Abstract
The misfolding and aggregation of specific proteins is a seminal occurrence in a remarkable variety of neurodegenerative disorders. In Alzheimer’s disease (the most prevalent cerebral proteopathy), the two principal aggregating proteins are β-amyloid (Aβ) and tau. The abnormal assemblies formed by conformational variants of these proteins range in size from small oligomers to the characteristic lesions that are visible by optical microscopy, such as senile plaques and neurofibrillary tangles. Pathologic similarities with prion disease suggest that the formation and spread of these proteinaceous lesions might involve a common molecular mechanism – corruptive protein templating. Experimentally, cerebral β-amyloidosis can be exogenously induced by exposure to dilute brain extracts containing aggregated Aβ seeds. The amyloid-inducing agent probably is Aβ itself, in a conformation generated most effectively in the living brain. Once initiated, Aβ lesions proliferate within and among brain regions. The induction process is governed by the structural and biochemical nature of the Aβ seed, as well as the attributes of the host, reminiscent of pathogenically variant prion strains. The concept of prion-like induction and spreading of pathogenic proteins recently has been expanded to include aggregates of tau, α-synuclein, huntingtin, superoxide dismutase-1, and TDP-43, which characterize such human neurodegenerative disorders as frontotemporal lobar degeneration, Parkinson’s/Lewy body disease, Huntington’s disease, and amyotrophic lateral sclerosis. Our recent finding that the most effective Aβ seeds are small and soluble intensifies the search in bodily fluids for misfolded protein seeds that are upstream in the proteopathic cascade, and thus could serve as predictive diagnostics and the targets of early, mechanism-based interventions. Establishing the clinical implications of corruptive protein templating will require further mechanistic and epidemiologic investigations. However, the theory that many chronic neurodegenerative diseases can originate and progress via the seeded corruption of misfolded proteins has the potential to unify experimental and translational approaches to these increasingly prevalent disorders.
Keywords: Alzheimer’s disease, amyloid, amyotrophic lateral sclerosis, frontotemporal lobar degeneration, Huntington’s disease, inclusions, neurofibrillary tangles, Parkinson’s disease, prion, proteopathy, senile plaques, tauopathy
It is a concept that is compelling in its power and simplicity: the misfolding and aggregation of specific proteins underlies many of the chronic neurodegenerative diseases that afflict aging humans. But what initiates this injurious cascade, and how does the pathology ramify throughout the nervous system? A wave of recent research, initially driven by tantalizing pathologic similarities between prion diseases and Alzheimer’s disease (AD)1-3, has begun to address these questions, and the findings increasingly implicate corruptive protein templating, or seeding, as a prime mover of the neurodegenerative process. The prion-like corruption of proteins may also be involved in the pathogenesis of such clinically and etiologically diverse neurological disorders as Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis, frontotemporal lobar degeneration, and chronic traumatic encephalopathy. Currently there is no evidence that these maladies are infectious in the same sense as are prion diseases. However, understanding protein misfolding and aggregation could reveal general principles, and thus similar therapeutic targets, of a pathogenic mechanism that impels some of the most devastating diseases of the elderly, AD foremost among them.
The proteopathic basis of Alzheimer’s disease
Alois Alzheimer linked senile plaques and neurofibrillary tangles to the dementia of AD over a century ago, but fundamental insights into the pathogenesis of the disorder emerged only with the identification of the principal proteins that comprise these lesions: Aβ in senile plaques and cerebral amyloid angiopathy (CAA), and tau in neurofibrillary tangles4 (for historical references see5). Whereas the degree of tauopathy correlates strongly with cognitive decline in AD6-8, genetic, pathologic and biochemical evidence implicates the aggregation of Aβ as a critical, early trigger in the chain of events that leads to tauopathy, neuronal dysfunction and dementia9.
Advancing age is the most prevalent risk factor for the accumulation of Aβ in the brain10, 11, possibly because of the decline of cellular protein quality control processes12. All firmly established genetic risk factors for AD promote the buildup of Aβ, either by increasing its production, promoting its aggregation, or impeding its elimination9, 13. Recent longitudinal imaging studies indicate that cerebral Aβ deposition precedes the clinical symptoms of AD by a decade or more4. How Aβ aggregates impair neuronal function remains uncertain, but evidence is growing that oligomeric forms of the protein, which can range in size from dimers to dodecamers or larger14-18, are more deleterious to brain function than are histologically obvious Aβ lesions such as senile plaques and CAA. Moreover, at least some of the Aβ toxicity appears to be tau-dependent19, 20.
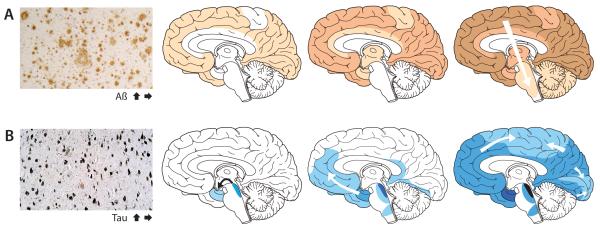
Cross-sectional analyses of postmortem human brains reveal a characteristic progression of β-amyloid plaques and a highly stereotypical appearance of neurofibrillary tangles (Fig 1). β-amyloid plaques develop first in the neocortex, followed by the allocortex and then the subcortex, and the progression of their appearance often corresponds to functionally and anatomically coupled brain regions21-23. Neurofibrillary tangles first arise in the locus coeruleus and entorhinal/limbic brain areas, and then spread to interconnected neocortical regions8, 24. The pattern that emerges from these studies implicates neuronal transport and synaptic exchange mechanisms in the spread of AD lesions within the brain24-26. Overall, the incidence of plaques and tangles correlates positively in AD, but a consistent anatomical relationship between the lesions is not apparent. Imaging ligands that bind selectively to pathogenic protein deposits in vivo, such as the β-amyloid binding agent Pittsburgh Compound B27, will increasingly enable the longitudinal analysis of lesion spread in AD patients28.
