottenuti alla Risonanza Magnetica, 108 casi sospetti di avere una vertigine di origine centrale sono stati selezionati da una popolazione di 580 pazienti con vertigine. Sono stati valutati i movimenti oculari saccadici e di inseguimento lento mediante l'elettronistagmografia ed i risultati sono stati comparati con i riscontri ottenuti con la Risonanza Magnetica cerebrale. Lo studio dei movimenti oculari saccadici e di inseguimento lento nei pazienti sospetti di avere una vertigine di origine centrale ha messo in evidenza una sensibilità dell'83,3% e una specificità dl 21,2%. Prendendo in considerazione solo le alterazioni gravi dei movimenti saccadici e di inseguimento lento come indicatori di una vertigine di origine centrale questo studio ha rilevato una sensibilità del 71,4% ed una specificità del 50%. In conclusione, lo studio delle alterazioni dei movimenti oculari nei pazienti con una vertigine di sospetta origine centrale ha dimostrato di essere un test con una buona sensibilità, confrontato anche con i risultati ottenuti con la Risonanza Magnetica cerebrale.
Introduction
Dizziness is a sensation of movement referred to the environment or to the individual. It is the main symptom of a disorder in the system of balance. The case history and objective examination may indicate whether the origin of the symptom is central or peripheral; however, in many cases, it is difficult to make this distinction without the use of neuroimaging. It is not practical to have all patients with dizziness of "suspected" central origin undergo magnetic resonance imaging (MRI): hence it is necessary to draw up further criteria of selection, in conjunction with clinical criteria, which make it possible to restrict suspected cases of a central lesion to a limited number of patients who would then undergo brain MRI.
Since dizziness stems from an alteration in a complex system which involves functions of a vestibular, proprioception and visual nature, reports in the literature have often proposed studying eye movements in order to diagnose whether balance disorders are of central origin.
Aims of this retrospective study were to analyse the diagnostic efficiency of the study of saccadic and smooth pursuit movements, compared to findings from brain MRI, in dizzy patients.
Materials and methods
This study comprises 580 outpatients recruited for symptoms of dizziness between January 2004 and July 2008. All the patients underwent vestibular evaluation in accordance with a pre-defined diagnostic procedure: case history, search for spontaneous nystagmus, head-shaking test, positional and positioning tests (Dix-Hallpike's test and Semont's diagnostic manoeuvre), otoscopic examination, caloric tests according to Freyss, objective neurotological examination including static and dynamic postural tests (e.g. Romberg's test, the star shaped test), cerebellar tests (index-nose and heel-knee), evaluation of cranial nerves, of sensitivity and of force, and tonal audiometry.
Dizziness was classified as of central origin if at least one of the following criteria was present: positive case history for TIA of the vertebrobasilar circle in the presence of risk factors for cardiovascular disease and co-existence of a form of dizziness with specific clinical features 1-3; presence of an up-beat or down-beat nystagmus (NY), disassociated NY, alternating NY, unfailing NY; presence of a persistent, stationary positional NY without latency; positive neurological examination; persistent bilateral hyper- or hypo-reflectivity to caloric tests, vestibular decruitment ("a unique phenomenon encountered when a weak vestibular caloric stimulation creates a stronger nystagmic response than a substantially stronger stimulus produces") 4; pathological alteration in the Visual Suppressor Test (VST) 5. Of the 580 cases, dizziness was classified as central in 166 (28.6%), and peripheral in 319 (55%). The 319 cases of peripheral dizziness were then subdivided into benign paroxismal positional vertigo (BPPV) in 253 cases (79.3%), Ménière's disease in 30 cases (9.4%) and vestibular neuronitis in 34 cases (10.65%). In 95 of the 580 patients (16.3%), no definitive diagnosis has yet been reached.
All 166 patients classified as central, besides undergoing the diagnostic procedures described above, also underwent electronystagmography to evaluate saccadic and pursuit eye movements. A digital electronystagmography from Micromedical Technologies Inc. (Chatam, Canada) was used for this purpose. The parameters used to evaluate horizontal saccadic movements were: latency time, maximum peak speed and accuracy. The pursuit system was analysed using the parameter gain which expresses the relationship between the speed of the eye movement and the speed of the target.
