Abstract
We describe a crystal structure, at atomic resolution (1.1 Å, 100 K), of a ruthenium polypyridyl complex bound to duplex DNA, in which one ligand acts as a wedge in the minor groove, resulting in the 51° kinking of the double helix. The complex cation Λ-[Ru(1,4,5,8-tetraazaphenanthrene)2(dipyridophenazine)]2+ crystallizes in a 1∶1 ratio with the oligonucleotide d(TCGGCGCCGA) in the presence of barium ions. Each complex binds to one duplex by intercalation of the dipyridophenazine ligand and also by semiintercalation of one of the orthogonal tetraazaphenanthrene ligands into a second symmetrically equivalent duplex. The result is noncovalent cross-linking and marked kinking of DNA.
Keywords: DNA oligonucleotide, ruthenium complex, X-ray crystal structure
Transcription, the first step leading to gene expression, is dependent on protein-induced distortions in DNA structure (1) to enhance its activation and rate. Protein molecules are known to bind sequence-specifically to DNA and can cause local bending or kinking (2). A DNA kink occurs when stacked base pairs become unstacked and allow the now strained DNA to make a sharp turn. It is a phenomenon almost exclusively observed with protein–DNA interactions (3, 4), with kinking caused by covalent binding of platinum drugs to adjacent guanine N7 positions in the major groove being a rare example of this effect caused by small molecules (5). Kinking by semiintercalation of a small molecule has been long suspected but has thus far proved difficult to demonstrate (6). In this article, we report the induction of a substantial kink in duplex DNA by a simple ruthenium polypyridyl complex.
The interactions between ruthenium polypyridyl complexes and nucleic acids have been studied for over 25 y (7, 8) because of the ability of these metallocompounds to recognize the handedness of DNA, their luminescent “light-switch” properties, and their photosensitization of reactions causing damage to DNA (9–12). Surprisingly, there are no examples to date of a full structural study of any ruthenium polypyridyl complexes with nucleic acids, even though the binding of [Ru(phen)3]2+ (phen, 1,10-phenanthroline) to DNA has been well studied via spectroscopic and hydrodynamic methods (7, 8, 13, 14), leading to the concept of binding via semiintercalation into the double helix (6). Although the enantiomers of this complex bind to DNA with modest binding constants, a much stronger interaction has been observed for [Ru(phen)2(dppz)]2+ (dppz, dipyridophenazine), which has attracted much attention because of its “light-switching” behavior when binding to duplex DNA. This enhanced binding was proposed to be due to intercalation of the dppz ligand between the base pairs of the DNA duplex, although in the absence of crystallographic data, there was initially considerable debate as to the precise nature of the binding site (9, 10, 15–20).
One of the motivations for our present study is the ability of ruthenium–dppz complexes to target photooxidation of nucleic acids (12, 21, 22), perhaps eventually within the living cell. It is very well established that incorporating electron-deficient ligands, such as 1,4,5,8-tetraazaphenanthrene (TAP), into octahedral ruthenium complexes results in excited states that are sufficiently oxidizing to cause direct oxidation of a guanine moiety and subsequent covalent adduct formation with nucleic acids (23). The [Ru(TAP)2(dppz)]2+ complex is nearly isostructural with the much studied [Ru(phen)2(dppz)]2+, which has been shown to bind strongly to a wide range of double-stranded DNA sequences. In contrast to its phenanthroline analogue, the excited state of [Ru(TAP)2(dppz)]2+ is quenched in the presence of guanine, and picosecond transient absorption experiments suggest that oxidation of guanine may proceed by proton-coupled electron transfer (21).
Recently, we reported the separation of dppz complexes on Sephadex ion-exchange columns (24) and have now used this technique to isolate the Λ and Δ enantiomers of [Ru(TAP)2(dppz)]2+. In the present paper, we report the binding of the Λ-enantiomer 1 (Fig. 1C) to the self-complementary decamer d(TCGGCGCCGA). This sequence was chosen for its ability to form both double-stranded B-DNA and Holliday junctions (25–27). Given recent literature reports demonstrating the ability of ruthenium polypyridyl complexes to bind to secondary structures of nucleic acids other than duplexes, such as quadruplex DNA and i-motif conformations (28), bulges and loops, etc. (29), we were intrigued to see how [Ru(TAP)2(dppz)]2+ would bind to this oligonucleotide.
Fig. 1.
Experimental design. (A) The stacked-X Holliday junction, from Protein Data Bank ID 3GOM. Ba2+ cation sites shown in gold, occupancies between 0.4 and 0.3. (B) Chemical formula of 1, Λ-dipyrido[3,2-a:2′,3′-c]phenazine-bis(1,4,5,8-tetraazaphenanthrene)-ruthenium(II).