Figure 1. The accumulation of misfolded proteins in AD follows characteristic and predictable patterns.
Cross-sectional autopsy studies indicate that β-amyloid plaques (A) first appear in the neocortex, followed by the allocortex and finally subcortical regions21. In the brain, neurofibrillary tangles (B) occur first in the locus coeruleus and transentorhinal area and then spread to the amygdala and interconnected neocortical brain regions8, 24. The relatively stereotyped patterns of expansion suggest the involvement of neuronal transport mechanisms in the spread of proteopathic seeds. Increasing density of shading indicates increasing pathology. The schemata with the progression of the Aβ and tau lesions have been modified from previous publications21.
Misfolded Proteins as Infectious Seeds: Prion Diseases
In humans, the prion diseases (spongiform encephalopathies) are relatively uncommon but uniformly fatal neurodegenerative disorders that include Creutzfeldt-Jakob disease (CJD), variant CJD, fatal familial insomnia, Gerstmann-Sträussler-Scheinker Syndrome and Kuru. In other mammals, spongiform encephalopathies can be more prevalent; the nonhuman prionoses include scrapie, chronic wasting disease, bovine spongiform encephalopathy (BSE), transmissible mink encephalopathy, and others29-31. Prion diseases are unusual in that they can be genetic, idiopathic, or infectious (transmissible) in origin29. Experimentally, prion disease has been transmitted to a broad range of species and models29, 32, 33. Fortunately, the transmission of prion disease to humans is unusual, having occurred in fewer than 700 known cases, most under extraordinary circumstances such as treatment with human growth hormone derived from cadaveric pituitary glands, or in conjunction with the BSE outbreak that peaked in the late 20th century34.
Prions ‘infect’ via an unconventional mechanism whereby misconformed, β-sheet-rich prion protein induces the templated misfolding of other prion protein molecules29, 30, 35-37. The biological functions of the normal (‘cellular’) prion protein (PrPC) remain indeterminate, but it is not essential for survival38. In the disease state, the replication of infectious particles is sustained because cells continually produce PrPC 29, 30, which serves as the raw material for templated conversion to the pathogenic form (‘PrP Scrapie’, or PrPSc). The conformationally corrupted molecules are predisposed to self-aggregation, in which form they can become injurious to neurons29, 30. Though the mechanism of spread remains uncertain, there is evidence that prions can be conveyed between neurons by trans-synaptic transport39. Clinically, pathologically, and molecularly, the prion diseases exhibit variability suggestive of polymorphic and polyfunctional strains of the agent35-37, 40. While prion disease is the only demonstrably infectious cerebral proteopathy, mounting evidence implicates prion-like molecular mechanisms in the initiation and spread of a variety of neurological and systemic diseases39, 41-45. This emerging principle of pathogenesis has the potential to unify experimental and therapeutic approaches to these seemingly disparate disorders.
Induction and spread of Aβ aggregates in experimental animals
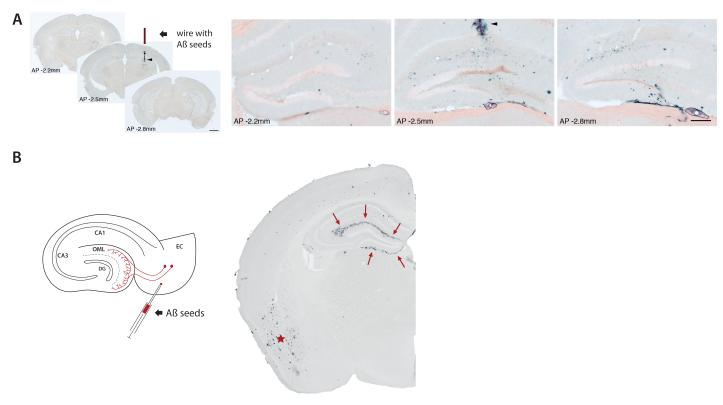
The pathologic similarities between prion disease and AD have long engendered speculation that AD might be inducible in a prion-like manner1-3. Early, long-term studies in nonhuman primates reported evidence both against46 and for47, 48 the exogenous inducibility of senile plaques. The introduction of transgenic mouse models of β-amyloidosis has provided a more efficient and definitive means of testing the hypothesis that AD-like lesions can be seeded in vivo. Studies in our laboratories and others have shown that the deposition of Aβ can be instigated in the brains of Aβ precursor protein (APP)-transgenic mice by the intracerebral infusion of brain extracts containing minute amounts of aggregated (multimeric) Aβ49-53 (Fig 2).
Figure 2. Induction and spread of Aβ lesions in a transgenic mouse model.
(A) Stainless-steel wire segments were coated with Aβ-rich brain extract, dried, and implanted unilaterally into the hippocampus of APP23 transgenic mice. Four months later, immunohistochemical analysis with an anti-Aβ-antibody revealed strong local induction of Aβ-deposits in the vicinity of the wire (arrowhead in the middle section of three coronal sections along the anterior-posterior (AP) axis through the hippocampus). Higher magnification of the dentate gyrus double-stained with anti-Aβ-antibody and Congo red revealed spreading of Aβ-deposition thoughout the dentate gyrus (distance between the sections shown: 600μm). Reproduced from52 with permission. (B) The injection of Aβ-rich brain extracts induces Aβ aggregation within the injected brain region, as shown here for the entorhinal cortex (EC) in APP23 transgenic mice (asterisk). However EC injections also induce β-amyloid deposition (arrows) in the outer molecular layer (OML) of the hippocampal dentate gyrus (DG), a region that is non-contiguous but is axonally interconnected with the injection site. For details of the methods, see52.
