Abstract
The process termed “epithelial–mesenchymal transition” (EMT) was originally discovered in ontogenic development, and has been shown to be one of the key steps in tumor cell progression and metastasis. Recently, we showed that the expression of some glycosphingolipids (GSLs) is down-regulated during EMT in human and mouse cell lines. Here, we demonstrate the involvement of GalNAc-type (or mucin-type) O-glycosylation in EMT process, induced with transforming growth factor β (TGF-β) in human prostate epithelial cell lines. We found that: (i) TGF-β treatment caused up-regulation of oncofetal fibronectin (onfFN), which is defined by mAb FDC6, and expressed in cancer or fetal cells/tissues, but not in normal adult cells/tissues. The reactivity of mAb FDC6 requires the addition of an O-glycan at a specific threonine, inside the type III homology connective segment (IIICS) domain of FN. (ii) This change is associated with typical EMT characteristics; i.e., change from epithelial to fibroblastic morphology, enhanced cell motility, decreased expression of a typical epithelial cell marker, E-cadherin, and enhanced expression of mesenchymal markers. (iii) TGF-β treatment up-regulated mRNA level of FN containing the IIICS domain and GalNAc-T activity for the IIICS domain peptide substrate containing the FDC6 onfFN epitope. (iv) Knockdown of GalNAc-T6 and T3 inhibited TGF-β–induced up-regulation of onfFN and EMT process. (v) Involvement of GSLs was not detectable with the EMT process in these cell lines. These findings indicate the important functional role of expression of onfFN, defined by site-specific O-glycosylation at IIICS domain, in the EMT process.
Keywords: O-glycosylated fibronectin, siRNA
Glycoconjugates, such as glycosphingolipids (GSLs) and N- and O-linked glycoproteins, have been shown to play important roles in embryogenesis (1), tumorigenesis, and cancer progression (2). Specific types of glycosyl residues modulate particular signaling pathways and regulate cell phenotypes. For example, GM3 inhibits epithelial growth factor receptor (EGFR) activation (3, 4); GM2 associated with tetraspanine CD82 inhibits the activation of the hepatocyte growth factor receptor cMet (5); and O-Fucose glycans modulate Notch signaling, which controls the fate of many cell types (6–8).
Epithelial–mesenchymal transition (EMT) was initially observed during early embryonic development and organ formation (9–11). Accumulating evidence has shown that the EMT process plays a key role in disease development, particularly in cancer progression to metastasis (10–14) and fibrosis (15). During EMT, cells lose their apical–basal polarity, change morphology to fibroblastic, display reduced expression of epithelial cell marker molecules such as E-cadherin (Ecad), and enhance expression of mesenchymal cell marker molecules such as fibronectin (FN),N-cadherin (Ncad), vimentin, and matrix-metalloproteinases (MMPs). When combined, these effects result in increased cell motility (10–15).
Our recent studies found a reduction in specific GSLs (Gg4 and/or GM2) in the EMT process induced by transforming growth factor β (TGF-β) in mouse and human epithelial cells (16). Further studies with mouse epithelial NMuMG cells showed that Gg4 synthase is down-regulated during the EMT process and that EMT induction is inhibited by transfection of Gg4 synthase gene or exogenous addition of Gg4 (17). These findings indicate the functional role of the GSL in EMT induction.
FN has often been used as one of the mesenchymal markers whose expression is strongly enhanced in EMT process. FN exists in multiple forms. There are isoforms that are produced through alternative splicing; some are from alternative splicing in the type III homology connective segment (IIICS) domain (18–20), whereas others consist of two different complete type III repeats, termed extra domain A (ED-A) and extra domain B (ED-B) (20, 21). In addition, our previous studies showed that mouse mAb FDC6, established by immunizing with FN isolated from a human cell line, HUH-7 (22), reacts strongly with FN in fetal and cancer cells and tissues, but not with FN in normal adult cells and tissues. Therefore, FDC6-positive FN was termed “oncofetal FN” (onfFN) (22, 23). The FDC6 epitope was based on the addition of an O-glycan (GalNAcα1-O-Ser/Thr or Galβ1-3GalNAcα1-O-Ser/Thr) (24) to the Thr residue of peptide sequence -Val-Thr-His-Pro-Gly-Tyr- (VTHPGY) at the IIICS domain. Because neither the O-GalNAc hapten nor the peptide in the IIICS domain were reactive with FDC6, the FDC6 epitope structure was assumed to be the O-glycopeptide with combined recognition of the O-glycan and the peptide backbone, VTHPGY (23, 25). Other mAbs with cancer-specific reactivity react with aberrant O-glycopeptide epitopes (26). Around 20 UDP-N-acetylgalactosamine:polypeptide N-acetylgalactosaminyltransferases (GalNAc-Ts) have been identified in humans, and GalNAc-T3 (27) and GalNAc-T6 (28) have been suggested as enzymes involved in the formation of onfFN (28, 29).
We addressed the possible functional correlation between onfFN carrying an FDC6 epitope and the EMT process by investigating the role of onfFN, defined by mAb FDC6, in EMT induction. For this study, we used the nonmalignant human prostate epithelial cell lines WPE and PNT1a.