Results
Barium Binding to the Holliday Junction Forming Sequence d(TCGGCGCCGA).
As part of an ongoing study of Holliday junction structure in the presence of metal ions, we determined the structure of this sequence in the presence of barium ions as the sole divalent cation (SI Text, Structure Determination for d(TCGGCGCCGA)4with Barium Ions). Crystals of d(TCGGCGCCGA)4 were obtained in the presence of 20 mM barium ions. Five barium sites are found in the asymmetric unit of two DNA strands, all with occupancies between 0.3 and 0.4 (Fig. 1A). There is no direct coordination of barium ion to any DNA base.
Binding of Λ-[Ru(TAP)2(dppz)]2+ to the Same d(TCGGCGCCGA) Sequence.
Crystallization of the decamer in the presence of the Λ-enantiomer 1 (Fig. 1B) was conducted under conditions closely similar to those needed to form the Holliday junction. Small, pink-orange, almost cubic crystals were seen after a few days at 277 K. No crystals are formed between the Δ-enantiomer and this sequence under any conditions tested as of yet. Diffraction data were obtained using in-house equipment from a crystal with longest dimension less than 0.1 mm. Atomic resolution data to 1.1 Å were subsequently obtained on beam line I02 at the Diamond Light Source, United Kingdom (SI Text, Structure Determination for d(TCGGCGCCGA) in the Presence of Barium Ions and Λ-[Ru(1,4,5,8-tetraazaphenanthrene)2(dipyridopyrazine)]2+, and Figs. S1 and S2).
Our structural analysis revealed a symmetrical duplex containing a twofold axis running through the midpoint. In each half-duplex, two different binding modes of 1 can be seen (Fig. 2 A and D). The dppz ligand of 1 is intercalated at the terminal G9-A10 step, whereas one TAP ligand (TAP1) is semiintercalated (wedged) at the G3-G4 step (Fig. 3). Each half-duplex also contains a barium ion, bound to G3 and G4 in the major groove (Fig. 3A), and 87 water molecules. The resulting overall arrangement is shown in Fig. 3A, which illustrates the way in which four symmetry-equivalent decamer strands provide the complete environment for a single chiral ruthenium complex. See the stereo pair Fig. S3 for a representation including the bound water. The stoichiometry of the interaction is 1∶1, thus four symmetry-equivalent ruthenium complexes, conversely, interact with a single DNA strand. The A10 terminal residue is flipped out (Fig. 2B), forming a reverse Watson–Crick base pair with T1 of another symmetry-equivalent duplex (Fig. 3A), giving the pairing shown in Fig. 4B, with the six-membered ring of adenine stacked with the dppz pyrazine ring. Most strikingly, the intercalation geometry of 1 results in distinct roles for the two chemically identical TAP ligands (distinguished as TAP1 and TAP2). Fig. 3 shows these differences, which also result in the enantiomeric specificity.
Fig. 2.
Structure of the d(TCGGCGCCGA)-[Ru(TAP)2(dppz)]2+ complex. (A) Side view of the complex with two symmetry-related strands colored pale green and blue, and the ruthenium cation 1 in pink. The twofold axis is horizontal. Electron density for the duplex and intercalated TAP ligands at 1.0σ (0.19e Å-3). (B) Residue numbering for the d(TCGGCGCCGA) strand, with the bases color coded. The same color code is used wherever the bases are distinguished by color. (C) Block sketch of the two binding modes (redrawn from ref. 6). (D) View into the minor groove of the assembly. The flipped-out adenine A10 can be seen stacking onto the dppz ligand.
Fig. 3.
Semiintercalation, kinking, and cross-linking—the environment of a ruthenium complex. (A) Architecture of the cross-linking: One d(TCGGCGCCGA)2 unit showing barium ions and a single intercalated cation of 1 with the ruthenium complex in space-filling mode. Ligands on the ruthenium are distinguished by color: dppz, pink; TAP1, purple; TAP2, white. Hydrogen atoms of the ligands are shown in calculated positions. All other ruthenium cations are omitted for clarity. A second symmetry-related duplex is shown to generate the complete environment of the cation. (B) TAP1 semiintercalated between G3 and G4. (C) Angles between the normals to the G3 and G4 purine and TAP1 planes.
Fig. 4.
Intercalation of the dppz ligand. (A) The C2-G9 base pair, showing the stacking of G9 onto the central pyrazine ring of the dppz ligand. (B) The reverse Watson–Crick T1-A10 base pair formed by the flipped-out A10. The purine ring of A10 is again stacked onto the pyrazine ring of the dppz. Bases are color coded as in Fig. 1, and the ruthenium ligands as in Fig. 3, but using a paler shade of pink for the dppz ligand for clarity.