Several lines of evidence49-52 collectively argue that aggregated Aβ in the brain extract is critical for in vivo seeding: (1) The extract is able to seed only if it contains aggregated, human-sequence Aβ; (2) Immunoneutralization/depletion of Aβ in the extract by anti-Aβ antibodies impedes seeding; (3) Seeding of Aβ deposition is ineffective in non-transgenic mice, which (because of three amino acid differences from human-sequence Aβ) do not develop cerebral β-amyloidosis; (4) The phenotype of the induced Aβ deposits mirrors that of the deposits in the extract, suggesting an Aβ-templating mechanism; (5) Aβ-rich brain extracts from transgenic mice seed as effectively as do AD brain extracts, ruling out such factors as a cross-species immune reaction or human-specific microbes; and (6) Seeding can be abrogated by denaturation of the extract with formic acid. Recent findings reveal that the β-amyloid-inducing seeds do not consist of a single type of Aβ aggregate, but rather can occur as proteinase K (PK)-resistant species in the pellet fraction and as a soluble, PK-sensitive species in the 100,000×g soluble fraction54. Sonication of the extract, and thus presumed fragmentation of the “insoluble” Aβ seeds into smaller “soluble” Aβ multimers, enhances seeding, and thus hints at a continuum of Aβ aggregates of various sizes that can act as amyloid-inducing agents54.
The induction of Aβ deposits following injection of Aβ-rich brain extracts initially is most evident within the injected brain area. However there is also spreading between non-contiguous but axonally interconnected regions50, 52 (Fig 2), suggesting that seeds can migrate along defined neuronal pathways. Interestingly, seed placement in one region also can foster CAA in separate locations51, implicating perivascular fluid drainage channels55 and/or vascular transport mechanisms in the dissemination of the seeds51. Moreover, the intraperitoneal injection of Aβ-rich brain extracts into APP-transgenic mice induces β-amyloidosis in the brain after prolonged incubation56. How the seeds travel from the periphery to the brain in this model is an important open question.
Aggregated synthetic Aβ thus far has not induced significant cerebral Aβ-deposition in APP-transgenic mice51. The failure of seeding by synthetic Aβ was not unanticipated, in that the induction of prion disease also has been difficult to achieve with PrP generated in vitro57. It is possible that the aggregation of synthetic Aβ under suitable (but as yet undefined) conditions will yield a more effective in vivo seed, as was recently demonstrated for PrP58. The differential seeding ability of synthetic vs. natural Aβ aggregates (which may contain additional factors) suggests the possibility that Aβ, like prions, can misfold into polymorphic and polyfunctional strains51, 59-62.
Induction and spread of tau filaments in experimental animals
Using a paradigm similar to in vivo Aβ-seeding, neurofibrillary (tau) tangles can be exogenously induced by the intracerebral infusion of brain extract containing abnormal tau filaments into mice bearing a human tau transgene63. This finding is remarkable in that the host (Alz17 mouse) expresses non-mutant human tau and does not normally develop tau filaments; moreover, the seeded tangles, unlike Aβ plaques and CAA, are intracellular. The induction of tauopathy is time- and brain region-dependent, and immunodepletion of tau from the donor brain extract prevents seeding63. Initially, the induced tau filaments are confined to the injected brain region, but over time, they extend to neighboring and/or axonally connected areas, suggesting directed spreading of seeds by neuronal transport processes. Although final evidence is still lacking that the in vivo induction of tau occurs via prion-like corruptive protein templating (versus, for example, activation of a signaling cascade that promotes tau aggregation), in vitro studies favor a prion-like mechanism64-66. Specifically, (1) aggregated tau is taken up via endocytosis from the cell medium and can induce the aggregation of soluble, endogenous tau in cells; (2) tau aggregates can transfer among co-cultured cells; and (3) distinct conformational properties of recombinant tau fibrils can be propagated. The latter finding, albeit achieved in a cell-free system65, is intriguing because tau inclusions characterize a variety of sporadic and genetic neurodegenerative diseases in which the aggregates display polymorphic conformations67, 68. Thus, as with other proteopathies, distinct tau strains may explain the pathogenic and phenotypic variations among the tauopathies45.
The coexistence of Aβ- and tau-lesions in AD still lacks a mechanistic explanation. Experiments in transgenic mice have shown that tauopathy can be augmented by aggregated, synthetic Aβ69 or by Aβ-rich brain extracts70. While this phenomenon may result from the direct cross-seeding of tau by aggregated Aβ71, 72, indirect pathways such as Aβ-induced tau phosphorylation, inflammation, and/or disruption of proteostasis20, 73-75 have not been ruled out.
Induction and spread of protein aggregates linked to other neurodegenerative diseases
Increasing evidence implicates the templated corruption of disease-specific proteins in other neurodegenerative diseases. In the brain, the α-synuclein-rich lesions that typify Lewy body disease/Parkinson’s disease first arise in the lower brainstem (notably the dorsal motor nucleus of the vagus nerve), and in the anterior olfactory nucleus and the olfactory bulb; they subsequently appear in a predictable sequence in mesencephalic and neocortical regions76, 77. The concept that α-synuclein lesions ramify within the CNS by a seeding-like process is bolstered by the observation that fetal dopaminergic neural transplants in the striatum of Parkinsonian patients can eventually exhibit α-synuclein-positive Lewy bodies in some cells, implying that synuclein seeds propagate from the host to the graft78, 79. In support of this observation, neural grafts placed into transgenic mice expressing human α-synuclein take up the human protein and form synuclein-positive aggregates80-82. The in vivo approaches in these studies could not discriminate between a prion-like corruptive templating mechanism, i.e. host-derived, misfolded α-synuclein inducing the misfolding of α-synuclein generated in the graft, versus the simple translocation of aggregated synuclein from the host to the graft. In cell culture, however, the prion-like propagation of α-synuclein lesions has been demonstrated80, 81, as has the induction of proteinaceous lesions associated with other neurodegenerative diseases, such as aggregates of superoxide dismutase 1 (SOD1)83, 84, which are characteristic of SOD1-mutant and some idiopathic cases of amyotrophic lateral sclerosis (ALS), cytosolic aggregates of TDP-4385, which are present in ALS and frontotemporal lobar degeneration with TDP-43-positive inclusions (FTLD-TDP), and aggregates of polyglutamine86, which typify Huntington’s disease and spinocerebellar ataxias.
