Abstract
Although ischemia-induced late preconditioning (PC) is known to be mediated by inducible nitric oxide (NO) synthase (iNOS), the role of this enzyme in pharmacologically induced late PC remains unclear. We tested whether targeted disruption of the iNOS gene abrogates late PC elicited by three structurally different NO donors [diethylenetriamine/NO (DETA/NO), nitroglycerin (NTG), and S-nitroso-N-acetyl-penicillamine (SNAP)], an adenosine A1 receptor agonist [2-chloro-N6-cyclopentyladenosine (CCPA)], and a δ1-opioid receptor agonist (TAN-670). The mice were subjected to a 30-min coronary occlusion followed by 24 h of reperfusion. In iNOS knockout (iNOS–/–) mice, infarct size was similar to wild-type (WT) controls, indicating that iNOS does not modulate infarct size in the absence of PC. Pretreatment of WT mice with DETA/NO, NTG, SNAP, TAN-670, or CCPA 24 h before coronary occlusion markedly reduced infarct size. In iNOS–/– mice, however, the late PC effect elicited by DETA/NO, NTG, SNAP, TAN-670, and CCPA was completely abrogated. Furthermore, in WT mice pretreated with TAN-670 or CCPA, the selective iNOS inhibitor 1400W also abolished the delayed PC properties of these drugs; 1400W had no effect in WT mice. These data demonstrate that iNOS plays an obligatory role in NO donor-induced, adenosine A1 receptor agonist-induced, and δ1-opioid receptor agonist-induced late PC, underscoring the critical role of this enzyme as a common mediator of cardiac adaptations to stress.
Keywords: nitric oxide, inducible nitric oxide synthase, myocardial ischemia, reperfusion injury
ISCHEMIC PRECONDITIONING (PC), the phenomenon whereby brief episodes of ischemia render the heart resistant to subsequent ischemic insults, is a powerful innate cardioprotective mechanism. The late phase of PC is particularly relevant because once induced, it confers cardioprotection for 3–4 days (1, 5). Research performed in the past 10 years has identified nitric oxide (NO) as both a trigger and a mediator of the late phase of ischemic PC (6). In particular, Guo et al. (12) have demonstrated that genetic disruption of the iNOS gene completely abrogates the infarct-sparing effect of ischemia-induced late PC, establishing inducible NO synthase (iNOS) as an obligatory mediator.
The cardioprotection afforded by ischemia-induced late PC can be mimicked by the administration of a variety of pharmacological agents, including NO-releasing agents (16, 19, 23, 24), adenosine A1 receptor agonists (2, 3, 22, 27), and δ1-opioid receptor agonists (10), in dogs, rabbits, and mice. However, the mechanism of pharmacologically induced late PC, and the role of iNOS in particular, remains unclear. Although iNOS is a necessary mediator of ischemia-induced late PC (4), its role in NO donor-induced late PC is presently unknown. A recent study (16) concluded that iNOS mediates morphine-induced late PC in mice, but the short duration of reperfusion (2 h) may not have afforded accurate assessment of final infarct size. In addition, controversy exists as to whether adenosine A1 agonist-induced late PC is mediated by iNOS. Bell et al. (3) concluded that iNOS is not obligatorily required for the development of late PC induced by adenosine A1 agonists, whereas Zhao et al. (27) arrived at the opposite conclusion. Both of these studies were performed with the use of in vitro models of global ischemia. To date, the role of iNOS in adenosine A1 agonist-induced late PC has not been tested in vivo.
Accordingly, the aims of this study were 1) to determine whether three structurally different NO [diethylenetriamine/NO (DETA/NO), nitroglycerin (NTG), and S-nitroso-N-acetylpenicillamine (SNAP)], an adenosine A1 receptor agonist (CCPA), and a δ1-opioid receptor agonist (TAN-670) induce delayed protection against infarction in an in vivo murine model; 2) to determine the effect of targeted disruption of the iNOS gene on NO donor-induced, adenosine A1 receptor agonist-induced, and δ1-opioid receptor agonist-induced late PC; and 3) to verify the effects of iNOS gene deletion by using a selective iNOS inhibitor (1400W) to block adenosine A1 receptor agonist-induced and δ1-opioid receptor agonist-induced late PC. All studies were conducted in a well-established in vivo murine model of myocardial infarction (13).
METHODS
Wild-type and iNOS knockout mice
The study was performed in male mice weighing 30.7 ± 0.4 g (age, 16.3 ± 0.5 wk). The experimental procedures and protocols used in this study were reviewed and approved by the Animal Care and Use Committee of the University of Louisville School of Medicine (Louisville, KY) and conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 86–23, Revised 1996). Breeding pairs of iNOS knockout (iNOS–/–) mice were purchased from Jackson Laboratory and maintained in the homozygous state. The iNOS–/– mice were of hybrid genetic background (C57BL/6J and 129/Sv [B6129/J]). Wild-type (WT) mice with a genetic background (B6129F2/J) as close as possible to the iNOS–/– mice (17) were used as controls. All mice were maintained in sterile microisolator cages under pathogen-free conditions. Food and water were autoclaved, and all handling was done under a laminar flow hood according to standard procedures for maintaining pathogen-free transgenic mice. The iNOS–/– mice were genotyped by PCR and Southern blot hybridization as previously described (17). The mice were typed as they were bred and then retyped with DNA prepared from tissue samples taken at the end of the experiments.
Experimental preparation
The experimental preparation has been described in detail previously (13). The mice were anesthetized with pentobarbital sodium (50 mg/kg iv), intubated, and ventilated with room air supplemented with oxygen at a rate of 105 strokes/min and with a tidal volume of 0.8 ml with the use of a small rodent ventilator. These respiratory settings were found to result in optimal values of arterial pH (7.39 ± 0.01) and PCO2 (31 ± 2 mmHg) (12, 13). Body temperature was carefully monitored with a rectal probe and maintained as close as possible to 37.0°C. To replace blood losses, blood from a donor mouse was given intravenously at 40 ml/kg divided into three equal boluses. After the administration of antibiotics, the chest was opened through a midline sternotomy and a nontraumatic balloon occluder was implanted around the middle left anterior descending coronary artery with the use of an 8-0 nylon suture. After the coronary occlusion/reperfusion protocol, the chest was closed in layers and the mice were allowed to recover.
