Abstract
The adult Drosophila copper cell region or “stomach” is a highly acidic compartment of the midgut with pH < 3. In this region, a specialized group of acid-secreting cells similar to mammalian gastric parietal cells has been identified by a unique ultrastructure and by copper-metallothionein fluorescence. However, the homeostatic mechanism maintaining the acid-secreting “copper cells” of the adult midgut has not been examined. Here, we combine cell lineage tracing and genetic analysis to investigate the mechanism by which the gastric epithelium is maintained. Our investigation shows that a molecularly identifiable population of multipotent, self-renewing gastric stem cells (GSSCs) produces the acid-secreting copper cells, interstitial cells, and enteroendocrine cells of the stomach. Our assays demonstrate that GSSCs are largely quiescent but can be induced to regenerate the gastric epithelium in response to environmental challenge. Finally, genetic analysis reveals that adult GSSC maintenance depends on Wnt signaling. Characterization of the GSSC lineage in Drosophila, with striking similarities to mammals, will advance the study of both homeostatic and pathogenic processes in the stomach.
The middle midgut of the Drosophila gastrointestinal (GI) tract has long been recognized for its distinctive biology. Strasburger (1) first noted the conspicuous morphology of the cells in the middle midgut or “Mitte” for their large size and peculiar packing arrangement within the midgut epithelium, which could be readily distinguished from both the anterior and posterior midgut by light microscopy. Based on this morphology, Strasburger called these cells “Calycocyten” or calycocytes by analogy to the acid-secreting cells in the larvae of the moth Deilephila (2). In addition, early studies of metal accumulation in the GI tract of Drosophila revealed that calycocytes of the middle midgut also accumulated copper and emitted orange florescence when the diet was supplemented with Cu++ (3, 4). Thus, “cuprophilic cells,” “copper-accumulating cells,” or simply “copper cells” ultimately emerged as the preferred nomenclature for this subset of cells in the midgut epithelium.
Subsequent ultrastructural analysis has revealed that the copper-accumulating region of the middle midgut is a mosaic consisting largely of copper cells and interstitial cells (5–7). Copper cells have a basally located nucleus, a deeply invaginated apical membrane covered with long microvilli, and a high density of mitochondria. In contrast, interstitial cells have a more apically localized nucleus, short microvilli, and a broad apical profile that appears to restrict exposure of adjacent copper cells to the luminal contents. It has been suggested that interstitial cells may have an active role in regulating copper cell function and thus together comprise a functional cellular unit (7, 8).
Of all of the differentiated cell types in the midgut, the function of copper cells is perhaps best understood. Feeding experiments using pH indicator dyes have demonstrated that the midgut can be subdivided into physiologically distinct compartments on the basis of pH (1, 6, 7, 9). These studies provide anatomical evidence that the copper cell region (CCR) in the middle midgut correlates with a region defined by an extremely low pH (pH < 3). These findings are also consistent with histological and ultrastructural observations suggesting that the apical membrane of copper cells is the site of vacuolar H+ ATPase proton-pump enrichment (6, 8). Moreover, feeding or perfusion of guts with acetazolamide, a carbonic anhydrase inhibitor, leads to the elimination of the acidic compartment of the midgut (7). These studies support the notion that the Drosophila vacuolar H+ ATPase pump employs a carbonic anhydrase-catalyzed pool of H+ to acidify the CCR. Finally, genetic studies show that mutations that disrupt copper cell formation result in a failure of midgut acidification (9). Altogether, these studies demonstrate that acid-secreting copper cells are responsible for generating an acidic compartment within the middle midgut.
The mammalian stomach is divided into anatomically and functionally distinct regions (10, 11). The majority of the stomach is called the corpus. This region is highly enriched in gastric parietal cells, the principal cell type specialized for regulated acid secretion in the stomach. In contrast, the smaller antral region of the stomach, which lies adjacent to the intestine, does not contain an abundance of acid-secreting parietal cells. It has been noted that copper cells and gastric parietal cells share strikingly similar cell morphology and function, suggesting that these two cell types are analogous (8, 12). For these reasons, the CCR has also been referred to as the “stomach” (6, 8).
Although considerable progress has been made in understanding the cell biology of acid secretion across the apical plasma membrane of parietal cells, the homeostatic mechanisms that maintain these cells is not well understood (10–13). Lineage-tracing studies in mammals have identified Lgr5+ stem cells in the stomach's antrum (14). However, the slowly proliferating gastric stem cells that give rise to the parietal cells of the corpus have not been identified with single-cell resolution. Thus, despite striking evolutionary conservation of form and function, the cellular mechanisms responsible for maintaining acid-secreting cells in the insect and mammalian GI tracts remain unknown. In this study, we use genetic lineage-tracing methods to investigate the cellular mechanism of homeostasis in the adult Drosophila CCR.