Results
Analysis of EMT Induction by TGF-β and EtDO-P4 in WPE and PNT1a Cells.
EMT induction in two human prostate epithelial cell lines, WPE and PNT1a, was assessed based on the changes in cell morphology, expression of epithelial vs. mesenchymal cell marker molecules, and cell motility. These criteria have been widely used for assessment of EMT in various types of cells (10, 11, 14) and human prostate cell lines (30, 31).
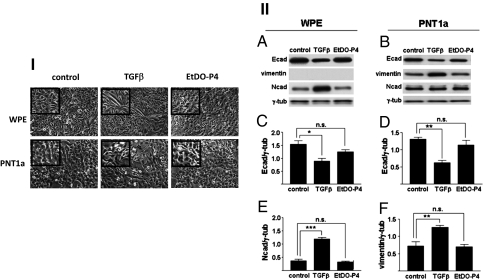
Treatment with TGF-β resulted in detectable morphological changes in both cell lines (Fig. 1I), with some differences between the two. In WPE cells, the typical flat, epithelial-type morphology was converted to a clear, bipolar, elongated, fibroblastic shape. In PNT1a cells, epithelial morphology was minimally changed to the fibroblastic shape in certain limited areas. In addition, TGF-β treatment reduced the expression of the epithelial cell marker Ecad in both cell lines and enhanced the expression of the mesenchymal markers Ncad and vimentin in WPE and PNT1a, respectively (Fig. 1II). Vimentin was not detected in WPE following TGF-β treatment, and Ncad expression was not enhanced in PNT1a (Fig. 1II). However, other mesenchymal cell markers, such as FN (Fig. 2), MMP-2, and MMP-9, were up-regulated in both cell lines. The MMPs released in the culture supernatants were assessed by Western blot analysis (Fig. S1II) and zymography using gelatin as the substrate (Fig. S1II). Next, cell motility was analyzed by phagokinetic assay on gold sol-coated plates and by cell scratch-wound assay. Both assays showed increased cell motility in both cell lines following TGF-β treatment (Fig. S2).
Fig. 1.
Analysis of EMT induction in human prostate epithelial cell lines WPE and PNT1a. Cells were treated with TGF-β or EtDO-P4, and analyzed for EMT induction as described in Materials and Methods and SI Materials and Methods. (I) Cell morphology changes. (Top) WPE cells. (Bottom) PNT1a cells. (Left) Control. (Center) TGF-β treatment. (Right) EtDO-P4 treatment. (II) Analysis of epithelial vs. mesenchymal cell markers by SDS/PAGE and Western blot. (A, C, and E) WPE cells. (B, D, and F) PNT1a cells. Samples containing 10 μg protein were used with γ-tubulin as loading control. (A and B) Experiments were performed in triplicate, and representative results are shown. (C–F) Signal intensities were normalized, and values are shown as relative intensity (mean ± SD) for Ecad (C and D), Ncad (E), and vimentin (F). n.s.: not significant; *P ≤ 0.05; **P ≤ 0.005; ***P ≤ 0.001.
Fig. 2.
Expression of total FN and onfFN after treatment with TGF-β or EtDO-P4. Cells were treated and cell lysates were prepared as described in SI Materials and Methods, and samples were subjected to SDS/PAGE and Western blot as described in Fig. 1. Total FN and onfFN were detected with mAb EP5 (A and C) and mAb FDC6 (B and D), respectively. Human plasma FN (0.5 μg) and HUH-7 cell lysate (10 μg) were used as positive controls. Western blot results were analyzed as described in Fig.1, and mean ± SD is shown. n.s.: not significant; **P ≤ 0.005; ***P ≤ 0.001.
In contrast, treatment of the cells with 1 μM EtDO-P4, the specific inhibitor of GlcCer synthase (32), did not cause a significant change in cell morphology, expression of EMT marker molecules, or cell motility (Fig. 1 and Fig. S2), though the majority of GSLs were essentially depleted under this condition, as assessed by orcinol/sulfuric acid staining after HPTLC separation of extracted GSLs (Fig. S3C).
Characterization of FN Up-Regulated During the EMT Process.
FN is one of the most studied mesenchymal markers and has been shown to be up-regulated in TGF-β–induced EMT (11, 13). Expression of FN, defined by mAb EP5 at ∼250 kDa in both cell lines, was strongly enhanced by TGF-β treatment, but not by EtDO-P4 treatment (Fig. 2 A and C). We characterized FN up-regulated during EMT process in these cells by using mAb FDC6, which reacts selectively with FN expressed in fetal and cancer tissue and cells and recognizes FN that carries O-glycan at its IIICS domain. Following TGF-β treatment, expression of FDC6-positive FN was enhanced in both cell lines (Fig. 2 B and D). This was paralleled to the total FN detected by mAb EP5, whereas human plasma FN, used as control, was detected by mAb EP5, but not by mAb FDC6. This result is consistent with our previous observations (22).
Comparison of GSLs Expressed in Control and TGF-β–Treated Cells.