Two tests were carried out for the saccades: one with 30 movements of the target, at a fixed range of 10 degrees, and one with 30 movements of the target, at a random range to a maximum of 30 degrees (in order to exclude automatic movements). In the smooth pursuit test, evaluation included at least 3 eye movements for frequencies of 0.1 H z, 6 movements for frequencies of 0.2 H z and 12 movements for frequencies of 0.4 H z of pendular oscillation of the target. In a previous step, the results of saccadic and smooth pursuit eye movements were standardised according to age range; a digital electronystagmography from Micromedical Technologies Inc. (Chatam, Canada) was used for this purpose. Normative data related to age band (18-40 years; 40-60 years; 60-70 years; 70-80 years and > 80 years) that take into consideration latency time, maximum peak speed and accuracy of saccadic movements and the parameter gain, which express the relationship between the speed of the eye movement and the speed of the target, for the smooth pursuit movements. All patients studied for the standardization of data were not affected by vertigo or dizziness. In evaluating the caloric tests, according to Freyss, the normative parameters usually applied in this Insitute were adopted. With regards toVST, a Fixation Index < 50 was considered normal.
Of the 166 central patients, 108 were selected for the study and underwent brain MRI with contrast medium. The remaining 56 cases were excluded from the study since, at the first check-up, carried out 30 days after electronystagmography, the clinical situation had returned to normal and/or on repeating the preliminary investigations obtained with the instrumentation tests and/or clinical evaluation. the situation was seen to have been resolved; for these patients, it was not considered appropriate, or necessary, to carry out brain MRI with contrast medium.
Statistical methods: calculations were made of sensitivity, specificity, the positive and negative predictive factors and the diagnostic accuracy of saccadic and smooth pursuit movements; MRI was taken as reference test (Gold Standard) in order to evaluate eyes movements as a diagnostic test.
Crude sensitivity and specificity were computed separately for each test. The number of valid cases for each combination Test/Gold Standard has been presented, and for continuous covariates the median, first and third quartile have been computed. Sensitivity and specificity have been evaluated for each Test, adjusting for the age of the patients. Adjustment has been performed estimating a GEE model 6 for sensitivity and specificity separately 7, inserting age as a covariate. P values have been reported, and significance assessed with a level reference of 0.05.
The diagnostic procedures adopted were in accordance with the ethical standards of the Committee on Human Experimentation of the Institution or in accordance with the Helsinki Declaration of 1975, as revised in 1983, and the study was approved by an Ethics Committee.
Results and analysis
The study included 108 patients (63 female and 45 male), aged between 18 and 88 years, mean age 68.6 years. The alterations indicative of central vertigo, highlighted during clinical evaluations, are outlined in Table I. The diagnostic procedure was completed with tonal audiometry in all cases, with an Echodoppler of the neck vessels in 12 cases (11.1%) and brainstem electric responses (BSER) in 8 cases (7.4%). All 108 patients in the study, underwent brain MRI. A total of 42 cases out of 108 (38.8%) were classified as positive following MRI (Table II). MRI findings which showed signs of general cerebral atrophy were considered negative (17 cases), whereas MRI findings showing signs of chronic cortical or subcortical ischaemia were considered positive (16 cases) 8 9.
Table I.
Alterations at the clinical evaluation.
| Objective symptomatology of vertigo | 32 (29.6%) |
| Dizzines such as predominant feature: | 49 (45.3%) |
| Positional vertigo persistent | 6 (13.6%) |
| Spontaneous nystagmus | 24 (22.2%) |
| Positional nystagmus with features of centrality | 30 (27.7%) |
| Signs of cerebellar unco-ordination (index-nose test) | 8 (18.1%) |
| Postural instability in gait and erect position (Romberg's test) | 6 (5.5%) |
| Positive for centrality in caloric tests according to Freyss: | 68 (62.9%) |
| - persistent hypereflectivity | 4 (13.7%) |
| - persistent hyporeflectivity | 25 (86.2%) |
| These 68 patients undewent the Visual Suppressor Test: | |
| - alterated Visual Suppressor Test | 40 (58.8%) |
Table II.