Semiintercalative Binding Mode of the TAP Ligand.
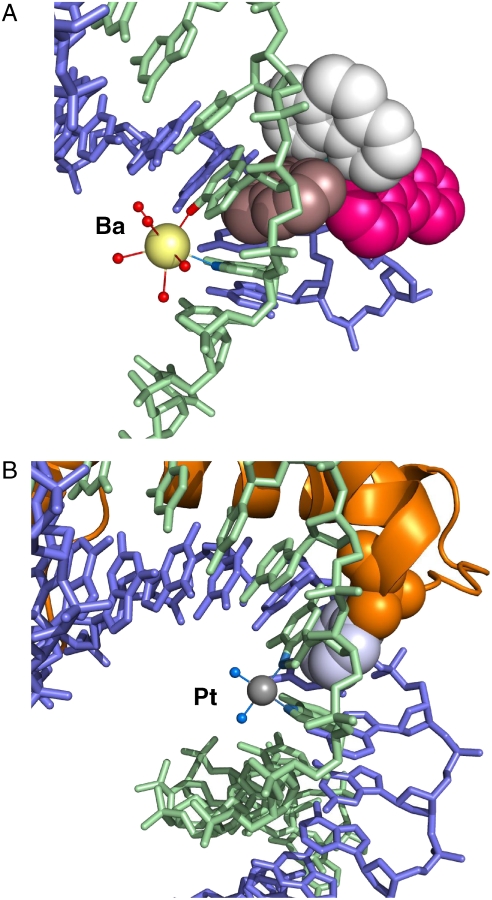
The TAP1 ligand semiintercalates from the minor groove at the G3-G4 step, interacting solely with the guanine component of the base pair but not the complementary C7-C8. TAP2 lies between the minor grooves of two duplexes and remains inert in terms of the supramolecular structure. The semiintercalation of TAP1 into the G3-G4 step, together with the flipping out creating the T1-A10 base pair generates the observed linking of symmetry-equivalent duplexes. TAP1 makes a series of close contacts with the six-membered purine rings of G3 and G4, generating an overall roll angle of 51°, similar to that of 52° in the catabolite activator protein-DNA complex (30). Inspection of the planes shows that the alignment here is not symmetrical, the G3-TAP1 plane angle being 37.5°, with the G4-TAP1 plane angle much smaller at 18.9° (Fig. 3C). The net effect of this asymmetry is that there is stronger stacking between residue G4 and TAP1. Additionally, semiintercalation creates a binding site for a barium ion quite unlike that seen in the absence of 1, with direct coordination to the N7 positions of G3 and O6 and N7 of G4 (Fig. 5A).
Fig. 5.
Kinking by platination in contrast to semiintercalation by 1. (A) Hydration of the barium ion and its coordination to N7 of guanine G3 and O6 of guanine G4 in the major groove. For Ba-ligand distances, see Table S2. The color scheme for 1 is as in Figs. 3A and 4. (B) Cisplatin-DNA adduct bound to high-mobility group B1 (HMGB1), Protein Data Bank ID 1CKT (37), in an approximately similar orientation. Platinum is shown as a small gray sphere, NH3 ligands as small blue spheres, HMGB1 as an orange ribbon, with the intercalating phenyl side chain of phenylalanine in space-fill mode with the phenyl group in gray. The platinum is directly coordinated to two N7 residues on adjacent guanine bases.
Intercalation of the Dppz Ligand.
As expected from spectroscopic and hydrodynamic data (9,10,15–20), the dppz ligand intercalates into the DNA duplex. As with the TAP semiintercalation, intercalation of the dppz ligand occurs from the minor groove. However, it does so in a “nonclassical” way because of the terminal T1-A10 pairing. The T1-A10 base pair has reverse Watson–Crick geometry with two full hydrogen bonds. The long axis of the dppz ligand is oriented at an angle of approximately 55° to the G9-C2 hydrogen bonds and at approximately 70° to the A10-T1 hydrogen bonds. (Fig. 4). The principal stacking interaction is between the pyrazine ring of the dppz ligand and the six-membered purine rings of G9 and A10, so that the ruthenium atom is approximately 6.5 Å distant from the helical axis of the duplex. The dppz ligand does not directly contact any of the surrounding water molecules.
Derived Parameters and Conformational Analysis.