Results
Molecular Markers of the Adult Middle Midgut.
Our initial studies confirmed that adult flies that were fed a copper-containing diet yield labeled copper cells in the middle midgut, yet the signal was quite labile under our experimental conditions (Fig. S1 A–C). Therefore, we first sought a more robust set of molecular markers to facilitate the study of the adult middle midgut. Ongoing screens led to the identification of candidate genes with expression domains in the middle of the midgut (Fig. 1A). To determine whether these markers were overlapping or mutually exclusive, we conducted a series of double-labeling experiments (Fig. 1 A–D and Figs. S1 A–F and S2 A–D). For example, defective proventriculus (dve) encodes a transcription factor with the broadest expression pattern and molecularly defines the region that we refer to as the middle midgut. The transcription factors encoded by cut (ct) and labial (lab) mark a subdomain that is largely coextensive with the acid-secreting CCR (Fig. 1 A and B and Figs. S1 D–F and S2B). The smallest region, defined by the NP2788 enhancer trap, falls within the CCR (Fig. S1 A–F). Thus, the middle midgut can be subdivided into molecularly distinct regions along the anterior–posterior axis.
Fig. 1.
Molecular and cellular organization of the adult Drosophila middle midgut. (A) Diagram of the adult GI tract. The middle midgut is indicated in color and corresponds to the dve-lacZ expression domain. Fine mapping reveals partially overlapping expression domains (A–E) and cell type expression in the middle midgut (CC, copper cell; ee, enteroendocrine cell; GSSC, gastric stem cell; IS, interstitial cell; ISC, intestinal stem cell; LFC, large flat cell). Anterior, Left: A dotted bar indicates markers that extend beyond the diagram. Regions B/C (copper cell region; CCR) and D (large flat cell region; LFC) are indicated in the diagram as red and gray, respectively. (B) Composite micrograph, dve-lacZ defines the middle midgut; anterior is to the left. A subdomain of cells expressing anti-Cut is nested within the dve-lacZ region. The posterior border of anti-Cut expression distinguishes the CCR from the LFC region. (C) Acid secreting copper cells (CC) have large basally positioned nuclei, a distinct apical invagination (arrows) and express both dve-lacZ and anti-Cut. Interstitial cells (IS) have large apically localized nuclei, are interspersed among CCs, and express both dve-lacZ and NP2788>GFP. (D) CCs express higher levels of Labial protein than IS cells. Scale bar: 50 μm in B, 20 μm in C and D.
A number of previously described cell types and morphological features of the CCR are also evident when using the molecular markers we identified (Fig. 1 A–D). For example, copper cells were found to be dve-lacZ+, anti-Cut+, and anti-Labhigh (Labhigh) (Fig. 1 C and D). In contrast, interstitial cells were dve-lacZ+ and anti-Lablow (Lablow), and expressed high levels of the NP2788Gal4 UAS-GFP (NP2788>GFP) driver line (Fig. 1 C and D). Note that the invaginated apical cell surface of the copper cell, which forms a microvilli-rich canaliculus, reproducibly stained strongly for Ct protein (Fig. 1C, arrows). However, the significance of this localization is currently unclear. Thus, a panel of molecular and morphological features distinguishes the differentiated cell types of the adult stomach.
Multipotent, Self-Renewing Lineages Maintain the Middle Midgut.
Cell lineage-tracing experiments were initiated to determine whether a proliferative compartment exists in the middle midgut. Mosaic analysis with a repressible cell marker (MARCM), a genetic strategy that uses mitotic recombination, selectively labels dividing cells with GFP (15). Using this method, we first asked whether labeled cells could be detected in the adult middle midgut. A total of 387 adult midgut samples were independently analyzed. These studies clearly show that marked lineages (or “clones”) can be generated in the middle midgut (Fig. 2 A–D and Fig. S3 A–F).
Fig. 2.
GSSCs in the CCR. The MARCM system was used to trace adult cell lineages in the CCR. (A–C) Cell lineages marked with GFP (anti-GFP; green) span differentiated cell types in the CCR at 7–14 d after induction. (A) A marked lineage contains both undifferentiated (DAPI only) and differentiated (dve-lacZ) cells. GB, gastroblast. (B) The same clone shown in A spans both copper cells (CC; Labhigh) and interstitial cells (IS; Lablow). (C) An example of a marked lineage in the CCR containing both copper cells (CC; anti-Cut) and a morphologically identifiable interstitial cell (IS). (D) Long-term lineage tracing shows that both stable (blue) and transient (red) cell lineages can be discriminated in the CCR (n = 9–33 samples scored at each time point). The number of cells per clone (green) reached a maximum at 10 d after induction. (Scale bars: 20 μm.)