Pathologies recorded by neuroimaging (MRI).
| Cortical/subcortical multi-infarction | 16 |
| Cerebellar infarction | 4 |
| Fronto-parietal infarction | 4 |
| Basal ganglia infarction | 4 |
| Meningioma | 2 |
| Temporo-occipital mass lesion | 1 |
| Fronto-parietal mass lesion | 1 |
| Suprasellar mass lesion | 1 |
| Brainstem mass lesion | 1 |
| Cerebellar mass lesion | 2 |
| Arachnoid cyst post cranial fossa | 1 |
| Cerebellar angioma | 1 |
| Right hemisphere oedema | 1 |
| Cerebellopontine atrophy | 1 |
| Thalamic lacunar lesion | 1 |
| Cerebellar haemmorrhage | 1 |
Eye movement responses were then correlated to MRI findings (Table III , 1st and 2nd row). Alterations in eye movements, taken as the finding of at least one parameter being altered among latency, accuracy and speed of saccades and gain of smooth pursuit correlates with a positive MRI in 35 cases (32.4%), whereas in 52 cases (48.1%), the MRI findings were negative. In 14 cases (12.1%), neuroimaging was carried out despite negative eye movement tests because of persistence or changes in the clinical situation and/or instrumentation tests: the results were negative for both tests. On the other hand, there were only 7 cases (6.4%) in which negative saccadic and pursuit results were in conflict with positive neuroimaging findings. The study of eye movements correlated with MRI findings offers a sensitivity of 83.3%; the low specificity (21.2%) is related to the large number of ENG false positives (52 cases).
Table III.
Results of the study of visuo-oculomotor movements.
| MRI + | MRI - | Sens. | Spec. | +PF | -PF | Diagn. Acc. | |
|---|---|---|---|---|---|---|---|
| + Eye movement | 35 (32.4%) | 52 (48.1%) | 83.3 | 21.2 | 40.2 | 66.6 | 45.3 |
| - Eye movement | 7 (6.4%) | 14 (12.9%) | |||||
| + Pursuit | 30 (27.7%) | 39 (36.1%) | 71.4 | 40.9 | 43.4 | 69.2 | 52.7 |
| - Pursuit | 12 (11.1%) | 27 (25.0%) | |||||
| + Saccades | 31 (28.7%) | 44 (40.7%) | 73.8 | 33.3 | 41.3 | 66.6 | 49.0 |
| - Saccades | 11 (10.1%) | 22 (20.3%) | |||||
| Serious + alterations in eye movements | 30 (27.7%) | 33 (30.5%) | 71.4 | 50.0 | 47.6 | 73.3 | 58.3 |
| Serious – alterations in eye movements | 12 (11.1%) | 33 (30.5%) |
Sens.: sensitivity; Spec.: specificity; +PF: positive predictive factor; -PF: negative predictive factor; Diagn. acc.: diagnostic accuracy
The correlations between saccades and smooth pursuit and neuroimaging were then evaluated separately (Table III, 3rd to 6th row). Evaluation of pursuit raises a sensitivity of 71.4%, a specificity of 40.9% and diagnostic accuracy of 52.7% whereas evaluation of saccades shows a slightly higher sensitivity (73.8%), but a lower specificity (33.3%) and accuracy (49.0%) compared to smooth pursuit (Fig. 1). Statistical analysis of the results gave a statistically significant difference only for the parameter specificity (p = 0.0003).
Fig. 1.
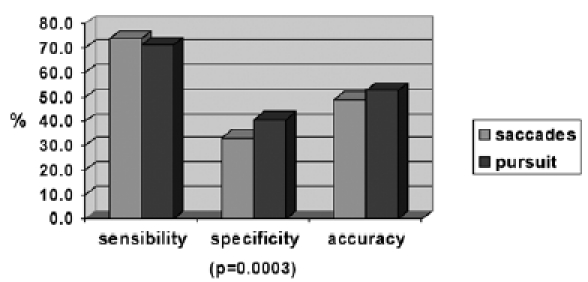
Evaluation of sensitivity, specificity and accuracy of saccadic movements compared to smooth pursuit movements.
Using the two evaluations in conjunction (saccades + smooth pursuit), significantly increases sensitivity from 73.8% to 83.3% (Fig. 2), with a decrease in specificity from 40.9% to 21.2%. When data are analysed, these differences are statistically significant for both parameters (p < 0.001).
Fig. 2.