These studies, along with those of Aβ and tau (above), imply that disease agents can be disseminated by cells, but the means whereby protein aggregates travel between cells, and the cellular domain(s) in which the templated conversion occurs, remain poorly understood39, 43, 44, 87. In vitro, aggregates of tau, α-synuclein, polyglutamine, and SOD1 all can be taken up by endocytosis and induce the misfolding of the corresponding intracellular proteins; moreover, cytoplasmic protein aggregates can translocate from one cultured cell to another64, 66, 80, 81, 83, 84, 86, 88. The mode of cell-to-cell transfer is unclear39, but at least α-synuclein and SOD1 are secreted into the cell medium89, 90. Furthermore, tau and α-synuclein have been measured at robust levels in the cerebrospinal fluid, suggesting secretion of these proteins in vivo91. The intercellular transfer of cytosolic protein aggregates may also occur through nanotubes, exosomes or microvesicles39. Like other pathogenic proteins, Aβ can be taken up, modified and secreted by cells in vitro92, 93, and it also is present in the CSF94.
Clinical, Practical and Theoretical Implications
The induction and proliferation of proteinaceous aggregates by corruptive protein templating appears to be a common feature of multiple, clinically diverse disorders, although many questions remain to be addressed (Table 1). The unexpected prevalence of this pathogenic mechanism raises a number of clinical and practical issues, and highlights the corruptive seeds as both potential biomarkers and therapeutic targets44 (Table 1).
Table 1.
Pathogenic protein seeding: Open questions
Mechanistic and theoretical issues:
|
Practical and clinical issues:
|
In AD, cerebral β-amyloid deposition begins in humans at least a decade prior to the onset of cognitive decline, and hence is an early and predictive indicator of the disease4. It is likely, therefore, that an effective disease-modifying therapy must be initiated prophylactically, before the disease has inflicted irreversible damage on the brain. Early intervention will require an early and informative biomarker4, 95. In vitro, the formation of corruptive seeds is a relatively slow, stochastic process96, though once a seed is present, aggregation proceeds quite rapidly. In vivo, then, the appearance of soluble Aβ seeds may precede appreciable Aβ-deposition in plaques and blood vessels. Because the most potent Aβ seeds, like prions97, appear to be relatively small54, soluble Aβ seeds could serve as informative biomarkers in bodily fluids.
The ability of Aβ-rich brain extracts injected into the peritoneal cavity to induce Aβ deposition in the brain56 indicates that Aβ seeds resemble prions in their ability to reach the central nervous system from the periphery. In the absence of direct evidence linking non-prion neurodegenerative diseases to seeds arising outside the central nervous system or taken up from the environment (e.g. in food or air), the practical implications of this finding are uncertain. It is probable, however, that a fuller understanding of the trafficking of pathogenic seeds will yield insights into the endogenous progression of disease, and hence denote novel points of intervention. For example, the early appearance of α-synuclein-containing Lewy bodies in the peripheral nervous system, and their relatively systematic spread within the brain98, 99, suggest that seeds-in-transit (i.e., those traveling between cells or from one region to another) might be profitable objectives for therapeutic interference44.
In some cases, protein misfolding and aggregation can be initiated by heterologous, β-sheet-rich proteins41, 100-102. This ‘cross-seeding’ is generally less potent than is homologous seeding, but the potential corruption of proteins by exogenous nanoscale materials, some of which may feature amyloid-like structural properties103, 104, should be factored into safety evaluations of such materials41. Furthermore, the cell-to-cell transfer of seeds and the induction of pathogenic protein aggregates in cellular grafts44, 79, 105 underscores the need for measures to protect grafted cells from host-induced corruptive protein templating, e.g., by selectively engineering the cells so that vulnerable proteins are either absent or resistant to seeding.
Beyond its importance as a therapeutic objective, there is a clear need for studies on the clinical and epidemiological implications of corruptive protein templating as a disease mechanism (Table 1). Given our current state of knowledge, we feel that it is unlikely that non-prion proteopathies are communicable under everyday circumstances. However, it is worth considering the possibility that non-prion proteopathies can be promoted under certain extraordinary circumstances. The most efficient induction of prion disease and Aβ deposition is achieved by direct introduction of the seeding agent into the brain30, 56. In rare instances, prion disease has been transmitted to humans by contaminated neurosurgical instruments34. Experimentally, β-amyloid induction can be triggered in transgenic mice by intracerebrally implanted stainless steel wires coated with minute amounts of brain extract rich in aggregated Aβ52 (Fig 2). While the transmission of non-prion proteopathies by tainted instruments thus is a theoretical possibility, proof of such a phenomenon (which could be obscured by a protracted incubation period) has not been demonstrated in humans. Nevertheless, when considered alongside the (slight) risk of prion transmission by instruments used on patients with undiagnosed prion disease106, the hypothetical risk that non-prion proteopathies might be similarly induced suggests a need for more research into the epidemiology of such disorders in long-term, post-neurosurgical patients. In addition, risk analysis of the incidence of proteopathic diseases in the recipients of donated organs, tissues, extracts or fluids is needed to establish with confidence the inclusion criteria for donors. For example, inasmuch as advancing age is a salient risk factor for most human neurodegenerative diseases, should there be an age-limit for organ and tissue donation? It is still premature to provide answers to these questions, but the growing prevalence of cerebral proteopathies necessitates a comprehensive quest to illuminate the causes and consequences of corruptive protein templating in human disease.
Conclusion
The seeded proliferation of misfolded proteins, a concept that arose and evolved in the prion field, holds considerable explanatory power for the pathogenesis of many of the neurodegenerative diseases that afflict our burgeoning elderly population. The theoretical framework underlying this paradigm recently has expanded to include an extraordinary array of neurological and systemic disorders. Moreover, the templated modification of protein structure also subserves the transfer and storage of useful biological information in systems ranging from microbes to mammals107-110. Unfortunately, much of the research on this far-reaching phenomenon is fragmentary, and the clinical implications remain uncertain. A concerted inquiry into the biophysics, biochemistry, and cell biology of protein aggregation is needed to decipher the molecular underpinnings of a large and growing constellation of age-related disorders of the nervous system, and thereby accelerate the discovery of efficacious therapies.