To test whether differentiated cells of the middle midgut arise from a common origin, we next examined the composition of marked clones in the adult at 7–14 d after induction. Analysis of molecular and morphological markers showed that individually marked cell lineages contain both copper and interstitial cells (Fig. 2 A–C and Fig. S3B), indicating that these cell types arise from a common progenitor. Similarly, labeling experiments revealed that marked lineages in the CCR also give rise to enteroendocrine cells, which are marked by the transcription factor prospero (pros) (Fig. S3 C and D). Altogether, these experiments demonstrate that a multipotent lineage exists in the CCR, which gives rise to the acid-secreting copper cells of the middle midgut.
Previous studies have indicated that the larval middle midgut contains a distinct region of large flat cells (LFCs) that lies immediately posterior to the larval CCR (16). Our molecular screens uncovered an enhancer trap line, NP6293, which indicated that the adult and larval midguts might share a similar organization. Double-labeling experiments in the adult confirmed that NP6293>GFP marks a distinct region of LFCs lying immediately posterior to the CCR (Fig. S2 C and D). Mosaic analysis indicated that clones recovered in the LFC region either contained dve+ LFCs alone or spanned both dve+ LFCs and Pros+ enteroendocrine cells (Fig. S3 E and F). Thus, a second multipotent lineage maintains the LFC region of the middle midgut.
We next sought to determine whether there is a self-renewing lineage in the CCR. Long-term retention of marked cells in a renewing tissue provides a powerful, unbiased method to identify self-renewing stem-cell lineages (17). Therefore, to define the kinetics of cell maintenance and turnover in the CCR, we next conducted a pulse–chase experiment in which cells in the CCR were labeled with the MARCM system early in adulthood and the resultant GFP+ lineages were followed over time. By using this approach, two different cell lineages are predicted. The first class corresponds to marked differentiated cells, which will only be transiently detectable in an actively renewing tissue. The second class corresponds to self-renewing lineages that will be stably detectable in the tissue.
Our lineage analysis revealed that both predicted classes of marked cells can be identified in the CCR based on their clonal composition (Fig. 2D). Transient clones contained one, or occasionally two, differentiated cells and had a half-life of 6 d. Stable clones contained both undifferentiated and differentiated cell types, were detected at every time point examined throughout adulthood, and had a half-life of 28 d in the gastric epithelium (Fig. 2D). In addition, we observed that stable clones showed an increase in the number of cells per clone after induction (Fig. 2D). Thus, long-term maintenance of marked cell lineages is observed in the stomach despite rapid loss of transient clones from the tissue. Altogether, our lineage-tracing studies support a model in which multipotent, self-renewing gastric stem cells (GSSCs) maintain the adult stomach.
Quiescent GSSCs Are Induced to Proliferate in Response to Challenge.
In the course of our lineage-tracing studies, we observed that marked clones in the middle midgut were often recovered at a much lower frequency than in either the anterior or posterior midgut (Fig. 3A). We hypothesized that this differential labeling might reflect lower stem-cell numbers in the region. To test this hypothesis, we examined expression of two stem-cell markers that have been used to identify intestinal stem cells of the posterior midgut, escargot (esg), and the Notch ligand encoded by Delta (Dl) (18, 19). A population of esg+ cells could be detected in the CCR, but Dl was not detected in the CCR (Fig. 3B and Fig. S4 A–C). Therefore, GSSCs and intestinal stem cells exhibit different marker gene expression in the Notch pathway. However, measurements of esg+ cell density in the CCR suggested that regional differences in clone detection could not be explained solely on the basis of stem-cell number (Fig. S4D).
Fig. 3.
