Abstract
The inhibition of thrombin is one of the important treatments of pathological blood clot formation. Variegin, isolated from the tropical bont tick, is a novel molecule exhibiting a unique ‘two-modes’ inhibitory property on thrombin active site (competitive before cleavage, noncompetitive after cleavage). For the better understanding of its function, we have determined the crystal structure of the human α-thrombin:synthetic-variegin complex at 2.4 Å resolution. The structure reveals a new mechanism of thrombin inhibition by disrupting the charge relay system. Based on the structure, we have designed 17 variegin variants, differing in potency, kinetics and mechanism of inhibition. The most active variant is about 70 times more potent than the FDA-approved peptidic thrombin inhibitor, hirulog-1/bivalirudin. In vivo antithrombotic effects of the variegin variants correlate well with their in vitro affinities for thrombin. Our results encourage that variegin and the variants show strong potential for the development of tunable anticoagulants.
Introduction
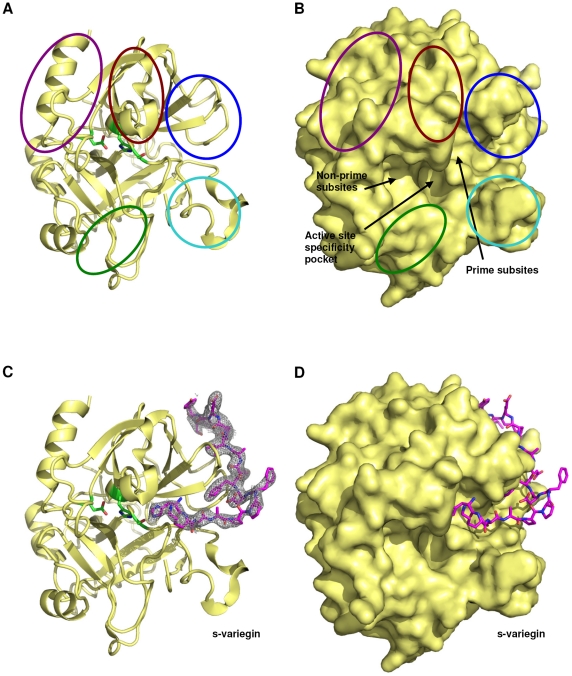
Serine proteinases in the blood coagulation cascade are important molecules in maintaining the integrity of hemostasis. Among them, thrombin (factor IIa) plays significant pro- and anti- coagulation roles. The active site contains the classical catalytic triad – His57, Asp102 and Ser195 (Figure 1A). Compared to other blood coagulation serine proteinases, thrombin has a prominent active site cleft, which is deep and narrow. Two insertion loops (called the 60-loop with residues Leu59-Asn62 and the autolysis-loop, residues Leu144-Gly150) form the wall of the cleft (Figure 1A–B) [1], [2]. The thrombin active site surfaces that interact with substrate residues, at N-terminal to the scissile bond, are described as ‘non-prime subsites’ (S subsites). Similarly, the surfaces of the active site which are in contact with substrate residues, at C-terminal to the scissile bond, are described as ‘prime subsites’ (S′ subsites) (Figure 1B).
Figure 1. Structure of thrombin:s-variegin complex.
(A) Thrombin (yellow) shown in the classical orientation in ribbon (without s-variegin). Side chains of catalytic triad, TAsp102, THis57 and TSer195 are shown in sticks (green). The 60-loop, autolysis loop and Na+-binding loop are circled in brown, cyan and green, respectively. Parts of thrombin forming the anion-binding exosite-I and exosite-II are circled in blue and purple, respectively. (B) Surface representation of thrombin (yellow) in the same orientation as (a). Locations of active site specificity pocket, non-prime and prime subsites are indicated by arrows. (C) The structure of thrombin (yellow) in the same orientation as above shown in complex with s-variegin (pink) together with its electron density map (2Fo-Fc) shown contoured at 0.9σ. (D) Surface representation of thrombin in complex with s-variegin (pink).
