Abstract
The Isua Supracrustal Belt, Greenland, of Early Archean age (3.81–3.70 Ga) represents the oldest crustal segment on Earth. Its complex lithology comprises an ophiolite-like unit and volcanic rocks reminiscent of boninites, which tie Isua supracrustals to an island arc environment. We here present zinc (Zn) isotope compositions measured on serpentinites and other rocks from the Isua supracrustal sequence and on serpentinites from modern ophiolites, midocean ridges, and the Mariana forearc. In stark contrast to modern midocean ridge and ophiolite serpentinites, Zn in Isua and Mariana serpentinites is markedly depleted in heavy isotopes with respect to the igneous average. Based on recent results of Zn isotope fractionation between coexisting species in solution, the Isua serpentinites were permeated by carbonate-rich, high-pH hydrothermal solutions at medium temperature (100–300 °C). Zinc isotopes therefore stand out as a pH meter for fossil hydrothermal solutions. The geochemical features of the Isua fluids resemble the interstitial fluids sampled in the mud volcano serpentinites of the Mariana forearc. The reduced character and the high pH inferred for these fluids make Archean serpentine mud volcanoes a particularly favorable setting for the early stabilization of amino acids.
Keywords: hydrothermal fluids, serpentinization, origin of life, subduction zone
The discovery of oceanic black smokers and their unique fauna prompted the idea that life may have sprung from hydrothermal vent fields at the bottom of the ocean (1–3). The highly reducing conditions of the vent fields associated with midocean ridges fulfill one of the most stringent conditions for the stabilization of biomolecules. These conditions are a consequence of the metamorphic hydration and oxidation of ultramafic rocks of the oceanic lithosphere—a series of reactions known as serpentinization—that release highly reduced hydrothermal fluids with high concentrations of methane, ammonia, and hydrogen (4, 5). Serpentinization also produces FeNi3, which catalyses formation of complex organic compounds (5). Serpentinization thus provides both a source of reduced carbon and a potential energy source, which, together, create an environment suitable for the emergence of the first biomolecules. The vast majority of hydrothermal vent fields, however, especially those hosted by midocean ridges, spout solutions with pH well below the pK of amino acids, which makes them unsuitable for Strecker synthesis (6, 7). Attention therefore shifted toward high-pH hydrothermal vent sites (8, 9) and notably toward the modern vent fluids from the unusual midocean ridge locality of Lost City.
The search for an Archean environment in which reducing and high-pH conditions coexist at temperatures appropriate for supporting early life prompted us to investigate Isua serpentinites and their associated hydrothermal carbonates (10). Precipitation of large amounts of carbonates suggests that carbonate ions were abundant in the parent fluid and therefore signals that the pH of this fluid was at least in the range of the second dissociation constant of carbonic acids, which for seawater at ambient temperature is approximately 9 (11). Recent work on isotope fractionation of Zn complexes in solution (hydrates, or aqua ions, chlorides, sulfides, sulfates, carbonates) (12, 13) indicates that Zn carbonates efficiently fractionate Zn isotopes. Measurements of Zn isotopes for Isua serpentinites revealed anomalous values (14). Therefore, a more systematic investigation of Zn isotopes in samples from this locality seemed promising. Focusing on this particular metal was reinforced by the proposition that, because transition elements are less mobile than the volatile elements commonly considered as potential biomarkers, Zn isotope compositions may reflect the original properties of Archean rocks more accurately than the isotope concentrations of carbon, sulfur, and nitrogen.
The complex lithology of the Isua Supracrustal Belt, Greenland (3.81–3.70 Ga old), includes metabasalts, which can be divided into an ophiolite-like unit and a second unit known as “Garbenschiefer,” the geochemistry of which is reminiscent of boninites (15) and, hence, ties Isua supracrustals to an island arc environment. Serpentinites are also abundant in the metabasalt series (16–18). Modern serpentinite samples from three representative geological settings also were investigated to provide a context for the Archean data. These are from (i) the magma-starved Gakkel Ridge (Arctic Ocean), which supplies well-serpentinized samples from a midocean ridge setting; (ii) Baja California and the Alps, which typify ophiolites obducted onto continents; and (iii) Mariana forearc serpentinite mud volcanoes (19), which represent a subduction zone setting far from continental influence.
Results and Discussion
The results are given in tabular form in SI Text and are plotted in Fig. 1. The data are reported in the conventional δ66Zn notation, which represents the fractional deviation in parts per one thousand of the 66Zn/64Zn sample ratio with respect to the ratio of the Lyon JMC Zn standard.