Quiescent esg+ cells are induced to regenerate the gastric epithelium. (A) The MARCM system was used to label adult cell lineages in the midgut (anti-GFP; green). Clones were analyzed at 5 d after induction. The anterior (A), middle (M), and posterior (P) midgut are indicated. Fewer clones are recovered in the middle midgut. (B) esg>GFP+ cells in the midgut can be distinguished by the extent of EdU incorporation. (Left) CCR. (Right) Posterior midgut. (C–F) Environmental challenge (heat shock) leads to regeneration of the gastric epithelium. (C) Strategy for directed cell lineage tracing of esg+ cells in the CCR. The conditional esgGal4, tubGal80ts, UAS-GFP (esgts) driver was used to activate the act>stop>lacZnuc;UAS-flp lineage tracer. esg+ cells were conditionally labeled in the adult by a shift to 29 °C, and guts were analyzed 5 d later. Challenged flies received a 37 °C heat shock (HS) of varying duration and were analyzed 2 d later. (D) In the absence of challenge, few labeled samples were recovered (No HS). However, a dose-dependent increase in the percentage of labeled samples was observed after challenge (15’ HS, 30’ HS, and 45’ HS) (n = 30–39 for each condition). (E and F) Representative micrographs from directed cell lineage-tracing studies in the CCR. (E) Unchallenged. Note that only individual esg+ GSSCs cells are labeled with lacZnuc. (F) Challenged. Newly formed GSSC daughter cells have completely regenerated the gastric epithelium. (Scale bars: 100 μm in A; 20 μm in B; 50 μm in E.)
A second possible explanation is that stem cells in the CCR divide infrequently. To test this hypothesis, we first conducted 5-ethynyl-2′-deoxyuridine (EdU) incorporation studies to label cells in S phase. In contrast to the posterior midgut, esg+ cells of the CCR did not readily incorporate EdU (Fig. 3B). We also performed directed cell lineage-tracing experiments with the driver line esgGal4,UAS-GFP,tub-Gal80ts (esgts) (18) to activate the act>stop>lacZnuc;UAS-flp lineage tracer (Fig. 3 C–F). This marking strategy relies on the conditional activation of FLPase enzyme under Gal4/Gal80 control to genetically mark a lineage of interest with lacZnuc. Control experiments indicate that this marking strategy was not associated with high background labeling in the CCR (Fig. S5D). Consistent with the EdU-labeling studies, directed cell lineage-tracing of esg+ cells produced a low percentage of guts containing labeled daughters in the CCR (Fig. 3 D and E, No HS). Altogether, these experiments demonstrate that GSSCs are normally quiescent under baseline conditions.
One difference between the lineage-tracing experiments performed with the MARCM system and the set of directed esg+ cell lineage-tracing experiments described above is that, in the case of MARCM, a brief 37 °C heat shock was administered to induce cell labeling. We reasoned that some of the variation in MARCM clone recovery observed in the CCR (e.g., Fig. 3A and Fig. S3A) might be because of the secondary effects of heat stress on the gastric epithelium during the MARCM induction protocol. To test this possibility, we combined directed esg+ lineage tracing with a heat shock of varying intensity. Challenging the flies with heat shock led to a dose-dependent increase in the percentage of samples containing labeled daughter cells (Fig. 3 C–F); an increase in the number of esg+, EdU+ cells (Fig. S5 A–C); and an increase in the number of new daughter cells produced in the CCR (Fig. S5D). Thus, esg+ cells in the CCR are normally quiescent but can be stimulated to divide and produce new daughter cells in a dose-sensitive manner in response to heat stress.
Although the level of heat stress is easily controlled and thus useful for testing the dose-sensitive effect of stress on the gastric epithelium, we wanted to extend our analysis to include the effect of a more physiologically relevant environmental challenge. Ingestion of the Gram-negative pathogen Pseudomonas entomophila (Pe), originally isolated from Drosophila, leads to enteric infection, destruction of gut cells, and subsequent regeneration of the epithelium (20, 21). As in the case of heat stress, flies challenged with Pe exposure displayed increases in both proliferation (Fig. S6 A–C) and the number of daughter cells produced by marked esg+ lineages in the middle midgut (Fig. S6 D–F). Altogether, our experiments demonstrate that quiescent esg+ cells in the CCR are induced to regenerate the gastric epithelium in response to a range of tissue-damaging insults.
esg Is a Marker for GSSCs.
The adaptive response of esg+ cells to challenge raised the possibility that esg might mark the GSSCs defined in our long-term lineage-tracing studies. To test this possibility, we first asked whether esg+ lineages could give rise to all of the differentiated cells of the stomach. Combining directed esg+ cell lineage tracing with molecular markers revealed that new copper, interstitial, and enteroendocrine cells are all generated in CCR after challenge (Fig. 4A and Fig. S7 A–F).
Fig. 4.