In view of our previous findings with mouse NMuMG cells, where the expression of certain GSLs, especially Gg4, are down-regulated by TGF-β treatment and exogenously added Gg4 inhibited TGF-β–induced EMT (16, 17), we analyzed GSLs expression in control and TGF-β–treated WPE and PNT1a cells. Expression patterns of total GSLs were essentially the same between control cells and TGF-β–treated cells in both cell lines (Fig. S3 A and B), but greatly reduced in EtDO-P4 treatment (Fig. S3C). Further analysis of GSLs from PNT1a cells showed that there are no detectable differences in neutral GSLs, monosialo-, disialo-, trisialo-, or polysialo (≥4)-gangliosides between control cells and TGF-β–treated cells (Fig. S3D). These results may be related to the lack of EMT induction after EtDO-P4 treatment on either cell line (Fig. 1 and Fig. S2).
Determination of GalNAc-T Enzyme Activity Toward the Peptide Substrate in FN IIICS Domain.
Because the expression of onfFN detected by mAb FDC6 was strongly enhanced by TGF-β treatment, we examined the effect of TGF-β treatment on GalNAc-T enzyme activity for the peptide substrate containing the VTHPGY sequence, whose O-glycosylation is required for the binding epitope of mAb FDC6 (25). The glycosyltransferase activity expressed as pmol/mg/hr in microsomal fractions prepared from control, and TGF-β–treated WPE cells were compared. The activity was much higher in TGF-β–treated cells than in control cells (Fig. S4). This increased enzyme activity in TGF-β–treated cells presumably contributes to the enhanced onfFN expression detected with mAb FDC6.
Effect of Knockdown of GalNAc-T6 and -T3 on the TGF-β–Induced EMT Process.
To elucidate the functional role of up-regulated expression of onfFN during the EMT process, we tried to block the expression using the siRNA approach. For this purpose, we knocked down expression of GalNAc-T6 and -T3 genes, which are indicated to be involved in O-glycosylation to synthesize FDC6-positive FN (28, 33). Preliminary experiments to assess the siRNA targeted for GalNAc-T6 and -T3 effectively reduced their mRNA level by more than 80% in both WPE and PNT1a cells. Because our preliminary experiments showed that the mixtures of siRNA targeted for T3 and T6 reduces the expression of onfFN more effectively than each siRNA for T3 or T6 alone, the mixture of the siRNAs was used. Selectivity of the siRNAs was evaluated by Western blot using mAbs for GalNAc-T3, -T6, and -T2 and quantitative RT-PCR (RT-qPCR). Western blot analysis showed that the enzyme protein levels for GalNAc-T3 and -T6 in the knockdown cells were significantly reduced compared with that in cells treated with control siRNA (Fig. 3I A, C, and D). No detectable change in protein level for GalNAc-T2 (Fig. 3I A and B) was observed, indicating the selective reduction with the siRNAs. RT-qPCR analysis showed that the transfection of the siRNAs resulted in a clear reduction of mRNAs for GalNAc-T3 and -T6, whereas the mRNA level of either GalNAc-T2 or IIICS domain of FN did not show any significant reduction, which further supports the selectivity (Fig. 3II). We did not detect a clear up-regulation of GalNAc-T3 and -T6 in TGF-β–treated cells, either at protein or at mRNA level, though the GalNAc-T activity assay using the peptide as a substrate did show clear up-regulation (Fig. S4). TGF-β treatment strongly up-regulated the mRNA level of FN containing IIICS domain (Fig. 3II). Because several alternative splicing within the IIICS domain were previously analyzed and reported (34), PCR products for the IIICS domain were analyzed to assess the size by agarose gel electrophoresis. Variants containing the hexapeptide VTHPGY, V120 and V89/V95, were strongly up-regulated in both cell lines, whereas variant V0, which does not contain the peptide sequence, was slightly up-regulated in WPE cells and was absent in PNT1a cells (Fig. S5).
Fig. 3.
Western blot analysis for GalNAc-Ts and RT-qPCR analysis for GalNAc-Ts and FN IIICS domain. WPE cells were transfected with control siRNA or siRNA for T3/T6, and then treated with TGF-β as described in Materials and Methods. Selectivity of the siRNA was evaluated by Western blot using specific mAbs. (I) Representative Western blot for T2, T3 and T6 from triplicate experiments (A) and mean ± SD (B–D) are shown. (II) Results from RT-qPCR for GalNAc-T2, T3, T6, and FN IIICS domain from triplicate experiments are normalized with values for actin and mean ± SD are shown. RQ, relative quantity. n.s.: not significant . *P ≤ 0.05; **P ≤ 0.005; ***P ≤ 0.001.
Our previous studies with glycopeptides containing the VTHPGY sequence indicated that mAb FDC6 binding was maintained by treatment with sialidase followed by β-galactosidase; however, the binding activity was completely lost by endo-α-N-acetylgalactosaminidase treatment following sialidase treatment (23). We confirmed this point by using HUH-7 cells treated with Benzyl-α-GalNAc, which blocks glycan elongation on O-GalNAc by competing as an acceptor (35). The treatment did not cause any reduction in the reactivity with FDC6; the accumulation of GalNAc residues induced by the treatment was clearly detected with Helix pomatia lectin, which binds terminal GalNAc residues (Fig. S6). Recently, we found that HUH-7 cells transfected with cDNA for GalNAc-T6 produce more FDC6-positive FN compared with the parent cells (Fig. S7). Together, these results indicate that the down-regulation of onfFN, detected with FDC6 in the cells transfected with the siRNAs, is accomplished through selective knockdown of the GalNAc-Ts.