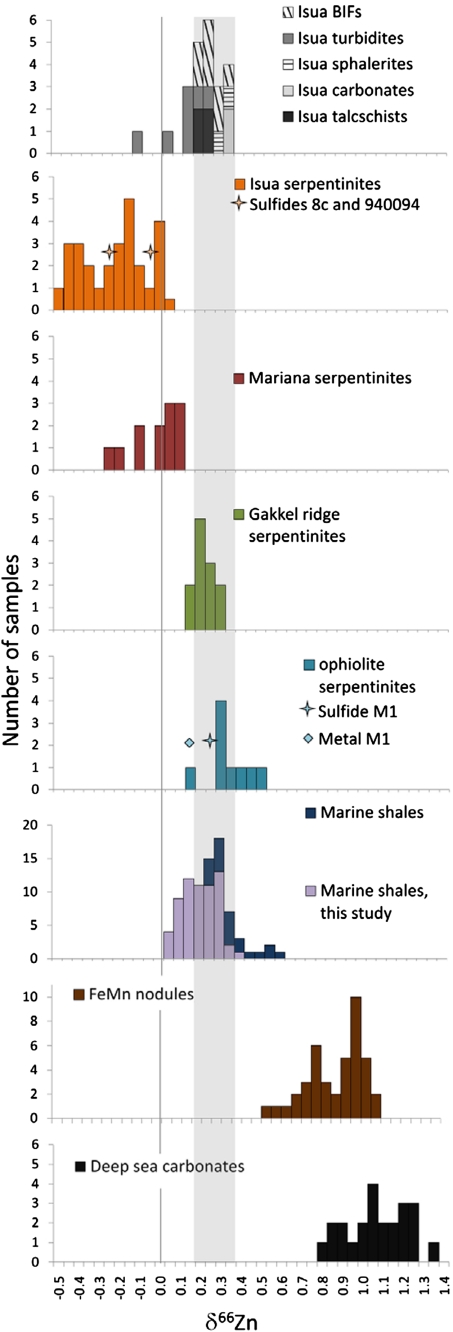
Fig. 1.
Zinc isotope compositions of Isua supracrustal rocks and serpentinites from the Mariana forearc, Gakkel Ridge, and Alpine ophiolites, compared with δ66Zn data in marine shales (this study and data from ref. 48), deep-sea carbonates (data from refs. 47 and 48), and FeMn nodules (data from ref. 31). The gray field represents the worldwide igneous average (20).
It is now well established (12, 20, 21) that the range of Zn isotope variations in the vast majority of igneous rocks and clastic sediments is fairly narrow (δ66Zn approximately 0.25–0.35‰). In contrast, Isua serpentinites are markedly depleted in the heavy Zn isotopes (δ66Zn = -0.48 to + 0.04‰ at the 95% confidence level with an average value of −0.19‰), whereas most δ66Zn values for serpentinites from two of the other types investigated here, the Baja California and Alpine ophiolites and the Gakkel Ridge, fall within the normal range of igneous rocks and clastic sediments (20). δ66Zn in serpentinites from the Marianas are shifted toward negative values by 0.2 to 0.5‰ relative to igneous samples, with an average δ66Zn value of −0.01‰, similar to that of Isua serpentinites within the reported analytical uncertainty. Pentlandite samples separated from the 8 c, 940094, and M1 serpentinites and metallic iron separated from sample M1 have Zn isotope compositions that are only slightly different from the corresponding whole-rock values. Sulfide and metal therefore seem to have formed or reequilibrated during serpentinization. In contrast, Isua sphalerite veins, talc schists, and hydrothermal (metasomatic) carbonates give igneous-like δ66Zn values (approximately 0.33, 0.24, and 0.35‰, respectively). Zn from Isua banded iron formations also fall within the general range of igneous and clastic rocks, whereas turbidites, with values ranging from -0.08 to + 0.28‰, can be interpreted as representing mixtures of igneous rock debris. Comparison of Zn serpentinite concentrations measured in this work with literature data (22) on peridotites suggests that about 30–60% Zn may be leached from the parent peridotite during serpentinization. Such an extent of Zn extraction requires that the nonigneous δ66Zn values of serpentinite reflect a strong partitioning of the light isotopes into the solid, whether Zn was present in the peridotite initially or was added later by fluid.
The Kinetic Isotope Effect.
