Abstract
Chemokines and chemokine receptors are extensively and broadly involved in cancer metastasis. Previously, we demonstrated that epigenetic silencing of the chemokine CXCL12 sensitizes breast and colon cancer cells to endocrine signaling and metastasis to distant tissues. Yet, the precise mechanism whereby CXCL12 production by tumor cells regulates dissemination remains unclear. Here, we show that administration of CXCL12 extended survival of tumor-bearing mice by potently limiting metastasis of colorectal carcinoma or murine melanoma. Because secreted CXCL12 is a mixture of monomeric and dimeric species in equilibrium, oligomeric variants that either promote (monomer) or halt (dimer) chemotaxis were used to dissect the mechanisms interrupting carcinoma metastasis. Monomeric CXCL12 mobilized intracellular calcium, inhibited cAMP signaling, recruited β-arrestin-2, and stimulated filamentous-actin accumulation and cell migration. Dimeric CXCL12 activated G-protein-dependent calcium flux, adenylyl cyclase inhibition, and the rapid activation of ERK1/2, but only weakly, if at all, recruited arrestin, stimulated actin polymerization, or promoted chemotaxis. NMR analyses illustrated that CXCL12 monomers made specific contacts with CXCR4 that were lost following dimerization. Our results establish the potential for inhibiting CXCR4-mediated metastasis by administration of CXCL12. Chemokine-mediated migration and β-arrestin responses did not dictate the antitumor effect of CXCL12. We conclude that cellular migration is tightly regulated by selective CXCR4 signaling evoked by unique interactions with distinct ligand quaternary structures.
Keywords: malignancy, functional selectivity, cellular idling, cancer therapeutics, chemokine oligomer
Chemokines are chemoattractant cytokines that bind G-protein–coupled receptors and are mediators for many physiological processes including cell trafficking, angiogenesis, and embryogenesis (1–3). Chemokine receptor signaling is linked with cancer metastasis as well as infiltration of tumor-associated immune cells, neoangiogenesis, and proliferation (4, 5). Chemokines as primary mediators of metastasis were first identified by Muller and colleagues who implicated the chemokine receptor CXCR4 in tumor cell trafficking (6–8). At least 23 different cancers have been shown to express elevated levels of CXCR4, sensitizing these cancers to CXCL12 gradients in distant tissues (9). CXCL12 is constitutively expressed in the bone marrow, lungs, and liver, which are common tissues of metastatic growth. Efforts to block metastatic dissemination have mainly used small molecule antagonists of CXCR4 (10) to limit cancer malignancy (11, 12). However, this avenue has proven difficult to move into the clinic (5, 12), suggesting alternative strategies to interfere with CXCR4-guided metastatic homing (for example, by the use of agonists rather than antagonists) are required. Our previous data indicate that epigenetic silencing of the Cxcl12 promoter enhances metastasis of colonic and mammary carcinoma, implicating the chemokine ligand as a key variable governing metastatic potential (13–15).
Chemokines coordinate cell migration within the context of stable adhesion to surrounding cells or extracellular matrix. A paradox of chemokines is that chemotaxis, but not proximal intracellular signaling, exhibits a biphasic response over a limited concentration range (16, 17). Chemokine ligands exist as a mixture of monomeric and higher-order species (18). We discovered that changes in the oligomeric state of CXCL12 dictated the migratory outcome of monocytic cells in vitro, with monomer, but not dimer, inducing cellular migration (17). Congruently, restoration of CXCL12 expression in colonic and mammary carcinoma cells markedly reduced their metastatic potential in vivo (14, 15), in part, through increased sensitivity to detachment-induced cell death (15, 19). We therefore speculate that local CXCL12 secretion acts as a tumor suppressor, preventing metastasis by shielding the primary tumor from remote chemotactic gradients. Here, we show that administration of wild-type CXCL12, a monomeric variant, or a constitutively dimeric mutant inhibits hepatic metastasis of colorectal carcinoma. Interestingly, dimeric CXCL12 exhibited receptor interactions, downstream signaling, and migration responses distinct from the monomeric chemokine. These data suggest the oligomeric states of CXCL12 mediate distinct biological effects through the same receptor.
Results
CXCL12 Treatment Blocks Colonic Carcinoma Metastasis.