Experimental protocol
Mice were assigned to 15 groups (Fig. 1). In control groups I (WT control) and II (iNOS–/– control), the mice received no pretreatment. In the treated groups, the mice received either DETA/NO [4 consecutive bolus doses (0.5 mg/kg iv) every 20 min (total dose, 2.0 mg/kg), groups III (WT DETA/NO) and IV (iNOS–/– DETA/NO)]; NTG [24 μg·kg–1·min–1 iv for 60 min, groups V (WT NTG) and VI (iNOS–/– NTG)]; SNAP [2.5 μg·kg–1·min–1 iv for 60 min, groups VII (WT SNAP) and VIII (iNOS–/– SNAP)]; TAN-670 [60 mg/kg ip, groups IX (WT TAN-670) and X (iNOS–/– TAN-670)]; or CCPA [10 μg·kg–1·min–1 iv for 10 min, groups XII (WT CCPA) and XIII (iNOS–/– CCPA)]. Twenty-four hours later (day 2), all mice underwent a 30-min coro nary occlusion followed by 24 h of reperfusion. Groups XI and XIII consisted of WT mice treated with the same doses of TAN-670 and CCPA as groups IX and XII; on day 2, these mice were given 1400W (10 mg/kg ip) 30 min before the 30-min coronary occlusion. Group XV (WT ischemic PC) was preconditioned with a sequence of six cycles of 4-min coronary occlusion/4-min reperfusion; 24 h later (day 2), this group underwent a 30-min coronary occlusion followed by 24 h of reperfusion.
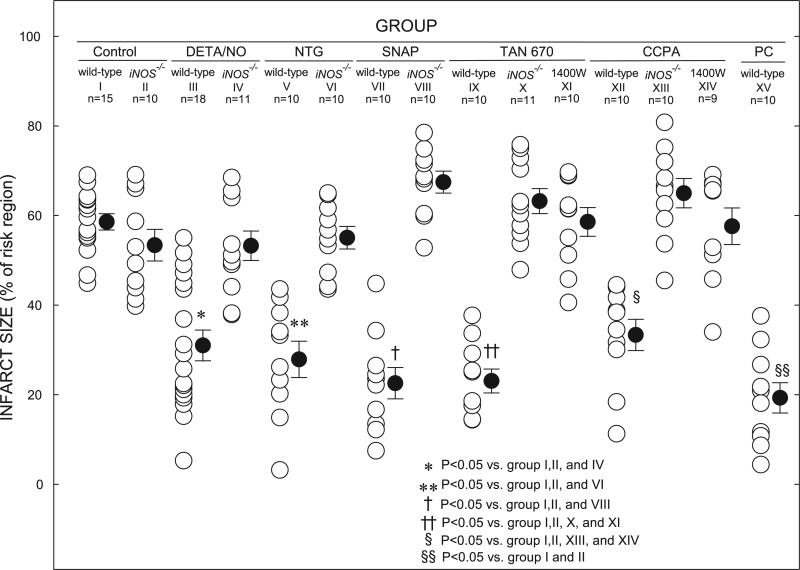
Fig. 1.
Myocardial infarct size in groups I-XV. Infarct is expressed as percentage of region at risk of infarction. Open circles represent individual mice; solid circles represent means ± SE for respective groups.
Postmortem tissue analysis
At the conclusion of the study, the heart was excised and perfused with Krebs-Henseleit solution through an aortic cannula. To delineate infarcted myocardium from viable myocardium, the heart was perfused with 1% triphenyltetrazolium chloride in phosphate buffer. To delineate the occluded/reperfused bed, the coronary artery was tied at the site of the previous occlusion and the aortic root was perfused with 10% Phthalo blue dye (13). As a result of this procedure, the region at risk was identified by the absence of blue dye, whereas the rest of the left ventricle was stained dark blue. The left ventricle was cut into five to seven transverse slices, which were fixed in 10% neutral buffered formaldehyde, weighed, and photographed under a microscope. The corresponding areas were measured by computerized videoplanimetry, and from these measurements the infarct size was calculated as a percentage of the region at risk (13).
Statistical analysis
Data are reported as means ± SE. Measurements were analyzed with a one-way or two-way repeated-measures ANOVA, as appropriate, followed by unpaired Student's t-tests with the Bonferroni correction.
RESULTS
A total of 234 mice were used. Seventy mice were excluded for the reasons specified in Table 1.
Table 1.
Reasons for excluding mice from study
| Groups |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VII | VIII | IX | X | XI | XII | XIII | XIV | XV | Total | |
| Bleeding | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 |
| Death | 5 | 1 | 8 | 6 | 3 | 1 | 1 | 4 | 1 | 0 | 1 | 0 | 1 | 0 | 7 | 39 |
| Technical problems | 0 | 3 | 1 | 2 | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 2 | 1 | 3 | 0 | 19 |
| Poor postmortem staining | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 1 | 1 | 0 | 10 |
| Mice instrumented | 21 | 14 | 28 | 21 | 14 | 12 | 12 | 15 | 15 | 13 | 13 | 12 | 13 | 14 | 17 | 234 |
| Mice excluded | 6 | 4 | 10 | 10 | 4 | 2 | 2 | 5 | 5 | 2 | 3 | 2 | 3 | 5 | 7 | 70 |
| Mice included in study | 15 | 10 | 18 | 11 | 10 | 10 | 10 | 10 | 10 | 11 | 10 | 10 | 10 | 9 | 10 | 162 |
| Mice included in study, % | 71 | 71 | 64 | 52 | 71 | 83 | 83 | 67 | 67 | 85 | 77 | 83 | 77 | 64 | 59 | 69 |
Pilot studies
Pilot studies were performed to identify doses of DETA/NO, NTG, SNAP, TAN-670, CCPA, and 1400W that would not significantly alter heart rate or blood pressure (Table 2). For each drug, heart rate and blood pressure measurements were taken carefully before, during, and after treatment. The doses were chosen on the basis of the maximum amount of drug that could be administered without significantly (≤20% from baseline) altering heart rate or mean arterial blood pressure. As shown in Table 2, at 4 consecutive bolus doses (0.5 mg/kg iv) every 20 min (total dose, 2.0 mg/kg), DETA/NO resulted in a 7% increase in heart rate and a 14% decrease in mean arterial pressure from baseline. NTG given at 24 μg·kg–1·min–1 iv for 60 min resulted in no significant change in heart rate or mean arterial pressure compared with baseline. SNAP at 2.5 μg·kg–1·min–1 iv for 60 min resulted in a 10% increase in heart rate and a 6% decrease in mean arterial pressure. A single 60 mg/kg ip dose of TAN-670 decreased baseline heart rate by 14% and had no appreciable effect on mean arterial pressure. CCPA at 10 μg·kg–1·min–1 iv for 10 min lowered heart rate by 15% and lowered mean arterial pressure by 9%. At 10 mg/kg ip, 1400W resulted in a 6% decrease in heart rate and a 9% decrease in mean arterial pressure.
