Abstract
Signal transducer and activator of transcription 3 (STAT3) plays a central role in the activation of multiple oncogenic pathways. Splicing variant STAT3β uses an alternative acceptor site within exon 23 that leads to a truncated isoform lacking the C-terminal transactivation domain. Depending on the context, STAT3β can act as a dominant-negative regulator of transcription and promote apoptosis. We show that modified antisense oligonucleotides targeted to a splicing enhancer that regulates STAT3 exon 23 alternative splicing specifically promote a shift of expression from STAT3α to STAT3β. Induction of endogenous STAT3β leads to apoptosis and cell-cycle arrest in cell lines with persistent STAT3 tyrosine phosphorylation compared with total STAT3 knockdown obtained by forced splicing-dependent nonsense-mediated decay (FSD-NMD). Comparison of the molecular effects of splicing redirection to STAT3 knockdown reveals a unique STAT3β signature, with a down-regulation of specific targets (including lens epithelium-derived growth factor, p300/CBP-associated factor, CyclinC, peroxisomal biogenesis factor 1, and STAT1β) distinct from canonical STAT3 targets typically associated with total STAT3 knockdown. Furthermore, similar in vivo redirection of STAT3 alternative splicing leads to tumor regression in a xenograft cancer model, demonstrating how pharmacological manipulation of a single key splicing event can manifest powerful antitumorigenic properties and validating endogenous splicing reprogramming as an effective cancer therapeutic approach.
Signal transducer and activator of transcription 3 (STAT3) is a member of the STAT family of transcription factors implicated in growth factor and cytokine signaling. Its activation is due to transient phosphorylation of cytoplasmic monomers that dimerize, translocate to the nucleus, and bind to specific promoter sequences, thereby regulating gene expression (1). Persistently phosphorylated STAT3 is observed in nearly 70% of human cancers. STAT3 is implicated in a vast range of normal and pathogenic processes including cellular proliferation, differentiation, survival, angiogenesis, metastasis, inflammation, and immune response, and acts as an oncogene to induce tumorigenesis while contributing to tumor-immune escape mechanisms (2–4).
STAT3's pivotal position at the convergence of many oncogenic pathways and dependence of some cancers on persistent STAT3 signaling (5, 6) makes it a prime target for cancer therapy (3, 5). Indeed, direct inhibition of STAT3 activity by overexpression of dominant-negative isoforms (7), antisense oligos (8), RNAi (9), peptides (10), and small drug inhibitors (11, 12) blocks growth and induces apoptosis in various model systems (3).
STAT3 exists in two main isoforms: full-length STAT3α and truncated STAT3β, generated by alternative splicing (AS) of exon 23 (13). STAT3β lacks the 55-residue C-terminal transactivation domain (TAD), replaced by seven unique amino acids. STAT3β can be efficiently phosphorylated at the key tyrosine 705 (Y705) but lacks serine 727 (S727), whose phosphorylation stimulates transcriptional activity (2). STAT3β can still bind to DNA as a homo- or heterodimer (with STAT3α or other transcription factors) (14). It was initially described as a dominant-negative factor and shown to inhibit gene transactivation, presumably by competitive promoter occupancy (15). In fact, STAT3β overexpression can induce apoptosis and inhibit tumor growth (7, 16). However, distinctive additional STAT3β properties have recently been described (6, 14) indicating that STAT3β is not simply a truncated version of STAT3α with dominant-negative properties but a protein whose unique biological functions contribute to the complex biology of STAT3. In particular, expression of STAT3β (without STAT3α) rescues embryonic lethality in STAT3-null mice (17). On the other hand, mice lacking only STAT3β have no apparent developmental abnormalities, but when challenged with endotoxins they exhibit impaired recovery and up-regulation of a subset of inflammation-related genes (18).
AS is a highly regulated process that generates functionally diverse protein isoforms, accounting for much of the complexity of the human genome, with >75% of human genes undergoing AS (19, 20). Aberrant AS leads to inappropriate expression levels of otherwise normal splice variants (21) and is particularly evident in cancer cells, with the appearance of hundreds of cancer-associated splicing isoforms that can provide growth advantage or play a determinative role in multiple aspects of the tumorigenic process (22).
Specific redirection of AS by antisense oligonucleotides constitutes a promising therapeutic approach (23) that shares with other antisense technologies (e.g., RNAi, RNAseH targeting) a high degree of specificity. However, rather than by degrading the encoding RNAs, the pathological target is reduced by inducing the expression of a different, potentially more desirable isoform.
Here we describe the use of phosphorodiamidate morpholino oligomers (morpholinos) to redirect endogenous STAT3 AS from STAT3α to STAT3β. This splicing switch is associated with increased cell death in cancer cells and with tumor regression in a xenograft model. Our results highlight STAT3β's ability to modulate the expression of a specific set of genes different from canonical STAT3 targets in breast cancer cells/tumors. The effectiveness of STAT3β induction in promoting tumor regression validates STAT3β's role as a powerful antitumorigenic molecule, and more in general showcases splicing redirection as a valid approach for cancer therapy.
Results
Deletion Analysis of STAT3 Exon 23.