In a number of cases, isotope fractionation is not the result of equilibrium processes, but rather the outcome of isotope-dependent reaction rates (known as the kinetic isotope effect or KIE). The role of KIE is well documented for hydrogen, carbon, and sulfur, especially in biologically mediated reactions (23). It has, in particular, been invoked to account for δ66Zn values down to −0.17‰ in sphalerite from the Irish Midlands ore field (21), but the negative correlation between δ66Zn and δ34S also observed at this locality would require an inverse rate isotope effect on sulfur (23), which has not so far been documented. Furthermore, the lack of fractionation of sulfur isotopes in Isua hydrothermal sulfides (24, 25) with respect to planetary abundances argues against a strong kinetic effect for Zn, which is much heavier than S.
Fractionation at Equilibrium.
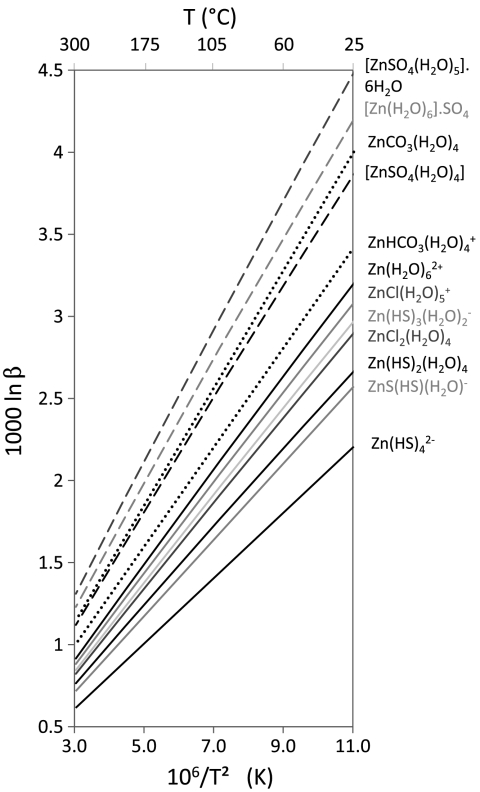
Recent theoretical work combining ab initio structure calculations and statistical mechanics (12, 13) is now allowing the role played by different species in solution in the fractionation of Zn isotopes to be determined over a broad range of temperatures. The ratio β of the partition functions for the 66Zn- and 64Zn-chloride, aqua ions, and sulfide isotopomers is very similar (Fig. 2), which discounts these species as being responsible for major isotope fractionation within the fluid. At temperatures of 100–350 °C and for solutions with carbonate and sulfate concentrations similar to that of seawater and other near-surface fluids, Zn2+ and Zn chloride and sulfide complexes are the dominant Zn species in low-pH solutions (26) reacting with peridotites. Solutions are dominated by Zn2+ at pH < 3, and, with increasing pH, by Zn(HS)2, 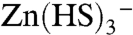 , and finally ZnS(HS)-. None of these species are expected to induce significant Zn isotope fractionation (Fig. 2), and this is exactly what is observed in solutions and sulfide ores from the hot low-pH environments of black-smoker vent fields (27). Progressive, Rayleigh-type leaching of sulfide can certainly account for the Zn depletion upon serpentinization but demands large and therefore unsupported isotope fractionation among S-rich species, typically > 1‰ for 50% Zn removal.
, and finally ZnS(HS)-. None of these species are expected to induce significant Zn isotope fractionation (Fig. 2), and this is exactly what is observed in solutions and sulfide ores from the hot low-pH environments of black-smoker vent fields (27). Progressive, Rayleigh-type leaching of sulfide can certainly account for the Zn depletion upon serpentinization but demands large and therefore unsupported isotope fractionation among S-rich species, typically > 1‰ for 50% Zn removal.
Fig. 2.
Theoretical isotope fractionation factors (β) between isotopomers as a function of temperature for different Zn species. Values are inferred from ab initio calculations for Zn in sulfate-rich (dashed lines, data from ref. 12), sulfide-rich (solid lines, data from ref. 13), and carbonate-rich fluids (dotted lines, data from ref. 13).
The Role of Sulfate.