A full analysis of torsion angle conformations has been conducted (31, 32) and shows that the duplex adopts a rather unusual conformation (Dataset S1). For the present purpose, a simpler analysis may be helpful for comparison, using the assignment of the sugar pucker (i.e., approximately C3′-endo and C2′-endo) to describe each residue in the five unique base pairs as “A” or “B” type. We observe, using this criterion, that each base pair in our sequence is composed of an A/B hybrid, and that the intercalation and semiintercalation steps are distinct in this respect (Table S1). The intercalation step can be written as 5′-AA-3′/3′-BB-5′, whereas the semiintercalation step can be written as 5′-AB-3′/3′-BA-5′. Examining the base pair and base pair step parameters in Table S2, the final T1-A10 base pair appears anomalous because of the flipped-out adenine and consequent reverse Watson–Crick base pairing, as shown by the opening angle of -170.9°. Otherwise, this base pair displays a rather neat matching around the intercalation cavity with the C2-G9 base pair, both showing buckling by 11–12° and propeller twisting of 7–8°. Thus, although it is nonclassical intercalation, these parameters highlight the balance in the stacking at this purine–purine interaction, with the pyrazine ring of the dppz group and illustrated by Fig. 4. In contrast, the TAP1 ligand causes the large positive roll angle of 50.8° at the semiintercalation (kink) step G3-G4/C8-C7, which is complemented by the low twist angle of 15.1° at this step. The adjacent G4-C5/C7-G6 step has a more typical small roll of -3°, but a larger twist of 38.7°. The TAP1 ligand interacting with the base pair G3-C8 gives the large positive buckle of 23.6°, whereas the other side of the semiintercalation step has the negative buckle of -9.4°, showing the greater asymmetry at this second purine–purine step.
Sequence Preference.
Another observation, arising from the binding of the dppz and TAP1 ligands, is the importance of adjacent purines in the oligonucleotide sequence. Writing the oligonucleotide sequence as py1-py2-pu3-pu4-py5-pu6-py7-py8-pu9-pu10 highlights the binding sites and shows that interaction of the complex with the duplex only occurs at these sites. The central C5-G6 step of the duplex, about the twofold rotation axis, forms no direct interaction with the bound 1.
Water Structure and Barium Coordination.
The major groove, although being highly compressed by the duplex kinking, contains nearly all the ordered water located in the analysis. Eighty-seven fully occupied water sites are observed per decamer single strand. The packing diagram shows a water channel running in the c axial direction right through the crystal lattice, surrounded by most of this ordered water (Fig. S1), which also includes the nine-coordinate barium ion. Six of the nine coordination positions are made up by this ordered water, the remaining being the direct coordination to G3 (N7) and G4 (O6 and N7), at distances of 2.852(7), 2.954(9), and 3.037(12) Å, respectively (Table S2). The high degree of ordering is possibly a consequence of the ordering created by the group II metal cation, as previously observed in our studies of water ordering in the Holliday junction cavity (26, 27).
Noncovalent Cross-Linking Interactions.
Another feature to arise from the binding of complex 1 to this oligonucleotide is the noncovalent cross-linking that occurs between the symmetry-equivalent DNA duplexes, which arises from several different interactions. As well as the semiintercalated TAP ligands, each duplex contains two AT base pairs (with reverse Watson–Crick geometry). In these base pairs, adenine A10 originates from another duplex, whereas the second adenine from the original duplex is donated to a third duplex, generating an infinite curved ladder in the c axial direction, arranged around the crystallographic 43 screw axis, which relates the duplexes.
Discussion
The work reported here allows a clear view of the binding of a polypyridyl ruthenium complex bound to an oligonucleotide. It remains to be seen whether the binding modes observed will be found with other ruthenium complexes and other DNA sequences. It is encouraging that, as outlined above, a range of biophysical data supports the semiintercalative binding mode with other ruthenium complexes, which is also a possible interpretation of NMR data on the binding of [Ru(phen)3]2+ enantiomers to d(GTGCAC)2 (33). For the purpose of structural comparison, there are three groups of crystallographic investigations that we consider particularly relevant. These deal with (i) noncovalent binding by chiral rhodium complexes, (ii) covalent platination of DNA, and (iii) a helicate of iron(II).
Barton and coworkers have reported detailed X-ray analyses of two rhodium compounds bound to dodecamer duplexes (34,35). The intercalation geometry we observe is, however, quite different from that observed for Δ-[Rh(bpy)2(chrysi)]2+(34), which showed intercalation into an AT/TA step from the major groove side, and also different to Δ-[Rh(Me2trien)phi]2+ (35), which intercalates into the major groove at the GC/CG step. Thus these rhodium complexes have both a different groove and different sequence preference from that in our study, which may be related to the Δ chirality of the compounds, although is it also to be expected that the nature of the organic ligand will be crucial in determining the sequence specificity of these inherently chiral cations.