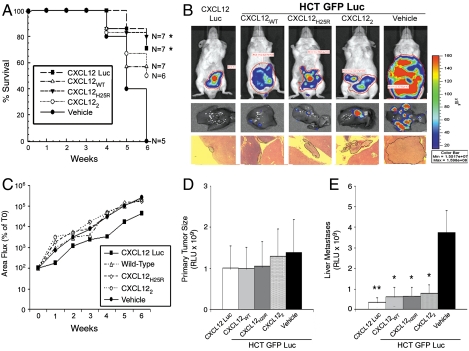
Previously, we demonstrated that colon cancer cells reexpressing CXCL12 had reduced primary tumor growth and hepatic metastasis, in part through elevated detachment-induced cell death termed anoikis (13, 15, 19). Here, we administered exogenous CXCL12 to immunodeficient mice orthotopically engrafted with human colonic carcinoma cells stably expressing firefly luciferase. To further dissect the roles for the monomeric and dimeric chemokine in metastasis, we used previously engineered CXCL12 variants (17). Mice were treated with intraperitoneal injections of wild-type (CXCL12WT), preferentially monomeric (CXCL12H25R) (18), or constitutively dimeric (CXCL122) (17) chemokine. Because our prior work indicates metastasis is markedly diminished in colon cancer cells (13) engineered to produce endogenous CXCL12 (CXCL12-Luc), those cells were used as a positive control (15, 19). As shown in Fig. 1A, treatment with each of the three CXCL12 proteins improved survival. Biophotonic imaging indicated little change in overall tumor formation between CXCL12 variants (Fig. 1B). Quantification of those data confirmed that observation, with little difference in whole body (Fig. 1C) or ex vivo primary tumor luminescence between any of the treatment groups. However, as highlighted in Fig. 1 B and E, ex vivo examination revealed markedly decreased tumor numbers in the liver of treated mice, suggesting the survival benefit reflected decreased metastasis. Indeed, administration of exogenous CXCL12 proteins was comparable to that of CXCL12 Luc cells in decreasing metastasis and improving survival.
Fig. 1.
CXCL12 treatment decreases metastasis of colorectal carcinoma cells. (A) CXCL12WT or CXCL12H25R or 100 μM CXCL122 chemokines were administered biweekly in a 200 μL volume at 50 μM starting 1 week after orthotopic engraftment of HCT116 cells. Statistical significance was determined by Mantel–Cox test. *, P ≤ 0.05. (B) Hepatic tumor formation was visualized in H&E stained liver (4× magnification) and ex vivo bioluminescence imaging after 5 weeks of treatment. (C) Tumor growth was slower in HCT-CXCL12 Luc expressing cells relative to cells treated with CXCL12 variants. Data are radiance units normalized to the week 0 (T0) values. (D) Primary tumor size and (E) level of hepatic metastasis was quantified (RLU) using ex vivo imaging 6 weeks after implantation. Data in C–E are mean ± SEM, n = 5–7 mice. Statistical significance was determined by one-way ANOVA followed by Tukey’s posttest. *, P ≤ 0.05; **, P ≤ 0.01.
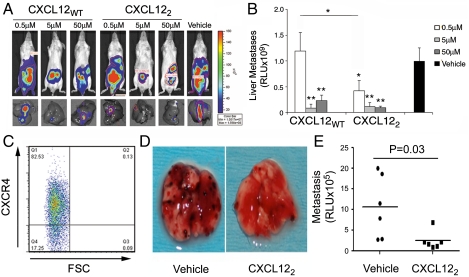
Next, we tested the CXCL12 variants across a range of concentrations. Mice orthotopically engrafted with HCT116 cells were treated with 0.5-, 5-, or 50-μM doses of CXCL12WT or CXCL22. Overall tumor burden was markedly diminished in animals treated with titrated doses of chemokine (Fig. 2A). Ex vivo quantification revealed that CXCL12WT or CXCL122 significantly decreased hepatic metastases at 5 and 50 μM, while the dimer also decreased metastasis at 0.5 μM (Fig. 2B). Wild-type and monomer CXCL12 were unable to inhibit liver metastasis at the lowest concentration (Fig. S1). We next tested if this response was limited to colonic carcinoma cells. CXCR4 enhances metastatic implantation of B16 murine melanoma cells to the lungs (20). CXCR4 expression was first confirmed in transfected B16 melanoma cells (Fig. 2C). Preincubation of those cells with the optimal 5-μM dose of CXCL122 abrogated pulmonary melanoma metastasis (Fig. 2 D and E). Together, these data indicate that CXCL12 limits the ability of either orthotopic colonic or intravenously implanted melanoma cells to metastasize. Increased potency of CXCL122 suggested the different oligomeric states of the chemokine inhibited metastasis by distinct mechanisms.
Fig. 2.
CXCL12 interferes with colonic carcinoma and melanoma tumor metastasis. (A) Liver metastasis was decreased in mice administered 5 and 50 μM CXCL12WT and CXCL122. Representative images at 4 weeks postengraftment show localized tumor growth and decreased hepatic metastasis. (B) Biweekly CXCL12WT or CXCL122 administration effectively blocked hepatic metastasis. CXCL122 effectively blocked metastasis at 0.5 μM dose. Values are mean ± SEM from six separate animals. Statistical significance was determined by one-way ANOVA/Tukey’s posttest. *, P ≤ 0.05; **, P ≤ 0.01. (C) APC-conjugated antibody confirmed expression of CXCR4 in transduced B16/F1 melanoma cells (CXCR4-B16). (D) CXCR4-B16 cells were premixed with 5 μM CXCL122 or vehicle immediately prior to tail-vein injection. The next day, mice received a second CXCL12 or control treatment. After 14 d, the lungs were recovered and photographed, revealing markedly less melanoma in CXCL122-treated animals. (E) Excised lungs were homogenized and assayed for luciferase activity (n = 6). Statistical significance was determined by Student’s t test. *, P = 0.03.