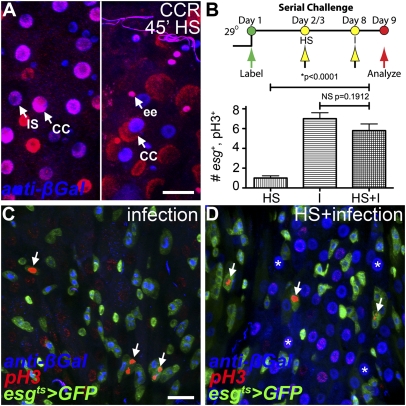
Evidence that esg+ cells are GSSCs. (A–D) Directed cell lineage tracing of esg+ cell lineages. The conditional esgGal4, tubGal80ts, UAS-GFP (esgts) driver was used to activate the act>stop>lacZnuc;UAS-flp lineage tracer. (A Left) Directed lineage tracing labels Labial+ daughter cells (red) in the CCR. Note that both copper cells (CC; Labhigh) and interstitial cells (IS; Lablow) are labeled. (Right) Directed lineage tracing labels Cut+ and Pros+ daughter cells (red) in the CCR. Note that both copper cells (CC) and enteroendocrine cells (ee) are labeled in the CCR. (B–D) Serial challenge assay. Flies were challenged with heat shock (HS), Pe infection (I), or sequentially with both (HS+I). The number of esg+, phospho-histone H3+ (pH3+) cells was scored in the CCR. Asterisks mark newly formed daughter cells, and arrows indicate pH3+ cells. (B) Quantification. No significant difference in the proliferative response was observed in flies that received serial challenge (n = 12–15 samples per condition). (C and D) Representative micrographs. (C) Infection only. Note that esg+, pH3+ cells are detectable, but anti-βGal+ daughter cells are not present in the stomach at 16 h after infection. (D) Heat shock and infection. Note that both anti-βGal+ daughter cells produced by the initial heat shock challenge and the esg+, pH3+ cells resulting from Pe challenge are detectable in the CCR. (Scale bars: 20 μm.)
It is possible that esg+ cells are committed progenitors of the CCR that do not self-renew. If so, their proliferative potential should be exhausted once the gastric epithelium has been replenished. To test this possibility, we examined the proliferative potential of esg+ cells in a serial challenge assay, where flies were successively presented with both heat shock and Pe challenge (Fig. 4B, Upper). Serial challenge experiments detected no significant difference in the ability of esg+ cells to divide in response to Pe infection, even after the gastric epithelium had been regenerated in response to the initial heat shock challenge (Fig. 4 B–D). These findings are consistent with the view that esg+ cells mark the GSSCs of the CCR.
Wnt Signaling Is Necessary for GSSC Maintenance.
The conserved Wnt signaling pathway plays a central role in GI homeostasis in both invertebrates and vertebrates (22–25). We therefore tested the relevance of Wnt signaling to maintain GSSCs in the adult Drosophila stomach. A dominant-negative form of pangolin, panΔN, the Drosophila lymphoid enhancer-binding factor 1/T cell factor (Lef1/TCF) homolog (26, 27), strongly abrogates Wnt signaling in the posterior midgut (23). To test the necessity of pan, we first conditionally activated panΔN with the esgts driver in adults and then scored the number of esg+ cells that persisted throughout adulthood. In contrast to WT, expression of dominant-negative pan led to a rapid decline in the number of esg+ cells (Fig. 5A and Fig. S8 A–D). Second, we tested whether pan is required to maintain esg+ cells in response to challenge. In contrast to unchallenged controls, we observed a reduction in the percentage of guts containing esg+ cells in panΔN mutants after challenge demonstrating that pan is required under both baseline and adaptive conditions (Fig. S8E). Finally, we used the MARCM system to compare the maintenance of GSSCs in WT and panΔN lineages that were generated in the adult. In contrast to WT lineages, GSSC lineages expressing panΔN were rapidly lost from the gastric epithelium (Fig. 5 B–D). Altogether, these experiments suggest that Wnt signaling is necessary for the maintenance of GSSCs in the gastric stem-cell niche.
Fig. 5.
Wnt signaling maintains gastric stem cells in the adult stomach. (A) The conditional esgGal4, tubGal80ts, UAS-GFP (esgts) driver was used to express a dominant-negative form of pangolin, panΔN. In control experiments, no difference in the number of esg+ cells was observed after 20 d at the restrictive temperature (29 °C; black). Conditional activation of panΔN led to a decline in the number of esg+ cells in the CCR (red) (n = 10 samples for each time point). (B–D) The MARCM system was used to label adult cell lineages in the CCR. (B) Clones were analyzed 5–20 d after induction (n = 27–32 samples for each time point). In contrast to WT (black), cell lineages expressing panΔN (red) were rapidly lost from the CCR. (C) WT 10 d control clones. (D) Cell lineages expressing panΔN. Rare mutant clones can be found in the CCR at 10 d after induction. (Scale bar: 20 μm.)
