SUMMARY
Narrow band imaging and high definition television are recent innovations in upper aero-digestive tract endoscopy. Aim of this prospective, non-randomized, unblinded study was to establish the diagnostic advantage of these procedures in the evaluation of squamous cell cancer arising from various upper aero-digestive tract sites. Between April 2007 and January 2010, 444 patients affected by upper aero-digestive tract squamous cell cancer, or previously treated for it, were evaluated by white light and narrow band imaging ± high definition television endoscopy, both in the pre-/intra-operative setting and during follow-up. Tumour resection was performed taking into account narrow band imaging and high definition television information to obtain histopathologic confirmation of their validity. Endoscopic and pathologic data were subsequently matched to obtain sensitivity, specificity, positive, negative predictive values, and accuracy. Overall, 110 (25%) patients showed adjunctive findings by narrow band imaging ± high definition television when compared to standard white light endoscopy. Of these patients, 98 (89%) received histopatological confirmation. The sensitivity, specificity, positive, negative predictive values, and accuracy for white light-high definition television were 41%, 92%, 87%, 82%, and 67%, for narrow band imaging alone 75%, 87%, 87%, 74%, and 80%, and for narrow band imaging-high definition television 97%, 84%, 88%, 96%, and 92%. The highest diagnostic gain was observed in the oral cavity and oropharynx (25%). Narrow band imaging and high definition television were of value in the definition of superficial tumour extension, and in the detection of synchronous lesions in the pre-/intra-operative settings. These technologies also played an important role during post-treatment surveillance for early detection of persistences, recurrences, and metachronous tumours.
KEY WORDS: Head and neck cancer, Squamous cell cancer, Upper aero-digestive tract, Narrow band imaging, High definition television, Endoscopy
RIASSUNTO
La narrow band imaging e la high definition television rappresentano recenti innovazioni nell'endoscopia delle vie aereo-digestive superiori. Lo scopo di questo studio prospettico, non randomizzato, non cieco, è stato quello di quantificare il guadagno diagnostico di queste metodiche nella valutazione del carcinoma squamocellulare ad origine da diverse sedi delle vie aereo-digestive superiori. Tra aprile 2007 e gennaio 2010, 444 pazienti affetti da carcinoma squamocellulare delle vie aereo-digestive superiori o precedentemente trattati per tale patologia sono stati valutati mediante endoscopia in luce bianca e narrow band imaging ± high definition television, sia in fase pre- ed intra-operatoria, che durante il follow-up. L'exeresi della neoplasia è stata eseguita tenendo in considerazione le informazioni derivanti dalla narrow band imaging e dalla high definition television al fine di ottenere una conferma istopatologica della loro validità. I dati endoscopici ed anatomo-patologici sono stati successivamente messi a confronto al fine di ottenere i relativi valori di sensibilità, specificità, valore predittivo positivo, negativo e accuratezza. Complessivamente, 110 (25%) pazienti hanno ottenuto reperti endoscopici aggiuntivi grazie alla narrow band imaging ± high definition television rispetto a quanto evidenziato dall'endoscopia standard in luce bianca. Tra questi, 98 (89%) hanno avuto una conferma istopatologica di tale dato endoscopico aggiuntivo. I valori di sensibilità, specificità, valore predittivo positivo, valore predittivo negativo ed accuratezza per la luce bianca-high definition television sono stati pari al 41%, 92%, 87%, 82% e 67%, per la sola narrow band imaging pari al 75%, 87%, 87%, 74% e 80% e per la narrow band imaging-high definition television pari al 97%, 84%, 88%, 96% e 92%. Il maggior guadagno diagnostico è stato osservato nell'ambito del cavo orale ed orofaringeo (25%). La narrow band imaging e la high definition television hanno dimostrato la loro efficacia nella definizione dell'estensione superficiale della neoplasia e nell'identificazione di lesioni sincrone, sia in fase pre- che intraoperatoria. Queste metodiche hanno anche svolto un ruolo durante il follow-up post-trattamento per la precoce identificazione di persistenze, recidive e tumori metacroni.