Table 2.
Blood pressure and heart rate before, during, and after drug administration
| During Infusion, min |
After Administration, min |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Baseline | 10 | 30 | 60 | 10 | 30 | 40 | 50 | 60 | |
| Mean arterial pressure, mmHg | ||||||||||
| DETA/NO | 3 | 95 ± 6 | 92 ± 8 | 90 ± 6 | 86 ± 5 | 88 ± 6 | 85 ± 8 | 82 ± 8 | ||
| NTG | 2 | 97 | 97 | 98 | 99 | 102 | 94 | |||
| SNAP | 3 | 87 ± 5 | 82 ± 6 | 82 ± 5 | 85 ± 6 | 83 ± 2 | 86 ± 6 | |||
| TAN-670 | 3 | 89 ± 2 | 86 ± 5 | 92 ± 3 | 88 ± 2 | 90 ± 2 | ||||
| CCPA | 4 | 92 ± 2 | 84 ± 2 | 84 ± 2 | 85 ± 5 | 87 ± 2 | 88 ± 2 | 92 ± 2 | ||
| 1400W | 3 | 88 ± 6 | 82 ± 5 | 83 ± 5 | 80 ± 6 | |||||
| Heart rate, beats/min | ||||||||||
| DETA/NO | 3 | 520 ± 18 | 522 ± 15 | 505 ± 26 | 532 ± 12 | 510 ± 9 | 508 ± 9 | 485 ± 12 | ||
| NTG | 2 | 504 | 510 | 500 | 510 | 490 | 488 | |||
| SNAP | 3 | 504 ± 8 | 508 ± 9 | 500 ± 5 | 530 ± 8 | 556 ± 8 | 504 ± 15 | |||
| TAN-670 | 3 | 510 ± 6 | 456 ± 9 | 440 ± 12 | 480 ± 14 | 485 ± 10 | ||||
| CCPA | 4 | 552 ± 15 | 470 ± 15 | 500 ± 16 | 515 ± 15 | 540 ± 15 | 550 ± 8 | 580 ± 8 | ||
| 1400W | 3 | 512 ± 17 | 515 ± 10 | 480 ± 16 | 484 ± 16 | |||||
Values are means ± SE. Measurements of blood pressure and heart rate were taken before administration of drugs and for up to 70 min after administration. Doses were diethylenetriamine (DETA/NO, 0.5 mg/kg iv every 20 min), nitroglycerine (NTG, 24 μg·kg–1·min–1 iv for 60 min), S-nitroso-N-acetyl-penicillamine/(SNAP, 2.5 μg·kg–1·min–1 iv for 60 min), TAN-670, (60 mg/kg ip), 2-chloro-N6-cyclopentyladenosine (CCPA, 10 μg·kg–1·min–1 iv for 10 min), and 1400W (10 mg/kg ip).
Body temperature and heart rate
By experimental design, rectal temperature remained within a narrow physiological range (36.8° to 37.3°C) in all groups (Table 3). Heart rate was measured 5 min before the 30-min occlusion, during the occlusion, and after reperfusion (Table 3). The average heart rate in groups I-XV ranged from 485 to 628 beats/min. Heart rate did not differ significantly among the 15 groups at any time during the 30-min occlusion or the ensuing reperfusion (Table 3).
Table 3.
Rectal temperature and heart rate on the day of the 30-min coronary occlusion
| Occlusion, min |
Reperfusion, min |
||||||
|---|---|---|---|---|---|---|---|
| Group | Preocclusion | 5 | 15 | 30 | 1 | 10 | 15 |
| Temperature, °C | |||||||
| I (B6129, AMI) | 36.8 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 | 37.2 ± 0.1 | 37.0 ± 0.1 | 36.8 ± 0.1 | 37.0 ± 0.1 |
| II (iNOS–/–, AMI) | 36.9 ± 0.1 | 37.2 ± 0.1 | 37.2 ± 0.1 | 37.0 ± 0.1 | 37.0 ± 0.1 | 37.0 ± 0.1 | 37.0 ± 0.1 |
| III (B6129, DETA/NO) | 37.1 ± 0.1 | 37.1 ± 0.0 | 37.2 ± 0.0 | 37.1 ± 0.0 | 37.1 ± 0.0 | 37.1 ± 0.1 | 37.1 ± 0.1 |
| IV (iNOS–/–, DETA/NO) | 37.0 ± 0.1 | 37.1 ± 0.1 | 37.2 ± 0.1 | 37.2 ± 0.1 | 37.2 ± 0.1 | 37.2 ± 0.1 | 37.1 ± 0.1 |
| V (B6129, NTG) | 37.0 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 | 37.0 ± 0.0 | 37.0 ± 0.1 | 37.1 ± 0.1 | 37.0 ± 0.0 |
| VI (iNOS–/–, NTG) | 37.0 ± 0.1 | 37.2 ± 0.1 | 37.2 ± 0.0 | 37.2 ± 0.1 | 37.1 ± 0.1 | 37.0 ± 0.1 | 37.1 ± 0.1 |
| VII (B6129, SNAP) | 37.1 ± 0.1 | 37.2 ± 0.1 | 37.2 ± 0.1 | 37.0 ± 0.1 | 37.1 ± 0.1 | 37.0 ± 0.0 | 37.1 ± 0.0 |
| VIII (iNOS–/–, SNAP) | 36.9 ± 0.1 | 37.2 ± 0.1 | 37.1 ± 0.1 | 37.2 ± 0.1 | 37.1 ± 0.1 | 36.9 ± 0.1 | 37.0 ± 0.1 |
| IX (B6129, TAN-670) | 36.9 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.0 | 37.0 ± 0.1 | 36.9 ± 0.1 |
| X (iNOS–/–, TAN-670) | 37.0 ± 0.1 | 37.0 ± 0.1 | 37.2 ± 0.0 | 37.2 ± 0.1 | 37.1 ± 0.0 | 37.0 ± 0.1 | 37.1 ± 0.0 |
| XI (1400W, TAN-670) | 37.1 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 | 37.0 ± 0.0 | 37.0 ± 0.0 | 36.9 ± 0.1 | 37.0 ± 0.1 |
| XII (B6129, CCPA) | 37.0 ± 0.1 | 37.1 ± 0.1 | 37.2 ± 0.1 | 37.1 ± 0.0 | 37.0 ± 0.1 | 37.1 ± 0.0 | 37.1 ± 0.1 |
| XIII (iNOS–/–, CCPA) | 37.0 ± 0.1 | 37.1 ± 0.0 | 37.0 ± 0.1 | 37.1 ± 0.0 | 37.0 ± 0.0 | 37.0 ± 0.0 | 37.1 ± 0.1 |
| XIV (1400W, CCPA) | 36.8 ± 0.1 | 37.2 ± 0.1 | 37.0 ± 0.1 | 37.1 ± 0.0 | 36.8 ± 0.1 | 37.0 ± 0.1 | 37.0 ± 0.1 |