Acknowledgement
We thank Yvonne Eisele (Tübingen, Germany), Rebecca Rosen (Washington, D.C.), Harry LeVine III (Lexington, Kentucky) and other members of our laboratories for experimental support and comments on this manuscript. The contributions of H. Braak and D. Thal (Ulm, Germany), M. Tolnay (Basel, Switzerland), and S. Eberle (Tübingen) to figures and text are greatly appreciated. Supported by the BMBF in the framework of ERA-Net NEURON (MIPROTRAN), Competence Network on Degenerative Dementias (BMBF-01GI0705), NIH RR-00165, PO1AG026423, P50AG025688, and the CART Foundation.
References
- 1.Brown P, Salazar AM, Gibbs CJ, Jr., Gajdusek DC. Alzheimer’s disease and transmissible virus dementia (Creutzfeldt-Jakob disease) Ann N Y Acad Sci. 1982;396:131–43. doi: 10.1111/j.1749-6632.1982.tb26849.x. [DOI] [PubMed] [Google Scholar]
- 2.Prusiner SB. Some speculations about prions, amyloid, and Alzheimer’s disease. N Engl J Med. 1984;310:661–3. doi: 10.1056/NEJM198403083101021. [DOI] [PubMed] [Google Scholar]
- 3.Gajdusek DC. Spontaneous generation of infectious nucleating amyloids in the transmissible and nontransmissible cerebral amyloidoses. Mol Neurobiol. 1994;8:1–13. doi: 10.1007/BF02778003. [DOI] [PubMed] [Google Scholar]
- 4.Holtzman DM, Morris JC, Goate AM. Alzheimer’s disease: the challenge of the second century. Sci Transl Med. 2011;3:77sr1. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jucker M, Beyreuther K, Haass C, Nitsch R, Christen Y, editors. Alzheimer: 100 Years and Beyond. Springer; 2006. [Google Scholar]
- 6.Wilcock GK, Esiri MM. Plaques, tangles and dementia. A quantitative study. J Neurol Sci. 1982;56:343–56. doi: 10.1016/0022-510x(82)90155-1. [DOI] [PubMed] [Google Scholar]
- 7.Crystal H, Dickson D, Fuld P, et al. Clinico-pathologic studies in dementia: nondemented subjects with pathologically confirmed Alzheimer’s disease. Neurology. 1988;38:1682–7. doi: 10.1212/wnl.38.11.1682. [DOI] [PubMed] [Google Scholar]
- 8.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 9.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 10.Thal DR, Capetillo-Zarate E, Del Tredici K, Braak H. The development of amyloid beta protein deposits in the aged brain. Sci Aging Knowledge Environ. 2006;2006:re1. doi: 10.1126/sageke.2006.6.re1. [DOI] [PubMed] [Google Scholar]
- 11.Price JL, McKeel DW, Jr., Buckles VD, et al. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging. 2009;30:1026–36. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem. 2009;78:959–91. doi: 10.1146/annurev.biochem.052308.114844. [DOI] [PubMed] [Google Scholar]
- 13.Castellano JM, Kim J, Stewart FR, et al. Human apoE Isoforms Differentially Regulate Brain Amyloid-{beta} Peptide Clearance. Sci Transl Med. 2011;3:89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lambert MP, Barlow AK, Chromy BA, et al. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95:6448–53. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lesné S, Koh MT, Kotilinek L, et al. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–7. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 16.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–12. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 17.Shankar GM, Li S, Mehta TH, et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–42. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin M, Shepardson N, Yang T, Chen G, Walsh D, Selkoe DJ. Soluble amyloid {beta}-protein dimers isolated from Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic degeneration. Proc Natl Acad Sci U S A. 2011;108:5819–24. doi: 10.1073/pnas.1017033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberson ED, Scearce-Levie K, Palop JJ, et al. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316:750–4. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 20.Ittner LM, Götz J. Amyloid-beta and tau--a toxic pas de deux in Alzheimer’s disease. Nat Rev Neurosci. 2011;12:65–72. doi: 10.1038/nrn2967. [DOI] [PubMed] [Google Scholar]
- 21.Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 22.Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–17. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker LC, Jucker M. Amyloid by default. Nat Neurosci. 2011;14:669–70. doi: 10.1038/nn.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braak H, Del Tredici K. The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol. 2011;121:171–81. doi: 10.1007/s00401-010-0789-4. [DOI] [PubMed] [Google Scholar]
- 25.Saper CB, Wainer BH, German DC. Axonal and transneuronal transport in the transmission of neurological disease: potential role in system degenerations, including Alzheimer’s disease. Neuroscience. 1987;23:389–98. doi: 10.1016/0306-4522(87)90063-7. [DOI] [PubMed] [Google Scholar]
- 26.Hardy J. An ‘anatomical cascade hypothesis’ for Alzheimer’s disease. Trends Neurosci. 1992;15:200–1. doi: 10.1016/0166-2236(92)90033-5. [DOI] [PubMed] [Google Scholar]
- 27.Klunk WE, Mathis CA. The future of amyloid-beta imaging: a tale of radionuclides and tracer proliferation. Curr Opin Neurol. 2008;21:683–7. doi: 10.1097/WCO.0b013e3283168e1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villemagne VL, Pike KE, Chetelat G, et al. Longitudinal assessment of Abeta and cognition in aging and Alzheimer disease. Ann Neurol. 2011;69:181–92. doi: 10.1002/ana.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prusiner SB. Shattuck lecture--neurodegenerative diseases and prions. N Engl J Med. 2001;344:1516–26. doi: 10.1056/NEJM200105173442006. [DOI] [PubMed] [Google Scholar]
- 30.Prusiner SB. Prion Biology and Diseases. 2nd ed Cold Spring Harbor Laboratory; 2004. [Google Scholar]
- 31.Brown K, Mastrianni JA. The prion diseases. J Geriatr Psychiatry Neurol. 2010;23:277–98. doi: 10.1177/0891988710383576. [DOI] [PubMed] [Google Scholar]