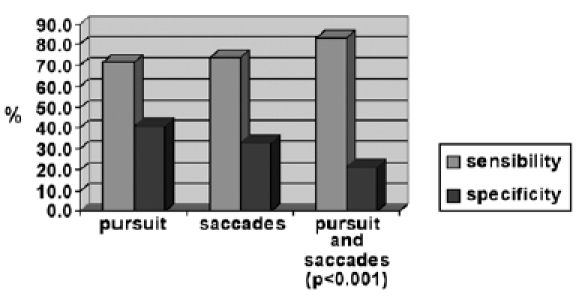
Evaluation of sensitivity and specificity with saccades and smooth pursuit in conjunction compared to single methods.
The entity of the alteration of visuo-oculomotor movements was then subdivided into severe and mild, and the findings of neuroimaging were then correlated to severe alterations in eye movements thereby obtaining new values for sensitivity, specificity and diagnostic accuracy which have been compared to the results shown in Table III (7th to 8th row). For the purpose of this study only severe alterations were taken into consideration. For saccades, it was arbitrarily decided to consider the alteration "severe", in the presence of at least 2 of the 3 parameters (speed, latency and accuracy), with values exceeding the normal limits by at least 20%. For smooth pursuit movements, the alteration was considered severe if gain values exceeded the normal limits by at least 20%. The 12 cases (11.1%) found positive by MRI were without severe alterations in saccades or smooth pursuit, making the method 71.4% sensitive. Compared to the evaluation of both mild and severe oculomotor alterations (Fig. 3), this test is less sensitive (71.4% vs. 83.3%), but more specific (50.0% vs. 21.2%). When data were analysed these differences were statistically significant both for parameter sensitivity (p < 0.001) and parameter specificity (p < 0.001).
Fig. 3.
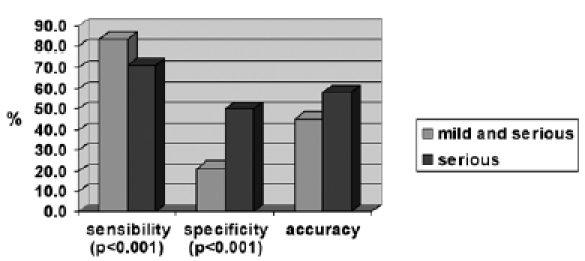
Evaluation of sensitivity, specificity and accuracy of severe alterations in oculomotor movements compared to alterations taken as a whole (serious and mild).
Discussion
In this study, the diagnostic efficiency of saccadic and pursuit eye movements were evaluated in relation to findings from brain MRI in patients with dizziness of a possibly central origin.
Our study arbitrarily assigns a diagnostic value of "gold standard" to MRI rather than eye movement findings: this enabled us to calculate the sensitivity and specificity of electronystagmographic methods compared to neuroimaging. Since a statistically significant correlation was recorded between patient age and positive neuroimaging findings, the sensitivity and specificity parameters were adjusted for the patients' age class: this allowed us to evaluate the results of the oculomotor tests regardless of the effect of the patient's age.
Previous studies, while throwing light on the role of ENG in vestibology, have studied the diagnostic value of visuo- oculomotor movements considering a positive neuroimaging response as a criterion of recruitment for the studies 4 8 10-14. This approach to recruitment, which only includes true positives in the study, makes it impossible to correctly verify the diagnostic sensitivity and specificity of a method. On the contrary, in our study, cases were recruited from a heterogeneous group of 580 outpatients who, at the time of our evaluation exhibited dizziness of an unknown origin, possibly of central or peripheral origin. Using inclusion criteria based on routine clinical practice (case history, search for nystagmus characteristic of central origin, caloric tests, VST, etc.), 166 patients were selected with features suggesting that the dizziness might be of central origin, regardless of whether the findings of neuroimaging, unknown at the time of recruitment, were positive or negative.
The eye movements of these patients were recorded by ENG with the aim of evaluating the diagnostic efficiency of visuo-oculomotor movements in the diagnosis of central dizziness. Among the patients with negative eye movements, 58 were excluded from the study since, at the check-up time, the clinical situation or instrumentation tests had improved or been resolved. Only patients with negative eye movements who, during the check-up, exhibited persistence or deterioration of the symptoms (19 cases) underwent MRI and were then included in the study. This approach of carefully selecting patients probably modified the "crude" values of sensitivity and specificity which can be obtained with oculomotor tests; however, it was not considered advisable, even within the framework of an experimental study, to perform radiological examinations using contrast media on patients whose clinical situation had improved and who did not present alterations in eye movements.