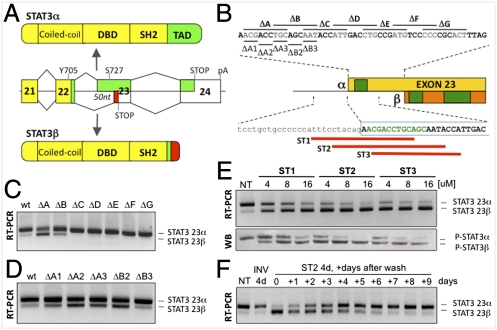
The use of an alternative acceptor site within STAT3 exon 23 causes an internal 50-nt deletion (Fig. 1A), but little is known about the regulation of this event (24). To identify exon 23 splicing regulatory cis-elements, we generated a STAT3 minigene containing exons 22 and 23, part of the large exon 24, and the intervening introns. Upon transient transfection, this minigene is spliced similarly to endogenous STAT3 (Fig. S1A), indicating that no essential regulatory elements were lost in the subcloning steps. A systematic deletion/transfection analysis of the region was then performed. A first set of seven partially overlapping 9-bp deletions (ΔA–ΔG) was introduced in the first 50 nt of exon 23 (α-specific region) from position +2 to +47 (Fig. 1B). Deletions ΔA and ΔB induced a switch from STAT3α to STAT3β (Fig. 1C), suggesting the presence of a putative exonic splicing enhancer (ESE), also predicted by ESEfinder and PESX algorithms (Fig. S1B). Smaller 3-bp deletions further restricted the putative ESE from position +2 to +12 (Fig. 1D). When the region of exon 23 common to both the α and β variants was similarly investigated with a set of 12-bp deletions (ΔH–ΔO), the low β baseline levels made it difficult to study β-inducing elements by deletion analysis (Fig. S1C). A second minigene was thus generated where the weak β pyrimidine tract was strengthened by a double mutation (βpy^), resulting in enhanced STAT3β splicing (Fig. S1D). ΔH–ΔO and other smaller deletion mutants were reanalyzed within this new context and two additional regulatory elements were identified (Fig. S1 E–G and Fig. 1B).
Fig. 1.
Modulation of STAT3 alternative splicing. (A) Alternative 3′ splice site use in exon 23 generates STAT3α or STAT3β, where the TAD (green) is substituted by a unique 7-aa tail (red). DBD, DNA binding domain. (B) STAT3 exon 23. Deletion mutants within the 50-nt α-specific region are indicated (Upper), as are the positions of the morpholinos (red lines) (Lower). The putative mapped ESEs are depicted in green. (C and D) RT-PCR analysis of STAT3 α/β levels in HeLa cells transfected with the 9-nt (ΔA–ΔG) or 3-nt (ΔA1–ΔB3) deletion mutants. (E) RT-PCR and Western blot analysis of STAT3 α/β levels in MDA-MB-435s cells treated with increasing concentrations of ST1, ST2, and ST3 for 4 d. NT, untreated. (F) RT-PCR analysis of STAT3α/β levels in MDA-MB-435s cells pretreated with either control (INV) or ST2 for 4 d and then grown in fresh media for up to 9 d.
Modulation of STAT3 Exon 23 Alternative Splicing.
The presence of a putative α-promoting ESE at the 5′ end of exon 23 prompted us to design a panel of three morpholinos directed at blocking the α 3′ splice site and/or the nearby putative ESE (Fig. 1B). MDA-MB-435s cells were treated with increasing concentrations (4, 8, and 16 μM) of ST1, ST2, and ST3 morpholinos. After 4 days (d), endogenous STAT3α and STAT3β were monitored at RNA and protein levels (Fig. 1E). All three oligonucleotides, but especially ST2 and ST3, induced a switch from STAT3α to STAT3β, indicating the importance of the identified ESE. A morpholino containing the inverted sequence (INV) was used as control (Fig. S2). ST2 was chosen for subsequent experiments, as it is 100% conserved in mouse and effective in human and murine cells (Fig. S3). This effect is sustainable, as the observed splicing switch could be maintained when MDA-MB-435s cells were treated with ST2 for 4 d, trypsinized, plated, and re-treated for two additional rounds, for a total of 12 d (Fig. S4). On the other hand, when cells treated for 4 d were placed in fresh media, the α:β ratio gradually reverted to its initial state (Fig. 1F).
Thus, morpholino antisense compounds can be used as tools to efficiently control STAT3 exon 23 AS at the endogenous level in multiple cell lines. This effect is reversible and depends on the continuous presence of the compounds in the media.
Knockdown of STAT3 by Forced Splicing-Dependent Nonsense-Mediated Decay.
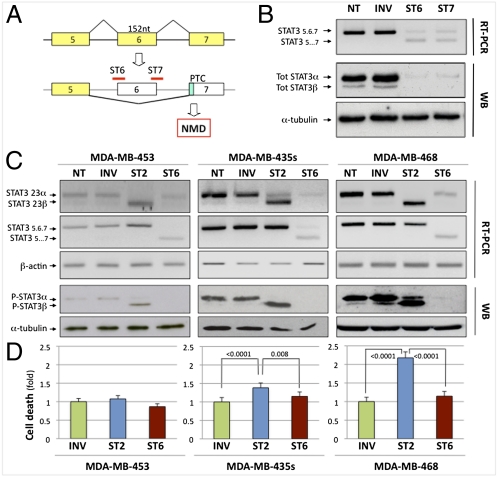
The described morpholinos allow us to induce the production of the STAT3β isoform while—simultaneously—reducing the level of the α isoform. It is, however, essential to distinguish between effects due to the appearance of the β isoform and those caused by the disappearance of the α one. We therefore designed two additional morpholinos to generate a null STAT3 mutant using a modified splicing redirection approach that exploits nonsense-mediated decay (NMD). To effectively knock down the target, forced splicing-dependent NMD (FSD-NMD) induces out-of-frame exon skipping that eventually triggers NMD (Fig. 2A). In this specific case, the two compounds (ST6 and ST7) were targeted to either splice sites of the 152 nt-long exon 6. Its skipping introduces a frameshift and thus a premature termination codon (PTC) in exon 7 (Fig. 2A), destabilizing STAT3 mRNA by NMD.
Fig. 2.
Effect of STAT3 alternative splicing modulation in vitro. (A) Knockdown of STAT3 by FSD-NMD. Morpholinos ST6 and ST7 cause skipping of exon 6, leading to a frameshift and a PTC, ultimately causing RNA degradation by NMD. (B) RT-PCR and WB analysis of total STAT3α/β levels in MDA-MB-435s cells treated with 16 μM ST6, ST7, or INV for 4 d. α-Tubulin was the loading control for WBs. (C) RT-PCR and WB analysis of STAT3 α/β levels in MDA-MB-453, MDA-MB-435s, and MDA-MB-468 cells treated with 16 μM INV, ST2, or ST6 for 4 d. β-Actin was used as control for RT-PCR. (D) Cell-death quantification (represented as fold change relative to control treatment) determined by trypan blue exclusion for treatments in C. The average of at least three independent experiments is represented, each in triplicate. P values were calculated by the stratified χ2 test; bars represent SE of the rate. See Figs. S7 and S8 for effects on cell cycle and apoptosis.