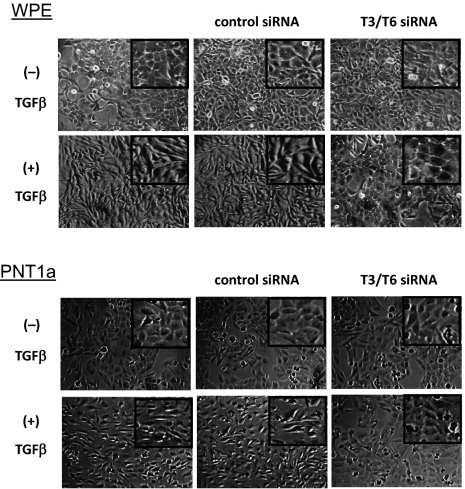
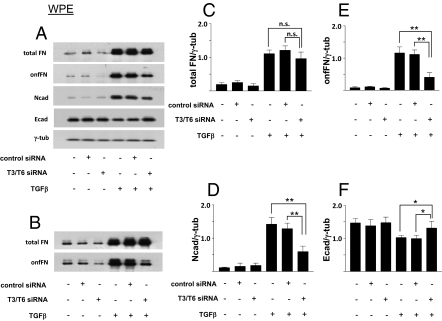
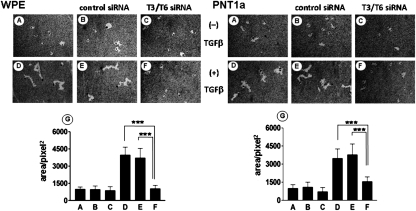
The effect of the reduction of FDC6-positive FN induced by the siRNAs, on EMT process, was assessed by change of cell morphology, expression of EMT marker molecules, and cell motility. Transfection of the targeted siRNAs inhibited the change of cell morphology induced by TGF-β treatment in the both cell lines, whereas transfection of the control siRNA had no significant effect (Fig. 4). As expected, the expression of total FN, defined by EP5, was not significantly affected by the knockdown, and was enhanced to similar degree by TGF-β treatment in both cell lines, whereas TGF-β–induced up-regulation of onfFN, defined by FDC6, was strongly inhibited in T6/T3 knockdown cells. In addition to FN in cell lysates, FN secreted in culture supernatants was analyzed, and similar results were obtained. In the knockdown cells, compared with the nontransfected controls and the negative siRNA-transfected controls, expression of the mesenchymal markers Ncad and vimentin was significantly lower, and the expression of the epithelial cell marker Ecad was higher in both WPE (Fig. 5) and PNT1a cells (Fig. S8). The enhanced cell motility induced by TGF-β treatment was strongly inhibited in the knockdown cells, but not in the nontransfected control cells or the negative control siRNA transfected cells for the both cell lines (Fig. 6).
Fig. 4.
Effect of knockdown of GalNAc-T3/T6 on TGF-β–induced EMT, assessed by cell morphology. WPE and PNT1a cells were transfected with a mixture of siRNA duplexes for human GalNAc-T3 and T6 to obtain double knockdown cells, or with negative siRNA for control cells, and then treated with TGF-β as described in SI Materials and Methods. Representative photos from triplicate experiments are shown.
Fig. 5.
Effect of knockdown of GalNAc-T3/T6 on TGF-β–induced EMT in WPE cells, assessed by expression of epithelial and mesenchymal cell markers. WPE cells were transfected with siRNA duplexes or negative siRNA, and treated with TGF-β, as in Fig. 4. Cell lysates were prepared for Western blot analysis as described in SI Materials and Methods. (A) Representative results from quadruplicate experiments. (C–F) Relative expression levels normalized with loading control were calculated, and shown as mean ± SD; n.s.: not significant; *P ≤ 0.05; **P ≤ 0.005. (B) Total FN and onfFN secreted in the culture supernatants was also analyzed with WPE cells. Representative results from triplicate experiments are shown.
Fig. 6.
Effect of knockdown of GalNAc-T3/T6 on TGF-β–induced EMT, assessed by cell motility. WPE and PNT1a cells were transfected with siRNA duplexes or negative siRNA, and treated with TGF-β, as in Fig. 3. Cell motility was analyzed by phagokinetic assay, as described in SI Materials and Methods. (A–C) No TGF-β treatment. (D–F) TGF-β treatment. (B and E) Negative control siRNA. (C and F) GalNAc-T3/T6 siRNA mixture. (G) Tracks from 50 individual cells were measured and quantified as described in SI Materials and Methods, and mean ± SD is shown. ***P ≤ 0.001.
Discussion
The EMT process was originally observed through in vivo studies of cells in tissues associated with early embryonic development (36). The process was later found to play a key role in tissue repair, to prevent apoptosis and senescence, and to induce characteristic properties of stem cells. EMT is also reported to be a cause of organ fibrosis and to promote cancer progression through enhancement of cell motility, acquisition of stem cell characteristics, and other mechanisms (10–15).