| XV (B6129, LPC) | 36.9 ± 0.1 | 37.3 ± 0.1 | 37.1 ± 0.1 | 37.0 ± 0.1 | 37.3 ± 0.1 | 36.8 ± 0.1 | 37.0 ± 0.1 |
| Heart rate, beats/min | |||||||
| I (B6129, AMI) | 544 ± 12 | 570 ± 14 | 585 ± 22 | 588 ± 22 | 584 ± 23 | 585 ± 20 | 600 ± 24 |
| II (iNOS–/–, AMI) | 523 ± 13 | 522 ± 14 | 515 ± 18 | 517 ± 15 | 502 ± 19 | 548 ± 21 | 567 ± 15 |
| III (B6129, DETA/NO) | 556 ± 12 | 573 ± 10 | 563 ± 10 | 569 ± 10 | 578 ± 9 | 597 ± 10 | 614 ± 13 |
| IV (iNOS–/–, DETA/NO) | 548 ± 17 | 567 ± 17 | 556 ± 16 | 547 ± 15 | 552 ± 12 | 570 ± 9 | 571 ± 13 |
| V (B6129, NTG) | 576 ± 17 | 592 ± 14 | 615 ± 16 | 612 ± 11 | 610 ± 14 | 628 ± 18 | 631 ± 18 |
| VI (iNOS–/–, NTG) | 540 ± 11 | 563 ± 18 | 575 ± 21 | 596 ± 20 | 593 ± 22 | 603 ± 20 | 603 ± 23 |
| VII (B6129, SNAP) | 556 ± 16 | 566 ± 19 | 570 ± 19 | 566 ± 14 | 562 ± 16 | 573 ± 17 | 576 ± 19 |
| VIII (iNOS–/–, SNAP) | 547 ± 8 | 588 ± 8 | 574 ± 17 | 584 ± 15 | 577 ± 15 | 563 ± 16 | 571 ± 14 |
| IX (B6129, TAN-670) | 485 ± 10 | 538 ± 12 | 547 ± 7 | 556 ± 11 | 543 ± 11 | 527 ± 13 | 527 ± 13 |
| X (iNOS–/–, TAN-670) | 517 ± 9 | 579 ± 13 | 594 ± 18 | 602 ± 21 | 601 ± 21 | 598 ± 19 | 597 ± 21 |
| XI (1400W, TAN-670) | 511 ± 15 | 546 ± 15 | 562 ± 11 | 573 ± 11 | 559 ± 15 | 556 ± 15 | 547 ± 17 |
| XII (B6129, CCPA) | 547 ± 14 | 560 ± 13 | 571 ± 20 | 580 ± 17 | 574 ± 20 | 573 ± 14 | 565 ± 18 |
| XIII (iNOS–/–, CCPA) | 513 ± 21 | 579 ± 13 | 596 ± 14 | 606 ± 15 | 599 ± 16 | 593 ± 15 | 578 ± 17 |
| XIV (1400W, CCPA) | 532 ± 17 | 536 ± 17 | 587 ± 23 | 606 ± 28 | 592 ± 29 | 595 ± 29 | 592 ± 28 |
| XV (B6129, LPC) | 551 ± 14 | 566 ± 16 | 565 ± 17 | 572 ± 16 | 572 ± 17 | 581 ± 21 | 576 ± 18 |
Values are means ± SE. Measurements of rectal temperature and heart rate were taken before 30-min coronary occlusion (preocclusion), at 5, 15 and 30 min into 30-min occlusion and at 1, 10, and 15 min after reperfusion. Rectal temperature was continuously monitored and carefully controlled throughout experiment. AMI, acute myocardial infarction; iNOS–/–, inducible nitric oxide synthase knockout mice; LPC, late preconditioning.
Infarct size
There were no significant differences among the 15 groups with respect to body weight or weight of the region at risk (Table 4). In control WT mice (group I), infarct size averaged 58.6 ± 1.8% of the region at risk (Fig. 1). In control iNOS–/– mice (group II), infarct size was similar (53.4 ± 3.5% of the region at risk), indicating that iNOS does not modulate the extent of cell death in the naive (nonpreconditioned) state. In groups III (DETA/NO), V (NTG), VII (SNAP), IX (TAN-670), and XII (CCPA), infarct size was significantly (P < 0.05) smaller (31.0 ± 3.4, 27.9 ± 4.1, 22.6 ± 3.5, 23.1 ± 2.7, and 33.3 ± 3.5% of the region at risk, respectively) compared with the control groups (groups I and II; Fig. 1), indicating a late PC effect. To compare NO donor-induced, TAN-670-induced, and CCPA-induced late PC with the late phase of ischemic PC, the mice in group XV (late PC) were subjected to a sequence of six 4-min occlusion/4-min reperfusion cycles on day 1 followed by a 30-min coronary occlusion on day 2. In these mice, infarct size was 19.3 ± 3.4% of the risk region, similar to that measured in the pharmacologically preconditioned mice (groups III, V, VII, IX, and XII). However, when DETA/NO (group IV), NTG (group VI), SNAP (group VIII), TAN-670 (group X), or CCPA (group XIII) was given to iNOS–/– mice, the reduction in infarct size seen in groups III, V, VII, IX, and XII was completely abrogated (53.3 ± 3.3, 55.1 ± 2.5, 67.5 ± 2.5, 63.2 ± 2.8, and 65.0 ± 3.3% of the region at risk, respectively; Fig. 1). When WT mice pretreated with TAN-670 (group XI) or CCPA (group XIV) were given the iNOS inhibitor 1400W 30 min before the 30-min coronary occlusion, infarct size (58.6 ± 3.2 and 57.6 ± 4.1%, respectively) was similar to that seen in the iNOS–/– mice pretreated with TAN-670 and CCPA and significantly (P < 0.05) greater than that seen in WT mice not treated with 1400W.