CXCL12 Monomers and Dimers Exert Opposing Effects on Migration.
To confirm the specific impact of CXCL12 variants on colorectal cancer cells, we examined migration in cell culture systems. CXCL12WT and CXCL12H25R induced HCT116 chemotaxis (Fig. 3A) and HT29 epithelial sheet migration (Fig. 3B). However, neither 10-nM CXCL122 or 1,000-nM CXCL12WT was able to stimulate migration (Fig. 3 A and B and Fig. S2 A and B). Native PAGE revealed the accumulation of homodimers at increasing concentrations of CXCL12WT, consistent with the notion that the lack of migration reflects formation of nonmotogenic CXCL12 dimers (Fig. S2C). Migration differences were not due to alterations in growth or programmed cell death because cell proliferation and apoptosis were not altered by CXCL12 variant stimulation (Fig. S3). Further, CXCL122 competitively blocked CXCL12WT-mediated (Fig. 3C), but not CXCL8-induced, carcinoma migration (Fig. S4).
Fig. 3.
Colonic carcinoma migration in vitro is mediated by monomeric CXCL12. (A) HCT116 chemotaxis was measured after exposure to 10 nM CXCL12WT, CXCL12H25R, CXCL122, or 1,000 nM CXCL12WT. Chemotaxis was quantified by fluorescence microscopy using a 10× objective 16 h after stimulation. (B) HT29 were wounded with an aspirator and stimulated daily for 3 d with 10 nM CXCL12WT, CXCL12H25R, CXCL122, and 1,000 nM CXCL12WT. Migration was quantified by comparing the difference in wound area between day 0 and day 3. Data are the average of eight wounds per treatment with experiments performed in triplicate. (C) CXCL12WT (10 nM) stimulated chemotaxis of HCT116 cells was competitively inhibited by increasing concentrations of CXCL122 dimer. Values in A–C are mean ± SEM, n = 4. Statistical significance was determined by unpaired Student’s t test. *, P ≤ 0.05. (D and E) Cells were exposed to 10 nM CXCL12WT, CXCL12H25R, CXCL122, and 1,000 nM CXCL12WT for 30 min, and F-actin polymerization and lamellipodia formation were visualized by Alexafluor-488 phalloidin staining. Lysophosphotidic acid (LPA) (1 μg/mL) was a positive control. Average fluorescence intensity (AFI) per cell was determined using software at 600× magnification. Thirty single cells were randomly selected and examined per treatment in three independent experiments. Statistical significance was determined by unpaired Student’s t test. *, P ≤ 0.05.
Because cytoskeletal rearrangement and formation of newly branched filamentous-actin (F-actin) are required for migration, we visualized actin polymerization in cells stimulated with CXCL12 variants. Lamellipodia formation and F-actin were elevated in HCT116 cells treated with motogenic 10-nM CXCL12WT or CXCL12H25R but unchanged by 10-nM CXCL122 or 1,000-nM CXCL12WT ligands (Fig. 3 D and E). Activated chemokine receptors undergo phosphorylation by G-protein–coupled receptor kinases, leading to a subsequent wave of G-protein–independent signaling involving β-arrestin (21). F-actin polymerization stimulated by CXCL12WT was decreased in HCT116 cells in which β-arrestin-1 and -2 were genetically depleted (Fig. S5A). Thus, knockdown of β-arrestin-1/-2 (Fig. S5E) significantly decreased wild-type (Fig. S5B) and monomer (Fig. S5C) F-actin accumulation. Pretreatment with pertussis toxin further prevented CXCL12WT- or CXCL12H25R-induced actin polymerization (Fig. S5D), suggesting G-proteins and arrestin play roles in monomer-induced actin rearrangement.
CXCL12 Monomers and Dimers Activate Discrete Signaling Profiles.
To uncover the mechanisms whereby CXCL12 suppresses tumor metastasis and regulates carcinoma cell migration, we defined the ability of CXCL12 variants to interact with its receptors CXCR4 and CXCR7 (22). Whole cell radioligand binding assays indicated that CXCL12WT and CXCL12H25R each bound to CXCR4 and CXCR7 with high affinity, whereas CXCL122 preferentially interacted with CXCR4 (Fig. 4 A and B). Complementary radioligand displacement on cell membrane preparations showed that, relative to CXCR4, CXCL122 binds to CXCR7 with approximately 1,000-fold lower affinity compared to CXCL12WT (Fig. S6), making CXCR7 an unlikely target for CXCL122.
Fig. 4.