Discussion
In this study, we have characterized epithelial homeostasis of the adult Drosophila stomach by using cell lineage-tracing methodologies. Independent approaches each provide direct evidence for a quiescent GSSC that maintains the acid-secreting cells of the CCR. The simplest interpretation of the data is that the adult Drosophila stomach is maintained by esg+ GSSCs (Fig. 6), although our studies do not formally exclude more complex alternatives. A range of environmental challenges tested robustly induced division of quiescent GSSCs to produce a daughter cell that can differentiate into a copper, interstitial, or enteroendocrine cell of the stomach. We call the undifferentiated GSSC daughter cell a gastroblast, in accord with previously established nomenclature conventions (18, 28, 29). Finally, the functional relationship of LFCs of the middle midgut to the acid-secreting CCR of the stomach still remains unclear. Although our data clearly indicate that a multipotent lineage renews the LFC region, our study did not specifically focus on measuring the self-renewal capacity of this progenitor cell population. To distinguish these progenitor cells from their GSSC neighbors, we designate them LFC stem cells.
Fig. 6.
Homeostatic maintenance of the adult middle midgut. The middle midgut contains a physiologically distinct cellular compartment characterized by an acidic pH and the presence of acid-secreting copper cells (red). A population of GSSCs produces the acid-secreting copper cells, interstitial cells, and enteroendocrine cells of the adult Drosophila stomach. GSSCs can be detected as single esg+ cells in the acidic CCR of the middle midgut in close apposition to the muscle and basement membrane surrounding the midgut. Wnt signals maintain GSSCs in their niche. Largely quiescent, GSSCs are induced to regenerate the gastric epithelium in response to environmental challenge.
The mammalian stomach performs several important functions, including temporary storage, movement, and sterilization of ingested food through secretion of hydrochloric acid, thus providing an important line of defense against ingested pathogens. In addition, secretion of enzymes and hormones promotes digestion and relays physiological status to other tissues in the organism. We have emphasized that many of these attributes appear to be functionally analogous to the adult Drosophila CCR, although perhaps not all. For example, the crop, a diverticulated sac descending from the GI tract at the foregut–midgut junction, is thought to be important for storage and determining satiety after meals in certain insects (30), a function that is not obviously represented in the CCR. Similarly, the proventriculus, which also lies at the foregut–midgut junction, is classically thought to function as a one-way valve, and the site of peritrophic membrane production in many insect species (31), may be analogous to the esophageal sphincter. Interestingly, a recent study has reported the presence of stem cells in the adult proventriculus (32). Thus, functional hallmarks of the mammalian stomach may be distributed among a variety of distinct anatomical locations in the insect GI tract.
A fundamental characteristic of the GI tract in many, if not all, multicellular organisms is the functional specialization found along the anterior–posterior axis. How the distinct homeostatic demands of each unique physiological compartment are met is currently not well understood. Genetic analysis of the adult Drosophila GI tract suggests that certain stem-cell niches share some aspects of their regulatory control. For example, analysis of the Wnt signaling pathway has uncovered a requirement for the pathway in maintenance of a number of different adult stem-cell populations, including those found in the proventriculus, posterior midgut, and hindgut (22–24, 32). Our study suggests that Wnt signaling is also required for the maintenance of GSSCs in the stomach. However, not all features of this genetic program appear to be shared. Notch/Dl signaling has been found to be a key regulator of cell fate specification in the intestinal stem cell lineage of the posterior midgut (18, 19, 28, 33). However, characterization of Notch pathway reporters performed in this study and in the context of the renal stem cells of the Malpighian tubules suggests that Notch signaling is regulated differently in these lineages (29). Together, these observations raise the hypothesis that homeostasis in the GI tract is achieved through the region-specific modification of a core niche program, which adapts each stem cell to a region-specific physiology. Future studies will define the distinct molecular and environmental factors that control GSSC quiescence in the adult Drosophila stomach.
In conclusion, the Drosophila stomach is a model for understanding fundamental mechanisms controlling stem cell behavior and homeostasis in the gastric lineage. The relative simplicity of the Drosophila system should facilitate an understanding of conserved processes and provide insight into the mechanism of tumorigenesis, Helicobacter pylori pathogenesis, and metabolic signaling in the stomach.
Materials and Methods
Lineage Analysis.
WT MARCM clones were induced by heat shock in female flies of the genotype y,w,UAS-GFP,hsflp/w;+;tubGal4,FRT82B,tubGal80/ FRT82BhsπM. To assess cell lineage, markers were analyzed at 7–14 d after induction, after transient lineages had cleared. To assess the long-term dynamics of lineage maintenance, samples were analyzed at 2–35 d after induction.
Directed esg+ Cell Lineage-Tracing Studies.