The creation of a DNA kink by the semiintercalating TAP ligand forms an interesting comparison to the effect of DNA platination, for example, by the well-known antitumor agents cis-platin (5, 36) and oxaliplatin (37), which bind by direct coordination of the platinum to adjacent guanines. It is this kink that confers their cytotoxic properties, because architectural proteins such as high-mobility group B1 and B2 can bind to such distorted DNA, preventing the action of repair enzymes, inhibiting transcription, resulting in cell apoptosis (36, 38). A comparison of the structure of the DNA-bound [Ru(TAP)2(dppz)]2+ complex with that of cis-platin adducted to an oligonucleotide in the presence of protein is instructive (Fig. 5) because there are marked similarities in the overall curvature and alignment of the semiintercalated aromatic ring. In the absence of any protein, the platinated DNA [e.g., the hybrid dodecamer 5′(CCTCTG*G*TCTCC/ GGAGACCAGAGG), where *G* is the platination site] shows A-DNA character duplex on the 5′ side of the cis-platin binding site and B-DNA on the 3′ side. Interestingly, later NMR solution studies showed that, in solution, the platinated duplex is in the B form, but with a much larger kink at the platination site, probably illustrating the effect of crystal packing forces in the earlier study. In the structure of the closely related oxaliplatin bound to DNA, the platinum binding caused DNA kinking of 30°. Importantly, in that same structure, a barium ion, which is bound to two guanine residues in the major groove, caused no kinking (37), confirming that barium alone should not create a semiintercalation site.
Oleksi et al. (39) have reported the crystallization of a three-way DNA junction around a dinuclear iron(II) supramolecular helicate. Each of the three DNA strands are kinked in the center by some 60° between adjacent T and A bases, with which the polypyridyl ligands of the complex make stacking interactions, thus opening up a cavity in which to accommodate the tetracationic helicate.
It is interesting to speculate about whether complex 1 will bind to natural DNA by both dppz intercalation and TAP semiintercalation. Previous measurements carried out in solution with racemic [Ru(TAP)2(dppz)]2+ (40) were consistent with intercalation of the dppz being the principal mode of binding with both natural DNA and with double-stranded polynucleotides. Spectroscopic studies in progress with the resolved compounds and natural double-stranded DNA indicate that there are significant differences in the binding affinity of the enantiomers. Whether this difference is due to TAP semiintercalation, in addition to dppz intercalation, for the Λ-enantiomer will require more detailed hydrodynamic and related experiments. It may also be noted that it has recently been shown that some Ru–phen compounds can penetrate into the cell nucleus, where the high concentration of DNA may favor this interaction (41). This behavior, and the pronounced kinking induced here by the metal complex, suggests that such complexes might be very effective in modulating the transcription process. This discovery suggests significant potential for these small molecules in photodynamic therapy.
Materials and Methods
Synthesis of Λ-[Ru(TAP)2(dppz)]2+.
Rac-[Ru(TAP)2(dppz)]Cl2 was prepared by a modification of the literature method (40). The two enantiomers were successfully resolved by introducing an aqueous solution of rac-[Ru(TAP)2(dppz)]Cl2 onto a Sephadex C-25 column with 0.1 M (-)-O, O′-dibenzoyl-l-tartrate as the eluent at 2 mL/ min flow rate recycled by a peristaltic pump (23). The resulting fractions (the Λ enantiomer elutes first) were collected separately, precipitated as  salts and characterized using CD spectroscopy (Fig. S4), by comparison with previous reported spectra (42). Both enantiomers were passed through a second Sephadex C-25 column with aqueous NaCl as the eluent to ensure purity and to exchange the counterion.
salts and characterized using CD spectroscopy (Fig. S4), by comparison with previous reported spectra (42). Both enantiomers were passed through a second Sephadex C-25 column with aqueous NaCl as the eluent to ensure purity and to exchange the counterion.
Crystallization, Data Collection, Structure Solution, and Refinement.
Crystals containing the complex Λ-[Ru(TAP)2(dppz)]Cl2 (1) were grown by vapor diffusion from sitting drops at 276 K. Reddish-yellow rhombohedral crystals appeared after a few days. A crystal of approximate dimensions 0.2 × 0.2 × 0.2 mm was mounted in a 0.2-mm loop. Data were collected on beam line I02 at Diamond Light Source, Ltd. Data were processed with MOSFLM (43) and SCALA (44), giving 15,135 unique reflections. Subsequent anisotropic refinement was performed using REFMAC5 (45), version 5.5, against all data. Manual model editing was done using Coot (46). Five percent of reflections were used for the Rfree test. The model gave a final Rcryst of 0.10 and Rfree of 0.12. The quality of the maps obtained after refinement is shown in Fig. S2, drawn with PyMol (47). Final coordinates and data are deposited as Protein Data Bank ID 3QRN. For refinement statistics, full experimental details, including the initial phasing with SHELXC/D/E (48), and model building carried out in-house, using programs from the CCP4 suite (49); see SI Text, Structure Determination for d(TCGGCGCCGA) in the Presence of Barium Ions and Λ-[Ru(1,4,5,8-tetraazaphenanthrene)2(dipyridopyrazine)]2+.