- 32.Brown P, Gibbs CJ, Jr., Rodgers-Johnson P, et al. Human spongiform encephalopathy: the National Institutes of Health series of 300 cases of experimentally transmitted disease. Ann Neurol. 1994;35:513–29. doi: 10.1002/ana.410350504. [DOI] [PubMed] [Google Scholar]
- 33.Wadsworth JD, Asante EA, Collinge J. Review: contribution of transgenic models to understanding human prion disease. Neuropathol Appl Neurobiol. 2010;36:576–97. doi: 10.1111/j.1365-2990.2010.01129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belay ED, Schonberger LB. The public health impact of prion diseases. Annu Rev Public Health. 2005;26:191–212. doi: 10.1146/annurev.publhealth.26.021304.144536. [DOI] [PubMed] [Google Scholar]
- 35.Aguzzi A, Heikenwalder M, Polymenidou M. Insights into prion strains and neurotoxicity. Nat Rev Mol Cell Biol. 2007;8:552–61. doi: 10.1038/nrm2204. [DOI] [PubMed] [Google Scholar]
- 36.Collinge J, Clarke AR. A general model of prion strains and their pathogenicity. Science. 2007;318:930–6. doi: 10.1126/science.1138718. [DOI] [PubMed] [Google Scholar]
- 37.Soto C. Prion hypothesis: the end of the controversy? Trends Biochem Sci. 2011;36:151–8. doi: 10.1016/j.tibs.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bueler H, Fischer M, Lang Y, et al. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature. 1992;356:577–82. doi: 10.1038/356577a0. [DOI] [PubMed] [Google Scholar]
- 39.Aguzzi A, Rajendran L. The transcellular spread of cytosolic amyloids, prions, and prionoids. Neuron. 2009;64:783–90. doi: 10.1016/j.neuron.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 40.Parchi P, Strammiello R, Giese A, Kretzschmar H. Phenotypic variability of sporadic human prion disease and its molecular basis: past, present, and future. Acta Neuropathol. 2011;121:91–112. doi: 10.1007/s00401-010-0779-6. [DOI] [PubMed] [Google Scholar]
- 41.Walker LC, Levine H, 3rd, Mattson MP, Jucker M. Inducible proteopathies. Trends Neurosci. 2006;29:438–43. doi: 10.1016/j.tins.2006.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soto C, Estrada L, Castilla J. Amyloids, prions and the inherent infectious nature of misfolded protein aggregates. Trends Biochem Sci. 2006;31:150–5. doi: 10.1016/j.tibs.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Frost B, Diamond MI. Prion-like mechanisms in neurodegenerative diseases. Nat Rev Neurosci. 2010;11:155–9. doi: 10.1038/nrn2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brundin P, Melki R, Kopito R. Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat Rev Mol Cell Biol. 2010;11:301–7. doi: 10.1038/nrm2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goedert M, Clavaguera F, Tolnay M. The propagation of prion-like protein inclusions in neurodegenerative diseases. Trends Neurosci. 2010;33:317–25. doi: 10.1016/j.tins.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 46.Goudsmit J, Morrow CH, Asher DM, et al. Evidence for and against the transmissibility of Alzheimer disease. Neurology. 1980;30:945–50. doi: 10.1212/wnl.30.9.945. [DOI] [PubMed] [Google Scholar]
- 47.Baker HF, Ridley RM, Duchen LW, Crow TJ, Bruton CJ. Evidence for the experimental transmission of cerebral beta-amyloidosis to primates. Int J Exp Pathol. 1993;74:441–54. [PMC free article] [PubMed] [Google Scholar]
- 48.Ridley RM, Baker HF, Windle CP, Cummings RM. Very long term studies of the seeding of beta-amyloidosis in primates. J Neural Transm. 2006;113:1243–51. doi: 10.1007/s00702-005-0385-2. [DOI] [PubMed] [Google Scholar]
- 49.Kane MD, Lipinski WJ, Callahan MJ, et al. Evidence for seeding of beta -amyloid by intracerebral infusion of Alzheimer brain extracts in beta -amyloid precursor protein-transgenic mice. J Neurosci. 2000;20:3606–11. doi: 10.1523/JNEUROSCI.20-10-03606.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walker LC, Callahan MJ, Bian F, Durham RA, Roher AE, Lipinski WJ. Exogenous induction of cerebral beta-amyloidosis in betaAPP-transgenic mice. Peptides. 2002;23:1241–7. doi: 10.1016/s0196-9781(02)00059-1. [DOI] [PubMed] [Google Scholar]
- 51.Meyer-Luehmann M, Coomaraswamy J, Bolmont T, et al. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science. 2006;313:1781–4. doi: 10.1126/science.1131864. [DOI] [PubMed] [Google Scholar]
- 52.Eisele YS, Bolmont T, Heikenwalder M, et al. Induction of cerebral beta-amyloidosis: intracerebral versus systemic Abeta inoculation. Proc Natl Acad Sci U S A. 2009;106:12926–31. doi: 10.1073/pnas.0903200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watts JC, Giles K, Grillo SK, Lemus A, DeArmond SJ, Prusiner SB. Bioluminescence imaging of Abeta deposition in bigenic mouse models of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2011;108:2528–33. doi: 10.1073/pnas.1019034108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Langer F, Eisele YS, Fritschi S, Staufenbiel M, Walker LC, Jucker M. Soluble Aβ seeds are potent inducers of cerebral β-amyloid deposition. J Neuroscience. 2011 doi: 10.1523/JNEUROSCI.3088-11.2011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weller RO, Subash M, Preston SD, Mazanti I, Carare RO. Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer’s disease. Brain Pathol. 2008;18:253–66. doi: 10.1111/j.1750-3639.2008.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eisele YS, Obermüller U, Heilbronner G, et al. Peripherally applied Abeta-containing inoculates induce cerebral beta-amyloidosis. Science. 2010;330:980–2. doi: 10.1126/science.1194516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Legname G, Baskakov IV, Nguyen HO, et al. Synthetic mammalian prions. Science. 2004;305:673–6. doi: 10.1126/science.1100195. [DOI] [PubMed] [Google Scholar]