Indeed, when we treated MDA-MB-435s cells with either ST6 or ST7, they both induced exon 6 skipping and mRNA degradation. As a consequence, full-length STAT3 protein was virtually undetectable, with almost complete loss of both isoforms (Fig. 2B). To quantify the extent of the knockdown, we performed a side-by-side comparison with the more standard siRNA approach. As shown in Fig. S5, FSD-NMD is at least as efficient as the standard RNAi approach, with the added advantage that its results can be directly compared and contrasted to splicing redirection (ST2) without having to use two separate treatments, thus resulting in more properly controlled experiments. Both ST6 and ST7 efficiently knock down total STAT3, and ST6 was chosen for subsequent experiments because, like ST2, it also targets murine STAT3.
Effects of STAT3 Alternative Splicing Modulation in Vitro.
When overexpressed, STAT3β can interfere with STAT3's role in tumor growth and survival. We therefore decided to compare the effect on cell viability of inducing STAT3β versus knocking down both isoforms.
We used ST2 or ST6 to treat three breast cancer cell lines with variable levels of phospho-STAT3 (11) (MDA-MB-453 < MDA-MB-435s < MDA-MB-468; Fig. S6). MDA-MB-435s and MDA-MB-468 express moderate to high levels of phospho-STAT3 and have been previously described to be dependent upon activated STAT3 (11). As expected, cells treated with ST2 showed induction of STAT3β and reduction of STAT3α, whereas cells treated with ST6 showed reduction of both isoforms at the RNA and protein levels (Fig. 2C). After 4 d, cell viability in both treatment groups was assayed. Induction of STAT3β in the ST2-treated cells caused an increase in cell death in the MDA-MB-435s and MDA-MB-468 cells (Fig. 2D) compared with controls (INV) (respectively, 1.384-fold ± 0.129 SE, P < 0.0001, and 2.179-fold ± 0.160 SE, P < 0.0001), but no effect in MDA-MB-453 cells (1.076-fold ± 0.089 SE, P = 0.34). However, treatment of MDA-MB-435s and MDA-MB-468 cells with ST6, resulting in ablation of both STAT3 isoforms (Fig. 2C), had a lower effect on cell death than their treatment with ST2 (respectively, 1.151-fold ± 0.119 SE, P = 0.001, and 1.147-fold ± 0.127 SE, P < 0.0001) (Fig. 2D).
We then evaluated the effect of ST2 or ST6 on the cell cycle by flow cytometric analysis (Fig. S7). After 60 h of treatment, both MDA-MB-435s and MDA-MB-468 cells treated with ST2 displayed an increase in the G2/M population compared with INV or ST6 treatments (MDA-MB-435s: ST2: 20.8% ± 0.28 SE; INV: 14% ± 1.4 SE; ST6: 12.5% ± 3.5 SE; MDA-MB-468: ST2: 25% ± 1.4 SE; INV: 9% ± 4.24 SE; ST6: 12% ± 0.7 SE). The observed G2 arrest following ST2 treatment was associated with an increase in apoptosis in both cell lines as evaluated by annexin V staining (Fig. S8), consistent with the STAT3β-dependent reduction in cell viability described above.
Modulation of STAT3 Alternative Splicing in Vivo.
We next set out to test whether STAT3 AS modulation could affect tumor growth in vivo. To this end, we chose to use morpholinos covalently linked to an octaguanidine dendrimer (Vivo-Morpholinos) (25), which in tissue culture can self-deliver to multiple cell lines as efficiently as Endo-Porter-delivered naked morpholinos (Fig. S9A).
As antisense delivery to tumors is notoriously difficult, we initially tested delivery of these compounds into tumors by intravenous (IV) or intratumoral (IT) administration. Athymic mice subcutaneously implanted with MDA-MB-435s cells were randomly assigned to one of four treatment groups (Control-IV, ST2-IV, Control-IT, and ST2-IT) once tumors reached an average volume of 100 mm3 (n = 3 per group). Animals were treated twice in 1 wk and, 4 d after the second treatment, tumors were collected and analyzed. No obvious histopathological changes or difference in tumor size were observed between control and treated tumors. However, when tumor RNAs were analyzed, a switch from STAT3α to STAT3β was observed in two-thirds of the tumors treated by IT injection (but not by IV; Fig. S9B).
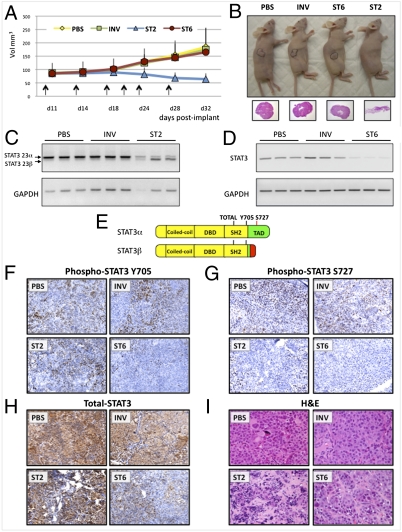
We then increased the length of the treatment and included the ST6 Vivo-Morpholino compound to better understand the relative contributions of the STAT3 isoforms to tumor growth. Mice were implanted as before (n = 5 per group) and Vivo-Morpholinos (INV, ST2, and ST6) or saline solution (PBS) were IT injected twice a week for a total of 3 wk while monitoring for tumor growth. Thirteen days after the first injections, tumors treated with the switching ST2 morpholino showed regression (Fig. 3 A and B), whereas tumors treated with the knockdown ST6 compound grew as well as PBS- and INV-treated tumors.
Fig. 3.