In addition, exosite-I is the surface near the prime subsites. The bottom of exosite-I is a deep, canyon-like cleft that extends from the prime subsites. The walls of the cleft are formed by two surface loops, Phe34-Leu39 (described as the 34-loop) and Lys70-Glu80 (the 70-loop) [2], [3]. In contrast to the apolar nature of the canyon-like cleft, the surface of exosite-I is dominated by several positively-charged residues [4]. Exosite-II, another surface near the ‘non-prime subsites’, is even more basic (Figure 1B). The occupancy of either exosites can induce allosteric changes to the active site to enhance catalysis. The binding of Na+ to the Na+ binding loop (Cys220-Trp225) (Figure 1A) favors procoagulant functions of thrombin whereas Na+-free thrombin favors anticoagulant functions such as increased protein C activation [5]. The activity and fate of thrombin is directed by competition for its exosites and differences in distribution of its substrates and cofactors [6].
Imbalances in blood coagulation may give rise to either loss of clotting activity, leading to hemorrhagic disorders, or unwanted clot formation, resulting in thrombosis. In particular, thrombosis causes high morbidity and mortality due to vascular occlusion and consequent myocardial infarction, stroke, pulmonary embolism, or deep-vein thrombosis. Increased atherosclerosis and thromboembolic disorders, associated with changing food habits and lifestyles, are increasing the demand for anticoagulant agents [7], [8]. Heparin and warfarin are the cornerstones of anticoagulation therapy. Unfortunately, both classes of drug have well-documented limitations such as a narrow therapeutic window and highly variable dose-response [9]. These limitations drive continual and intense efforts to develop new, efficacious and safe anticoagulants, especially for targeting specific coagulation factors [9]. Thrombin is one of the main targets for inhibition, owing to its pivotal role in coagulation. Several direct thrombin inhibitors, such as hirudin [10], hirulog-1/bivalirudin [11], argatroban [12] and dabigatran [13], are currently available in the market. Among them, hirudin and hirulog-1/bivalirudin are both developed from hematophagous parasites and their success continues to inspire the search for more novel anticoagulants from these sources [14], [15].
Recently, we described variegin, a novel, fast and tight-binding competitive inhibitor of thrombin (refers to the α form of thrombin unless otherwise stated) isolated from the tropical bont tick, Amblyomma variegatum [16]. Like hirudin/hirulog, variegin targets the thrombin catalytic site and exosite-I. However, unlike other naturally occurring thrombin inhibitors, variegin interacts with the thrombin prime subsites in addition to exosite-I [17]. Variegin has several potential advantages over hirulog-1/bivalirudin: (i) synthetic variegin (s-variegin) contains only L-amino acids, while hirulog-1/bivalirudin has a D-Phe [16]; (ii) inhibition by variegin is about 9 times greater than hirulog-1/bivalirudin; (iii) the cleavage product of variegin (MH22) remains tightly bound to thrombin (about 400 fold stronger than cleaved hirulog-1/bivalirudin). Most importantly, MH22 potently and noncompetitively inhibits thrombin whereas cleaved hirulog-1/bivalirudin, paradoxically, activates the function of the thrombin active site [17].
We solved the crystal structure of the thrombin:s-variegin complex at 2.4 Å resolution in order to understand the molecular interactions between thrombin and variegin. Based on the structure and data on thrombin inhibitors, a series of peptides was designed to analyse the structure-function relationships. These peptides cover a diverse spectrum of properties: potency, kinetics, mechanism of inhibition, affinities (ranging from nanomolar to picomolar values) with fast, slow, tight-binding and competitive and noncompetitive inhibition. Finally, in vivo activities of selected peptides were examined using a venous thrombosis model involving zebrafish larvae.
Methods
Synthesis, purification and mass spectrometry of peptides
Synthesis, purification and mass spectrometry analysis of all peptides were performed as described elsewhere [16]. Peptides were named with two alphabets representing the first two residues in their sequence, followed by a number representing their respective length. Position of point mutants are added after the number and italicized. Modifications to amino acids are indicated by superscript. As sulfate groups are acid labile, the peptides containing sulfotyrosine (DV24Ysulf, DV24K10RYsulf and MH18Ysulf) were cleaved from resins with 90% aqueous trifluoroacetic acid on ice for 5 h.