Table 4.
Size of left ventricle, risk region, and infarct
| Group | Body Weight, g | Heart Weight, mg | LV Weight, mg | Heart Weight/Body Weight | Risk Region, mg | Infarct Weight, mg | Risk Region, % of LV | Infarct, % of Risk Region | Infarct, % of LV |
|---|---|---|---|---|---|---|---|---|---|
| I | 29.2 ± 1.0 | 161.1 ± 7.8 | 118.6 ± 6.3 | 0.55 ± 0.01 | 43.2 ± 3.0 | 25.6 ± 2.3 | 36.8 ± 2.1 | 58.6 ± 1.8 | 21.7 ± 1.5 |
| II | 30.8 ± 2.4 | 166.6 ± 14.6 | 120.7 ± 9.4 | 0.54 ± 0.02 | 44.3 ± 2.4 | 23.5 ± 1.9 | 38.0 ± 2.8 | 53.4 ± 3.5 | 20.4 ± 2.1 |
| III | 32.3 ± 0.8 | 166.8 ± 6.5 | 124.1 ± 5.4 | 0.52 ± 0.02 | 42.0 ± 2.8 | 13.3 ± 2.0a,b | 33.9 ± 1.7 | 31.0 ± 3.4a,b | 10.4 ± 1.3a,b |
| IV | 31.6 ± 1.7 | 161.4 ± 12.3 | 121.8 ± 9.8 | 0.51 ± 0.03 | 40.1 ± 3.5 | 21.7 ± 2.5 | 33.1 ± 1.8 | 53.3 ± 3.3 | 17.6 ± 1.4 |
| V | 33.7 ± 1.1 | 195.1 ± 8.1 | 150.7 ± 7.5 | 0.58 ± 0.02 | 48.6 ± 3.9 | 14.1 ± 2.4a,c | 33.0 ± 3.4 | 27.9 ± 4.1a,c | 9.5 ± 1.8a,c |
| VI | 27.5 ± 0.8 | 150.8 ± 6.6 | 110.9 ± 6.1 | 0.55 ± 0.02 | 40.6 ± 2.7 | 22.7 ± 2.2 | 36.9 ± 2.0 | 55.1 ± 2.5 | 20.5 ± 1.9 |
| VII | 30.1 ± 0.7 | 160.2 ± 7.7 | 117.1 ± 5.1 | 0.53 ± 0.02 | 35.5 ± 3.7 | 7.92 ± 1.4a,d | 30.1 ± 2.5 | 22.6 ± 3.5a,d | 6.8 ± 1.1a,d |
| VIII | 28.4 ± 1.2 | 167.4 ± 10.7 | 127.1 ± 7.3 | 0.59 ± 0.03 | 42.0 ± 4.1 | 28.6 ± 3.1 | 33.0 ± 2.6 | 67.5 ± 2.5 | 22.4 ± 2.1 |
| IX | 30.1 ± 0.8 | 160.1 ± 6.0 | 119.3 ± 4.5 | 0.53 ± 0.01 | 36.1 ± 3.1 | 8.0 ± 0.83a,e | 30.1 ± 1.9 | 23.1 ± 2.7a,e | 6.8 ± 0.8a,e |
| X | 31.5 ± 0.9 | 177.2 ± 5.6 | 130.7 ± 3.7 | 0.57 ± 0.02 | 38.1 ± 3.9 | 24.9 ± 3.3 | 29.2 ± 2.9 | 63.2 ± 2.8 | 19.1 ± 2.5 |
| XI | 31.8 ± 1.0 | 174.4 ± 5.5 | 129.3 ± 4.1 | 0.55 ± 0.01 | 40.7 ± 3.1 | 24.3 ± 2.6 | 31.4 ± 2.1 | 58.6 ± 3.2 | 18.8 ± 2.0 |
| XII | 31.0 ± 1.0 | 158.1 ± 5.4 | 115.6 ± 4.4 | 0.51 ± 0.01 | 40.7 ± 2.1 | 14.1 ± 2.87a,f | 35.15 ± 1.2 | 33.3 ± 3.5a,f | 12.0 ± 1.4a,f |
| XIII | 31.8 ± 0.9 | 186.1 ± 7.9 | 143.7 ± 6.4 | 0.59 ± 0.02 | 47.1 ± 3.9 | 30.6 ± 2.8 | 32.59 ± 1.9 | 65.0 ± 3.3 | 21.2 ± 1.7 |
| XIV | 28.7 ± 0.72 | 162.2 ± 7.3 | 121.3 ± 5.4 | 0.57 ± 0.03 | 40.8 ± 2.5 | 23.2 ± 1.7 | 34.0 ± 2.0 | 57.6 ± 4.1 | 19.5 ± 1.8 |
| XV | 31.7 ± 1.5 | 145.2 ± 8.6 | 109.4 ± 6.1 | 0.46 ± 0.02 | 37.7 ± 4.4 | 6.32 ± 0.8a | 34.2 ± 2.76 | 19.3 ± 3.4a | 6.2 ± 1.0a |
Values are means ± SE. Heart weight, total heart weight (ventricles and atria); LV, left ventricle
P < 0.05 vs. groups I and II
P < 0.05 vs. group IV
P < 0.05 vs. group VI
P < 0.05 vs. group VIII
P < 0.05 vs. groups X and XI
P < 0.05 vs. group XIII and group XIV.