Effect of STAT3 alternative splicing modulation in vivo. (A) Following s.c. injection of 10 × 106 MDA-MB-435s cells, tumors of ∼100 mm3 were treated IT with 0.12 mg of Vivo-Morpholino (INV, ST2, and ST6) twice a week for 3 wk (arrows), and tumor volumes were measured. (B) Representative image of mice in each treatment group at time of sacrifice, with H&E staining of tumor sections underneath. (C and D) RT-PCR analysis of STAT3 exon 23 and exon 6 levels in three tumors for each treatment group. GAPDH was used as control. Primers recognize both human and mouse genes. (E) Epitope location of STAT3 antibodies used for IHC staining. (F–I) Representative images of tumor sections from the four different treatment groups following IHC using P-Y705, P-S727, and total STAT3 antibodies (F–H) or H&E staining.
RT-PCR for either STAT3 exon 23 (Fig. 3C) or STAT3 exon 6 (Fig. 3D) confirmed the efficacy of both ST2 and ST6 treatments in inducing the α-to-β splicing switch or total STAT3 knockdown. Tumor sections were analyzed by immunohistochemistry (IHC) with three STAT3 antibodies. Staining for total-STAT3 and P-Y705 (present in both STAT3α and STAT3β) was strongly decreased in ST6-treated tumors (Fig. 3 F and H), confirming that ST6 effectively knocked down STAT3. On the contrary, the level of both total STAT3 and P-Y705 in ST2- and control-treated tumors was comparable, consistent with the persistent expression of STAT3β and the maintenance of this phosphorylation site (Fig. 3 F and H). IHC for P-S727 (present only in STAT3α) was negative for both ST2- and ST6-treated tumors, further confirming the efficacy of these treatments at the protein level (Fig. 3G). Blinded analysis of tumor sections stained for hematoxylin and eosin (Fig. 3I) for estimated area of tumor necrosis and highest mitotic count showed that the ST2-induced splicing switch is the only treatment associated with a significant effect on tumor morphology. Histopathological examination of the ST2 tumors revealed that they had the lowest mitotic index, in agreement with the virtual absence of cancer cells (Fig. S10A), which could also account for lower scoring for necrosis and apoptosis indices (Fig. S10 B and C). Of particular interest is that whereas STAT3 knockdown was effective at the molecular level, it did not have any notable effects on tumor morphology, mitosis, apoptosis, and growth compared with controls, except for increased cytologic alterations (Fig. 3I and Fig. S10A).
Taken together, these data demonstrate that AS redirection of STAT3 in tumors is feasible and generates a potent inhibitor of tumorigenesis. Furthermore, it reveals the importance of context, as the effects of STAT3β induction in vivo are only partially recapitulated in vitro.
STAT3β Unique Transcriptional Signature.
To understand how switching from STAT3α to STAT3β by splicing modulation can decrease cancer cell viability more efficiently than knocking down both STAT3 isoforms, we set out to determine which STAT3 target genes may be mediating this process.
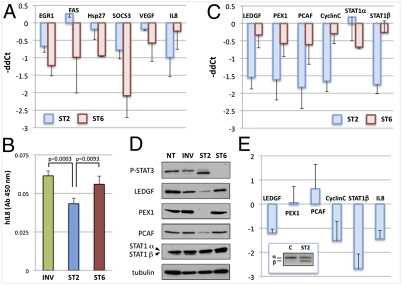
Following treatment for 4 d with ST2, ST6, or INV, cDNAs from MDA-MB-435s cells were analyzed by quantitative (q)PCR for expression of a panel of STAT3-established targets. In agreement with previous results, the treatments resulted in robust β switching or knockdown of STAT3 (Fig. S11). Total STAT3 knockdown by ST6 resulted in a somewhat modest but consistent down-regulation of the known STAT3 targets analyzed. However, ST2-induced STAT3β did not behave as a “dominant-negative” regulator of transcription (Fig. 4A). Indeed, even upon quantitative α-to-β switch, the expression of the analyzed canonical targets is minimally affected. An exception is represented by interleukin 8 (IL8), whose expression mRNA is down-regulated more by the splicing switch than by total STAT3 knockdown both at the RNA (Fig. 4A) and protein level, as confirmed by ELISA on media from treated MDA-MB-435s cells (Fig. 4B).
Fig. 4.
STAT3β switch modulates specific target genes. (A) MDA-MB-435s cells treated with 16 μM ST2, ST6, or INV for 4 d were analyzed by qPCR. Results are represented as a comparison between ST2 and INV (blue) or between ST6 and INV treatments (red), expressed as −ddC(t) values after normalization to hypoxanthine phosphoribosyl transferase (HPRT). Data represent the average of at least three independent experiments. (B) IL8 quantification by ELISA on conditioned media from MDA-MB-435s cells treated as in A (n = 4). P values were calculated by two-tailed Student's t test. To validate STAT3β targets identified by microarray analysis, cDNAs from A were analyzed as above by qPCR (C) and lysates were analyzed by WB, with α-tubulin as control (D). (E) Quantitative PCR analysis of STAT3β target genes from tumor RNAs from xenograft experiments in which tumors were injected IT twice in a week with vehicle (C) or ST2s. Results are represented as a comparison between ST2 and control, expressed as −ddC(t) values after normalization to β-2-microglobulin. Data are from at least three independent experiments. Bars represent SD. (Inset) RT-PCR analysis of STAT3 levels in the same RNAs.