Introduction
Endoscopy is still considered the first-choice for examination and the most reliable way to obtain precise bidimensional assessment of the superficial extension of a squamous cell cancer (SCC) arising within the upper aero- digestive tract (UADT). In fact, its role, as the cornerstone of every diagnostic step, in the evaluation of head and neck cancer patients to be treated or followed-up after any kind of treatment, cannot be overemphasized. Consequently, the pivotal responsibility of the head and neck surgeon in correctly performing this first-line evaluation remains unchanged, both during pre-treatment assessment, as well as throughout all the various phases of the treatment planning, or during post-treatment surveillance, at the end of the treatment itself 1.
The importance of endoscopy, in head and neck surgery, is confirmed by the fact that, in the last two decades, a number of approaches and technological improvements have been introduced to enhance conventional white light (WL) endoscopy of the UADT. Starting from the use of toluidine blue staining 2, up to the most recent light-based detection systems, such as chemiluminescence 3, autofluorescence 4, and narrow band imaging (NBI) 5-8, researchers have focused studies on the identification of specific biologic properties of neoplastic tissues to be targeted by new endoscopic tools. These aids should help the surgeon to establish a more reliable distinction between normal and neoplastic or precancerous mucosa, within a wide range of inflammatory and iatrogenic modifications. Even if direct comparison of the efficacy of these instrumentations is seldom feasible, due to their site-specific application, different advantages and disadvantages, economic constrains, and limited diffusion in daily practice, it is, nevertheless, clear that the future of endoscopy of the UADT SCC will be strongly influenced by these new technologies.
The aim of the present report was to describe personal experience with a light-based detection system, namely NBI, together with high definition television (HDTV) (Olympus Medical System Corporation, Tokyo, Japan). NBI is an optical technique that applies narrow-band spectrum filters to enhance the visualization of mucosal and submucosal microvascular patterns, based on the principle of the different penetration depth of light according to its wavelength. NBI filters select the blue and green light with wavelengths of 415 and 540 nm, respectively, corresponding to the peaks of absorption of haemoglobin. These filtered wavelengths penetrate the superficial layers of the mucosa, thus highlighting the capillary network, and deeper levels, enhancing the sub-mucosal vessels. Furthermore, the best image definition both for conventional WL and NBI endoscopy is achieved with the use of a HDTV camera, which provides 1080 lines of resolution, thus allowing a signal definition that is 4.26 times better than standard endoscopy 6.
Even though the diagnostic value of NBI±HDTV, in the UADT, has already been demonstrated by our group and by others 7-15, its usefulness in the pre-, intra-operative, and post-treatment settings, in relation to the different anatomical sites, has still to be confirmed. The present study was, therefore, designed to prospectively evaluate the role of NBI ± HDTV in these various settings, and to quantify the diagnostic improvement following its use in the assessment of UADT SCC.
Patients and methods
The present not randomized, unblinded, prospective study was carried out, between April 2007 and January 2010, at the Department of Otorhinolaryngology – Head and Neck Surgery of the University of Brescia, Italy. A total of 444 patients (369 males, 75 females; mean age 64 years; range, 27-86) affected by UADT SCC or previously treated for this pathological condition, underwent endoscopic evaluation by WL and NBI ± HDTV by 3 of the Authors (G.P., C.P., and D.C.).
Patients were divided into 2 groups according to the timing of endoscopic evaluation in relation to treatment: a staging group including 170 patients (140 males, 30 females; mean age, 63 years; range, 27-86) submitted to endoscopic evaluation during the hospitalisation period, before surgical treatment, and a follow-up group comprising 274 patients (230 males, 44 females; mean age, 65 years; range, 35-85) evaluated at a minimum of 6 months after treatment (either surgery, RT, or CHT-RT). Among the latter, 77 (28%) patients were evaluated 2 or more times during follow-up for a total of 402 endoscopic examinations.