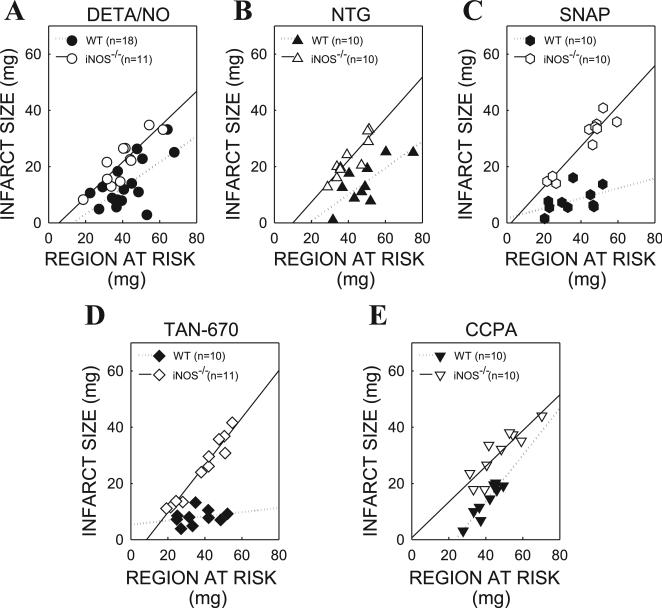
In all groups, infarct size was positively and linearly related to the risk region (Figs. 2 and 3). In the DETA/NO-, NTG-, SNAP-, TAN-670-, and CCPA-treated groups, the regression line for the iNOS–/– mice (groups IV, VI, VIII, X, and XIII) was shifted to the left compared with the WT mice (groups III, V, VII, IX, and XII), indicating that for any given size of the region at risk, the resulting infarction was smaller in WT mice than in iNOS–/– mice. Similarly, in the TAN-670- and CCPA-treated groups, the regression line for the 1400W-treated mice (groups XI and XIV) was shifted to the left compared with the untreated WT mice (groups IX and XII), confirming the absence of a late PC effect (Fig. 3).
Fig. 2.
Relationship between size of region at risk and size of infarction in diethylenetriamine/nitric oxide (DETA/NO)-, nitroglycerin (NTG)-, S-nitroso-N-acetylpenicillamine (SNAP)-, TAN-670-, and 2-chloro-N6-cyclopentyladenosine (CCPA)-treated wild-type (WT) and inducible NO synthase knockout (iNOS–/–) mice. Individual values and regression lines obtained by linear regression analysis are shown. In all groups, infarct size was positively and linearly related to risk region size. A: DETA/NO-treated WT (group III) and iNOS–/– mice (group IV). B: NTG-treated WT (group V) and iNOS–/– mice (group VI). C: SNAP-treated WT (group VII) and iNOS–/– mice (group VIII). D: TAN-670-treated WT (group IX) and iNOS–/– mice (group X). E: CCPA-treated WT (group XII) and iNOS–/– mice (group XIII). Linear regression equations were as follows: group III, y = 0.63x – 3.51, r = 0.88; group IV, y = 0.46x – 6.1, r = 0.65; group V, y = 0.47x – 8.8, r = 0.75; group VI, y = 0.74x – 7.43, r = 0.90; group VII, y = 0.18x + 1.65, r = 0.47; group VIII, y = 0.72x – 1.61, r = 0.94; group IX, y = 0.07x + 5.32, r = 0.27; group X, y = 0.84x – 7.1, r = 0.97; group XII, y = 0.635x – 0.68, r = 0.88; group XIII, y = 0.83x – 19.55, r = 0.93.
Fig. 3.
Relationship between size of region at risk and size of infarction in TAN-670-, CCPA-treated WT, and 1400W-treated mice. In all groups, infarct size was positively and linearly related to risk region size. A: TAN-670-treated WT (group IX) and 1400W-treated mice (group XI). B: CCPA-treated WT (group XII) and 1400W-treated mice (group XIV). Linear regression equations were as follows: group IX, y = 0.07x + 5.32, r = 0.27; group XI, y = 0.75x – 6.31, r = 0.88; group XII, y = 0.64x + 0.68, r = 0.88; group XIV, y = 0.60x + 0.17, r = 0.79.
DISCUSSION
This study demonstrates that three structurally different NO donors (DETA/NO, SNAP, and NTG), an adenosine A1 receptor agonist (CCPA), and a δ1-opioid receptor agonist (TAN-670) given 24 h before ischemia-reperfusion injury attenuate myocardial infarct size in an in vivo murine model. The magnitude of the infarct size reduction was similar to that attained during the late phase of ischemic PC. The cardioprotection afforded by these drugs cannot be attributed to hemo-dynamic effects because the drugs produced no appreciable changes in heart rate or arterial pressure. The infarct-sparing properties of TAN-670 and CCPA were abolished by the iNOS selective inhibitor 1400W, suggesting that iNOS is an essential mediator of TAN-670- and CCPA-induced late PC. This was confirmed by studies with genetically targeted animals, which demonstrated that the infarct size reduction produced by NO donor-induced, adenosine A1 receptor agonist-induced, and δ1-opioid receptor agonist-induced late PC was completely abrogated in iNOS–/– mice, providing definitive evidence that iNOS is indeed an essential mediator of this cardioprotective response. Our data identify iNOS as a final common effector of pharmacological PC. In addition, to our knowledge, this is the first report that NO donor-induced late PC is iNOS dependent. This is also the first report that administration of adenosine A1 agonists mitigates myocardial infarct size in an in vivo murine model.
Previous studies have identified iNOS as an obligatory mediator of late PC induced by ischemia (12), physical exercise (14), and endotoxin derivatives (9, 25, 26). The finding that iNOS is also a mediator of NO donor-induced, adenosine A1 receptor agonist-induced, and a δ1-opioid receptor agonist-induced delayed cardioprotection further corroborates the concept that this enzyme is a central mediator of cardioprotection in response to diverse stimuli. Whether other proteins found to be essential for ischemia-induced late PC, such as cyclooxygenase-2 (11, 21), heme oxygenase-1 (7, 8), and aldose reductase (20), are also involved in pharmacologically induced late PC remains to be determined. Future studies will also be needed to further investigate the role of other NOS isoforms, namely endothelial and neuronal NOS, that have been found to play a role in ischemia-induced PC. These studies will delineate important aspects of the pathways by which these clinically relevant pharmacological agents induce cardioprotection and further clarify the roles the various NOS isoforms play in delayed preconditioning.