In view of the many documented examples of aberrant glycosylation associated with cancer progression (37–40), we assumed that glycosylation changes in GSLs or glycoproteins occur during the EMT process. Our previous studies using the mouse mammary epithelial cell line NMuMG demonstrated a functional role of Gg4: Expression of Gg4 was reduced by down-regulation of Gg4 synthase gene expression during EMT, and enhancement of Gg4 level inhibited EMT (16, 17). In contrast, the present study using EtDO-P4, the inhibitor of GlcCer synthase (32), did not show involvement of GSLs in EMT process in prostate epithelial cell lines WPE and PNT1a. However, we cannot rule out the possible involvement of GSL having GalCer as core structure; for example, Galα1–4Galβ1-Cer (diGalCer) (41), Galβ-sphingosine (psychosine), two types of plasmalopsychosine (42), and NeuAcα2–3Galβ1-Cer (GM4 ganglioside) (43) have been shown to be present in some types of mammalian cells.
In both prostate cell lines, TGF-β treatment down-regulated the expression of epithelial marker Ecad and up-regulated the expression of mesenchymal markers FN, MMP-2, and MMP-9. Mesenchymal marker Ncad was enhanced in WPE but not in PNT1a, whereas mesenchymal marker vimentin was enhanced in PNT1a, but not in WPE. Such variation in expression patterns of molecular EMT markers has been reported previously, and appears to depend on type, origin, and history of the cell lines (10, 11, 14, 44). Other epithelial markers, ZO-1 and occluding, were not detected, whereas cytokeratin-18 was unchanged in either cell line, indicating that Ecad is the principal epithelial marker down-regulated during EMT process in prostate cells. This finding is consistent with previous studies (45–48).
The focus of this study is the functional role of a site-specific single O-glycosylation event in the IIICS domain of human FN following EMT induction. FN is widely used for assessment of EMT process, but is not yet clearly characterized. For the characterization, we focused on onfFN, defined by mAb FDC6, which reacts with a specific O-glycosylated peptide sequence in IIICS domain (22, 23, 25). The onfFN expression and mRNA level of FN with IIICS domain was strongly up-regulated with TGF-β treatment. TGF-β treatment was found to enhance GalNAc-T enzyme activity for the synthetic peptide substrate derived from the IIICS domain defining the FDC6 epitope. Surprisingly, no significant change in GalNAc-T3 or -T6 was detected in either protein or mRNA level. Although there are several possible explanations for the discrepancy in these results (e.g., TGF-β treatment might stabilize GalNAc-T enzyme activity), further extensive studies are needed.
The presence of three to four biantennary N-linked glycans at the ∼30-KDa collagen-binding domain of hamster and human FN was reported (49, 50). The nonreducing terminal structures of these biantennary structures of FN are modified by oncogenic transformation of human fibroblasts (51). However, the functional significance of the N-linked glycan in EMT remains unknown. The expression of ED-B or ED-A domains in FN is reported to be correlated with disease, including cancer formation and progression (52–54). ED-B or ED-A domains may be expressed in both FDC6-positive and -negative FNs; future studies may clarify this point.
We found that blocking the expression of onfFN through knockdown of GalNAc-Ts inhibits the TGF-β–induced EMT process. This finding indicates that onfFN, but not norFN plays a key role in EMT induction, which is probably due to the different cell signaling induced by them. The next step is to elucidate the mechanism underlying the functional role of onfFN. We hypothesize that onfFN is involved in the “mesenchymal adhesion process” (44, 55), whereby extracellular matrix (ECM)-dependent integrin activity, and associated signal transduction are altered (for review, see ref. 56). FN is known to activate integrin α5β1; one possibility is that onfFN and normal adult FN activate α5β1 differently.
Recent reports indicate that FN is capable of inducing EMT process. Two studies on lung fibrosis, which occurs in certain respiratory diseases, showed that mouse and human primary alveolar epithelial cells undergo EMT when they are in contact with FN in ECM (57, 58). Also, O-glycosylation of FN through GalNAc-T6 was reported to be associated in an EMT-like process (59), though the O-glycosylation FN was not characterized for FDC6 reactivity.
In summary, the results presented here demonstrate the essential role of onfFN in TGF-β–induced EMT process, which involves a site-specific O-glycosylation event of FN that induces the FDC6 epitope. These results in human prostate cells, together with our previous results that showed the functional role of GSLs in EMT in mouse mammary cells, indicate that different types of glycosylations are involved in EMT process in different cell types. Further studies will clarify the role of other types of glycosylation in EMT process.
Materials and Methods
Treatment of Cells with TGF-β and EtDO-P4.
WPE and PNT1a cells (2 × 105 cells per well) were grown in culture medium as described in SI Materials and Methods in six-well plates (3.5-cm-diameter wells; Corning), and incubated overnight. Medium was changed to fresh medium alone (control), medium containing TGF-β 5.0 ng/mL, or medium containing EtDO-P4 1.0 μM, and the cells were incubated for an additional 72 h. Photographs were taken for cell morphology, and the cells were used for analysis for the expression of proteins, or mRNAs and for cell motility, as described in SI Materials and Methods.