Dimeric and monomeric CXCL12 functionally activate CXCR4. (A) The affinities of CXCL12 variants for CXCR4 were determined by 125I-CXCL12 displacement. Kd values for binding of CXCL12WT, CXCL12H25R, and the CXCL122 were calculated as 25, 25, and 150 nM, respectively, from their corresponding logEC50s of -7.59 ± 0.07, -7.62 ± 0.10, and -6.78 ± 0.11. (B) Radioligand displacement of CXCR7-bound 125I-CXCL12 resulted in weaker affinity of CXCL122 (Kd > 1 mM) compared to CXCL12WT (Kd = 28 nM) and CXCL12H25R (Kd = 15 nM). (C) Intracellular calcium mobilization of HCT116 cells stimulated by 10 nM CXCL12 variants was measured in the presence or absence of the Gαi inhibitor, pertussis toxin (PTX). (D) Inhibition of forskolin-induced cAMP production was measured in HEK293 cells cotransfected with CXCR4 and an intramolecular Epac-BRET2 sensor. Measurements were performed 5 min after incubation with CXCL12 variants. (E) Stimulation of HCT116 cells with 10 nM CXCL12WT, (white bar), CXCL12H25R (gray bar), or CXCL122 (black bar) evoked phosphorylation of ERK1/2. Wild-type and monomer resulted in a slower increase in ERK1/2 phosphorylation that was statistically greater than unstimulated (NS) cells starting at 10 min and continuing through 30 min of stimulation. In contrast, dimer resulted in rapid and transient ERK1/2 phosphorylation at 5 min and 10 min and returned to baseline after 15 min and 30 min of treatment. Thirty-minute stimulation with 5 nM epidermal growth factor (EGF) served as a positive control. Data are the ratio of phosphorylated and total ERK1/2 protein normalized to the unstimulated control. Values are mean ± SEM, n = 3. Asterisk indicates statistically significant (P ≤ 0.05) differences. (F) HEK293 cells transiently coexpressing β-arrestin-2-RLuc as a BRET donor and CXCR4-YFP as BRET acceptor were stimulated with the indicated concentrations of CXCL12 variants. Data are the mean ± SD, n = 3. Statistical significance was determined by one-way ANOVA/Dunnett posttest. *, P ≤ 0.01. (G) Time-course measurements of CXCR4 β-arrestin-2 recruitment following stimulation 200 nM CXCL12WT (gray line), CXCL12H25R (dashed line), or CXCL122 (black line). Values are mean ± SD of three separate experiments. (H) Surface CXCR4 levels were measured by flow cytometry after stimulation with CXCL12WT, CXCL12H25R, or CXCL122 for the indicated times (n = 2).
Dimeric CXCR4 activates heterotrimeric G-proteins, leading to inhibition of adenylyl cyclase and mobilization of intracellular calcium. As predicted, CXCL12WT mobilized intracellular calcium in a pertussis toxin sensitive fashion, with CXCL12H25R and CXCL122 each evoking comparable fluxes in calcium (Fig. 4C). Calcium mobilization was dose dependent and inhibited by CXCR4 specific inhibitors (Fig. S7). We had previously demonstrated that CXCL12 binding to CXCR4 on colonic carcinoma cells inhibited forskolin-induced cAMP production (23). To further assess G-protein activation, we next measured inhibition of forskolin-stimulated cAMP production using an Epac bioluminescence resonance energy transfer (BRET) sensor (24). As shown in Fig. 4D, CXCL12 variants inhibited cAMP-dependent activation of Epac, providing further evidence for Gαi activation via CXCR4.
Lastly, arrestin-dependent and -independent signaling through the G-protein –coupled receptor was examined. As shown in Fig. 4E and illustrated in Fig. S8, CXCL12H25R stimulated a slower increase in ERK1/2 phosphorylation relative to CXCL122, which evoked a more rapid and transient activation. In agreement with those data, BRET analyses demonstrated that stimulation with CXCL12WT and CXCL12H25R elicited the dose-dependent (Fig. 4F) and time-dependent (Fig. 4G) recruitment of β-arrestin-2 to CXCR4. There was substantially less CXCL122-induced recruitment of β-arrestin-2 (Fig. 4 F and G). Nevertheless, as shown in Fig. 4H, each of the CXCL12 variants stimulated CXCR4 internalization. Thus, monomeric and dimeric chemokine functionally activate discrete signaling pathways through CXCR4.
CXCL12 Dimers Have Distinct Interactions with CXCR4.
We next asked if the differential migration and signaling responses resulted from unique interactions between the CXCL12 variants and CXCR4. In the “two-step, two-site” model for chemokine receptor activation (25), CXCL12 binds first to the CXCR4 N terminus. Our prior work indicated that CXCR4 N terminus (CXCR41–38) bridges the CXCL12 dimer interface and forms contacts that are absent in the monomer, suggesting the oligomers interact differently with the receptor (17). NMR measurements of CXCR41–38 dynamics indicate that residues 5–10 of the receptor are flexible in complex with CXCL122 but become more ordered when bound to CXCL12H25R (Fig. 5A). Based on these structural data, we speculated that oligomer-specific CXCR4 binding modes could lead to qualitatively different signaling.
Fig. 5.