To investigate whether STAT3β might control additional targets similarly to IL8, we compared ST2- or ST6-treated versus INV-treated MDA-MB-435s cells by microarray analysis. Genes with a significant fold change ≥2 (Fig. S12) were further investigated by qPCR and Western blot (WB; Fig. 4 C and D). Five genes were confirmed to be down-regulated both at the RNA and protein level by the switch to STAT3β, but not by the knockdown: lens epithelium-derived growth factor (LEDGF/PSIP1, a chromatin-binding protein and transcriptional coactivator; a prosurvival and growth factor) (26); peroxisomal biogenesis factor 1 (PEX1; part of the AAA ATPase subfamily, required for peroxisomal import) (27); CyclinC (CCNC; controls transcription by associating with Cdk8 and modulating RNA pol II activity and regulates both G0/G1 and G2/M transitions) (28); p300/CBP-associated factor (PCAF; histone acetyltransferase and transcriptional coactivator, promotes growth, invasion, and drug resistance) (29); and STAT1β (proliferative antagonist isoform of tumor suppressor STAT1) (30). In the case of STAT1β, the effect is indirect or posttranscriptional, as STAT1α is not affected (Fig. 4C) and the two variants are generated from the same pre-mRNA by AS. Notably, the microarray analysis confirmed that none of the canonical STAT3 targets, except for IL8, were strongly down-regulated by the STAT3β induction in MDA-MB-435s cells. In MDA-MB-468 cells, the switch from STAT3α to STAT3β resulted in down-regulation of LEDGF, PEX1, CyclinC, and STAT1β but not PCAF or IL8 (Fig. S13). More importantly, these genes were affected by the switch to STAT3β also in vivo. In fact, expression of LEDGF, CyclinC, STAT1β, and IL8, but not PEX1 and PCAF, was down-regulated in tumors treated for 1 wk with ST2 (Fig. 4E).
Altogether, these data do not support STAT3β's role as a classical dominant-negative regulator of STAT3α but rather as a transcriptional regulator associated with its own specific set of target genes.
Knockdown and Rescue of STAT3β Targets.
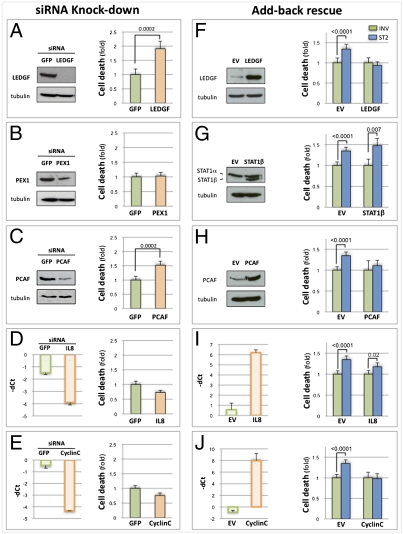
The set of target genes specifically down-regulated when STAT3 splicing is redirected from STAT3α to STAT3β has not been previously associated with STAT3β functions. To get a better understanding of their contribution to the observed biological effects, we took two different approaches. First, we tested whether their individual down-regulation was able to recapitulate the decrease in cell viability observed with the ST2 treatment. MDA-MD-435s cells were treated with siRNA against each of five target genes (no siRNAs are available to specifically target STAT1β but not STAT1α). Effective knockdown was verified by qPCR and WB, when suitable antibodies were available (Fig. 5 A–E), and cell viability was measured. Individual down-regulation of PEX1, IL8, or CyclinC did not lead to an increase in cell death (Fig. 5 B, D, and E). On the other hand, knockdown of LEDGF (Fig. 5A) and PCAF (Fig. 5C) was associated with a significant decrease in cell viability (respectively, 1.908-fold ± 0.261 SE, P = 0.0002, and 1.521-fold ± 0.139 SE, P = 0.0002).
Fig. 5.
Knockdown and overexpression of STAT3β target genes. (A–E) (Left) MDA-MB-435s cells were treated for 72 h with siRNA against GFP or LEDGF (A), PEX1 (B), PCAF (C), IL8 (D), or CyclinC (E). Knockdown was verified by WB (A–C, with α-tubulin as control) or by qPCR analysis (D and E), displayed as −dC(t) values after normalization to HPRT. (Right) Cell-death quantification was determined by trypan blue exclusion for MDA-MB-435s cells treated as described. Data are displayed as fold change of treated samples (orange) relative to control (green). Data are from three independent experiments (each in triplicate). P values were calculated by the stratified χ2 test; bars represent SE of the rate. (F) (Left) Lysates from MDA-MB-435s cells transiently transfected with LEDGF or empty vector were immunoblotted using an antibody to LEDGF. (Right) Transfected cells were concurrently treated with 16 μM INV or ST2 for 4 d. Cell death was quantified as above. (G–J) (Left) Stable MDA-MB-435s clones overexpressing STAT1β (G), PCAF (H), IL8 (I), CyclinC (J), or selected for the EV were treated with 16 μM INV or ST2 for 4 d. Overexpression levels were determined by WB (G and H, with α-tubulin as control) or qPCR analysis (I and J), and displayed as −dC(t) values normalized to HPRT. (Right) Cell death was quantified as above.
Next, we asked whether forced reexpression of the STAT3β target genes could protect against the increase in cell death induced by the α-to-β switch. We thus generated MDA-MB-435s-derived cell lines stably expressing four of the STAT3β target genes (STAT1β, PCAF, IL8, and CyclinC). We were unable to generate a stable LEDGF-expressing line, and therefore the equivalent experiment was performed under transient expression conditions. As PEX1 was not down-regulated in the treated tumors and its down-regulation in MDA-MB-435s cells did not have any effect on cell viability, we did not pursue it further. As above, expression was verified by qPCR and/or WB (Fig. 5 F–J).
Cells were concurrently treated with either ST2 or INV compounds, and cell viability was measured after 4 d. Forced expression of STAT1β did not protect against cell death induced by the splicing switch (Fig. 5G) compared with cells selected for integration of the empty vector (EV) (1.347-fold increase following ST2 treatment, ±0.089 SE, P < 0.0001; STAT1β, 1.472-fold increase, ±0.175 SE, P = 0.007). Expression of IL8 (Fig. 5I) showed a reduced but still significant increase in cell death (1.178-fold increase, ±0.094 SE, P = 0.02). However, overexpression of LEDGF, PCAF, and CyclinC all protected cells from the α-to-β shift (Fig. 5 F and H–J), as ST2 treatment had no effect on their viability, unlike what was observed with control cells (EVtransient, 1.337-fold increase, ±0.119 SE, P < 0.0001; LEDGFtransient, 0.935-fold increase, ±0.092 SE, P = 0.36; PCAF, 1.108-fold increase, ±0.13 SE, P = 0.3; CCNC, 0.978-fold increase, ±0.122 SE, P = 0.3).