Knockdown of GalNAc-T3 and -T6 Through Small Interfering RNA (siRNA).
WPE and PNT1a cells (7.5 × 104 cells per well) were seeded in a 12-well plate in culture medium. After 24 h, cells were transfected with the mixture of siRNA duplexes, 100 nM each, for human GalNAc-T3 and T6 (respectively CCAUAGAUCUGAACACGUU and CCAUCGACCUUAAUACUUU; Dharmacon Research), or a negative control siRNA (Qiagen), using Lipofectamine 2000 reagent (Invitrogen) following the manufacturer's instructions.
Effect of GalNAc-T3 and -T6 Knockdown on TGF-β–Induced EMT.
A total of 1.0 to 1.5 × 105 WPE or PNT1a cells were seeded onto a six-well plate and transfected with the mixture of the two siRNAs at 100 nM each, or negative control siRNA at 100 nM, as described above. After 24 h of incubation, the cells in each well were detached with trypsin/EDTA, and seeded into two wells of 12-well plate. After 6 h of incubation, TGF-β was added to one of the wells and incubated for 48 h. After taking photos, the cells were used for analysis of the expression of proteins or mRNAs and for cell motility activity, as described in SI Materials and Methods.
GSL Extraction and Analysis.
Determination of GalNAc-T Activity and Effect of Benzyl-α-GalNAc on FDC6 Binding.
RT-qPCR for GalNAc-T2, -T3, -T6, and IIICS Domain of FN.
Supplementary Material
Acknowledgments
We thank Dr. James A. Shayman for the kind donation of EtDO-P4; Marlin Lobaton for technical assistance; and Drs. Wai Cheu Lai and Steve Anderson for the preparation of the manuscript. This work was funded by the 2 R01 CA080054 “Glycosynapse in cancer cell adhesion and signaling” grant and the Biomembrane Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1115191108/-/DCSupplemental.
References
- 1.Haltiwanger RS, Lowe JB. Role of glycosylation in development. Annu Rev Biochem. 2004;73:491–537. doi: 10.1146/annurev.biochem.73.011303.074043. [DOI] [PubMed] [Google Scholar]
- 2.Hakomori S. Traveling for the glycosphingolipid path. Glycoconj J. 2000;17:627–647. doi: 10.1023/a:1011086929064. [DOI] [PubMed] [Google Scholar]
- 3.Miljan EA, Bremer EG. Regulation of growth factor receptors by gangliosides. Science STKE. 2002;2002:11–10. doi: 10.1126/stke.2002.160.re15. RE15. [DOI] [PubMed] [Google Scholar]
- 4.Rebbaa A, Chou PM, Bremer EG. Modulation of Growth Factor response in brain tumors by complex carbohydrates. Bull Cancer. 2004;91:E15–E60. [PubMed] [Google Scholar]
- 5.Todeschini AR, Dos Santos JN, Handa K, Hakomori SI. Ganglioside GM2-tetraspanin CD82 complex inhibits met and its cross-talk with integrins, providing a basis for control of cell motility through glycosynapse. J Biol Chem. 2007;282:8123–8133. doi: 10.1074/jbc.M611407200. [DOI] [PubMed] [Google Scholar]
- 6.Stanley P. Regulation of Notch signaling by glycosylation. Curr Opin Struct Biol. 2007;17:530–535. doi: 10.1016/j.sbi.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brückner K, Perez L, Clausen H, Cohen S. Glycosyltransferase activity of Fringe modulates Notch-Delta interactions. Nature. 2000;406:411–415. doi: 10.1038/35019075. [DOI] [PubMed] [Google Scholar]
- 8.Moloney DJ, et al. Fringe is a glycosyltransferase that modifies Notch. Nature. 2000;406:369–375. doi: 10.1038/35019000. [DOI] [PubMed] [Google Scholar]
- 9.Hay ED. The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev Dyn. 2005;233:706–720. doi: 10.1002/dvdy.20345. [DOI] [PubMed] [Google Scholar]
- 10.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: New insights in signaling, development, and disease. J Cell Biol. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinberg RA. Mechanisms of malignant progression. Carcinogenesis. 2008;29:1092–1095. doi: 10.1093/carcin/bgn104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 14.Hugo H, et al. Epithelial—mesenchymal and mesenchymal—epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213:374–383. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 15.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guan F, Handa K, Hakomori SI. Specific glycosphingolipids mediate epithelial-to-mesenchymal transition of human and mouse epithelial cell lines. Proc Natl Acad Sci USA. 2009;106:7461–7466. doi: 10.1073/pnas.0902368106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan F, Schaffer L, Handa K, Hakomori SI. Functional role of gangliotetraosylceramide in epithelial-to-mesenchymal transition process induced by hypoxia and by TGF-beta. FASEB J. 2010;24:4889–4903. doi: 10.1096/fj.10-162107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White ES, Muro AF. Fibronectin splice variants: Understanding their multiple roles in health and disease using engineered mouse models. IUBMB Life. 2011;63:538–546. doi: 10.1002/iub.493. [DOI] [PubMed] [Google Scholar]