X-ray crystallography
Lyophilized recombinant human thrombin [18], [19] and s-variegin were dissolved and mixed in buffer containing 50 mM HEPES (pH 7.4) and 375 mM NaCl to final concentrations of 20 mg/ml and 3 mg/ml (1∶1.5 molar ratio), respectively. Crystallization was achieved using the hanging drop vapor diffusion method. Typically, 1 µl of protein solution was mixed with 1 µl of precipitant buffer (100 mM HEPES buffer pH 7.4, containing 20 to 25% PEG 8000) and equilibrated against 1 ml of the precipitant buffer at 4°C. X-ray diffraction data were collected at Beamline X29 (National Synchrotron Light Source, NY, USA). Prior to data collection, crystals were briefly soaked in a cryoprotectant solution containing the mother liquor, supplemented with 25% (v/v) glycerol. Data sets were collected using the Quantum 4 CCD detector and were processed using HKL2000 [20]. As the χ2 values for the P1 unit-cell were better than those for the C2 unit-cell during data integration (around 1.0 against 3.5 and above), data were first processed under the P1 unit-cell orientation and then transformed to the C2 orientation using the transformation A = 2c+a, B = a and C = b, where a,b,c and A,B,C are the P1 and C2 unit-cell vectors, respectively.
The structure of thrombin:s-variegin complex was solved by the molecular replacement method using PHASER [21] at 2.4 Å resolution. The coordinates of the thrombin-hirulog-3 structure (PDB code: 1ABI) [22] were used as a search model. Several cycles of map fitting using program COOT [23] and refinement using program REFMAC5 [24] with one TLS [25] group per chain of thrombin led to convergence of R-values. The crystallographic and refinement statistics are listed in Table 1. The correctness of stereochemistry of the model was verified using PROCHECK [26] and MolProbity [27]. The geometry of the thrombin molecule is comparable to that of other structures at this resolution. The peptide is relatively more flexible. The coordinates of the structure were deposited with the RCSB Protein Data Bank under the entry code 3B23. Online server PISA [28] was used to analyze the protein-peptide interface. Throughout the manuscript, the residues of thrombin and s-variegin are marked with superscripted prefixes ‘T’ and ‘V’, respectively. The chymotrypsinogen numbering system is used for numbering the thrombin residues, as first described here [29].
Table 1. Crystallographic data and refinement statistics.
| Data set | Thrombin:s-variegin complex | |
| Crystal | ||
| Space Group | P1 | C2 |
| Unit Cell Parameters (Å, °) | a = 50.8 | A = 124.7 |
| b = 61.58 | B = 50.8 | |
| c = 67.3 Å | C = 61.5 Å | |
| α = 98.1 | AL = 90 | |
| β = 112.2 | BE = 98.7 | |
| γ = 89.9° | GA = 90° | |
| Data collection | ||
| Resolution range (Å) | 50−2.4 | |
| Wavelength (Å) | 0.9795 | |
| Total number of reflections | 52,825 | |
| Unique reflections | 29,154 | 15,137 |
| Completeness (%) | 88.1 (56.8) | 98.1 (97.0) |
| I/σI | 25.1 (7.0) | 20.0 (5.4) |
| Redundancy | 1.9 (1.7) | 3.6 (3.1) |
| Rmerge (%) | 2.4 (8.5) | 5.3 (15.4) |
| Refinement and quality | ||
| Resolution range (Å) I>σ(I) | 8−2.4 | |
| Rwork | 0.208 | |
| Rfree | 0.259 | |
| RMSD bond lengths (Å) | 0.01 | |
| RMSD bond angles(°) | 1.22 | |
| Average B-factors (Å2) | ||
| Protein atoms (2450 atoms) | 67.4 | |
| Water molecules (51 atoms) | 66.2 | |
| Ramachandran plot | ||
| Most favored regions (%) | 86.1 | |
| Additional allowed regions (%) | 13.9 | |
| Generously allowed regions (%) | 0 | |
| Disallowed regions (%) | 0 | |
Values in parentheses are for the last resolution shell (2.46−2.40 Å). The diffraction data were processed under the space group P1 and transformed to the space group C2 using the transformation A = 2c+a, B = a and C = b, where a,b,c and A,B,C are the P1 and C2 unit-cell vectors, respectively.