All patients in the staging group were evaluated pre-operatively by pan-endoscopy of the UADT after local anaesthesia with 1% oxybuprocaine chlorohydrate using a transnasal flexible videoendoscope (ENF-VQ-High Resolution, Olympus Medical Systems Corporation, Tokyo, Japan) connected to an Evis Exera II CLV -180B light source (Olympus Medical Systems Corporation, Tokyo, Japan). At present, this instrumentation cannot be integrated with HDTV for technical reasons, mainly due to miniaturization of the distal microchips (already available for trans-oral and trans-rectal flexible videoendoscopes). The oral cavity and oropharynx were evaluated with trans-oral 0° and 70° rigid endoscopes (Karl Storz, Tuttlingen, Germany) coupled to an Evis Exera II HD TV camera (Olympus Medical Systems Corporation, Tokyo, Japan) by both WL and NBI. All examinations were recorded and stored for subsequent re-evaluation. Attention was focused on the superficial extension of the primary lesion, and special emphasis was given to margins, involvement of adjacent subsites, multifocality, and detection of synchronous lesions of the UADT. According to the literature 8-10 13-16, any well-demarcated brownish area with thick dark spots and/or winding vessels was considered as a positive lesion at NBI (Fig. 1a-b). Furthermore, the presence of one or more afferent hypertrophic vessels branching out in small vascular loops within the lesion was considered as indicative of malignancy (Fig. 2a-b). Intra-operatively, all anatomical sites were endoscopically re-evaluated by WL and NBI using rigid 0° and angled telescopes coupled to a HDTV camera. Surgical resection was performed taking into account pre- and intra-operative information obtained by NBI ± HDTV. All specimens were oriented, stained with black ink on one surgical margin, and submitted to a dedicated pathologist for histopathologic examination in order to obtain specific information regarding the positive areas identified by NBI±HDTV.
Fig. 1.
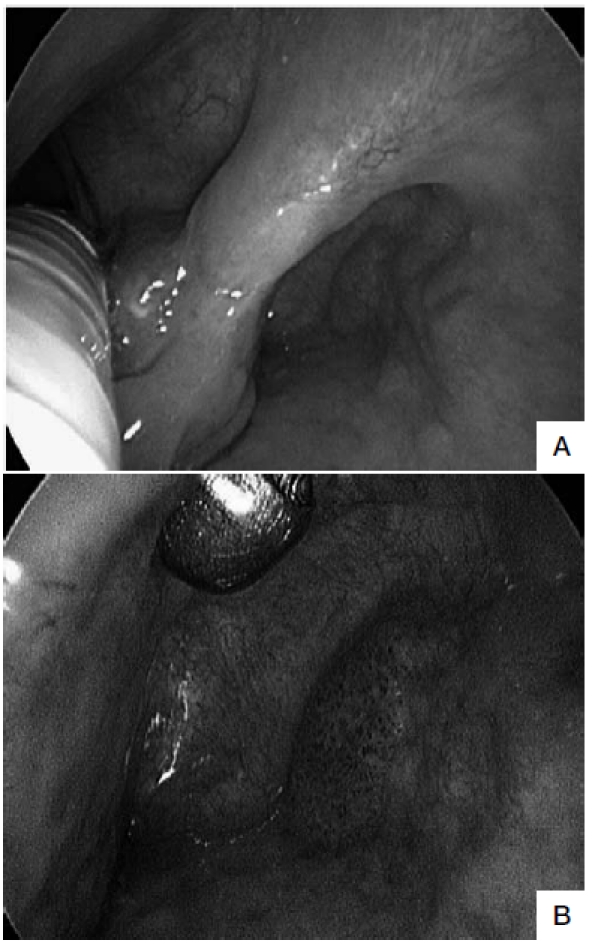
A. Intra-operative HDTV-WL examination by rigid 0° telescope 3 months after trans-oral laser resection of SCC of the right ary-epiglottic fold shows an exophytic lesion at this level. On the lateral wall of the piriform sinus, a second erythroplakia is visible. B. Same patient evaluated by HDTV-NBI (closer view), better enhancing the typical neoangiogenic vascular pattern of the piriform sinus erythroplakia (suspicious for persistence of multifocal disease, then confirmed by histopathologic evaluation of the excisional biopsy specimen to be a micro-invasive carcinoma). In contrast, the exophytic lesion of the ary-epiglottic fold was found to be a granuloma.