Supplementary Material
Acknowledgments.
We thank Professor Andrée Kirsch-De Mesmaeker (Chimie Organique et Photochimie, Université Libre de Bruxelles, Brussels, Belgium) for very helpful comments and for the gift of the TAP ligand, Howard Colquhoun (Reading) for critical reading of the manuscript, James Sandy for assistance with the data collection at Diamond Light Source, Ltd., and Graeme Winter and Thomas Sorensen (Diamond Light Source) for helpful discussions and support with the project. We acknowledge financial support from The Royal Society, The Royal Irish Academy, Diamond Light Source, Ltd., The University of Reading, Science Foundation Ireland (Grant 06/RF/CHP035), Irish Research Council for Science Engineering and Technology, and European Cooperation in Science and Technology COST Action D35.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 3QRN, 3QF8, and 3GOM).
See Commentary on page 17573.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1108685108/-/DCSupplemental.
References
- 1.Crick FHC, Klug A. Kinky helix. Nature. 1975;255:530–533. doi: 10.1038/255530a0. [DOI] [PubMed] [Google Scholar]
- 2.Werner MH, Gronenborn AM, Clore GM. Intercalation DNA kinking, and the control of transcription. Science. 1996;271:778–784. doi: 10.1126/science.271.5250.778. [DOI] [PubMed] [Google Scholar]
- 3.Kim JL, Nikolov DB, Burley SK. Co-crystal structure of TBP recognizing the minor-groove of a TATA element. Nature. 1993;365:520–527. doi: 10.1038/365520a0. [DOI] [PubMed] [Google Scholar]
- 4.Schultz SC, Shields GC, Steitz TA. Crystal structure of a CAP-DNA complex: The DNA is bent by 90 degrees. Science. 1991;253:1001–1007. doi: 10.1126/science.1653449. [DOI] [PubMed] [Google Scholar]
- 5.Todd RC, Lippard SJ. Structure of duplex DNA containing the cisplatin 1,2 - {Pt(NH3)2}2+-d(GpG) cross-link at 1.77 Å resolution. J Inorg Bio. 2010;104:902–908. doi: 10.1016/j.jinorgbio.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lincoln P, Nordén B. DNA binding geometries of ruthenium(II) complexes with 1,10-phenanthroline and 2,2′-bipyridine ligands studied with linear dichroism spectroscopy. Borderline cases of intercalation. J Phys Chem B. 1998;102:9583–9594. [Google Scholar]
- 7.Kelly JM, Tossi AB, McConnell DJ, O’hUigin CA. Study of the interactions of some polypyridyl ruthenium(II) complexes with DNA using fluorescence spectroscopy, topoisomerization and thermal-denaturation. Nucleic Acids Res. 1985;13:6017–6034. doi: 10.1093/nar/13.17.6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barton JK. Metals and DNA—molecular left-handed complements. Science. 1986;233:727–734. doi: 10.1126/science.3016894. [DOI] [PubMed] [Google Scholar]
- 9.Hartshorn RM, Barton JK. Novel dipyridophenazine complexes of ruthenium(II)—exploring luminescent reporters of DNA. J Am Chem Soc. 1992;114:5919–5925. [Google Scholar]
- 10.Zeglis BM, Pierre VC, Barton JK. Metallo-intercalators and metallo-insertors. Chem Commun. 2007;44:4565–4579. doi: 10.1039/b710949k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vos JG, Kelly JM. Ruthenium polypyridyl chemistry; from basic research to applications and back again. Dalton Trans. 2006;41:4869–4883. doi: 10.1039/b606490f. [DOI] [PubMed] [Google Scholar]
- 12.Elias B, Kirsch-De Mesmaeker A. Photo-reduction of polyazaaromatic Ru(II) complexes by biomolecules and possible applications. Coord Chem Rev. 2006;250:1627–1641. [Google Scholar]
- 13.Barton JK, Danishefsky AT, Goldberg JM. Tris(phenanthroline)ruthenium(II)—stereoselectivity in binding to DNA. J Am Chem Soc. 1984;106:2172–2176. [Google Scholar]
- 14.Satyanarayana S, Dabrowiak JC, Chaires JB. Neither delta-tris(phenanthroline)ruthenium(II) nor lambda-tris(phenanthroline)ruthenium(II) binds to DNA by classical intercalation. Biochemistry. 1992;31:9319–9324. doi: 10.1021/bi00154a001. [DOI] [PubMed] [Google Scholar]