The rationale for the extensive investigation of pharmacological PC performed in the present study with five different PC-mimetic agents (DETA/NO, NTG, SNAP, CCPA, and TAN-670) was to determine whether the results obtained with one drug can be applied to other forms of pharmacologically induced protection. An additional reason for selecting these five agents was that the role of iNOS in mediating pharmacologically induced late PC is either unknown or unclear. Specifically, no data exist regarding the contributions of iNOS to the delayed infarct-sparing effects elicited by NO donors. With regard to δ1-opioid receptor-induced late PC, a recent study (16) has concluded that iNOS mediates the delayed infarct-sparing actions of morphine. However, morphine is not a specific δ1-opioid receptor agonist, and, in that investigation, the duration of coronary reperfusion was limited to 2 h. It is unknown whether a 2-h reperfusion period is sufficient for accurate assessment of infarct size in mice. With regard to the role of iNOS in adenosine A1 receptor agonist-induced late PC, no data are available in in vivo murine models, and the data available in vitro are conflicting (3, 28). Consequently, we felt it was important to perform a careful analysis of NO donor-induced, δ1-opioid receptor-induced, and adenosine A1 receptor-induced late PC with the use of a well-established in vivo murine model in which fundamental physiological variables (heart rate, blood pressure, body temperature, and arterial blood gases) are kept within normal limits and coronary reper-fusion is extended to 24 h. We reasoned that by comparing the effects of the deletion of iNOS on three different types of pharmacological PC in the same in vivo model, we would be able to establish whether this enzyme plays a broad obligatory role or whether its involvement differs in relation to the stimulus applied.
The murine model utilized in this investigation has been extensively tested in our laboratory (13). In particular, physiological variables that affect infarct size, such as body temperature, heart rate, arterial blood pressure, and arterial blood PO2 and pH, are carefully monitored and kept within normal limits (13). The measurements of infarct size are highly reproducible (13), and surgical mortality is <20%. Thus we believe that this preparation represents an excellent model for testing the role of iNOS in late PC.
In conclusion, this study expands our understanding of the significance of iNOS in the cardiovascular system by identifying this enzyme as a common mediator of cardioprotection in three different forms of pharmacological PC. From a protein previously thought to play a detrimental role in inflammatory states, iNOS has recently emerged as a powerful, ubiquitous cardioprotectant that is obligatorily required for the response of the heart to stress (4). The ultimate goal of studying PC is to utilize this powerful phenomenon for the protection of ischemic myocardium in patients with coronary artery disease. Despite our extensive understanding of the mechanisms underlying ischemic PC, no applicable therapies have been developed. PC mimetics such as NO donors (15, 18), TAN-670, and CCPA have obvious clinical potential. Combined with previous studies (6, 16, 25–27), the present data support the development of strategies aimed at selectively upregulating iNOS in cardiac myocytes to induce a chronic PC state.
ACKNOWLEDGMENTS
We thank Hiroshi Nagase for generously supplying TAN-670.
GRANTS
This study was supported in part by National Heart, Lung, and Blood Institute Grants HL-55757, HL-68088, HL-70897, and HL-76794, American Heart Association Awards 0265087-B, 0325372-B, and 0575035-N, the University of Louisville Research Foundation, and the Jewish Hospital Research Foundation at Louisville.
REFERENCES
- 1.Baxter GF, Ferdinandy P. Delayed preconditioning of myocardium: current perspectives. Basic Res Cardiol. 2001;96:329–344. doi: 10.1007/s003950170041. [DOI] [PubMed] [Google Scholar]
- 2.Baxter GF, Marber MS, Patel VC, Yellon DM. Adenosine receptor involvement in a delayed phase of myocardial protection 24 hours after ischemic preconditioning. Circulation. 1994;90:2993–3000. doi: 10.1161/01.cir.90.6.2993. [DOI] [PubMed] [Google Scholar]
- 3.Bell RM, Smith CC, Yellon DM. Nitric oxide as a mediator of delayed pharmacological (A1 receptor triggered) preconditioning: is eNOS masquerading as iNOS? Cardiovasc Res. 2002;53:405–413. doi: 10.1016/s0008-6363(01)00472-2. [DOI] [PubMed] [Google Scholar]
- 4.Bolli R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J Mol Cell Cardiol. 2001;33:1897–1918. doi: 10.1006/jmcc.2001.1462. [DOI] [PubMed] [Google Scholar]
- 5.Bolli R. The late phase of preconditioning. Circ Res. 2000;87:972–983. doi: 10.1161/01.res.87.11.972. [DOI] [PubMed] [Google Scholar]
- 6.Bolli R, Dawn B, Tang XL, Qiu Y, Ping P, Xuan YT, Jones WK, Takano H, Guo Y, Zhang J. The nitric oxide hypothesis of late preconditioning. Basic Res Cardiol. 1998;93:325–338. doi: 10.1007/s003950050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark JE, Foresti R, Sarathchandra P, Kaur H, Green CJ, Motterlini R. Heme oxygenase-1-derived bilirubin ameliorates postischemic myocardial dysfunction. Am J Physiol Heart Circ Physiol. 2000;278:H643–H651. doi: 10.1152/ajpheart.2000.278.2.H643. [DOI] [PubMed] [Google Scholar]
- 8.Csonka C, Varga E, Kovacs P, Ferdinandy P, Blasig IE, Szilvassy Z, Tosaki A. Heme oxygenase and cardiac function in ischemic/reperfused rat hearts. Free Radic Biol Med. 1999;27:119–126. doi: 10.1016/s0891-5849(99)00077-5. [DOI] [PubMed] [Google Scholar]