Distinct interactions between monomeric and dimeric CXCL12 and CXCR4. (A) 15N-1H heteronuclear nuclear Overhauser effect (NOE) of 250 μM [U-15N]-CXCR41–38 in the absence (gray) and presence (black) of 500 μM CXCL122 (Upper) or CXCL12H25R (Lower). CXCR41–38 residues 4–9 (red) are weakly associated with CXCL122, but interact strongly with CXCL12H25R, indicating a unique 1∶1 complex stoichiometry. (B) Heteronuclear single-quantum coherence titration of [U-15N]-CXCR41–38 with CXCL12WT at pH 5.5 shows two-stage binding behavior, with CXCR4 N-terminus residue signals retreating toward their original position at elevated CXCL12 concentrations. (C) Stoichiometry of the CXCL12–-CXCR41–38 complex shifts from 1∶1 to 2∶2 as the CXCL12 concentration exceeds the Kd for dimerization in the presence of CXCR41–38 (50 μM).
In an NMR titration of [U-15N]-labeled CXCR41–38 peptide with unlabeled CXCL12WT, many residues shift in response to the addition of substoichiometric CXCL12WT, but some peaks exhibited unusual behavior at higher chemokine concentrations (Fig. 5B). In particular, the signals for S5 and I6 reversed direction and shifted back toward their original positions before broadening beyond detection. After initially moving to lower 1H and 15N frequencies, the T13 amide signal shifted in a different direction. In an equivalent titration using CXCL122, only simple shift perturbations were observed, consistent with a single complex (17). However, we showed previously that binding to CXCR41–38 promotes CXCL12 dimerization (26). Thus, the biphasic progression of chemical shift perturbations induced by CXCL12WT denotes the formation of distinct CXCL12–CXCR41–38 complexes during the titration as the CXCL12 equilibrium shifts from monomer to dimer. Together with the differences in backbone mobility, and as summarized in Fig. 5C, these data demonstrate that the CXCL12 monomer makes specific contacts with CXCR4 that are lost when CXCL12 dimerizes.
Discussion
Overexpression of CXCR4 has been correlated with tumor malignancy (6). Our refined model for chemokine-directed metastasis includes epigenetic silencing of the ligand that confers a leukocyte migratory phenotype to carcinoma cells that is diminished by reexpression of the suppressed chemokine (13–15, 25). The purpose of our study was to test whether exogenous CXCL12 treatment would also reduce metastasis. In contrast to early studies focused largely on CXCR4 receptor antagonism, our data establish that exogenous CXCL12 is a potent inhibitor of colorectal and melanoma metastasis. Because CXCL12 exists in a monomer–dimer equilibrium, we also assessed the therapeutic potential of preferentially monomeric and constitutively dimeric CXCL12 variants. We demonstrated that CXCL12, independent of its oligomerization, reduced metastasis. In combination with our prior work, we have discovered that CXCL12 produced by cancer cells or administered in a preclinical setting provides a strong antimetastatic cue.
Our work indicates that CXCL12WT exists in a complex equilibrium in the presence of CXCR41–38, consistent with our previous results (18). Using CXCL12H25R and CXCL122, we isolated the equilibrium components and observed stable contacts between the CXCR4 N terminus and monomeric CXCL12 that are lost upon dimerization. CXCR4 forms constitutive homodimers in live cells (27), and the recently solved crystal structure revealed a dimeric arrangement compatible with either 1∶2 or 2∶2 ligand∶receptor complexes (28). Thus, ligand dimerization might correspond to single- versus double-agonist occupancy of the dimeric receptor, with double occupancy by the forced dimeric ligand evoking an immotile cellular arrest response, as noted for other class A G-protein–coupled receptors (29). If so, single versus double receptor-occupancy might indeed provide a general mechanism for the as yet unexplained ubiquitous biphasic dose-responses of chemotaxis, regardless of the capacity of the chemoattractant ligand to dimerize.
We previously demonstrated that CXCL12H25R extended the chemotactic response to higher concentrations and that CXCL122 was unable to stimulate leukocyte migration (17). With this report, we demonstrate that colon carcinoma cells follow a similar biphasic migration response driven almost exclusively by monomeric ligand. Examination of the receptors and signaling pathways regulating these opposing functions revealed that monomeric and dimeric chemokine bind to and drive internalization of CXCR4. Both ligands mobilize intracellular calcium and inhibit adenylyl cyclase signaling. Interestingly, CXCL12H25R stimulated F-actin polymerization, mediated, at least in part, through β-arrestin, and evoked a prolonged phosphorylation of ERK1/2. In contrast, CXCL122 stimulated a rapid and transient increase in ERK1/2 with minimal, if any, recruitment of β-arrestin or induction of actin mobilization. Thus, a clue to the absent chemotactic response to CXCL122 may rest in altered β-arrestin-2 signaling. The requirement for β-arrestin-2 in CXCR4-mediated migration has been documented using knockout mice (30), siRNA (31), and in the context of hypermigratory CXCR4 mutations (32). Depletion of both β-arrestin-1 and β-arrestin-2 significantly inhibited CXCL12WT- or CXCL12H25R-stimulated F-actin polymerization, suggesting arrestin was, in part, regulating migration. In contrast to arrestin knockdown, pertussis toxin abrogated HCT116 migration, calcium signaling, and cytoskeletal remodeling. Thus, we postulate dimer-induced signaling induces a nonmotile cellular idling response. The differential signaling activated by CXCL12H25R and CXCL122 mirrors prior reports for the CCR7 ligands CCL19 and CCL21, as well as monomeric and dimeric forms of CXCL8 (33, 34).