- 19.White ES, Baralle FE, Muro AF. New insights into form and function of fibronectin splice variants. J Pathol. 2008;216:1–14. doi: 10.1002/path.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115:3861–3863. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- 21.Zardi L, et al. Transformed human cells produce a new fibronectin isoform by preferential alternative splicing of a previously unobserved exon. EMBO J. 1987;6:2337–2342. doi: 10.1002/j.1460-2075.1987.tb02509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuura H, Hakomori S. The oncofetal domain of fibronectin defined by monoclonal antibody FDC-6: Its presence in fibronectins from fetal and tumor tissues and its absence in those from normal adult tissues and plasma. Proc Natl Acad Sci USA. 1985;82:6517–6521. doi: 10.1073/pnas.82.19.6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuura H, et al. The oncofetal structure of human fibronectin defined by monoclonal antibody FDC-6. Unique structural requirement for the antigenic specificity provided by a glycosylhexapeptide. J Biol Chem. 1988;263:3314–3322. [PubMed] [Google Scholar]
- 24.Schachter H, Brockhausen I. In: Glycoconjugates: Composition, Structure, and Function. Allen H, Kisailus E, editors. New York: Marcel Dekker; 1992. pp. 263–332. [Google Scholar]
- 25.Matsuura H, Greene T, Hakomori S. An alpha-N-acetylgalactosaminylation at the threonine residue of a defined peptide sequence creates the oncofetal peptide epitope in human fibronectin. J Biol Chem. 1989;264:10472–10476. [PubMed] [Google Scholar]
- 26.Tarp MA, et al. Identification of a novel cancer-specific immunodominant glycopeptide epitope in the MUC1 tandem repeat. Glycobiology. 2007;17:197–209. doi: 10.1093/glycob/cwl061. [DOI] [PubMed] [Google Scholar]
- 27.Bennett EP, Hassan H, Clausen H. cDNA cloning and expression of a novel human UDP-N-acetyl-alpha-D-galactosamine. Polypeptide N-acetylgalactosaminyltransferase, GalNAc-t3. J Biol Chem. 1996;271:17006–17012. doi: 10.1074/jbc.271.29.17006. [DOI] [PubMed] [Google Scholar]
- 28.Bennett EP, et al. Cloning and characterization of a close homologue of human UDP-N-acetyl-alpha-D-galactosamine:Polypeptide N-acetylgalactosaminyltransferase-T3, designated GalNAc-T6. Evidence for genetic but not functional redundancy. J Biol Chem. 1999;274:25362–25370. doi: 10.1074/jbc.274.36.25362. [DOI] [PubMed] [Google Scholar]
- 29.Wandall HH, et al. Molecular basis for the presence of glycosylated onco-foetal fibronectin in oral carcinomas: The production of glycosylated onco-foetal fibronectin by carcinoma cells. Oral Oncol. 2007;43:301–309. doi: 10.1016/j.oraloncology.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Kong D, et al. Epithelial to mesenchymal transition is mechanistically linked with stem cell signatures in prostate cancer cells. PLoS ONE. 2010;5:e12445. doi: 10.1371/journal.pone.0012445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marian CO, et al. Evidence of epithelial to mesenchymal transition associated with increased tumorigenic potential in an immortalized normal prostate epithelial cell line. Prostate. 2011;71:626–636. doi: 10.1002/pros.21278. [DOI] [PubMed] [Google Scholar]
- 32.Lee L, Abe A, Shayman JA. Improved inhibitors of glucosylceramide synthase. J Biol Chem. 1999;274:14662–14669. doi: 10.1074/jbc.274.21.14662. [DOI] [PubMed] [Google Scholar]
- 33.Wandall HH, et al. Substrate specificities of three members of the human UDP-N-acetyl-alpha-D-galactosamine:Polypeptide N-acetylgalactosaminyltransferase family, GalNAc-T1, -T2, and -T3. J Biol Chem. 1997;272:23503–23514. doi: 10.1074/jbc.272.38.23503. [DOI] [PubMed] [Google Scholar]
- 34.Kilian O, et al. Expression of EDA+ and EDB+ fibronectin splice variants in bone. Bone. 2004;35:1334–1345. doi: 10.1016/j.bone.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 35.Kuan SF, Byrd JC, Basbaum C, Kim YS. Inhibition of mucin glycosylation by aryl-N-acetyl-alpha-galactosaminides in human colon cancer cells. J Biol Chem. 1989;264:19271–19277. [PubMed] [Google Scholar]
- 36.Sugrue SP, Hay ED. Response of basal epithelial cell surface and cytoskeleton to solubilized extracellular matrix molecules. J Cell Biol. 1981;91:45–54. doi: 10.1083/jcb.91.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hakomori S. Tumor-associated carbohydrate antigens. Annu Rev Immunol. 1984;2:103–126. doi: 10.1146/annurev.iy.02.040184.000535. [DOI] [PubMed] [Google Scholar]
- 38.Hakomori S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer Res. 1996;56:5309–5318. [PubMed] [Google Scholar]