- 15.Jenkins Y, Friedman AE, Turro NJ, Barton JK. Characterization of dipyridophenazine complexes of ruthenium(II)—the light-switch effect as a function of nucleic-acid sequence and conformation. Biochemistry. 1992;31:10809–10816. doi: 10.1021/bi00159a023. [DOI] [PubMed] [Google Scholar]
- 16.Hiort C, Lincoln P, Nordén B. DNA-binding of delta-Ru(phen)2dppz2+ and lambda-Ru(phen)2dppz2+ J Am Chem Soc. 1993;115:3448–3454. [Google Scholar]
- 17.Haq I, et al. Interaction of delta- Ru(phen)2dppz2+ and lambda- Ru(phen)2dppz2+ with DNA—a calorimetric and equilibrium binding study. J Am Chem Soc. 1995;117:4788–4796. [Google Scholar]
- 18.Tuite E, Lincoln P, Nordén B. Photophysical evidence that Delta- and Lambda-Ru(phen)2(dppz)2+ intercalate DNA from the minor groove. J Am Chem Soc. 1997;119:239–240. [Google Scholar]
- 19.Dupureur CM, Barton JK. Structural studies of Λ- and Δ-[Ru(phen)2dppz]2+ bound to d(GTCGAC)2: Characterization of enantioselective intercalation. Inorg Chem. 1997;36:33–43. [Google Scholar]
- 20.Holmlin RE, Stemp EDA, Barton JK. Ru(phen)2dppz2+ luminescence: Dependence on DNA sequences and groove-binding agents. Inorg Chem. 1998;37:29–34. doi: 10.1021/ic970869r. [DOI] [PubMed] [Google Scholar]
- 21.Elias B, et al. Photooxidation of guanine by a ruthenium dipyridophenazine complex intercalated in a double-stranded polynucleotide monitored directly by picosecond visible and infrared transient absorption spectroscopy. Chem Eur J. 2008;14:369–375. doi: 10.1002/chem.200700564. [DOI] [PubMed] [Google Scholar]
- 22.Smith JA, George MW, Kelly JM. Transient spectroscopy of dipyridophenazine metal complexes which undergo photo-induced electron transfer with DNA. Coord Chem Rev. 2011;253:2021–2035. [Google Scholar]
- 23.Jacquet L, Davies RJH, Kirsch-De Mesmaeker A, Kelly JM. Photoaddition of Ru(tap)2(bpy)2+ to DNA: A new mode of covalent attachment of metal complexes to duplex DNA. J Am Chem Soc. 1997;119:11763–11768. [Google Scholar]
- 24.Vasudevan S, et al. Substituted dipyridophenazine complexes of Cr(III): Synthesis, enantiomeric resolution and binding interactions with calf thymus DNA. Dalton Trans. 2010;39:3990–3998. doi: 10.1039/c000150c. [DOI] [PubMed] [Google Scholar]
- 25.Hays FA, et al. How sequence defines structure: A crystallographic map of DNA structure and conformation. Proc Natl Acad Sci USA. 2005;102:7157–7162. doi: 10.1073/pnas.0409455102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thorpe JH, Gale BC, Teixeira SCM, Cardin CJ. Conformational and hydration effects of site-selective sodium, calcium and strontium ion binding to the DNA Holliday junction structure d(TCGGTACCGA)4. J Mol Biol. 2003;327:97–109. doi: 10.1016/s0022-2836(03)00088-3. [DOI] [PubMed] [Google Scholar]
- 27.Brogden AL, Hopcroft NH, Searcey M, Cardin CJ. Ligand bridging of the DNA Holliday junction: Molecular recognition of a stacked-X four-way junction by a small molecule. Angew Chem. 2007;119:3924–3928. doi: 10.1002/anie.200603760. [DOI] [PubMed] [Google Scholar]
- 28.Shi S, et al. Molecular “light switch” for G-quadruplexes and i-motif of human telomeric DNA: [Ru(phen)2(dppz)]2+ Dalton Trans. 2010;39:2490–2493. doi: 10.1039/b916094a. [DOI] [PubMed] [Google Scholar]
- 29.Keene FR, Smith JA, Collins JG. Metal complexes as structure-selective binding agents for nucleic acids. Coord Chem Rev. 2009;253:2021–2035. [Google Scholar]
- 30.Parkinson G, et al. Structure of the CAP-DNA complex at 2.5 Å resolution: A complete picture of the protein–DNA interface. J Mol Biol. 1996;260:395–408. doi: 10.1006/jmbi.1996.0409. [DOI] [PubMed] [Google Scholar]