Taken together, these data suggest that the effect of STAT3β on cell viability is likely mediated by the combined down-regulation of a specific set of target genes rather than exerted through a single major effector, in agreement with STAT3 pleiotropic functions.
Discussion
Persistent STAT3 activation contributes to multiple aspects of tumorigenesis. Overexpression of its main splicing variant, STAT3β, inhibits cancer cell growth in vitro and in vivo, and these antitumorigenic properties are frequently ascribed to dominant-negative properties (7, 16), although recent studies have revealed new biological properties of STAT3β independent of its dominant-negative functions (17, 18). The data presented in this work support this view also for cancer cells wherein the relative abundance of STAT3α and STAT3β is maintained within a physiological range.
By specifically redirecting STAT3 splicing with antisense compounds, we were able to elucidate STAT3β functions in a physiological context, avoiding the artifactual results often associated with overexpression. In addition, knockdown of STAT3 by FSD-NMD, a modified splicing redirection approach that purposely destabilizes target mRNAs by triggering NMD, allowed us to uncouple the effects of the induction of STAT3β from the down-regulation of STAT3α using a single experimental approach.
Our study demonstrates that the induction of the β variant has a much greater effect on cell growth/viability compared with the knockdown of both isoforms, particularly in vivo, where it causes tumor regression. Further, the expression profile of a panel of STAT3 canonical target genes was not impacted by the switch from STAT3α to STAT3β (unlike what was observed when STAT3β is exogenously overexpressed at high levels). Rather, a unique expression signature seems to be associated with the physiological α-to-β splicing shift.
The observation that the STAT3β switch has a more profound effect in vivo than in vitro resembles what was described for STAT3 knockdown and abrogation of gp130 signaling (9, 31), and can be explained by STAT3 involvement in various aspects of the oncogenic process, including angiogenesis. In addition, the morpholino target sequences are conserved in mouse, so STAT3 splicing could potentially be redirected also in the surrounding murine cells. Although it is clear that the treatments were effective on human tumor cells (Fig. 3 F–I), additional studies will be required to address this aspect, as STAT3β induction in the tumor microenvironment, including stromal and immune cells, could amplify the effects of the splicing switch in tumor cells and may represent an essential contribution to the in vivo efficacy of the approach.
The modest-to-null effect on cell viability of STAT3 knockdown in vitro and in vivo, in contrast to some previous reports (8–12), is noteworthy. However, different cell type, cellular context, STAT3 activation status, and inhibitory approach and experimental design could account for these differences. For example, we acutely down-regulated endogenous STAT3 expression in tumors using antisense compounds, whereas other studies used stable down-regulation or kinase inhibition to limit tumor growth. Clearly, kinase inhibitors may affect more than just STAT3 activity, and could account for the observed difference. Indeed, a pan-JAK inhibitor (P6) (12) blocks STAT3 activation and inhibits proliferation in MDA-MB-468 cells (as does STAT3β induction by ST2), whereas specific knockdown by ST6 under similar conditions has no effect compared with control (Fig. S14), indicating that lack of phospho-STAT3 is not sufficient, per se, to inhibit growth even in cells described to be highly sensitive to its depletion such as MDA-MB-468.
The surprising lack of inhibition of the analyzed canonical STAT3 targets upon STAT3β induction, contrasted with their down-regulation when total STAT3 is similarly knocked down, indicates that, at physiological levels, their expression does not depend on the presence of STAT3's C-terminal TAD. Alternatively, dimers between STAT3β and other transcription factors (such as other STATs or c-jun) can sustain the expression of these genes (32). However, the inhibitory effect of STAT3β on the cell cycle (Fig. S7) and the associated cell death/apoptosis (Fig. S8 and Fig. 2D) must be mediated by either the repression of genes that promote oncogenesis/survival or by the activation of genes that inhibit growth/promote cell death. Microarray analysis revealed a specific expression signature associated with the acute switch from α to β that differs from that identified in other contexts (14, 17, 18). Validated genes that are specifically repressed by the induction of STAT3β include LEDGF, PCAF, IL8, CyclinC, PEX1, and STAT1β. In particular, LEDGF and PCAF are capable of both inducing cell death when down-regulated by themselves (Fig. 5 A and C) and of rescuing STAT3β's effect on cell viability when overexpressed (Fig. 5 F and H). LEDGF (PSIP1) is a chromatin-associated protein that has been implicated in transcriptional regulation, leukemogenesis, lymphangiogenesis, autoimmunity, and HIV integration (26). Intriguingly, LEDGF can control IL6 expression and STAT3 activation (33), possibly supporting an autocrine/paracrine loop. Moreover, LEDGF is described as a growth factor and a cancer-associated survival protein involved in control of the lysosomal cell-death pathway (26, 34). PCAF regulates gene transcription by acetylation (29) and can promote cell-cycle progression, epithelial-to-mesenchymal transition, invasion, and chemoresistance (35, 36).
The other identified targets could also contribute to the STAT3β-mediated phenotype, particularly in vivo. For example, whereas CyclinC down-regulation alone does not mimic ST2-induced cell death, its overexpression does protect from it, suggesting that its role might become evident only when in association with other STAT3β-dependent events. Similarly, the robust and selective down-regulation of STAT1β caused by ST2 could still potentiate STAT1α tumor suppressor activity, even if its re-expression in cells was not sufficient to rescue cell viability. The antitumorigenic properties displayed by STAT3β are thus likely the combinatorial result of a context-dependent, orchestrated control of the expression of these and presumably additional targets.