- 39.Hakomori S. Glycosylation defining cancer malignancy: New wine in an old bottle. Proc Natl Acad Sci USA. 2002;99:10231–10233. doi: 10.1073/pnas.172380699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varki A, Kannagi R, Toole BP. Glycosylation changes in cancer. In: Varki A, et al., editors. Essentials of Glycobiology. 2nd Ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2009. pp. 617–632. [Google Scholar]
- 41.Gahmberg CG, Hakomori S. Surface carbohydrates of hamster fibroblasts. I. Chemical characterization of surface-labeled glycosphingolipids and aspecific ceramide tetrasaccharide for transformants. J Biol Chem. 1975;250:2438–2446. [PubMed] [Google Scholar]
- 42.Nudelman ED, Levery SB, Igarashi Y, Hakomori S. Plasmalopsychosine, a novel plasmal (fatty aldehyde) conjugate of psychosine with cyclic acetal linkage. Isolation and characterization from human brain white matter. J Biol Chem. 1992;267:11007–11016. [PubMed] [Google Scholar]
- 43.Ledeen RW, Yu RK, Eng LF. Gangliosides of human myelin: Sialosylgalactosylceramide (G7) as a major component. J Neurochem. 1973;21:829–839. doi: 10.1111/j.1471-4159.1973.tb07527.x. [DOI] [PubMed] [Google Scholar]
- 44.Turley EA, Veiseh M, Radisky DC, Bissell MJ. Mechanisms of disease: Epithelial-mesenchymal transition—does cellular plasticity fuel neoplastic progression? Nat Clin Pract Oncol. 2008;5:280–290. doi: 10.1038/ncponc1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barnett P, et al. Snail-mediated regulation of reactive oxygen species in ARCaP human prostate cancer cells. Biochem Biophys Res Commun. 2011;404:34–39. doi: 10.1016/j.bbrc.2010.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gan Y, et al. Differential roles of ERK and Akt pathways in regulation of EGFR-mediated signaling and motility in prostate cancer cells. Oncogene. 2010;29:4947–4958. doi: 10.1038/onc.2010.240. [DOI] [PubMed] [Google Scholar]
- 47.Wallerand H, et al. The epithelial-mesenchymal transition-inducing factor TWIST is an attractive target in advanced and/or metastatic bladder and prostate cancers. Urol Oncol. 2010;28:473–479. doi: 10.1016/j.urolonc.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 48.Jiang YG, et al. Role of Wnt/beta-catenin signaling pathway in epithelial-mesenchymal transition of human prostate cancer induced by hypoxia-inducible factor-1alpha. Int J Urol. 2007;14:1034–1039. doi: 10.1111/j.1442-2042.2007.01866.x. [DOI] [PubMed] [Google Scholar]
- 49.Hakomori S, et al. In: Extracellular matric biochemistry. Piez KA, Reddi AH, editors. New York: Elsevier; 1984. pp. 230–275. [Google Scholar]
- 50.Fukuda M, Hakomori S. Carbohydrate structure of galactoprotein a, a major transformation-sensitive glycoprotein released from hamster embryo fibroblasts. J Biol Chem. 1979;254:5451–5457. [PubMed] [Google Scholar]
- 51.Murayama K, et al. The carbohydrate structure of human fibronectins: A comparison between normal embryonic lung fibroblasts WI-38 and the SV40 virus transformed cell line VA13. Glycoconj J. 1984;1:155–169. [Google Scholar]
- 52.Sekiguchi K, Siri A, Zardi L, Hakomori S. Differences in domain structure between human fibronectins isolated from plasma and from culture supernatants of normal and transformed fibroblasts. Studies with domain-specific antibodies. J Biol Chem. 1985;260:5105–5114. [PubMed] [Google Scholar]
- 53.Borsi L, et al. Monoclonal antibodies in the analysis of fibronectin isoforms generated by alternative splicing of mRNA precursors in normal and transformed human cells. J Cell Biol. 1987;104:595–600. doi: 10.1083/jcb.104.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borsi L, Balza E, Allemanni G, Zardi L. Differential expression of the fibronectin isoform containing the ED-B oncofetal domain in normal human fibroblast cell lines originating from different tissues. Exp Cell Res. 1992;199:98–105. doi: 10.1016/0014-4827(92)90466-l. [DOI] [PubMed] [Google Scholar]
- 55.Moustakas A, Heldin CH. Signaling networks guiding epithelial-mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci. 2007;98:1512–1520. doi: 10.1111/j.1349-7006.2007.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hynes RO. Integrins: Versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 57.Kim KK, et al. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci USA. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Câmara J, Jarai G. Epithelial-mesenchymal transition in primary human bronchial epithelial cells is Smad-dependent and enhanced by fibronectin and TNF-alpha. Fibrogenesis Tissue Repair. 2010;3:2. doi: 10.1186/1755-1536-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park JH, Katagiri T, Chung S, Kijima K, Nakamura Y. Polypeptide N-acetylgalactosaminyltransferase 6 disrupts mammary acinar morphogenesis through O-glycosylation of fibronectin. Neoplasia. 2011;13:320–326. doi: 10.1593/neo.101440. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.