Previous studies reported conflicting values regarding the sensitivity of visuo-oculomotor movements 7-9 15 16, whereas our study showed a high sensitivity of visuo-oculomotor movements (83.3%). The 7 false negative cases could have been caused by organic lesions which can be identified by MRI but do not involve the cerebral areas investigated in functional terms by ENG, hence giving false negatives in oculomotor tests 17. On the other hand, the small number of false negatives observed could be due to exclusion from the study of 58 patients who were negative for visuo-oculomotor movements but did not undergo brain MRI because, at the time of the check-up, they had either improved or recovered: it cannot be ruled out that some cases, albeit negative in clinical and/or instrumental tests at the time of the check up, would have shown radiographic lesions which were not clinically evident, possibly because the patients were already in a phase of clinical - functional recovery.
Regarding the parameter specificity of eye movements reported in our study, this was low (21.2%) compared to the results obtained by Herr who reports a specificity of 92% 15. The large number of false positives in our study (52 out of 108 cases) might be due to a number of reasons. First and foremost, it is possible, as several Authors maintain 17 18, that some proportion of central pathologies, responsible for oculomotor alterations, cannot be identified by neuroimaging. This hypothesis of understaging by neuroimaging would appear to be further validated by the coexistence, in some patients, of electronystagmographic alterations which are associated with alterations found in a neurological examination and a protracted, significant or deteriorating clinical situation. These findings would appear to support the suspicion that there is a central pathology which cannot be pinpointed radiologically.
Regarding the low specificity obtained, it must be pointed out that in a number of cases the results of the recordings of eye movements might have been altered because some patients, especially the elderly, had difficulty in understanding the task to be carried out. Moreover, the many false positives, which characterise results of visuooculomotor movements, might also be correlated with having applied excessively wide criteria for oculographic abnormality. A wide variability in the range of normal responses has been reported in the literature, which at times makes it difficult to evaluate results 19. To investigate the efficiency of the abnormality criteria applied, we decided to re-evaluate the results on the basis of stricter oculographic criteria, i.e., only cases of severe alterations of eye movements were considered positive for ENG (Table II , 7th to 8th row). Compared to a decrease in sensitivity from 83.3% to 71.4% (p < 0.001), there was a significant drop in the number of false positives, from 52 to 33 (specificity 50.0%), with an increase in diagnostic accuracy (Fig. 3). These results suggest that in order to diagnose central vertigo, the study of oculomotor alterations is more efficient if only severe alterations are considered 20. If, on the other hand, we wish to use visuo-oculomotor movements as a screening test, it is preferable, in order to maintain a better sensitivity, to consider positive patients with mild alterations.
With specific regard to saccadic and smooth pursuit movements, several Authors have reported a larger number of alterations in smooth pursuit movements compared to saccades for patients suffering from central lesions 7 9 17 18 21-25. Our experience indicates that evaluation of pursuit shows a similar sensitivity (71.4% vs. 73.8%) but higher specificity compared to evaluation of saccades (40.9% vs. 33.3%) (Fig. 1). Using the two evaluations together (saccades + smooth pursuit) significantly increases sensitivity from 73.8% to 83.3%, making it a more efficient diagnostic method, especially for screening purposes, than the study of single movements (p = 0.0013 and p < 0.001) (Fig. 2).
MRI abnormalities reported in Table I were the findings obtained with MRI in patients with vertigo; however, these abnormalities are not necessarily related to vertigo or any oculomotor system disorder. To clarify this aspect, the lesions reported by MRI were correlated anatomotopographically to the anatomical cerebral structures involved in the electrophysiology of ocular movements. The lesions highlighted by MRI and reported in Table I all involved at least one anatomical subunit correlated to visuoculomotor movements. In particular, the saccadic system depends on higher structures, such as the frontal and parietal lobes, basal ganglia, superior colliculus and cerebellum, which are important to determine how the brain stem circuits, that generate saccades, are triggered. Moreover, other anatomical structures involved are the caudal pons which is important for horizontal saccades and the rostral mesencephalon for vertical saccades 26-28. Smooth pursuit movements, on the other hand, depend on the striate cortex (primary visual cortex-area 17 Brodmann), extra-striate visual areas (such as middle temporal visual area, middle superior temporal visual area and posterior parietal cortex), dorsolateral pontine nuclei, flocculus and dorsal vermis (lobules VI-VII) of the cerebellum, vestibular nuclei, ocular motor nuclei and ocular nerves (III, IV, VI) 29.
