Abstract
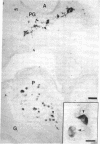
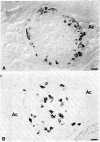
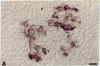
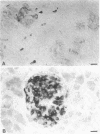
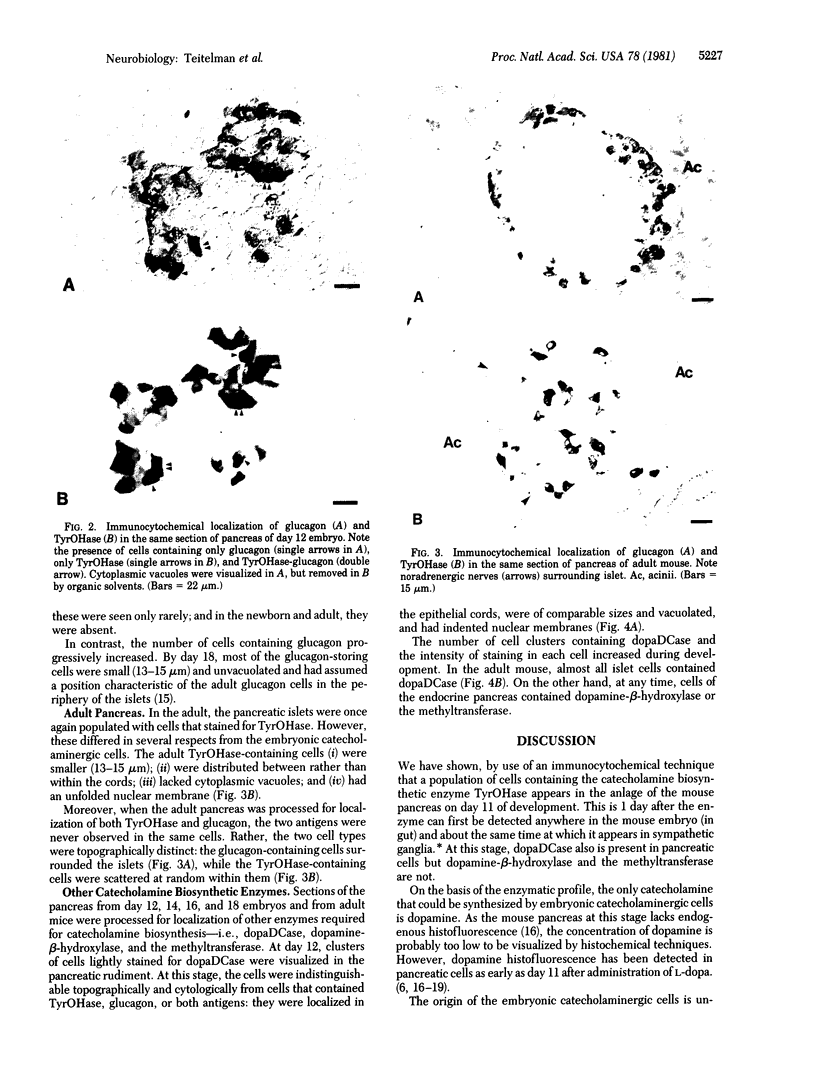
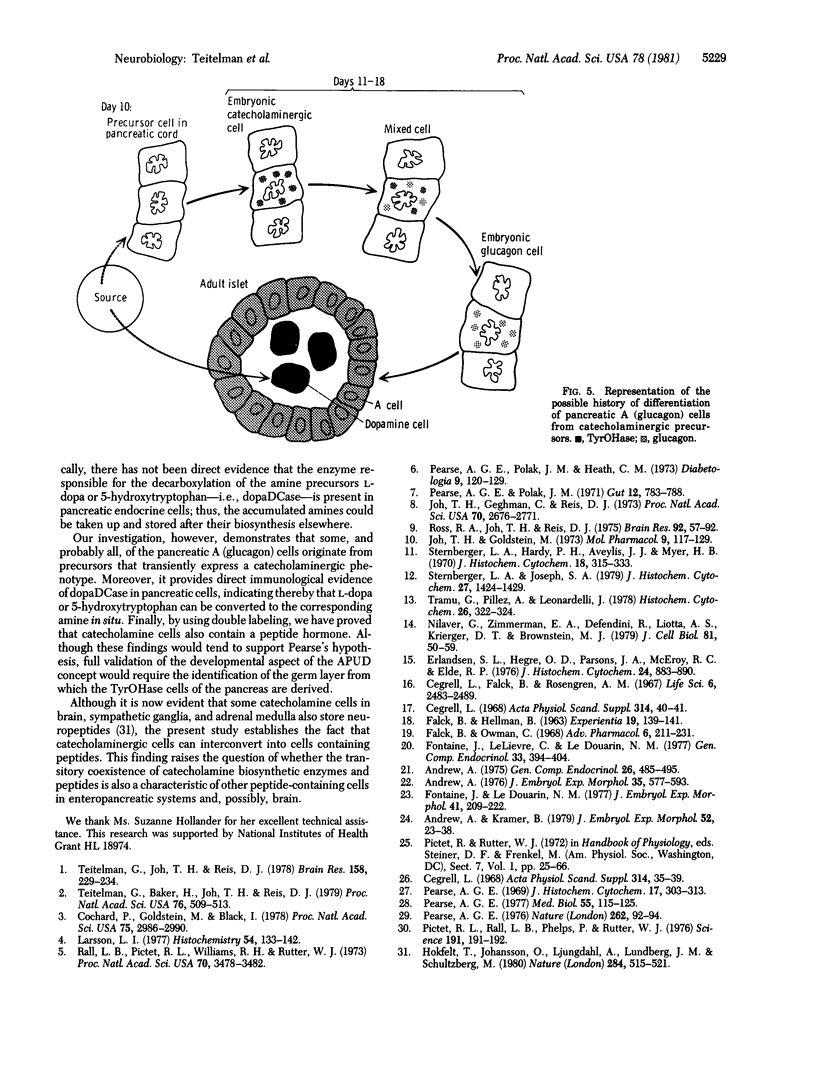
In embryonic mice, the catecholamine biosynthetic enzyme tyrosine hydroxylase [L-tyrosine, tetrahydropteridine:oxygen oxidoreductase (3-hydroxylating), EC 1.14.16.2] can be visualized immunocytochemically in a population of cells in epithelial cords of the developing pancreas. These embryonic catecholamine cells, first seen by day 11, are large and vacuolated and have a folded nuclear membrane. One day later, at day 12, glucagon is first detected immunocytochemically in pancreatic cells similar in location and morphology to the embryonic catecholamine cells. By use of a method for detecting both antigens in the same cell, both the hydroxylase and glucagon can be visualized between day 12 and day 14 in 10-40% of stained cells. From day 14, the number of cells stained for hydroxylase decreases; they cannot be detected after day 18. In contrast, the cells containing glucagon increase during development and persist throughout life. Endocrine cells of the embryonic pancreas also contain dopa decarboxylase but not dopamine-beta-hydroxylase or phenylethanolamine-N-methyl transferase. In adult mice, small cells containing tyrosine hydroxylase but differing in location and morphology from the embryonic catecholaminergic cells are seen in pancreatic islets. The adult catecholaminergic cells never store glucagon. We suggest that adult glucagon (A)-containing cells arise from transformation in situ of cells that transiently express a catecholaminergic (probably dopaminergic) phenotype. These results suggest that one class of peptidergic cells may arise from transformation of an aminergic precursor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrew A. APUD cells in the endocrine pancreas and the intestine of chick embryos. Gen Comp Endocrinol. 1975 Aug;26(4):485–495. doi: 10.1016/0016-6480(75)90171-9. [DOI] [PubMed] [Google Scholar]
- Andrew A. An experimental investigation into the possible neural crest origin of pancreatic APUD (islet) cells. J Embryol Exp Morphol. 1976 Jun;35(3):577–593. [PubMed] [Google Scholar]
- Andrew A., Kramer B. An experimental investigation into the possible origin of pancreatic islet cells from rhombencephalic neurectoderm. J Embryol Exp Morphol. 1979 Aug;52:23–38. [PubMed] [Google Scholar]
- Cegrell L., Falck B., Rosengren A. M. Dopamine and 5-hydroxytryptamine in the guinea-pig pancreas. Life Sci. 1967 Dec 1;6(23):2483–2489. doi: 10.1016/0024-3205(67)90311-6. [DOI] [PubMed] [Google Scholar]