Fig. 2.
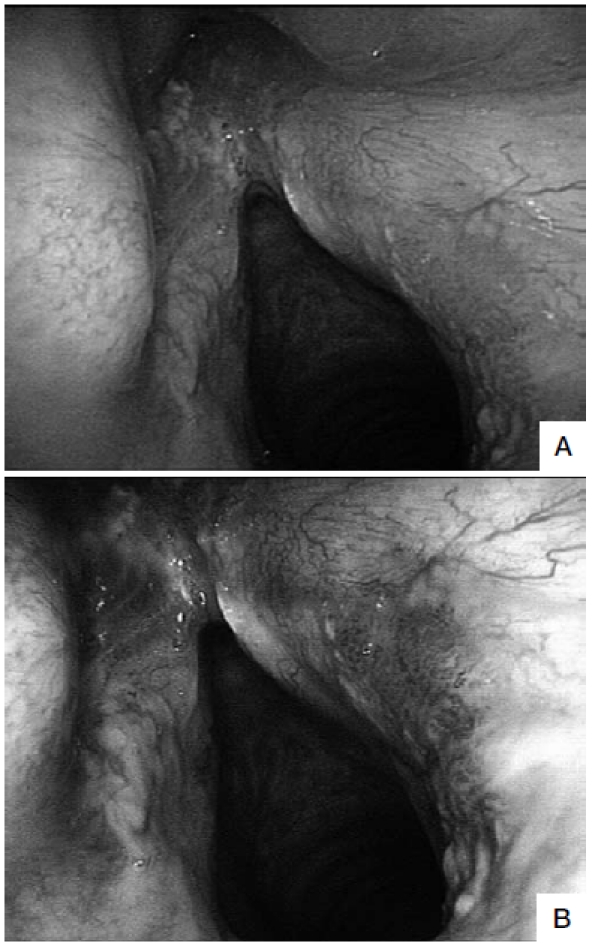
A. Follow-up examination by flexible WL endoscopy 12 months after trans-oral laser extended cordectomy (Type Va) showing a recurrent leucoplakia in the anterior commissure and a diffuse erythroplakia of the posterior third of the right vocal cord (negative at WL examination). B. The same view at flexible NBI videoendoscopy shows that the erythroplakia is a well-demarcated, brownish area with thick dark spots and an afferent hypertrophic vessel that branches out in small vascular loops in the context of the lesion (positive at NBI). Histopathological evaluation of the leukoplakia was consistent with mild dysplasia, while the erythroplakia was found to be a micro-invasive carcinoma.
In the follow-up group, all patients underwent endoscopic evaluation of the UADT by trans-nasal flexible video-endoscopy with WL and NBI at least 6 months after treatment. In the event of more than one endoscopic examination, these were performed with a minimum interval of 6 months. In this group, attention was focused on the early detection of persistent or recurrent disease, and the identification of metachronous lesions. In the event of positive WL and/or NBI findings, patients were scheduled for re-assessment of the UADT under general anaesthesia. In this setting, patients underwent intra-operative HDTVWL and HDTV-NBI endoscopy and excisional biopsy of the positive areas, as described above.
Pre-, intra-operative, and post-treatment videos were reevaluated separately by 3 of the Authors (G.P., C.P., and D.C.) who rated them as positive or not positive. The examination was considered as positive when at least 2 of the 3 Authors were in agreement. All endoscopic evaluations were assessed in relation to the definitive histopathologic report in order to calculate sensitivity (Se) and positive predictive values (PPV). Although lesions judged as negative by NBI±HDTV did not receive histological confirmation, attention was focused on the group of 77 patients submitted to multiple evaluations during follow-up in order to calculate the specificity (Sp), negative predictive value (NPV), and accuracy (Ac) of the technology.
To compare the diagnostic gain, Se, Sp, PPV, NPV, as well as Ac of HDTV-WL and NBI±HDTV findings were analyzed according to the different anatomical sites involved by the SCC. As a result, 2 site-specific subgroups (larynxhypopharynx, oral-oropharyngeal cavities) were obtained and considered separately.