Because Zn sulfate complexes (12) stand out as particularly enriched in heavy isotopes, the effect of Zn complexation by sulfate in hydrothermal fluids must be considered. At ambient temperature and for sulfate concentration typical of modern seawater (28.6 mmol kg-1), Zn-sulfate complexes are subordinate, making up less than a few percent of the metallic ion and chloride complexes (12, 28). Seawater-like abundances are too low to induce a substantial isotopic shift of the sulfide species, and therefore of the sulfide minerals precipitated from the solutions, toward negative δ66Zn values. Sulfate usually is absent from black-smoker vent solutions but may be present at low concentration levels in white smoker fluids such as at the TAG (290 °C) (27) and Lost City (40–70 °C) (29) vent fields on the Mid-Atlantic ridge. However, correlated Mg excesses strongly suggest that the white smoker sulfate originates from subsurface mixing of seawater and hydrothermal fluids. Regardless, the limited data on white smoker fluids do not indicate anomalous Zn isotope compositions (27). More generally, the range of variations of δ66Zn in sulfide ores from a wide variety of depositional environments (20, 21, 27, 30, 31) and, as shown in this study, also in Alpine ophiolites and Gakkel serpentinites, is quite narrow: The striking lack of negative δ66Zn values indicates that if Zn sulfate complexes were present in the parent hydrothermal fluid, they were not abundant enough to create major Zn isotope fractionation in hydrothermal solutions and sulfide deposits. Moreover, sulfate is widely thought to be missing from the Archean ocean (32), and the dominant sulfur species in Isua serpentinites is sulfide, not sulfate.
The Role of Carbonate.
For want of a strong isotopic effect induced by sulfates, complexation by carbonate ions is a potential alternative. In seawater and in other hydrous fluids equilibrated under surface conditions, carbonate complexes are not abundant (13, 28). High carbonate concentrations are unlikely along midocean ridges because there is no other source of CO2 than mantle outgassing and even that CO2 is largely reduced to methane by hydrogen. In addition, the pH of hydrothermal fluids is usually very low (< 5) and under such conditions, H2CO3 is not significantly dissociated. High carbonate concentrations can, however, be achieved at depths typical of arc environments, where subduction of carbonated basalts and calcareous sediments provides a potential source of CO2. Fuji et al. (13) considered the case for a CO2 pressure of 5 × 106 Pa, which may be equated with a depth of 1 km below the seafloor and 15% CO2 in the fluid. They concluded that, under these conditions and at temperatures < 150 °C, ZnCO3 dominates Zn species for pH > 8. Thus, Zn in high-pH, medium-temperature fluids is largely in the form of carbonate complexes. Carbonate concentrations in hydrothermal fluids can be estimated from pH and alkalinity, with the caveat that precipitation of hydrothermal carbonates, for which there is plenty of observational support (33), reduces alkalinity. In addition to the low δ66Zn observed, the widespread occurrence of carbonates at Isua (10) and in Mariana mud volcanoes away from the trench by > 70 km (33, 34), is a feature common to both sites and indicates that fluids in these localities were rich in carbonates. The presence of aragonite, a mineral species unstable under the conditions prevalent at the local seafloor and in Mariana serpentine mud volcanoes (34), strongly supports decarbonation as a CO2 source. The associated fluids have high alkalinity and H2S contents, and their carbon isotope compositions confirm that CO2 does not derive from the atmosphere but from the breakdown of subducted carbonates (33, 35, 36).
Isua Serpentine Mud Volcanoes and the Origin of Life.
The association of Isua serpentinites with carbonates and negative δ66Zn suggests that these rocks formed in conditions similar to those of the Mariana forearc, which is in line with previous conclusions about the Isua environment (15, 37). Of all the parameters that this comparison with mud volcanoes (33, 37) entails, the temperature range of 100–300 °C (19) and the high pH of fluids (9–12.6) are the most noticeable. Metamorphic transformation of ultramafic rocks requires massive CO2 uptake (10), while boiling of CO2-rich fluids causes a sharp increase of the pH of hydrothermal solutions and promotes crystallization of calcite, ankerite, and dolomite (38). Because, at depth, ZnCO3 accounts for most of the Zn dissolved in hydrothermal fluids (13), its δ66Zn is essentially unfractionated with respect to the bulk of the fluid. The igneous-like δ66Zn of Isua carbonate veins, therefore, are explained by precipitation from high-pH fluids percolating through serpentinites. In contrast, any sulfide precipitating from the same fluid must show markedly negative δ66Zn, which is what Isua and Mariana serpentinites do. In contrast, Isua talc schists and sphalerite veins contain Zn processed by hydrothermal transformation of peridotites at metamorphic temperatures around 500 °C (10), causing their δ66Zn to be essentially unfractionated with respect to the igneous range and, hence, reflect δ66Zn of the fluid.