Use of antisense compounds is a well-established approach for gene expression regulation, and it normally relies on the destabilization of target mRNAs, either by triggering RNase H degradation or by taking advantage of RNAi machinery. Redirection of AS differs from standard antisense-based technologies in that the objective is not the degradation of the target RNA but its repurposing. The possibility of eliminating a target while—at the same time—inducing the expression of a favorable isoform represents the most relevant advantage of the splicing redirection approach (23). This in fact ensures that even a moderate success at the targeting level translates into higher treatment effectiveness, and might in part account for the better performance of the ST2 treatment in vivo. Specific and persistent splice switching has been obtained in vivo in multiple systems (37). For example, antisense compounds directed at inducing the proapoptotic splicing variant Bcl-XS at the expense of the more abundant survival Bcl-XL isoform resulted in reduced tumor load in a B16F10 murine melanoma model (38), and splicing redirection compounds are showing promising results in clinical trials to treat Duchenne muscular dystrophy (39) and other diseases.
Our work demonstrates how the modulation of a single endogenous splicing event in tumors can have powerful antioncogenic effects and lead to tumor regression. Moreover, because of the intrinsic plasticity of this approach, the same strategy can be used to induce skipping of other STAT3 exons (e.g., encoding for DNA-binding or SH2 dimerization domains), allowing the dissection of their contribution to STAT3 functions and possibly the identification of additional variants of therapeutic potential.
In summary, the development of means to manipulate endogenous STAT3 alternative splicing in a physiological context allowed us to validate STAT3β as a potent antitumorigenic molecule that does not act as a dominant-negative mechanism but modulates targets not typically associated with STAT3 activity. The flexibility and efficiency of this strategy make it readily translatable to any other cancer genes with splicing isoforms involved in different aspects of tumorigenesis, and open exciting therapeutic perspectives.
Materials and Methods
For a description of reagents and experimental details see SI Materials and Methods. These include cloning, mutants, RNA extraction, PCR analysis, WB, tissue-culture conditions, morpholino, siRNA or P6 treatments, microarray, overexpression, and in vivo experiments. Additional details regarding statistical analysis are also found in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank K. Manova and A. Viale for assistance and M. Jäättelä for providing the LEDGF construct. This work was supported by Department of Defense Grant BC074961. F.Z. is supported by an American-Italian Cancer Foundation fellowship.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1108482108/-/DCSupplemental.
References
- 1.Darnell JE., Jr Transcription factors as targets for cancer therapy. Nat Rev Cancer. 2002;2:740–749. doi: 10.1038/nrc906. [DOI] [PubMed] [Google Scholar]
- 2.Haura EB, Turkson J, Jove R. Mechanisms of disease: Insights into the emerging role of signal transducers and activators of transcription in cancer. Nat Clin Pract Oncol. 2005;2:315–324. doi: 10.1038/ncponc0195. [DOI] [PubMed] [Google Scholar]
- 3.Yue P, Turkson J. Targeting STAT3 in cancer: How successful are we? Expert Opin Investig Drugs. 2009;18:45–56. doi: 10.1517/13543780802565791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inghirami G, et al. New and old functions of STAT3: A pivotal target for individualized treatment of cancer. Cell Cycle. 2005;4:1131–1133. doi: 10.4161/cc.4.9.1985. [DOI] [PubMed] [Google Scholar]
- 6.Huang Y, et al. Stat3 isoforms, α and β, demonstrate distinct intracellular dynamics with prolonged nuclear retention of Stat3β mapping to its unique C-terminal end. J Biol Chem. 2007;282:34958–34967. doi: 10.1074/jbc.M704548200. [DOI] [PubMed] [Google Scholar]
- 7.Niu G, et al. Overexpression of a dominant-negative signal transducer and activator of transcription 3 variant in tumor cells leads to production of soluble factors that induce apoptosis and cell cycle arrest. Cancer Res. 2001;61:3276–3280. [PubMed] [Google Scholar]
- 8.Gritsko T, et al. Persistent activation of Stat3 signaling induces survivin gene expression and confers resistance to apoptosis in human breast cancer cells. Clin Cancer Res. 2006;12:11–19. doi: 10.1158/1078-0432.CCR-04-1752. [DOI] [PubMed] [Google Scholar]
- 9.Ling X, Arlinghaus RB. Knockdown of STAT3 expression by RNA interference inhibits the induction of breast tumors in immunocompetent mice. Cancer Res. 2005;65:2532–2536. doi: 10.1158/0008-5472.CAN-04-2425. [DOI] [PubMed] [Google Scholar]
- 10.Zhao W, Jaganathan S, Turkson J. A cell-permeable Stat3 SH2 domain mimetic inhibits Stat3 activation and induces antitumor cell effects in vitro. J Biol Chem. 2010;285:35855–35865. doi: 10.1074/jbc.M110.154088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song H, Wang R, Wang S, Lin J. A low-molecular-weight compound discovered through virtual database screening inhibits Stat3 function in breast cancer cells. Proc Natl Acad Sci USA. 2005;102:4700–4705. doi: 10.1073/pnas.0409894102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pedranzini L, et al. Pyridone 6, a pan-Janus-activated kinase inhibitor, induces growth inhibition of multiple myeloma cells. Cancer Res. 2006;66:9714–9721. doi: 10.1158/0008-5472.CAN-05-4280. [DOI] [PubMed] [Google Scholar]