Results
Of the 444 patients in the present cohort, 333 (75%) were affected by SCC of the larynx (L), 68 (15%) of the oral cavity (OC), 29 (7%) of the oropharynx (OP), and 14 (3%) of the hypopharynx (HP). Results obtained by HDTV-WL and NBI±HDTV are recorded according to the 2 site-specific subgroups previously defined.
Larynx-hypopharynx
Of the 347 patients affected by L-HP SCC, 135 (39%) were evaluated in the pre- and intra-operative setting, and 212 (61%) as part of routine oncologic follow-up. Overall, 61 (17%) patients showed additional pre-operative findings with flexible NBI, which are undetectable by standard WL. HDTV-NBI showed adjunctive findings in 86 (25%) patients, while only 34 (9%) of these were also evident upon HDTV-WL evaluation. Overall, 74 (86%) of the 86 lesions judged as positive at HDTV-NBI were histologically confirmed as moderate to severe dysplasia in 36 cases, micro-invasive carcinoma in 25, and invasive carcinoma in 13. The remaining 12 (14%) positive lesions were found to be false positives.
From a practical point of view, use of HDTV-NBI in L-HP showed a diagnostic gain (compared to standard WL endoscopy) in 21% of patients. This was in terms of better definition of the tumour category (upstaging of 33 neoplasms), superficial surgical margins (13 patients), and detection of synchronous tumours (2 patients), recurrences/ persistences (22 patients), and metachronous lesions (4 patients). The Se, Sp, PPV, NPV, and Ac for the L-HP sites are outlined in Table I.
Table I.
Se and PPV of NBI, HDTV-NBI, and HDTV-WL endoscopy calculated for the entire cohort of patients. Sp, NPV and Ac of NBI, HDTV-NBI, and HDTV-WL calculated for patients submitted to multiple evaluations during follow-up.
| L-HP % (n= 347) |
O-OP % (n= 97) |
Entire cohort % (n= 444) |
||
|---|---|---|---|---|
| Se | NBI | 69 | 96 | 75 |
| HDTV-NBI | 98 | 96 | 97 | |
| HDTV-WL | 39 | 56 | 41 | |
| Sp | NBI | 87 | 98 | 87 |
| HDTV-NBI | 83 | 98 | 84 | |
| HDTV-WL | 93 | 100 | 92 | |
| PPV | NBI | 85 | 96 | 87 |
| HDTV-NBI | 86 | 96 | 88 | |
| HDTV-WL | 85 | 100 | 87 | |
| NPV | NBI | 73 | 98 | 74 |
| HDTV-NBI | 98 | 98 | 96 | |
| HDTV-WL | 59 | 87 | 82 | |
| Ac | NBI | 78 | 97 | 80 |
| HDTV-NBI | 91 | 97 | 92 | |
| HDTV-WL | 65 | 89 | 67 |
L-HP: larynx-hypopharynx; O-OP: oral cavity-oropharynx.
Oral cavity-oropharynx
Of the 97 patients affected by O-OP SCC, 35 (36%) were evaluated in the pre- and intra-operative setting, and 62 (64%) as part of their surveillance protocol. Overall, 25 (26%) patients showed adjunctive findings during pre-operative NBI endoscopy that were undetectable by standard WL evaluation. HDTV-NBI confirmed these findings in all patients, but only 13 (52%) were also evident during HDTV-WL. A total of 24 (96%) of the 25 lesions judged as positive at HDTV-NBI were histologically confirmed as moderate to severe dysplasia in 13 cases, micro-invasive carcinoma in 9, and invasive carcinoma in 2. Only one patient (4%) was found to be a false positive.
The application of HDTV-NBI, in the O-OP, led to a diagnostic gain for 25% of the patients in this subgroup. In particular, the technique was useful to better define superficial margins (6 patients), detect synchronous tumours (4 patients), identify tumour progression in surveillance of pre-cancerosis (3 patients), early diagnosis of persistences/ recurrences (7 patients), and metachronous tumours (5 patients). The Se, Sp, PPV, NPV, and Ac for O-OP are shown in Table I.