The Isua environment is best interpreted as an equivalent of the Mariana forearc (19) with the Isua serpentinites being the Archean analogue of modern mud volcanoes. Modern active serpentine mud volcanoes are an unusual geological feature restricted to the Mariana and Izu-Bonin arc because the appropriate conditions at nonaccretionary, intraoceanic subduction zones are simply infrequent. The seismic structure of the Izu-Bonin underneath serpentine mud volcanoes shows low-strength serpentinite diapirs rising from the topmost layer of the mantle wedge above the subduction zone (39). This mechanism is consistent with the field observations at Isua, where serpentinite bodies occur as tectonized podded structures within pillow basalt units of the ophiolite sequence. An interpretation as a sea floor mud volcano is thus consistent with the overall forearc geologic environment (15, 37), with the composition of the ultramafic protolith of serpentines (40), and with the occurrence of a few δ66Zn values lower than the igneous average in Isua turbidites, for which a dacitic or andesitic protolith has been acknowledged (18). An intriguing implication is that carbonate-rich seafloor lithologies were being subducted by 3.8 Ga.
Our data favor the existence of warm, highly reducing hydrothermal fluids with high pH in early Archean serpentinite mud volcanoes. In a world endowed with plate tectonics but with smaller continental expanses than today, intraoceanic arcs such as the Mariana arc must have been common and, hence, also serpentine mud volcanoes. The presence of extremophilic Archaea on a Mariana forearc serpentinite mud volcano and their role in oxidizing methane from the ascending fluid to carbonate ion and organic carbon has been previously noted (41). Forearcs have the added appeal that, in addition to serpentinization being a major source of hydrogen, subaerial volcanoes provide a proximal source of phosphorus, an indispensable nutrient for all forms of life, in a world where continents had not yet reached their modern surface areas (42). Unlike midocean ridges, which are generally deepwater structures and not a source but a sink for phosphorus (43), weathering of nearby aerial volcanic edifices from the arc system provides forearc vent field oceanic environments with a sustainable supply of phosphorus. The onset of plate tectonics, which presumably took place sometime during the Hadean, in addition to the existence of a water ocean with carbonate sedimentation and the resulting ocean-continent dichotomy, may have fostered the emergence of life on our planet in mud volcanoes.
Materials and Methods
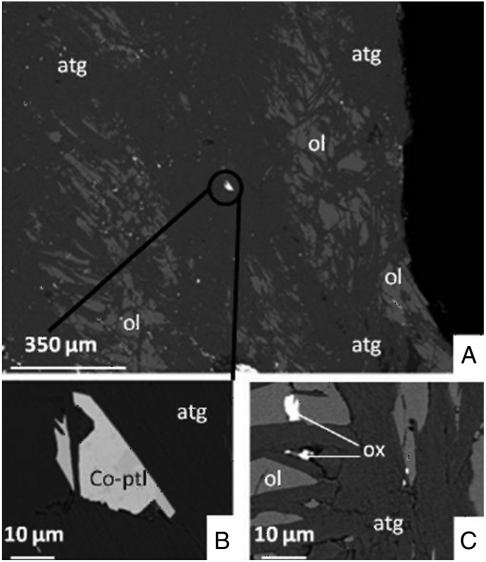
Samples analyzed from Isua are serpentinites, talc schists, veins of sphalerite (ZnS), metasomatic carbonates, and banded iron formations (BIF). Sulfides from two Isua serpentinites (samples 8c and 940094) and from Baja California sample M1 were purified by sieving and heavy-liquid separation. Analysis of Isua serpentinites by secondary electron microscopy (SEM) coupled with energy-dispersive X-ray spectroscopy (EDX) did not reveal the presence of sphalerite (Fig. 3). Instead, the dominant sulfide mineral in Isua serpentinites is cobalt-rich pentlandite, a common mineral in Archean mafic and ultramafic rocks (44). Pentlandite is also the sulfide mineral present in the M1 serpentinite. A metal phase was extracted from sample M1 using a hand magnet. The analytical techniques used for the chemical separation of Zn and for the isotopic analyses have been described elsewhere (45, 46) and are only repeated briefly here. Zinc was separated on Dowex AG1-X8 anion-exchange resin using HBr. Because ion-exchange resins fractionate Zn isotopes, full yields are required. By analyzing all the recovered fractions, we were able to demonstrate that the yield was better than 99%. All reagents were distilled in PFA stills. Zinc isotope ratios were measured by multiple collection inductively-coupled plasma mass spectrometry (MC-ICP-MS) at ENS-Lyon on a Nu Plasma 500 HR. The samples were taken up in 0.05 N HNO3 and run in wet plasma mode with free-aspiration using a glass microconcentric nebulizer (uptake rate: 80 μL/ min) and a glass cyclonic spray chamber. Peak intensities (M = 64, 66, 67, and 68) were measured in Faraday detectors in static mode with a spectral resolution of M/ΔM = 300. We used Cu NIST-SRM976 for doping the samples and an exponential mass fractionation law to correct for the instrumental mass bias. Samples were bracketed with standards, randomized, and the measurements duplicated. Sample solutions were diluted to match the concentration of the standard mixture (Zn 0.5 ppm–Cu 0.5 ppm). The total procedural blank including sample dissolution, chemical purification steps, and mass spectrometry measurements was 15 ng of Zn, which represents less than 0.5% of the total sample signal. The external reproducibility on δ66Zn based on repeated measurements of the Zn standard is 0.05‰.