- 9.Elliott GT, Sowell CG, Walker EB, Weber PA, Moore J, Gross GJ. The novel glycolipid RC-552 attenuates myocardial stunning and reduces infarct size in dogs. J Mol Cell Cardiol. 2000;32:1327–1339. doi: 10.1006/jmcc.2000.1166. [DOI] [PubMed] [Google Scholar]
- 10.Gross GJ. Role of opioids in acute and delayed preconditioning. J Mol Cell Cardiol. 2003;35:709–718. doi: 10.1016/s0022-2828(03)00135-4. [DOI] [PubMed] [Google Scholar]
- 11.Guo Y, Bao W, Wu WJ, Shinmura K, Tang XL, Bolli R. Evidence for an essential role of cyclooxygenase-2 as a mediator of the late phase of ischemic preconditioning in mice. Basic Res Cardiol. 2000;95:479–484. doi: 10.1007/s003950070024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo Y, Jones WK, Xuan YT, Tang XL, Bao W, Wu WJ, Han H, Laubach VE, Ping P, Yang Z, Qiu Y, Bolli R. The late phase of ischemic preconditioning is abrogated by targeted disruption of the inducible NO synthase gene. Proc Natl Acad Sci USA. 1999;96:11507–11512. doi: 10.1073/pnas.96.20.11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo Y, Wu WJ, Qiu Y, Tang XL, Yang Z, Bolli R. Demonstration of an early and a late phase of ischemic preconditioning in mice. Am J Physiol Heart Circ Physiol. 1998;275:H1375–H1387. doi: 10.1152/ajpheart.1998.275.4.H1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo Y, Xuan YT, Wu WJ, Li Q, Tang XL, Bolli R. Nitric oxide plays a dual role in exercise-induced late preconditioning (Abstract). Circulation. 2001;104(Suppl II):60. [Google Scholar]
- 15.Heusch G. Nitroglycerin and delayed preconditioning in humans: yet another new mechanism for an old drug? Circulation. 2001;103:2876–2878. doi: 10.1161/01.cir.103.24.2876. [DOI] [PubMed] [Google Scholar]
- 16.Jiang X, Shi E, Nakajima Y, Sato S. Inducible nitric oxide synthase mediates delayed cardioprotection induced by morphine in vivo: evidence from pharmacologic inhibition and gene-knockout mice. Anesthesiology. 2004;101:82–88. doi: 10.1097/00000542-200407000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Laubach VE, Shesely EG, Smithies O, Sherman PA. Mice lacking inducible nitric oxide synthase are not resistant to lipopolysaccharide-induced death. Proc Natl Acad Sci USA. 1995;92:10688–10692. doi: 10.1073/pnas.92.23.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leesar MA, Stoddard MF, Dawn B, Jasti VG, Masden R, Bolli R. Delayed preconditioning-mimetic action of nitroglycerin in patients undergoing coronary angioplasty. Circulation. 2001;103:2935–2941. doi: 10.1161/01.cir.103.24.2935. [DOI] [PubMed] [Google Scholar]
- 19.Qiu Y, Rizvi A, Tang XL, Manchikalapudi S, Takano H, Jadoon AK, Wu WJ, Bolli R. Nitric oxide triggers late preconditioning against myocardial infarction in conscious rabbits. Am J Physiol Heart Circ Physiol. 1997;273:H2931–H2936. doi: 10.1152/ajpheart.1997.273.6.H2931. [DOI] [PubMed] [Google Scholar]
- 20.Shinmura K, Bolli R, Liu SQ, Tang XL, Kodani E, Xuan YT, Srivastava S, Bhatnagar A. Aldose reductase is an obligatory mediator of the late phase of ischemic preconditioning. Circ Res. 2002;91:240–246. doi: 10.1161/01.res.0000029970.97247.57. [DOI] [PubMed] [Google Scholar]
- 21.Shinmura K, Tang XL, Wang Y, Xuan YT, Liu SQ, Takano H, Bhatnagar A, Bolli R. Cyclooxygenase-2 mediates the cardioprotective effects of the late phase of ischemic preconditioning in conscious rabbits. Proc Natl Acad Sci USA. 2000;97:10197–10202. doi: 10.1073/pnas.97.18.10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takano H, Bolli R, Black RG, Jr, Kodani E, Tang XL, Yang Z, Bhattacharya S, Auchampach JA. A1 or A3 adenosine receptors induce late preconditioning against infarction in conscious rabbits by different mechanisms. Circ Res. 2001;88:520–528. doi: 10.1161/01.res.88.5.520. [DOI] [PubMed] [Google Scholar]
- 23.Takano H, Tang XL, Qiu Y, Guo Y, French BA, Bolli R. Nitric oxide donors induce late preconditioning against myocardial stunning and infarction in conscious rabbits via an antioxidant-sensitive mechanism. Circ Res. 1998;83:73–84. doi: 10.1161/01.res.83.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang G, Liem DA, Vondriska TM, Honda HM, Korge P, Pantaleon DM, Qiao X, Wang Y, Weiss JN, Ping P. Nitric oxide donors protect murine myocardium against infarction via modulation of mitochondrial permeability transition. Am J Physiol Heart Circ Physiol. 2005;288:H1290–H1295. doi: 10.1152/ajpheart.00796.2004. [DOI] [PubMed] [Google Scholar]
- 25.Xi L, Salloum F, Tekin D, Jarrett NC, Kukreja RC. Glycolipid RC-552 induces delayed preconditioning-like effect via iNOS-dependent pathway in mice. Am J Physiol Heart Circ Physiol. 1999;277:H2418–H2424. doi: 10.1152/ajpheart.1999.277.6.H2418. [DOI] [PubMed] [Google Scholar]
- 26.Zhao L, Weber PA, Smith JR, Comerford ML, Elliott GT. Role of inducible nitric oxide synthase in pharmacological “preconditioning” with monophosphoryl lipid A. J Mol Cell Cardiol. 1997;29:1567–1576. doi: 10.1006/jmcc.1997.0390. [DOI] [PubMed] [Google Scholar]
- 27.Zhao T, Xi L, Chelliah J, Levasseur JE, Kukreja RC. Inducible nitric oxide synthase mediates delayed myocardial protection induced by activation of adenosine A1 receptors: evidence from gene-knockout mice. Circulation. 2000;102:902–907. doi: 10.1161/01.cir.102.8.902. [DOI] [PubMed] [Google Scholar]
- 28.Zhao TC, Hines DS, Kukreja RC. Adenosine-induced late preconditioning in mouse hearts: role of p38 MAP kinase and mitochondrial KATP channels. Am J Physiol Heart Circ Physiol. 2001;280:H1278–H1285. doi: 10.1152/ajpheart.2001.280.3.H1278. [DOI] [PubMed] [Google Scholar]