Entire cohort of patients
Overall, of the 110 patients with positive lesions at NBI ± HDTV, 98 (89%) were confirmed to be true positives, while 12 (11%) were false positives. Therefore, the overall diagnostic gain in the present study was 22%.
Of the 288 endoscopic examinations judged as negative (and, therefore, not submitted to excisional biopsy with consequent histopathologic evaluation), we considered as true negatives 72 (25%) persistently negative patients, based on the negative findings at NBI evaluations, repeated at 6-month intervals. In contrast, 3 negative cases (1%) were found to be false negatives during the same followup period. Of these, 2 (66%) became positive 6 months after the first endoscopic examination and were confirmed as carcinoma in situ (one in the L and another in the OC) while one (33%) was pre-operatively considered to be a benign lesion even though histologically diagnosed as a micro-invasive carcinoma. The Se, Sp, PPV, NPV, and Ac for the entire cohort of patients are outlined in Table I.
Discussion
Despite the progress made in the diagnosis and treatment of the UADT SCC, the advanced stages of disease frequently encountered at the first clinical consultation, and the not negligible rates of local-regional persistence/ recurrence and distant metastases still have a negative impact on patient survival 17. In addition, according to the "field cancerization" phenomenon, multiple SCC frequently occur within the UADT, either synchronously or metachronously, resulting in a definite reduction in overall survival 18.
Panendoscopy of the UADT offers a higher diagnostic rate of superficial synchronous lesions compared to either physical examination or routine radiological investigations 19. However, the possibility of standard WL endoscopy detecting lesions, at an earlier stage, can be extremely difficult in some subsites of the UADT (e.g., OP and HP) even after many repeated manoeuvers of the endoscope by experienced physicians 7. This problem becomes even more evident when considering the post-treatment scenario, particularly if iatrogenic and actinic changes contribute together in masquerading potential persistences/recurrences. In this perspective, NBI has been demonstrated to significantly improve the efficacy of screening, initial evaluation, and surveillance of head and neck cancer, even in areas traditionally considered "difficult" to adequately assess by means of endoscopy, or after organ preservation protocols 7 8 13-15.
Muto et al. 7 were the first to recognize the potential advantages of NBI in otolaryngology. In particular, during endoscopic post-treatment surveillance of patients, previously treated for oesophageal cancer, using this instrumentation they were able to identify 34 metachronous lesions in OC, OP, and HP (only 5 of which were also evident by means of standard WL endoscopy). Since then, many groups, from independent institutions, have confirmed these encouraging findings in prospective series of patients 8 10-15.
The main end-point of the present study was, therefore, to compare the diagnostic gain of NBI and HDTV in the different sites of the UADT, thus confirming, on a large series, the overall accuracy of these techniques already observed by our group 13-15. For example, in the O-OP sites, NBI is always feasible in conjunction to HDTV, both in the pre- and post-treatment settings, even under local anaesthesia: this translates into a diagnostic gain of 25%, with early detection of synchronous and metachronous UADT tumours (9.3%), as well as of early persistences/ recurrences (7.2%). Moreover, in these anatomical sites, we observed the highest values of Se, Sp, PPV, NPV, and Ac, compared to other UADT sites.
Watanabe et al. 8 11 were the first to report that the use of NBI endoscopy in the assessment of laryngeal cancer leads to early detection of abnormal microvascular changes and is useful in distinguishing between low- and high-grade dysplasia (with Se and Sp rates of 91.3% and 91.6%, respectively). Our results confirm these data, since the application of NBI in the pre-operative setting allowed the detection of 52 lesions that were not visible at routine WL endoscopy. Nonetheless, in the L-HP sites, we observed that NBI reaches the highest diagnostic accuracy during intra-operative rigid endoscopy when coupled with a HDTV camera. In this setting, the rate of Se significantly improved from 69% (without HDTV) to 98%. The application of NBI and HDTV in the L-HP sites, with its diagnostic gain set at 21%, showed the greatest usefulness in the better definition of neoplastic superficial spreading, with consequent improvement in control of the peripheral narrow-margin obtained by trans-oral microsurgical resection of glottic and supra-glottic early tumours. Future improvements in technology, in terms of distal microchip miniaturization, should further improve the accuracy of NBI even under local anaesthesia, both in the pre- and post-treatment scenarios.