- 13.Schaefer TS, Sanders LK, Park OK, Nathans D. Functional differences between Stat3α and Stat3β. Mol Cell Biol. 1997;17:5307–5316. doi: 10.1128/mcb.17.9.5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dewilde S, Vercelli A, Chiarle R, Poli V. Of &alphas and &betas: Distinct and overlapping functions of STAT3 isoforms. Front Biosci. 2008;13:6501–6514. doi: 10.2741/3170. [DOI] [PubMed] [Google Scholar]
- 15.Caldenhoven E, et al. STAT3β, a splice variant of transcription factor STAT3, is a dominant negative regulator of transcription. J Biol Chem. 1996;271:13221–13227. doi: 10.1074/jbc.271.22.13221. [DOI] [PubMed] [Google Scholar]
- 16.Xu G, Zhang C, Zhang J. Dominant negative STAT3 suppresses the growth and invasion capability of human lung cancer cells. Mol Med Report. 2009;2:819–824. doi: 10.3892/mmr_00000178. [DOI] [PubMed] [Google Scholar]
- 17.Maritano D, et al. The STAT3 isoforms α and β have unique and specific functions. Nat Immunol. 2004;5:401–409. doi: 10.1038/ni1052. [DOI] [PubMed] [Google Scholar]
- 18.Yoo JY, Huso DL, Nathans D, Desiderio S. Specific ablation of Stat3β distorts the pattern of Stat3-responsive gene expression and impairs recovery from endotoxic shock. Cell. 2002;108:331–344. doi: 10.1016/s0092-8674(02)00636-0. [DOI] [PubMed] [Google Scholar]
- 19.Cartegni L, Chew SL, Krainer AR. Listening to silence and understanding nonsense: Exonic mutations that affect splicing. Nat Rev Genet. 2002;3:285–298. doi: 10.1038/nrg775. [DOI] [PubMed] [Google Scholar]
- 20.Nilsen TW, Graveley BR. Expansion of the eukaryotic proteome by alternative splicing. Nature. 2010;463:457–463. doi: 10.1038/nature08909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ward AJ, Cooper TA. The pathobiology of splicing. J Pathol. 2010;220:152–163. doi: 10.1002/path.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.David CJ, Manley JL. Alternative pre-mRNA splicing regulation in cancer: Pathways and programs unhinged. Genes Dev. 2010;24:2343–2364. doi: 10.1101/gad.1973010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bauman J, Jearawiriyapaisarn N, Kole R. Therapeutic potential of splice-switching oligonucleotides. Oligonucleotides. 2009;19:1–13. doi: 10.1089/oli.2008.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shao H, Quintero AJ, Tweardy DJ. Identification and characterization of cis elements in the STAT3 gene regulating STAT3 α and STAT3 β messenger RNA splicing. Blood. 2001;98:3853–3856. doi: 10.1182/blood.v98.13.3853. [DOI] [PubMed] [Google Scholar]
- 25.Morcos PA, Li Y, Jiang S. Vivo-Morpholinos: A non-peptide transporter delivers Morpholinos into a wide array of mouse tissues. Biotechniques. 2008;45:613–614. doi: 10.2144/000113005. 616, 618 passim. [DOI] [PubMed] [Google Scholar]
- 26.Llano M, Morrison J, Poeschla EM. Virological and cellular roles of the transcriptional coactivator LEDGF/p75. Curr Top Microbiol Immunol. 2009;339:125–146. doi: 10.1007/978-3-642-02175-6_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crane DI, Maxwell MA, Paton BC. PEX1 mutations in the Zellweger spectrum of the peroxisome biogenesis disorders. Hum Mutat. 2005;26:167–175. doi: 10.1002/humu.20211. [DOI] [PubMed] [Google Scholar]
- 28.Liu ZJ, et al. A critical role for cyclin C in promotion of the hematopoietic cell cycle by cooperation with c-Myc. Mol Cell Biol. 1998;18:3445–3454. doi: 10.1128/mcb.18.6.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagy Z, Tora L. Distinct GCN5/PCAF-containing complexes function as co-activators and are involved in transcription factor and global histone acetylation. Oncogene. 2007;26:5341–5357. doi: 10.1038/sj.onc.1210604. [DOI] [PubMed] [Google Scholar]
- 30.Baran-Marszak F, et al. Differential roles of STAT1α and STAT1β in fludarabine-induced cell cycle arrest and apoptosis in human B cells. Blood. 2004;104:2475–2483. doi: 10.1182/blood-2003-10-3508. [DOI] [PubMed] [Google Scholar]
- 31.Selander KS, et al. Inhibition of gp130 signaling in breast cancer blocks constitutive activation of Stat3 and inhibits in vivo malignancy. Cancer Res. 2004;64:6924–6933. doi: 10.1158/0008-5472.CAN-03-2516. [DOI] [PubMed] [Google Scholar]
- 32.Sasse J, et al. Mutational analysis of acute-phase response factor/Stat3 activation and dimerization. Mol Cell Biol. 1997;17:4677–4686. doi: 10.1128/mcb.17.8.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeichi T, et al. Overexpression of LEDGF/DFS70 induces IL-6 via p38 activation in HaCaT cells, similar to that seen in the psoriatic condition. J Invest Dermatol. 2010;130:2760–2767. doi: 10.1038/jid.2010.203. [DOI] [PubMed] [Google Scholar]
- 34.Rammer P, et al. BAMLET activates a lysosomal cell death program in cancer cells. Mol Cancer Ther. 2010;9:24–32. doi: 10.1158/1535-7163.MCT-09-0559. [DOI] [PubMed] [Google Scholar]
- 35.Shiota M, et al. P300/CBP-associated factor regulates Y-box binding protein-1 expression and promotes cancer cell growth, cancer invasion and drug resistance. Cancer Sci. 2010;101:1797–1806. doi: 10.1111/j.1349-7006.2010.01598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirano G, et al. Enhanced expression of PCAF endows apoptosis resistance in cisplatin-resistant cells. Mol Cancer Res. 2010;8:864–872. doi: 10.1158/1541-7786.MCR-09-0458. [DOI] [PubMed] [Google Scholar]
- 37.Moulton HM, et al. Peptide-morpholino conjugate: A promising therapeutic for Duchenne muscular dystrophy. Ann N Y Acad Sci. 2009;1175:55–60. doi: 10.1111/j.1749-6632.2009.04976.x. [DOI] [PubMed] [Google Scholar]
- 38.Bauman JA, Li SD, Yang A, Huang L, Kole R. Anti-tumor activity of splice-switching oligonucleotides. Nucleic Acids Res. 2010;38:8348–8356. doi: 10.1093/nar/gkq731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cirak S, et al. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: An open-label, phase 2, dose-escalation study. Lancet. 2011;378:595–605. doi: 10.1016/S0140-6736(11)60756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.