Fig. 3.
SEM images of a section of Isua peridotite 8c. atg: antigorite; ol: olivine; Co-ptl: cobaltian pentlandite; ox: oxide (Fe-Ni-Cr). (A) Only one sulfide (black circle) is present. (B) The sulfide is cobaltian-pentlandite (Fe19Ni29Co4S48). (C) Other white spots in A are not sulfides but oxides as analyzed by EDS.
Supplementary Material
Acknowledgments.
We are grateful to Henry Dick, who provided the Gakkel Ridge samples with support from the National Science Foundation to the Woods Hole Oceanographic Institution collection; Bertrand Van de Moortèle and the Centre Lyonnais de Microscopie for the SEM images; Philippe Télouk and Emmanuelle Albalat for maintaining an efficient and well-functioning work environment; Janne Blichert-Toft for generously editing the English text; Mike Mottl for reviewing an earlier version of the manuscript; and anonymous reviewers for providing very helpful scientific and editorial comments. We thank the Institut des Sciences de l’Univers for steady support to F.A., in particular through the Origine des Planètes et de la Vie Program and the Agence Nationale pour la Recherche through the Begdy grant. Grants from the Carslberg Foundation and the Danish National Research Foundation to M.R. supported field work in Greenland. Support of the Danish National Research Fund to NordCEE is gratefully acknowledged. The International Ocean Drilling Project provided samples from the Marianas. The Lyon ICP-MS facility is supported by Institut National des Sciences de l'Univers and the Ecole Normale Supérieure de Lyon.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1108061108/-/DCSupplemental.
References
- 1.Corliss JB. The flow of energy, natural learning systems and the creation of life on Earth. Acta Astronaut. 1989;19:869–873. [Google Scholar]
- 2.Wächtershäuser G. Groundworks for an evolutionary biochemistry: The iron-sulphur world. Progr Biophys Mol Biol. 1992;58:85–201. doi: 10.1016/0079-6107(92)90022-x. [DOI] [PubMed] [Google Scholar]
- 3.Cody GD, et al. Geochemical roots of autotrophic carbon fixation: hydrothermal experiments in the system citric acid, H2O-( ± FeS)-( ± NiS) Geochim Cosmochim Acta. 2001;65:3557–3576. [Google Scholar]
- 4.Holm NG, Charlou JL. Initial indications of abiotic formation of hydrocarbons in the Rainbow ultramafic hydrothermal system, Mid-Atlantic Ridge. Earth Planet Sci Lett. 2001;191:1–8. [Google Scholar]
- 5.Sleep NH, Meibom A, Fridriksson T, Coleman RG, Bird DK. H2-rich fluids from serpentinization: Geochemical and biotic implications. Proc Nat Acad Sci USA. 2004;101:12818–12823. doi: 10.1073/pnas.0405289101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferris JP, Joshi PC, Edelson EH, Lawless JG. HCN: A plausible source of purines, pyrimidines and amino acids on the primitive earth. J Mol Evol. 1978;11:293–311. doi: 10.1007/BF01733839. [DOI] [PubMed] [Google Scholar]
- 7.Cleaves H, Chalmers J, Lazcano A, Miller S, Bada J. A reassessment of prebiotic organic synthesis in neutral planetary atmospheres. Orig Life Evol Biosph. 2008;38:105–115. doi: 10.1007/s11084-007-9120-3. [DOI] [PubMed] [Google Scholar]
- 8.Shibuya T, Komiya T, Nakamura K, Takai K, Maruyama S. Highly alkaline, high-temperature hydrothermal fluids in the early Archean ocean. Precambrian Res. 2010;182:230–238. [Google Scholar]
- 9.Russel MJ, Hall AJ. The onset and early evolution of life. In: Kesler SE, Ohmoto H, editors. Evolution of Early Earth’s Atmosphere, Hydrosphere, and Biosphere—Constraints from Ore Deposits. Boulder, CO: Geol Soc Am; 2006. pp. 1–28. (GSA Memoir 198). [Google Scholar]
- 10.Rosing MT, Rose NM. The role of ultramafic rocks in regulating the concentrations of volatile and non-volatile components during deep crustal metamorphism. Chem Geol. 1993;108:187–200. [Google Scholar]