- Cochard P., Goldstein M., Black I. B. Ontogenetic appearance and disappearance of tyrosine hydroxylase and catecholamines in the rat embryo. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2986–2990. doi: 10.1073/pnas.75.6.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlandsen S. L., Hegre O. D., Parsons J. A., McEvoy R. C., Elde R. P. Pancreatic islet cell hormones distribution of cell types in the islet and evidence for the presence of somatostatin and gastrin within the D cell. J Histochem Cytochem. 1976 Jul;24(7):883–897. doi: 10.1177/24.7.60437. [DOI] [PubMed] [Google Scholar]
- Falck B., Owman C. 5-hydroxytryptamine and related amines in endocrine cell systems. Adv Pharmacol. 1968;6(Pt A):211–231. doi: 10.1016/s1054-3589(08)61175-8. [DOI] [PubMed] [Google Scholar]
- Fontaine J., Le Douarin N. M. Analysis of endoderm formation in the avian blastoderm by the use of quail-chick chimaeras. The problem of the neurectodermal origin of the cells of the APUD series. J Embryol Exp Morphol. 1977 Oct;41:209–222. [PubMed] [Google Scholar]
- Fontaine J., Le Lièvre C., Le Douarin N. M. What is the developmental fate of the neural crest cells which migrate into the pancreas in the avian embryo? Gen Comp Endocrinol. 1977 Nov;33(3):394–404. doi: 10.1016/0016-6480(77)90055-7. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Johansson O., Ljungdahl A., Lundberg J. M., Schultzberg M. Peptidergic neurones. Nature. 1980 Apr 10;284(5756):515–521. doi: 10.1038/284515a0. [DOI] [PubMed] [Google Scholar]
- Joh T. H., Geghman C., Reis D. Immunochemical demonstration of increased accumulation of tyrosine hydroxylase protein in sympathetic ganglia and adrenal medulla elicited by reserpine. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2767–2771. doi: 10.1073/pnas.70.10.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joh T. H., Goldstein M. Isolation and characterization of multiple forms of phenylethanolamine N-methyltransferase. Mol Pharmacol. 1973 Jan;9(1):117–129. [PubMed] [Google Scholar]
- Larsson L. I. Ontogeny of peptide-producing nerves and endocrine cells of the gastro-duodeno-pancreatic region. Histochemistry. 1977 Oct 22;54(2):133–142. doi: 10.1007/BF00489671. [DOI] [PubMed] [Google Scholar]
- Nilaver G., Zimmerman E. A., Defendini R., Liotta A. S., Krieger D. T., Brownstein M. J. Adrenocorticotropin and beta-lipotropin in the hypothalamus. Localization in the same arcuate neurons by sequential immunocytochemical procedures. J Cell Biol. 1979 Apr;81(1):50–58. doi: 10.1083/jcb.81.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse A. G., Polak J. M., Heath C. M. Development, differentiation and derivation of the endocrine polypeptide cells of the mouse pancreas. Immunofluorescence, cytochemical and ultrastructural studies. Diabetologia. 1973 Apr;9(2):120–129. doi: 10.1007/BF01230691. [DOI] [PubMed] [Google Scholar]