- 58.Wang F, Wang X, Yuan CG, Ma J. Generating a prion with bacterially expressed recombinant prion protein. Science. 2010;327:1132–5. doi: 10.1126/science.1183748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Petkova AT, Leapman RD, Guo Z, Yau WM, Mattson MP, Tycko R. Self-propagating, molecular-level polymorphism in Alzheimer’s beta-amyloid fibrils. Science. 2005;307:262–5. doi: 10.1126/science.1105850. [DOI] [PubMed] [Google Scholar]
- 60.Nilsson KP, Aslund A, Berg I, et al. Imaging distinct conformational states of amyloid-beta fibrils in Alzheimer’s disease using novel luminescent probes. ACS Chem Biol. 2007;2:553–60. doi: 10.1021/cb700116u. [DOI] [PubMed] [Google Scholar]
- 61.Rosen RF, Ciliax BJ, Wingo TS, et al. Deficient high-affinity binding of Pittsburgh compound B in a case of Alzheimer’s disease. Acta Neuropathol. 2010;119:221–33. doi: 10.1007/s00401-009-0583-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Levine H, 3rd, Walker LC. Molecular polymorphism of Abeta in Alzheimer’s disease. Neurobiol Aging. 2010;31:542–8. doi: 10.1016/j.neurobiolaging.2008.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clavaguera F, Bolmont T, Crowther RA, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11:909–13. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Frost B, Jacks RL, Diamond MI. Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem. 2009;284:12845–52. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frost B, Ollesch J, Wille H, Diamond MI. Conformational diversity of wild-type Tau fibrils specified by templated conformation change. J Biol Chem. 2009;284:3546–51. doi: 10.1074/jbc.M805627200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guo JL, Lee VM. Seeding of Normal Tau by Pathological Tau Conformers Drives Pathogenesis of Alzheimer-like Tangles. J Biol Chem. 2011;286:15317–31. doi: 10.1074/jbc.M110.209296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crowther RA, Goedert M. Abnormal tau-containing filaments in neurodegenerative diseases. J Struct Biol. 2000;130:271–9. doi: 10.1006/jsbi.2000.4270. [DOI] [PubMed] [Google Scholar]
- 68.Wegmann S, Jung YJ, Chinnathambi S, Mandelkow EM, Mandelkow E, Muller DJ. Human Tau isoforms assemble into ribbon-like fibrils that display polymorphic structure and stability. J Biol Chem. 2010;285:27302–13. doi: 10.1074/jbc.M110.145318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Götz J, Chen F, van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science. 2001;293:1491–5. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- 70.Bolmont T, Clavaguera F, Meyer-Luehmann M, et al. Induction of tau pathology by intracerebral infusion of amyloid-beta - containing brain extract and by amyloid-beta deposition in APP × Tau transgenic mice. Am J Pathol. 2007;171:2012–20. doi: 10.2353/ajpath.2007.070403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guo JP, Arai T, Miklossy J, McGeer PL. Abeta and tau form soluble complexes that may promote self aggregation of both into the insoluble forms observed in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2006;103:1953–8. doi: 10.1073/pnas.0509386103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miller Y, Ma B, Nussinov R. Synergistic interactions between repeats in tau protein and Abeta amyloids may be responsible for accelerated aggregation via polymorphic states. Biochemistry. 2011;50:5172–81. doi: 10.1021/bi200400u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Busciglio J, Lorenzo A, Yeh J, Yankner BA. beta-amyloid fibrils induce tau phosphorylation and loss of microtubule binding. Neuron. 1995;14:879–88. doi: 10.1016/0896-6273(95)90232-5. [DOI] [PubMed] [Google Scholar]
- 74.Yoshiyama Y, Higuchi M, Zhang B, et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–51. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 75.Hernandez F, de Barreda E Gomez, Fuster-Matanzo A, Lucas JJ, Avila J. GSK3: a possible link between beta amyloid peptide and tau protein. Exp Neurol. 2010;223:322–5. doi: 10.1016/j.expneurol.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 76.Braak H, Del Tredici K, Rub U, de Vos RA, Steur EN Jansen, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 77.Braak H, Del Tredici K. [Pathophysiology of sporadic Parkinson’s disease] Fortschr Neurol Psychiatr. 2010;78(Suppl 1):S2–4. doi: 10.1055/s-0029-1245179. [DOI] [PubMed] [Google Scholar]
- 78.Li JY, Englund E, Holton JL, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–3. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 79.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 2008;14:504–6. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 80.Desplats P, Lee HJ, Bae EJ, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 2009;106:13010–5. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hansen C, Angot E, Bergstrom AL, et al. alpha-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest. 2011;121:715–25. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kordower JH, Dodiya HB, Kordower AM, et al. Transfer of host-derived alpha synuclein to grafted dopaminergic neurons in rat. Neurobiol Dis. 2011;43:552–7. doi: 10.1016/j.nbd.