- 11.Patterson CS, Busey RH, Mesmer RE. Second ionization of carbonic acid in NaCl media to 250 °C. J Solution Chem. 1984;13:647–661. [Google Scholar]
- 12.Black JR, Kavner A, Schauble EA. Calculation of equilibrium stable isotope partition function ratios for aqueous zinc complexes and metallic zinc. Geochim Cosmochim Acta. 2011;75:769–783. [Google Scholar]
- 13.Fujii T, Moynier F, Pons ML, Albarède F. The origin of Zn isotope fractionation in sulfides. Geochim Cosmochim Acta. 10.1016/j.gca.2011.09.036. [Google Scholar]
- 14.Ben Othman D, Luck JM, Bodinier JL, Albarède F. Cu-Zn isotopic variations in Precambrian and present-day mantle. Geophys Res Abstr. 2005;7:06732. [Google Scholar]
- 15.Polat A, Hofmann AW, Rosing MT. Boninite-like volcanic rocks in the 3.7–3.8 Ga Isua greenstone belt, West Greenland: Geochemical evidence for intra-oceanic subduction zone processes in the early Earth. Chem Geol. 2002;184:231–254. [Google Scholar]
- 16.Dymek RF, Boak JL, Brothers SC. Titanian chondrodite- and titanian clinohumite-bearing metadunite from the 3800 Ma Isua supracrustal belt, West Greenland; chemistry, petrology and origin. Am Mineral. 1988;73:547–558. [Google Scholar]
- 17.Dymek RF, Brothers SC, Schiffries CM. Petrogenesis of ultramafic metamorphic rocks from the 3800 Ma Isua supracrustal belt, West Greenland. J Petrol. 1988;29:1353–1397. [Google Scholar]
- 18.Nutman AP, Friend CRL. New 1∶20,000 scale geological maps, synthesis and history of investigation of the Isua supracrustal belt and adjacent orthogneisses, southern West Greenland: A glimpse of Eoarchaean crust formation and orogeny. Precambrian Res. 2009;172:189–211. [Google Scholar]
- 19.Fryer P, Gharib J, Ross K, Savov I, Mottl MJ. Variability in serpentinite mudflow mechanisms and sources: ODP drilling results on Mariana forearc seamounts. Geochem Geophy Geosy. 2006;7 10.1029/2005GC001201. [Google Scholar]
- 20.Albarède F. Johnson CM, Beard BL, Albarede F, editors. The stable isotope geochemistry of copper and zinc, in Geochemistry of Non-Traditional Stable Isotopes. Rev Mineral Geochem. 2004;55:409–427. [Google Scholar]
- 21.Wilkinson JJ, Weiss DJ, Mason TFD, Coles BJ. Zinc isotope variation in hydrothermal systems: Preliminary evidence from the Irish Midlands ore field. Econ Geol. 2005;100:583–590. [Google Scholar]
- 22.Le Roux V, Lee C-TA, Turner SJ. Zn/Fe systematics in mafic and ultramafic systems: Implications for detecting major element heterogeneities in the Earth’s mantle. Geochim Cosmochim Acta. 2010;74:2779–2796. [Google Scholar]
- 23.Wolfsberg M, Hook WA, Paneth P, Rebelo LPN. Isotope Effects in the Chemical, Geological, and Bio Sciences. New York: Springer; 2009. [Google Scholar]
- 24.Monster J, et al. Sulfur isotope studies in early Archaean sediments from Isua, West Greenland: Implications for the antiquity of bacterial sulfate reduction. Geochim Cosmochim Acta. 1979;43:405–413. [Google Scholar]
- 25.Strauss H. Sulphur isotopes and the early Archaean sulphur cycle. Precambrian Res. 2003;126:349–361. [Google Scholar]
- 26.Tagirov BR, Seward TM. Hydrosulfide/sulfide complexes of zinc to 250 °C and the thermodynamic properties of sphalerite. Chem Geol. 2010;269:301–311. [Google Scholar]
- 27.John SG, Rouxel OJ, Craddock PR, Engwall AM, Boyle EA. Zinc stable isotopes in seafloor hydrothermal vent fluids and chimneys. Earth Planet Sci Lett. 2008;269:17–28. [Google Scholar]
- 28.Long DT, Angino EE. Chemical speciation of Cd, Cu, Pb, and Zn in mixed freshwater, seawater, and brine solutions. Geochim Cosmochim Acta. 1977;41:1183–1191. [Google Scholar]