Although most Authors agree that NBI, with or without HDTV, seems to be a very promising diagnostic tool, the method certainly has some limitations. The most relevant is the possibility of generating, at least in the early phase of the learning curve, an increased number of false positives with consequently unnecessary biopsies. This fact is related primarily to acute or chronic inflammation as well as to post-actinic changes. However, Nonaka et al. 9 found that although intraepithelial papillary capillary loops can be modified by inflammation, it is generally possible to distinguish them from those observed in neoplastic lesions on the basis of their ill-defined margins and relatively low density. Recently, Lin and Wang 20 showed that even though post-actinic mucosal fibrosis, nasopharyngitis, and osteoradionecrosis can mimic recurrences during endoscopic follow-up of nasopharyngeal SCC patients, treated by RT, NBI can reliably distinguish them from early recurrences compared to conventional WL endoscopy. In a previous study from our group, we found that NBI allows a 20% higher detection rate of persistences/recurrences in patients affected by UADT SCC and previously treated by RT and/or CHT-RT. The high specificity of NBI helped to significantly reduce the number of unjustified biopsies. Moreover, our results showed that there is no statistically significant difference between the NBI false positive rate after RT or CHT-RT compared to that of a cohort of untreated patients 15. In contrast, we found that the major factor influencing the rate of false positives, at NBI, was related to our learning curve, being highest in the first 6 months of use and negligible in the last 6 months.
The second potential drawback of NBI concerns the estimation of its genuine Sp. In fact, it would be both nonethical and unfeasible to perform random biopsies in every patient with negative WL and NBI examinations. In order to overcome this limit, we judged as true negatives only those patients who underwent more than one endoscopic NBI evaluation during their follow-up and were found to be persistently negative.
In conclusion, although further technological advancements, such as the possibility to incorporate HDTV in a flexible trans-nasal videoendoscope are needed in order to optimize the diagnostic gain of NBI in the L-HP sites, its overall Sp, as well as NPV and Ac, allows us to consider this endoscopic tool as an essential aid in diagnosis, treatment, and post-therapeutic surveillance of UADT SCC.
Footnotes
Presented at the 90th Annual Meeting of the American Broncho-Esophagological Association April 28-29, 2010, Las Vegas, Nevada, USA
References
- 1.Wong RJ, Shaha JP. The role of the head and neck surgeon in contemporary multidisciplinary treatment programs for advanced head and neck cancer. Curr Opin Otolaryngol Head Neck Surg. 2010;18:79–82. doi: 10.1097/MOO.0b013e32833782f0. [DOI] [PubMed] [Google Scholar]
- 2.Niebel HH, Chomet B. In vivo staining test for delineation of oral intraepithelial neoplastic change: preliminary report. J Am Dent Assoc. 1964;68:801–806. doi: 10.14219/jada.archive.1964.0190. [DOI] [PubMed] [Google Scholar]
- 3.Ram S, Siar CH. Chemiluminescence as a diagnostic aid in the detection of oral cancer and potentially malignant epithelial lesions. Int J Oral Maxillofac Surg. 2005;34:521–527. doi: 10.1016/j.ijom.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Zellweger M, Grosjean P, Goujon D, et al. In vivo autofluorescence spectroscopy of human bronchial tissue to optimize the detection and imaging of early cancers. J Biomed Opt. 2001;6:41–51. doi: 10.1117/1.1332774. [DOI] [PubMed] [Google Scholar]