Differential activation of CXCR4 provides an exciting avenue for therapeutic intervention using biologic agonists tailored to the monomer or dimer. Chemokine-mediated inhibition of tumorigenesis has been attributed to trafficking of immune cells to the tumor (9, 35, 36). Our work supports a cancer progression model in which locally produced CXCL12 limits the migration potential by either desensitizing cells to endocrine ligand or by exceeding the optimal level for chemotaxis, resulting in the absence of actin polymerization and cell migration characteristic of dimeric CXCL12 (13–15). Alternatively, tumor-cell–produced CXCL12 limits spread of cells by nullifying chemokine gradients produced in distant tissues (13, 15). Here, we show that administration of CXCL12 prevents metastasis of colonic carcinomas or murine melanomas lacking endogenous ligand expression. Although monomeric and dimeric ligands activate opposing cell migratory responses in vitro, each markedly inhibited metastasis in a preclinical animal model. Our data suggest CXCL12 interruption in metastasis results from formation of dimers at elevated concentrations activating distinct “cellular idling” signaling pathways, desensitizing tumor cell-expressed CXCR4, and/or ligand-induced disruption in chemoattractant gradients. Our data therefore fill the gap in the current model defining increased expression of CXCR4 as a key regulatory step in tumor metastasis and explain how pathologic silencing of the Cxcl12 gene during neoplasia (i) confers a metastatic phenotype to those cells and (ii) enhances metastasis through the loss of autocrine ligand formation that limits emigration. These data establish CXCL12 as a potential avenue for the next generation of biologic therapies that specifically target metastasis and suggest ligand administration is a viable neoadjuvant intervention in colon cancer and melanoma.
Materials and Methods
Cell Culture.
HCT116 (CCL-247) and HT-29 (HTB-38) human colorectal carcinoma cells were purchased from ATCC and maintained as previously described (13). The HCT double stable cell lines (GFP Luc or CXCL12 Luc) were defined in our prior report (15).
Protein Expression and Purification.
CXCL12WT, CXCL122, and CXCL12H25R were expressed and purified as previously described (17, 18, 26, 37).
Bioluminescence Imaging.
HCT GFP-Luc cells (2 × 106) were suspended in 50 μL sterile phosphate-buffered saline (PBS) and implanted to the subserosal layer of the cecum in 8-week-old male SCID mice (cr-Prdkcscid, Charles River) using a Medical College of Wisconsin Institutional Animal Care and Use Committee approved protocol. CXCL12 Luc cells (2 × 106) were used as a control. Tumor burden was quantified as previously described (15). See details in SI Materials and Methods.
Melanoma Metastasis.
B16 murine melanoma cells in logarithmic phase of growth were premixed with vehicle or CXCL12 variant peptides in vitro and injected intravenously via the tail vein of mice. Prior to injection, cells were ≥90% viable as assessed by trypan blue exclusion. After 14 d, mice were euthanized and tumor burden quantified by in vitro luciferase assay as previously described (20).
Chemotactic Migration.
Transwell plates (8 μM pore size polycarbonate membrane, 6.5-mm diameter) were coated with 15 μg/mL collagen for 30 min at 37 °C (Corning Inc.). HCT116 cells were serum-starved 24 h and then released from the plate using cell dissociation buffer. Cells were collected, washed, and 50,000 cells/100 μL plated in serum-free medium in the top chamber and chemokine in the bottom chamber. After 16 h, cells on the top transwell membrane were removed using a cotton swab, fixed, and stained with 4′,6-diamidino-2-phenylindole. Chemotaxis was quantified by counting the migrated cells on the lower surface of the membrane of at least three fields per chamber at 10× objective.
BRET Measurements.
β-arrestin-2 recruitment to CXCR4 was measured by an intermolecular BRET assay performed as described previously (27, 38). The values were corrected to net BRET by subtracting the background BRET signal obtained in cells transfected with the Rluc construct alone. Intracellular cAMP levels were measured using an intramolecular Epac BRET2 sensor as described previously (24). BRET signal was calculated as the ratio of light emitted by acceptor over the light emitted by donor. See details in SI Materials and Methods.
Flow Cytometry.
Cells of the T-cell lymphoblastic line CEM were incubated with 100 nM of wild-type, monomeric, or dimeric CXCL12 in complete media at 37 °C for the indicated time. Cells were washed in PBS and then incubated with phycoerythrin-conjugated mouse monoclonal antibody to CXCR4 (clone 12G5; R&D Systems) and fluorescence intensity measured by flow cytometry (FACS Calibur, BD Biosciences).
Supplementary Material
Acknowledgments.