Most of the patients (16 cases) had cortical/subcortical multi-infarction and this is often seen in elderly patients as asymptomatic infarct or lacunes; to exclude this, in our study, the parameters of ocular movements were standardised according to age band; hence it is probable that patients' symptoms and altered ocular movements do not depend on age, which has been taken into account, but probably on the lesions highlighted by neuroimaging. The typical alterations in saccade movements, correlated to an advanced age, result in an increase in latency time, lower maximum peak speed and accuracy, and, with regard to pursuit movements, a reduced capacity to reach the speed of movement of the target.
Conclusions
In this study, single parameters which characterized saccadic movements (i.e., latency time, maximum peak speed and accuracy) were not evaluated since they were investigated exclusively together.
So the most important parameters in order to make a diagnosis of central vertigo are:
the study of alterations in oculomotor movements in patients with suspected central lesions proved to be a test with good sensitivity (83.3%);
the diagnostic efficiency of saccadic and pursuit movements is higher if only severe alterations in eye movements are considered indicative of a central origin;
the evaluation of smooth pursuit movements gives a comparable sensitivity but a higher specificity than evaluation of saccades, hence giving a better diagnostic performance;
using the study of saccadic and pursuit movements together provides statistically better results than when the methods are used on their own.
Acknowledgement
Acknowledgment of grant support and of individuals who were directly involved in the preparation of the study: Prof. Giancarlo Tirelli: conception and design of the study, analysis and interpretation of data; revising the study for important intellectual content and final approval of the version to be published.
Dr.ssa Cristina Meneguzzi: analysis and interpretation of data.
Dr. Stefano Rigo: analysis and interpretation of data.
Dr.ssa Federica Bullo: analysis and interpretation of data. Prof. Dario Gregori: statistical analysis of data.
References
- 1.Graad A, Baloh RW. Vertigo of vascular origin. Arch Neurol. 1989;46:281–284. doi: 10.1001/archneur.1989.00520390047014. [DOI] [PubMed] [Google Scholar]
- 2.Nelson JA, Viirre E. The clinical differentation of cerebellar infarction from common vertigo syndromes. West J Emerg Med. 2009;10:273–277. [PMC free article] [PubMed] [Google Scholar]
- 3.Kattah JC, Talkad AV, Wang DZ, et al. Three-step bedside oculomotor examination more sensitive than early MRI diffusion- weighted imaging. Stroke. 2009;40:3504–3510. doi: 10.1161/STROKEAHA.109.551234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mafee MF. CT in the evaluation of the vestibulocochlear nerves and their central pathways. Radiol Clin North Am. 1984;22:45–66. [PubMed] [Google Scholar]
- 5.Pagnini P. Visual suppression test and ocular dysmetria: some electronystagmographic findings relevant to the assessment of size of cerebellopontine angle tumor. Ital J Neurol Sci. 1984;V:177–183. doi: 10.1007/BF02043220. [DOI] [PubMed] [Google Scholar]
- 6.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 7.Ojala M. Electonystagmographic findings among 127 dizzy patients: correlation with the aetiology of dizziness. Clin Otolaryngol. 1989;14:343–348. doi: 10.1111/j.1365-2273.1989.tb00382.x. [DOI] [PubMed] [Google Scholar]
- 8.Silvoniemi P. Electronystagmographic findings in patients with acute cerebral vascular disease and vertigo. Acta Otolaryngol (Suppl) 2000;543:41–43. doi: 10.1080/000164800453919-1. [DOI] [PubMed] [Google Scholar]
- 9.Torn M. Testing the central vestibular factions: a clinical survey. Clin Otolaryngol. 2000;25:298–304. doi: 10.1046/j.1365-2273.2000.00371.x. [DOI] [PubMed] [Google Scholar]