- 29.Kelley DS, et al. An off-axis hydrothermal vent field near the Mid-Atlantic Ridge at 30 °N. Nature. 2001;412:145–149. doi: 10.1038/35084000. [DOI] [PubMed] [Google Scholar]
- 30.Maréchal CN, Nicolas E, Douchet C, Albarède F. Abundance of zinc isotopes as a marine biogeochemical tracer. Geochem Geophy Geosy. 2000;1 10.1029/1999GC000029. [Google Scholar]
- 31.Mason TFD, et al. Zn and Cu isotopic variability in the Alexandrinka volcanic-hosted massive sulphide (VHMS) ore deposit, Urals, Russia. Chem Geol. 2005;221:170–187. [Google Scholar]
- 32.Canfield DE. A new model for Proterozoic ocean chemistry. Nature. 1998;396:450–453. [Google Scholar]
- 33.Mottl MJ, Wheat CG, Fryer P, Gharib J, Martin JB. Chemistry of springs across the Mariana forearc shows progressive devolatilization of the subducting plate. Geochim Cosmochim Acta. 2004;68:4915–4933. [Google Scholar]
- 34.Fryer P, Mottl MJ. Lithology, mineralogy and origin of serpentine muds drilled at Conical Seamount and Torishima Forearc Seamount; Proc Ocean Drill Prog Sci Results; College Station, TX: Ocean Drilling Program; 1992. pp. 343–362. 10.2973/odp.proc.sr.125.126.1992. [Google Scholar]
- 35.Lupton J, et al. Submarine venting of liquid carbon dioxide on a Mariana Arc volcano. Geochem Geophy Geosy. 2006;7 10.1029/2005GC001152. [Google Scholar]
- 36.Fryer P, Wheat CG, Mottl MJ. Mariana blueschist mud volcanism: Implications for conditions within the subduction zone. Geology. 1999;27:103–106. [Google Scholar]
- 37.Furnes H, Rosing M, Dilek Y, de Wit M. Isua supracrustal belt (Greenland)—a vestige of a 3.8 Ga suprasubduction zone ophiolite, and the implications for Archean geology. Lithos. 2009;113:115–132. [Google Scholar]
- 38.Drummond SE, Ohmoto H. Chemical evolution and mineral deposition in boiling hydrothermal systems. Econ Geol. 1985;80:126–147. [Google Scholar]
- 39.Kamimura A, et al. Crustal structure study at the Izu-Bonin subduction zone around 31 °N: Implications of serpentinized materials along the subduction plate boundary. Phys Earth Planet Int. 2002;132:105–129. [Google Scholar]
- 40.Friend CRL, Nutman AP. Dunites from Isua, Greenland: A ca. 3720 Ma window into subcrustal metasomatism of depleted mantle. Geology. 2011;39:663–666. [Google Scholar]
- 41.Mottl MJ. Deep-slab fluids fuel extremophilic Archaea on a Mariana forearc serpentinite mud volcano: Ocean Drilling Program Leg 195. Geochem Geophy Geosy. 2003;4 10.1029/2003GC000588. [Google Scholar]
- 42.Albarède F, Blichert-Toft J. The cradle of life. In: Gérin M, Maurel MC, editors. Origins of Life: Self-Organization and/or Biological Evolution?; EDP Sciences; 2009. pp. 1–12. [Google Scholar]
- 43.Wheat CG, McManus J, Mottl MJ, Giambalvo E. Oceanic phosphorus imbalance: Magnitude of the mid-ocean ridge flank hydrothermal sink. Geophys Res Lett. 2003;30 [Google Scholar]
- 44.Ripley EM. Sulfide petrology of basal chilled margins in layered sills of the Archean Deer Lake Complex, Minnesota. Contrib Mineral Petrol. 1979;69:345–354. [Google Scholar]
- 45.Moynier F, Albarède F, Herzog GF. Isotopic composition of zinc, copper, and iron in lunar samples. Geochim Cosmochim Acta. 2006;70:6103–6117. [Google Scholar]
- 46.Maréchal CN, Télouk P, Albarède F. Precise analysis of copper and zinc isotopic compositions by plasma-source mass spectrometry. Chem Geol. 1999;156:251–273. [Google Scholar]
- 47.Bentahila Y, Ben Othman D, Luck JM. Strontium, lead and zinc isotopes in marine cores as tracers of sedimentary provenance: A case study around Taiwan orogen. Chem Geol. 2008;248:62–82. [Google Scholar]
- 48.Pichat S, Douchet C, Albarède F. Zinc isotope variations in deep-sea carbonates from the eastern equatorial Pacific over the last 175 ka. Earth Planet Sci Lett. 2003;210:167–178. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.