This work was supported by grants from the National Institutes of Health [AI058072 (B.F.V.) and DK062066 (M.B.D.)], the State of Wisconsin Income Tax Breast Cancer Check Box Program (B.F.V.), the Wisconsin Breast Cancer Showhouse (M.B.D.), and a Medical College of Wisconsin Interdisciplinary Cancer Fellowship (T.T.), as well as Advancing a Healthier Wisconsin (S.T.H.), an Ann’s Hope Foundation for Melanoma grant (T.T. and S.T.H.), the American Cancer Society Spin Odyssey Postdoctoral Fellowship from the New England Division and the University of Wisconsin–Whitewater (C.T.V.), the Bobbie Nick Voss Charitable Foundation (M.B.D.), the Canadian Institutes of Health Research (HOP-93431), the Fondation de l’Hôpital Sainte-Justine, and the Groupe de Recherche Universitaire sur le Médicament (N.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1101133108/-/DCSupplemental.
References
- 1.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 2.Wang JM, Deng X, Gong W, Su S. Chemokines and their role in tumor growth and metastasis. J Immunol Methods. 1998;220:1–17. doi: 10.1016/s0022-1759(98)00128-8. [DOI] [PubMed] [Google Scholar]
- 3.Strieter RM. Chemokines: Not just leukocyte chemoattractants in the promotion of cancer. Nat Immunol. 2001:285–286. doi: 10.1038/86286. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Baruch A. The multifaceted roles of chemokines in malignancy. Cancer Metastasis Rev. 2006:357–371. doi: 10.1007/s10555-006-9003-5. [DOI] [PubMed] [Google Scholar]
- 5.Sun X, et al. CXCL12/CXCR4/CXCR7 chemokine axis and cancer progression. Cancer Metastasis Rev. 2010;29:709–722. doi: 10.1007/s10555-010-9256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muller A, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 7.Murphy PM. Chemokines and the molecular basis of cancer metastasis. N Engl J Med. 2001;345:833–835. doi: 10.1056/NEJM200109133451113. [DOI] [PubMed] [Google Scholar]
- 8.Lee CH, et al. Sensitization of B16 tumor cells with a CXCR4 antagonist increases the efficacy of immunotherapy for established lung metastases. Mol Cancer Ther. 2006;5:2592–2599. doi: 10.1158/1535-7163.MCT-06-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dell’Agnola C, Biragyn A. Clinical utilization of chemokines to combat cancer: The double-edged sword. Expert Rev Vaccines. 2007;6:267–283. doi: 10.1586/14760584.6.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu X, Lee VC, Chevalier E, Hwang ST. Chemokine receptors as targets for cancer therapy. Curr Pharm Des. 2009;15:742–757. doi: 10.2174/138161209787582165. [DOI] [PubMed] [Google Scholar]
- 11.Epstein RJ. The CXCL12-CXCR4 chemotactic pathway as a target of adjuvant breast cancer therapies. Nat Rev Cancer. 2004;4:901–909. doi: 10.1038/nrc1473. [DOI] [PubMed] [Google Scholar]
- 12.Horuk R. Chemokine receptor antagonists: Overcoming developmental hurdles. Nat Rev Drug Discov. 2009;8:23–33. doi: 10.1038/nrd2734. [DOI] [PubMed] [Google Scholar]
- 13.Wendt MK, et al. Silencing of epithelial CXCL12 expression by DNA hypermethylation promotes colonic carcinoma metastasis. Oncogene. 2006;25:4986–4997. doi: 10.1038/sj.onc.1209505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wendt MK, Cooper AN, Dwinell MB. Epigenetic silencing of CXCL12 increases the metastatic potential of mammary carcinoma cells. Oncogene. 2008;27:1461–1471. doi: 10.1038/sj.onc.1210751. [DOI] [PubMed] [Google Scholar]
- 15.Wendt MK, Drury LJ, Vongsa RA, Dwinell MB. Constitutive CXCL12 expression induces anoikis in colorectal carcinoma cells. Gastroenterology. 2008;135:508–517. doi: 10.1053/j.gastro.2008.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith JM, Johanesen PA, Wendt MK, Binion DG, Dwinell MB. CXCL12 activation of CXCR4 regulates mucosal host defense through stimulation of epithelial cell migration and promotion of intestinal barrier integrity. Am J Physiol Gastrointest Liver Physiol. 2005;288:G316–G326. doi: 10.1152/ajpgi.00208.2004. [DOI] [PubMed] [Google Scholar]
- 17.Veldkamp CT, et al. Structural basis of CXCR4 sulfotyrosine recognition by the chemokine SDF-1/CXCL12. Sci Signal. 2008;1:ra4. doi: 10.1126/scisignal.1160755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veldkamp CT, Peterson FC, Pelzek AJ, Volkman BF. The monomer-dimer equilibrium of stromal cell-derived factor-1 (CXCL 12) is altered by pH, phosphate, sulfate, and heparin. Protein Sci. 2005;14:1071–1081. doi: 10.1110/ps.041219505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drury LJ, Wendt MK, Dwinell MB. CXCL12 chemokine expression and secretion regulates colorectal carcinoma cell anoikis through Bim-mediated intrinsic apoptosis. PLoS One. 2010;5:e12895. doi: 10.1371/journal.pone.0012895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murakami T, et al. Expression of CXC chemokine receptor-4 enhances the pulmonary metastatic potential of murine B16 melanoma cells. Cancer Res. 2002;62:7328–7334. [PubMed] [Google Scholar]