- 5.Gono K, Obi T, Yamaguchi M, et al. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt. 2004;9:568–577. doi: 10.1117/1.1695563. [DOI] [PubMed] [Google Scholar]
- 6.Uedo N, Ishihara R, Iishi H, et al. A new method of diagnosing gastric intestinal metaplasia: narrow-band imaging with magnifying endoscopy. Endoscopy. 2006;38:819–824. doi: 10.1055/s-2006-944632. [DOI] [PubMed] [Google Scholar]
- 7.Muto M, Nakane M, Katada C, et al. Squamous cell carcinoma in situ at oropharyngeal and hypopharyngeal mucosal sites. Cancer. 2004;101:1375–1381. doi: 10.1002/cncr.20482. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe A, Taniguchi M, Tsujie H, et al. The value of narrow band imaging endoscope for early head and neck cancers. Otolaryngol Head Neck Surg. 2008;38:446–451. doi: 10.1016/j.otohns.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 9.Nonaka S, Saito Y. Endoscopic diagnosis of pharyngeal carcinoma by NBI. Endoscopy. 2008;40:347–351. doi: 10.1055/s-2007-995433. [DOI] [PubMed] [Google Scholar]
- 10.Ugumori T, Muto M, Hayashi R, et al. Prospective study of early detection of pharyngeal superficial carcinoma with the narrowband imaging laryngoscope. Head Neck. 2009;31:189–194. doi: 10.1002/hed.20943. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe A, Taniguchi M, Tsujie H, et al. The value of narrow band imaging for early detection of laryngeal cancer. Eur Arch Otorhinolaryngol. 2009;266:1017–1023. doi: 10.1007/s00405-008-0835-1. [DOI] [PubMed] [Google Scholar]
- 12.Lin YC, Watanabe A, Chen WC, et al. Narrowband imaging for early detection of malignant tumors and radiation effect after treatment of head and neck cancer. Arch Otolaryngol Head Neck Surg. 2010;136:234–239. doi: 10.1001/archoto.2009.230. [DOI] [PubMed] [Google Scholar]
- 13.Piazza C, Cocco D, Benedetto L, et al. Narrow band imaging and high definition television in the assessment of laryngeal cancer: a prospective study on 279 patients. Eur Arch Otorhinolaryngol. 2010;267:409–414. doi: 10.1007/s00405-009-1121-6. [DOI] [PubMed] [Google Scholar]
- 14.Piazza C, Cocco D, Bon F, et al. Narrow band imaging and high definition television in evaluation of oral and oropharyngeal squamous cell cancer: a prospective study. Oral Oncol. 2010;46:307–310. doi: 10.1016/j.oraloncology.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 15.Piazza C, Cocco D, Benedetto L, et al. Role of narrow-band imaging and high-definition television in the surveillance of head and neck squamous cell cancer after chemo- and/or radiotherapy. Eur Arch Otorhinolaryngol. 2010;267:1423–1428. doi: 10.1007/s00405-010-1236-9. [DOI] [PubMed] [Google Scholar]
- 16.Katagiri A, Fu KI, Sano Y, et al. Narrow band imaging with magnifying colonoscopy as diagnostic tool for predicting histology of early colorectal neoplasia. Aliment Pharmacol Ther. 2008;27:1269–1274. doi: 10.1111/j.1365-2036.2008.03650.x. [DOI] [PubMed] [Google Scholar]
- 17.Lingen MW, Kalmar JR, Karrison T, et al. Critical evaluation of diagnostic aids for the detection of oral cancer. Oral Oncol. 2008;44:10–22. doi: 10.1016/j.oraloncology.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slaughter DP, Southwick HW, Smejakal W. Field cancerization in oral stratified squamous epithelium: clinical implications of multicentric origin. Cancer. 1953;6:963–968. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 19.Kerawala CJ, Bisase B, Lee J. Panendoscopy and simultaneous primary tumours in patients presenting with early carcinoma of the mobile tongue. Br J Oral Maxillofac Surg. 2009;47:363–365. doi: 10.1016/j.bjoms.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Lin YC, Wang WH. Narrow-band imaging for detecting early recurrent nasopharyngeal carcinoma. Head Neck. 2011;33:591–594. doi: 10.1002/hed.21310. [DOI] [PubMed] [Google Scholar]