- 10.Dumas G, Charachon R, Hommel M, et al. Apport de l'oculographie et de l'THE.R.M. aux correlations clinico-topographiques dans les accidents vasculaires ischèmiques du tronc cèrèbral. Ann Otolaryngol Chir Cervicofac. 1988;105:47–57. [PubMed] [Google Scholar]
- 11.Mafee MF. Central vestibulocochlear pathology: role of MRI and CT. Scand Audiol (Suppl) 1988;30:153–159. [PubMed] [Google Scholar]
- 12.Hamid A. ENG- MRI correlates in cerebellar oculomotor dysfaction. Otolaryngol Head Neck Surg. 1988;99:302–302. doi: 10.1177/019459988809900307. [DOI] [PubMed] [Google Scholar]
- 13.Munaro G, Ferreira da Silveira A, Garcia Rossi A, et al. Results of brainstem evoked response in patients with vestibular complaints. Braz J Otorhinolaryngol. 2010;76:384–391. doi: 10.1590/S1808-86942010000300019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campos ED, Carceller MA, Guzman RB. Electrooculography. Its value in the diagnosis of the patient with a balance disorder. Acta Otorrinolaringol Esp. 2005;56:12–16. doi: 10.1016/s0001-6519(05)78563-0. [DOI] [PubMed] [Google Scholar]
- 15.Herr RD. Immediate electronystagmography in the diagnosis of the dizzy patient. Ann Emerg Med. 1993;22:1182–1189. doi: 10.1016/s0196-0644(05)80986-2. [DOI] [PubMed] [Google Scholar]
- 16.Kinney WC. Retrospective blinded review of MRI in patients with central electronystagmography findings. Am J Otol. 1998;19:341–344. [PubMed] [Google Scholar]
- 17.Stoddart RL. MRI results in patients with central electronystagmography findings. Clin Otolaryngol. 2000;25:293–297. doi: 10.1046/j.1365-2273.2000.00369.x. [DOI] [PubMed] [Google Scholar]
- 18.Tjell C. Otoneurophysiologic diagnosis of vascular disturbances in the central nervous system. Scand Audiol (Suppl) 1988;30:205–209. [PubMed] [Google Scholar]
- 19.Schalen L. Quantification of tracking eye movements in patients with neurologic disorders. Acta Otolaryngol. 1982;93:387–395. doi: 10.3109/00016488209130896. [DOI] [PubMed] [Google Scholar]
- 20.Schmal F. Value of smooth pursuit evaluation within the scope of clinical computerized nystagmography. HNO. 1997;45:385–388. doi: 10.1007/s001060050114. [DOI] [PubMed] [Google Scholar]
- 21.Lekwuwa GU. Cerebral control of eye movements. The relationship between cerebral lesion sites and smooth pursuit deficits. Brain. 1996;119(Pt 2):473–490. doi: 10.1093/brain/119.2.473. [DOI] [PubMed] [Google Scholar]
- 22.Morrow MJ. Cerebral hemispheric localization of smooth pursuit asymmetry. Neurology. 1990;40:284–292. doi: 10.1212/wnl.40.2.284. [DOI] [PubMed] [Google Scholar]
- 23.Sakata AND. Positional nystagmus of benign paroxysmal type (BPPN) due to cerebellar vermis lesions. Acta Otolaryngol (Suppl) 1991;481:254–257. doi: 10.3109/00016489109131394. [DOI] [PubMed] [Google Scholar]
- 24.Leisenring W. A marginal regression modelling framework for evaluating medical diagnostic test statistics. Stat Med. 1997;16:1263–1291. doi: 10.1002/(sici)1097-0258(19970615)16:11<1263::aid-sim550>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 25.Bertholon P, Bronstein A, Davies RA, et al. Positional down beating nystagmus in 50 patients: cerebellar disorders and possible anterior semicircular canalithiasis. J Neural Neurosurg Psychiatry. 2002;72:366–372. doi: 10.1136/jnnp.72.3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leigh RJ, Zee DS. The neurology of the eye movements. Philadelphia, PA: F.A. Davis Company; 1991. pp. 97–108. [Google Scholar]
- 27.Strupp M, Brandt T. Current treatment of vestibular, ocular motor disorders and nystagmus. Ther Adv Neural Disord. 2009;2:223–239. doi: 10.1177/1756285609103120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johkura K. Central paroxysmal positional vertigo: isolated dizzines caused by small cerebellar hemorrhage. Stroke. 2007;38:26–27. doi: 10.1161/STROKEAHA.106.480319. [DOI] [PubMed] [Google Scholar]
- 29.Leigh RJ, Zee DS. The neurology of the eye movements. Philadelphia, PA: F.A. Davis Company; 1991. pp. 152–158. [Google Scholar]