- 21.Kendall RT, Luttrell LM. Diversity in arrestin function. Cell Mol Life Sci. 2009;66:2953–2973. doi: 10.1007/s00018-009-0088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balabanian K, et al. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280:35760–35766. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- 23.Dwinell MB, Ogawa H, Barrett KE, Kagnoff MF. SDF-1/CXCL12 regulates cAMP production and ion transport in intestinal epithelial cells via CXCR4. Am J Physiol Gastrointest Liver Physiol. 2004;286:G844–G850. doi: 10.1152/ajpgi.00112.2003. [DOI] [PubMed] [Google Scholar]
- 24.Leduc M, et al. Functional selectivity of natural and synthetic prostaglandin EP4 receptor ligands. J Pharmacol Exp Ther. 2009;331:297–307. doi: 10.1124/jpet.109.156398. [DOI] [PubMed] [Google Scholar]
- 25.Tessema M, et al. Re-expression of CXCL14, a common target for epigenetic silencing in lung cancer, induces tumor necrosis. Oncogene. 2010;29:5159–5170. doi: 10.1038/onc.2010.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veldkamp CT, Seibert C, Peterson FC, Sakmar TP, Volkman BF. Recognition of a CXCR4 sulfotyrosine by the chemokine stromal cell-derived factor-1alpha (SDF-1alpha/CXCL12) J Mol Biol. 2006;359:1400–1409. doi: 10.1016/j.jmb.2006.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Percherancier Y, et al. Bioluminescence resonance energy transfer reveals ligand-induced conformational changes in CXCR4 homo- and heterodimers. J Biol Chem. 2005;280:9895–9903. doi: 10.1074/jbc.M411151200. [DOI] [PubMed] [Google Scholar]
- 28.Wu B, et al. Structures of the CXCR4 Chemokine GPCR with Small-Molecule and Cyclic Peptide Antagonists. Science. 2010;330:1066–1071. doi: 10.1126/science.1194396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han Y, Moreira IS, Urizar E, Weinstein H, Javitch JA. Allosteric communication between protomers of dopamine class A GPCR dimers modulates activation. Nat Chem Biol. 2009:688–695. doi: 10.1038/nchembio.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun Y, Cheng Z, Ma L, Pei G. Beta-arrestin2 is critically involved in CXCR4-mediated chemotaxis, and this is mediated by its enhancement of p38 MAPK activation. J Biol Chem. 2002;277:49212–49219. doi: 10.1074/jbc.M207294200. [DOI] [PubMed] [Google Scholar]
- 31.Fong AM, et al. Defective lymphocyte chemotaxis in beta-arrestin2- and GRK6-deficient mice. Proc Natl Acad Sci USA. 2002;99:7478–7483. doi: 10.1073/pnas.112198299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lagane B, et al. CXCR4 dimerization and beta-arrestin-mediated signaling account for the enhanced chemotaxis to CXCL12 in WHIM syndrome. Blood. 2008;112:34–44. doi: 10.1182/blood-2007-07-102103. [DOI] [PubMed] [Google Scholar]
- 33.Zidar DA, Violin JD, Whalen EJ, Lefkowitz RJ. Selective engagement of G protein coupled receptor kinases (GRKs) encodes distinct functions of biased ligands. Proc Natl Acad Sci USA. 2009;106:9649–9654. doi: 10.1073/pnas.0904361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nasser MW, et al. Differential activation and regulation of CXCR1 and CXCR2 by CXCL8 monomer and dimer. J Immunol. 2009;183:3425–3432. doi: 10.4049/jimmunol.0900305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luster AD, Leder P. IP-10, a -C-X-C- chemokine, elicits a potent thymus-dependent antitumor response in vivo. J Exp Med. 1993;178:1057–1065. doi: 10.1084/jem.178.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fushimi T, O’Connor TP, Crystal RG. Adenoviral gene transfer of stromal cell-derived factor-1 to murine tumors induces the accumulation of dendritic cells and suppresses tumor growth. Cancer Res. 2006;66:3513–3522. doi: 10.1158/0008-5472.CAN-05-1493. [DOI] [PubMed] [Google Scholar]
- 37.Veldkamp CT, et al. On-column refolding of recombinant chemokines for NMR studies and biological assays. Protein Expr Purif. 2007;52:202–209. doi: 10.1016/j.pep.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gravel S, et al. The peptidomimetic CXCR4 antagonist TC14012 recruits {beta}-arrestin to CXCR7 roles of receptor domains. J Biol Chem. 2010;285:37939–37943. doi: 10.1074/jbc.C110.147470. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.