- Pearse A. G., Polak J. M. Neural crest origin of the endocrine polypeptide (APUD) cells of the gastrointestinal tract and pancreas. Gut. 1971 Oct;12(10):783–788. doi: 10.1136/gut.12.10.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse A. G. The cytochemistry and ultrastructure of polypeptide hormone-producing cells of the APUD series and the embryologic, physiologic and pathologic implications of the concept. J Histochem Cytochem. 1969 May;17(5):303–313. doi: 10.1177/17.5.303. [DOI] [PubMed] [Google Scholar]
- Pearse A. G. The diffuse neuroendocrine system and the apud concept: related "endocrine" peptides in brain, intestine, pituitary, placenta, and anuran cutaneous glands. Med Biol. 1977 Jun;55(3):115–125. [PubMed] [Google Scholar]
- Pictet R. L., Rall L. B., Phelps P., Rutter W. J. The neural crest and the origin of the insulin-producing and other gastrointestinal hormone-producing cells. Science. 1976 Jan 16;191(4223):191–192. doi: 10.1126/science.1108195. [DOI] [PubMed] [Google Scholar]
- Rall L. B., Pictet R. L., Williams R. H., Rutter W. J. Early differentiation of glucagon-producing cells in embryonic pancreas: a possible developmental role for glucagon. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3478–3482. doi: 10.1073/pnas.70.12.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. A., Joh T. H., Reis D. J. Reversible changes in the accumulation and activities of tyrosine hydroxylase and dopamine-beta-hydroxylase in neurons of nucleus locus coeruleus during the retrograde reaction. Brain Res. 1975 Jul 4;92(1):57–72. doi: 10.1016/0006-8993(75)90527-2. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Joseph S. A. The unlabeled antibody method. Contrasting color staining of paired pituitary hormones without antibody removal. J Histochem Cytochem. 1979 Nov;27(11):1424–1429. doi: 10.1177/27.11.92498. [DOI] [PubMed] [Google Scholar]
- Teitelman G., Baker H., Joh T. H., Reis D. J. Appearance of catecholamine-synthesizing enzymes during development of rat sympathetic nervous system: possible role of tissue environment. Proc Natl Acad Sci U S A. 1979 Jan;76(1):509–513. doi: 10.1073/pnas.76.1.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramu G., Pillez A., Leonardelli J. An efficient method of antibody elution for the successive or simultaneous localization of two antigens by immunocytochemistry. J Histochem Cytochem. 1978 Apr;26(4):322–324. doi: 10.1177/26.4.207771. [DOI] [PubMed] [Google Scholar]