- 31.Berman HM, et al. The nucleic acid database. Acta Crystallogr D Biol Crystallogr. 2002;58:889–898. doi: 10.1107/s0907444902003487. [DOI] [PubMed] [Google Scholar]
- 32.Svozil D, Kalina J, Omelka M, Schneider B. DNA conformations and their sequence preferences. Nucleic Acids Res. 2008;36:3690–3706. doi: 10.1093/nar/gkn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rehmann JP, Barton JK. 1H nmr studies of tris(phenanthroline) metal complexes bound to oligonucleotides: Characterisation of binding modes. Biochemistry. 1990;29:1701–1709. doi: 10.1021/bi00459a006. [DOI] [PubMed] [Google Scholar]
- 34.Pierre VC, Kaiser JT, Barton JK. Insights into finding a mismatch through the structure of a mispaired DNA bound by a rhodium intercalator. Proc Natl Acad Sci USA. 2007;104:429–434. doi: 10.1073/pnas.0610170104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kielkopf CL, Erkkila KE, Hudson BP, Barton JK, Rees DC. Structure of a photoactive rhodium complex intercalated into DNA. Nat Struct Biol. 2000;7:117–121. doi: 10.1038/72385. [DOI] [PubMed] [Google Scholar]
- 36.Ohndorf UM, Rould MA, He Q, Pabo CO, Lippard SJ. Basis for recognition of cis-platin-modified DNA by high-mobiity-group proteins. Nature. 1999;399:708–712. doi: 10.1038/21460. [DOI] [PubMed] [Google Scholar]
- 37.Spingler B, Whittington DA, Lippard SJ. 2.4 Å crystal structure of an oxaliplatin 1,2-d(GpG) intrastrand cross-link in a DNA dodecamer duplex. Inorg Chem. 2001;40:5596–5602. doi: 10.1021/ic010790t. [DOI] [PubMed] [Google Scholar]
- 38.Wang D, Zhu G, Huang X, Lippard SJ. X-ray structure and mechanism of RNA polymerase II stalled at an antineoplastic monofunctional platinum-DNA adduct. Proc Natl Acad Sci USA. 2010;107:9584–9589. doi: 10.1073/pnas.1002565107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oleksi A, et al. Molecular recognition of a three-way DNA junction by a metallosupramolecular helicate. Angew Chem. 2006;118:1249–1253. doi: 10.1002/anie.200503822. [DOI] [PubMed] [Google Scholar]
- 40.Ortmans I, Elias B, Kelly JM, Moucheron C, Kirsch-De Mesmaeker A. [Ru(TAP)2(dppz)]2+: A DNA intercalating complex, which luminesces strongly in water and undergoes photo-induced proton-coupled electron transfer with guanosine-5′-monophosphate. Dalton Trans. 2004:668–676. doi: 10.1039/b313213g. [DOI] [PubMed] [Google Scholar]
- 41.Puckett CA, Barton JK. Fluorescein redirects a ruthenium-octaarginine conjugate to the nucleus. J Am Chem Soc. 2009;131:8738–8739. doi: 10.1021/ja9025165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ardhammar M, Lincoln P, Rodger A, Nordén B. Absolute configuration and electronic state properties of light-switch complex [Ru(phen)2dppz]2+ deduced from oriented circular dichroism in a lamellar liquid crystal host. Chem Phys Lett. 2002;354:44–50. [Google Scholar]
- 43.Leslie AGW. Recent changes to the MOSFLM package for processing film and image plate data. Joint CCP4 + ESF-EAMCB Newsletter on Protein Crystallography. 1992. No. 26.
- 44.Evans PR. Scaling and assessment of data quality. Acta Crystallogr D Biol Crystallogr. 2005;62:72–82. doi: 10.1107/S0907444905036693. [DOI] [PubMed] [Google Scholar]
- 45.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 46.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DeLano WL. The PyMol Molecular Graphics System. San Carlos, CA: DeLano Scientific; 2002. [Google Scholar]
- 48.Sheldrick GM. Experimental phasing with SHELXC/D/E: Combining chain tracing with density modification. Acta Crystallogr D Biol Crystallogr. 2010;66:479–485. doi: 10.1107/S0907444909038360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Collaborative Computational Project, Number 4. The CCP4 suite: Programs for protein crystallography. Acta Crystallogr D Biol Crystallogr. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.