Female progeny of the genotype w;esgts/act>stop>lacZnuc;UAS-flp/+ were grown to adulthood at 18 °C and shifted to 29 °C to induce labeling. Flies received an environmental challenge (heat shock or enteric infection) on days 2 and 3 after the shift to 29 °C. All flies were dissected at 5 d postshift to 29 °C and analyzed. For serial challenge, flies received a 45-min heat shock (three times) on days 2 and 3 postshift, a 16 h infection with Pe (OD20) on day 8, or both.
Wnt Pathway Analysis.
Female progeny of the genotype w;esgts/+ (WT controls) and w/y,w;esgts/UAS-panΔ4 (pandn experimental) were grown to adulthood at 18 °C and shifted to 29 °C for a defined period. MARCM clones were induced as described above in the genotypes y,w,UAS-GFP,hsflp/w;+;tubGal4,FRT82B,tubGal80/ FRT82BhsπM (WT control), and y,w,UAS-GFP,hsflp/w;UAS-PanΔ4/+;tubGal4,FRT82B,tubGal80/ FRT82BhsπM (pandn experimental). Flies were dissected 5, 10, and 20 d after induction.
Infection.
Flies were infected ad libitum by mixing Pe cultures into food media. Flies were dry-starved for 3 h, then exposed to food supplemented with 0.5 mL of Pe at OD20 in 5% sucrose. Mock-infected flies were dry-starved for 3 h, then placed on food supplemented with 0.5 mL of 5% sucrose only.
See SI Materials and Methods for detailed methods.
Supplementary Material
Acknowledgments
We thank Drs. M. Affolter and S. Merabet, Bloomington Drosophila Stock Center, and GetDB for providing fly strains; Drs. T. Kaufman and H. Reichert and the Developmental Studies Hybridoma Bank for generously providing valuable antibodies; and Dr. B. Lemaitre for sharing Pe isolates. We wish to acknowledge J. Wong and J. Geahlen for their contribution to marker gene identification and K. Beebe for advice on the infection protocol. We thank J. Rusch for help translating German texts to English. Finally, we are indebted to our colleagues Drs. S. Johnson and J. Mills and members of C.A.M.'s laboratory for constructive discussions. M.S. is supported by a National Institutes of Health Cellular and Molecular Biology Training Grant. C.A.M. is a Pew Scholar. This work was also supported in part by the American Cancer Society and the National Institute of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1109794108/-/DCSupplemental.
References
- 1.Strasburger M. Structure, function and variability of the intestinal tract of Drosophila melanogaster Meigen (Translated from German) Z Wiss Zool. 1932;140:539–649. [Google Scholar]
- 2.Deegener P. The intestinal tract and its appendages (Translated from German) In: Schroder C, editor. Handbuch der Entomologie. Vol 1. Jena, Germany: Fischer; 1928. pp. 234–315. [Google Scholar]
- 3.Poulson DF, Bowen VT. Organization and function of the inorganic constituents of nuclei. Exp Cell Res Suppl. 1952;2:161–180. [Google Scholar]
- 4.McNulty M, Puljung M, Jefford G, Dubreuil RR. Evidence that a copper-metallothionein complex is responsible for fluorescence in acid-secreting cells of the Drosophila stomach. Cell Tissue Res. 2001;304:383–389. doi: 10.1007/s004410100371. [DOI] [PubMed] [Google Scholar]
- 5.Filshie BK, Poulson DF, Waterhouse DF. Ultrastructure of the copper-accumulating region of the Drosophila larval midgut. Tissue Cell. 1971;3:77–102. doi: 10.1016/s0040-8166(71)80033-2. [DOI] [PubMed] [Google Scholar]
- 6.Dubreuil RR, Grushko T, Baumann O. Differential effects of a labial mutation on the development, structure, and function of stomach acid-secreting cells in Drosophila melanogaster larvae and adults. Cell Tissue Res. 2001;306:167–178. doi: 10.1007/s004410100422. [DOI] [PubMed] [Google Scholar]
- 7.Shanbhag S, Tripathi S. Epithelial ultrastructure and cellular mechanisms of acid and base transport in the Drosophila midgut. J Exp Biol. 2009;212:1731–1744. doi: 10.1242/jeb.029306. [DOI] [PubMed] [Google Scholar]
- 8.Dubreuil RR. Copper cells and stomach acid secretion in the Drosophila midgut. Int J Biochem Cell Biol. 2004;36:745–752. doi: 10.1016/j.biocel.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Dubreuil RR, et al. Mutations of α spectrin and labial block cuprophilic cell differentiation and acid secretion in the middle midgut of Drosophila larvae. Dev Biol. 1998;194:1–11. doi: 10.1006/dbio.1997.8821. [DOI] [PubMed] [Google Scholar]