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chia R, Tattum MH, Jones S, Collinge J, Fisher EM, Jackson GS. Superoxide dismutase 1 and tgSOD1 mouse spinal cord seed fibrils, suggesting a propagative cell death mechanism in amyotrophic lateral sclerosis. PLoS One. 2010;5:e10627. doi: 10.1371/journal.pone.0010627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Munch C, O’Brien J, Bertolotti A. Prion-like propagation of mutant superoxide dismutase-1 misfolding in neuronal cells. Proc Natl Acad Sci U S A. 2011;108:3548–53. doi: 10.1073/pnas.1017275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Furukawa Y, Kaneko K, Watanabe S, Yamanaka K, Nukina N. A seeding reaction recapitulates intracellular formation of Sarkosyl-insoluble transactivation response element (TAR) DNA-binding protein-43 inclusions. J Biol Chem. 2011;286:18664–72. doi: 10.1074/jbc.M111.231209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ren PH, Lauckner JE, Kachirskaia I, Heuser JE, Melki R, Kopito RR. Cytoplasmic penetration and persistent infection of mammalian cells by polyglutamine aggregates. Nat Cell Biol. 2009;11:219–25. doi: 10.1038/ncb1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee SJ, Desplats P, Sigurdson C, Tsigelny I, Masliah E. Cell-to-cell transmission of non-prion protein aggregates. Nat Rev Neurol. 2010;6:702–6. doi: 10.1038/nrneurol.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Luk KC, Song C, O’Brien P, et al. Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc Natl Acad Sci U S A. 2009;106:20051–6. doi: 10.1073/pnas.0908005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee HJ, Patel S, Lee SJ. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci. 2005;25:6016–24. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Urushitani M, Sik A, Sakurai T, Nukina N, Takahashi R, Julien JP. Chromogranin-mediated secretion of mutant superoxide dismutase proteins linked to amyotrophic lateral sclerosis. Nat Neurosci. 2006;9:108–18. doi: 10.1038/nn1603. [DOI] [PubMed] [Google Scholar]
- 91.Mollenhauer B, Locascio JJ, Schulz-Schaeffer W, Sixel-Doring F, Trenkwalder C, Schlossmacher MG. alpha-Synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: a cohort study. Lancet Neurol. 2011;10:230–40. doi: 10.1016/S1474-4422(11)70014-X. [DOI] [PubMed] [Google Scholar]
- 92.Hu X, Crick SL, Bu G, Frieden C, Pappu RV, Lee JM. Amyloid seeds formed by cellular uptake, concentration, and aggregation of the amyloid-beta peptide. Proc Natl Acad Sci U S A. 2009;106:20324–9. doi: 10.1073/pnas.0911281106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rajendran L, Honsho M, Zahn TR, et al. Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A. 2006;103:11172–7. doi: 10.1073/pnas.0603838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bibl M, Mollenhauer B, Esselmann H, et al. CSF amyloid-beta-peptides in Alzheimer’s disease, dementia with Lewy bodies and Parkinson’s disease dementia. Brain. 2006;129:1177–87. doi: 10.1093/brain/awl063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Golde TE, Schneider LS, Koo EH. Anti-abeta therapeutics in Alzheimer’s disease: the need for a paradigm shift. Neuron. 2011;69:203–13. doi: 10.1016/j.neuron.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lansbury PT., Jr Structural neurology: are seeds at the root of neuronal degeneration? Neuron. 1997;19:1151–4. doi: 10.1016/s0896-6273(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 97.Silveira JR, Raymond GJ, Hughson AG, et al. The most infectious prion protein particles. Nature. 2005;437:257–61. doi: 10.1038/nature03989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bloch A, Probst A, Bissig H, Adams H, Tolnay M. Alpha-synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol Appl Neurobiol. 2006;32:284–95. doi: 10.1111/j.1365-2990.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- 99.Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett. 2006;396:67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 100.Yagi H, Kusaka E, Hongo K, Mizobata T, Kawata Y. Amyloid fibril formation of alpha-synuclein is accelerated by preformed amyloid seeds of other proteins: implications for the mechanism of transmissible conformational diseases. J Biol Chem. 2005;280:38609–16. doi: 10.1074/jbc.M508623200. [DOI] [PubMed] [Google Scholar]
- 101.Westermark GT, Westermark P. Prion-like aggregates: infectious agents in human disease. Trends Mol Med. 2010;16:501–7. doi: 10.1016/j.molmed.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 102.Waxman EA, Giasson BI. Induction of intracellular tau aggregation is promoted by alpha-synuclein seeds and provides novel insights into the hyperphosphorylation of tau. J Neurosci. 2011;31:7604–18. doi: 10.1523/JNEUROSCI.0297-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cherny I, Gazit E. Amyloids: not only pathological agents but also ordered nanomaterials. Angew Chem Int Ed Engl. 2008;47:4062–9. doi: 10.1002/anie.200703133. [DOI] [PubMed] [Google Scholar]
- 104.Knowles TPJ, Buehler MJ. Nanomechanics of functional and patholological amyloid materials. Nature Nanotechnology. 2011;6:469–79. doi: 10.1038/nnano.2011.102. [DOI] [PubMed] [Google Scholar]
- 105.Meyer-Luehmann M, Stalder M, Herzig MC, et al. Extracellular amyloid formation and associated pathology in neural grafts. Nat Neurosci. 2003;6:370–7. doi: 10.1038/nn1022. [DOI] [PubMed] [Google Scholar]
- 106.Ward HJ, Everington D, Croes EA, et al. Sporadic Creutzfeldt-Jakob disease and surgery: a case-control study using community controls. Neurology. 2002;59:543–8. doi: 10.1212/wnl.59.4.543. [DOI] [PubMed] [Google Scholar]
- 107.Shorter J, Lindquist S. Prions as adaptive conduits of memory and inheritance. Nat Rev Genet. 2005;6:435–50. doi: 10.1038/nrg1616. [DOI] [PubMed] [Google Scholar]
- 108.Wickner RB, Edskes HK, Shewmaker F, Nakayashiki T. Prions of fungi: inherited structures and biological roles. Nat Rev Microbiol. 2007;5:611–8. doi: 10.1038/nrmicro1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tuite MF, Serio TR. The prion hypothesis: from biological anomaly to basic regulatory mechanism. Nat Rev Mol Cell Biol. 2010;11:823–33. doi: 10.1038/nrm3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Norrby E. Prions and protein-folding diseases. J Intern Med. 2011;270:1–14. doi: 10.1111/j.1365-2796.2011.02387.x. [DOI] [PubMed] [Google Scholar]