- 10.Mills JC, Shivdasani RA. Gastric epithelial stem cells. Gastroenterology. 2011;140:412–424. doi: 10.1053/j.gastro.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khurana S, Mills JC. The gastric mucosa: Development and differentiation. In: Kaestner KH, editor. Progress in Molecular Biology and Translational Science. Vol 96. Burlington, MA: Academic; 2010. pp. 93–115. [DOI] [PubMed] [Google Scholar]
- 12.Yao X, Forte JG. Cell biology of acid secretion by the parietal cell. Annu Rev Physiol. 2003;65:103–131. doi: 10.1146/annurev.physiol.65.072302.114200. [DOI] [PubMed] [Google Scholar]
- 13.Forte JG, Zhu L. Apical recycling of the gastric parietal cell H,K-ATPase. Annu Rev Physiol. 2010;72:273–296. doi: 10.1146/annurev-physiol-021909-135744. [DOI] [PubMed] [Google Scholar]
- 14.Barker N, et al. Lgr5+ve stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 16.Poulson DF, Waterhouse DF. Experimental studies on pole cells and midgut differentiation in Diptera. Aust J Biol Sci. 1960;13:541–567. [Google Scholar]
- 17.Fox DT, Morris LX, Nystul T, Spradling AC, editors. StemBook. January 31, 2009. Lineage analysis of stem cells. The Stem Cell Research Community, 10.3824/stembook.1.33.1. [PubMed] [Google Scholar]
- 18.Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- 19.Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- 20.Vodovar N, et al. Drosophila host defense after oral infection by an entomopathogenic Pseudomonas species. Proc Natl Acad Sci USA. 2005;102:11414–11419. doi: 10.1073/pnas.0502240102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang H, et al. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin G, Xu N, Xi R. Paracrine Wingless signalling controls self-renewal of Drosophila intestinal stem cells. Nature. 2008;455:1119–1123. doi: 10.1038/nature07329. [DOI] [PubMed] [Google Scholar]
- 23.Lee WC, Beebe KB, Sudmeier L, Micchelli CA. Adenomatous polyposis coli regulates Drosophila intestinal stem cell proliferation. Development. 2009;136:2255–2264. doi: 10.1242/dev.035196. [DOI] [PubMed] [Google Scholar]
- 24.Takashima S, Mkrtchyan M, Younossi-Hartenstein A, Merriam JR, Hartenstein V. The behaviour of Drosophila adult hindgut stem cells is controlled by Wnt and Hh signalling. Nature. 2008;454:651–655. doi: 10.1038/nature07156. [DOI] [PubMed] [Google Scholar]
- 25.Spence JR, Lauf R, Shroyer NF. Vertebrate intestinal endoderm development. Dev Dyn. 2011;240:501–520. doi: 10.1002/dvdy.22540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brunner E, Peter O, Schweizer L, Basler K. pangolin encodes a Lef-1 homologue that acts downstream of Armadillo to transduce the Wingless signal in Drosophila. Nature. 1997;385:829–833. doi: 10.1038/385829a0. [DOI] [PubMed] [Google Scholar]
- 27.van de Wetering M, et al. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell. 1997;88:789–799. doi: 10.1016/s0092-8674(00)81925-x. [DOI] [PubMed] [Google Scholar]
- 28.Ohlstein B, Spradling AC. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- 29.Singh SR, Liu W, Hou SX. The adult Drosophila Malpighian tubules are maintained by multipotent stem cells. Cell Stem Cell. 2007;1:191–203. doi: 10.1016/j.stem.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deithier VG. The Hungry Fly. New York: Harvard Univ Press; 1976. [Google Scholar]
- 31.King DG. The origin of an organ: Phylogenetic analysis of evolutionary innovation in the digestive tract of flies (Insecta:Diptera) Evolution. 1991;45:568–588. doi: 10.1111/j.1558-5646.1991.tb04330.x. [DOI] [PubMed] [Google Scholar]
- 32.Singh SR, Zeng X, Zheng Z, Hou SX. The adult Drosophila gastric and stomach organs are maintained by a multipotent stem cell pool at the foregut/midgut junction in the cardia (proventriculus) Cell Cycle. 2011;10:1109–1120. doi: 10.4161/cc.10.7.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beebe K, Lee WC, Micchelli CA. JAK/STAT signaling coordinates stem cell proliferation and multilineage differentiation in the Drosophila intestinal stem cell lineage. Dev Biol. 2010;338:28–37. doi: 10.1016/j.ydbio.2009.10.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.