Thrombin inhibition
All peptides were assayed for their abilities to inhibit thrombin amidolytic activity on chromogenic substrate S2238 (Chromogenix, Milano, Italy) as described previously [16], [17]. Values for concentration of peptide needed for 50% inhibition (IC50) and inhibition constant (Ki) were calculated from data obtained were fitted using Origin software (MicroCal, Northampton, MA, USA). A detailed account for the selection and use of equations to fit the data is available in Materials and Methods S1.
Zebrafish larvae venous thrombosis model
Zebrafish and the larvae were maintained as previously described [30]. Intravenous microinjections of peptides were performed using Nanoject II (Drummond, Broomall, PA, USA) with glass injection needles. Ten nanolitres of peptides or phosphate buffered saline (PBS) were injected into 4 days post-fertilization larvae through the posterior cardinal vein. Laser ablation of larval veins were performed with a pulsed nitrogen laser light pumped through coumarin 440 dye (445 nm) (MicroPoint Laser system, Photonic Instrument, St Charles, IL, USA) at 10 pulses/s with laser intensity setting at 10. Laser ablation of each larvae was carried out 20 min after microinjection of the peptide or PBS. The laser beam was aimed at the caudal vein around five somites towards the caudal end from the anal pore and triggered for 3 s. Thrombus formation following vein injury, due to laser ablation, was monitored and the time taken for complete occlusion of the injured vein was recorded.
Results
Thrombin:s-variegin structure
The crystal structure of the thrombin:s-variegin complex was determined at 2.4 Å resolution (Table 1 and Figure 1C–D). The electron density of the complex structure is well defined except for termini residues of chain A [T(1HTFGSGE1C) and TArg15]. The structure of thrombin in the complex superimposes well with other thrombin structures.
Only 17 out of the 32 residues (VHis12 to VLeu28) of s-variegin have well-defined density (Figure 1C). The first seven N-terminal residues do not make direct contact with thrombin [16] and s-variegin is cleaved by thrombin between VLys10 and VMet11 [16]. It is likely that the N-terminal fragment V(1SDQGDVAEPK10) has dissociated from thrombin after cleavage before crystallization. In contrast, the C-terminal fragment MH22 V(11MHKTAPPFDFEAIPEEYLDDES32) remains bound to thrombin after cleavage [17]. The N-terminal VMet11 and the last five residues V(28LDDES32) of the fragment are not observed, reflecting disorder in the termini.
The C-termini of hirulog-1/-3, hirugen and hirudin have the following sequence DFEEIPEEYL(Q), with the Gln only present in hirudin. s-Variegin has an almost identical sequence V(19DFEAIPEEYLDDES 32), with four extra residues in the C-terminus. Despite the identity, there are large differences between their conformations. The C-terminus is disordered in the hirulog-1/bivalirudin structure (PDB code: 2HGT) [4], forming a 310 helix in hirulog-3 (PDB code: 1ABI) [22] and hirugen (PDB code: 1HGT [4]) and forming a full α-helical turn in sulfo-hirudin (PDB code: 2PW8) [31]. In s-variegin, these residues remain in an extended conformation until the last observed residues (VLeu28). The extra residues in C-terminus, although not observed in the present structure may cause the peptide to adopt the fully extended conformation (Figure S1).
Interactions with thrombin catalytic residues
The active site of thrombin in the crystal structure was compared to the published data for the thrombin:hirugen structure (unoccupied active site) (Figure 2). Of the three catalytic residues, the most striking differences are with the Oγ atom of TSer195 and the orientation of the imidazole ring of THis57. In the thrombin:s-variegin structure, TSer195 Oγ is displaced by 1.19 Å, pointing towards s-variegin. Distance between TSer195 Oγ and the side chain Nε of VHis12 is 3.35 Å, possibly forming hydrogen bond (Table S1). At the same time, the distance between Nε of THis57 and Oγ of TSer195 increases to 3.60 Å from 2.79 Å, breaking the crucial strong hydrogen bond needed to form the catalytic charge relay system. Without stabilization by the strong hydrogen bond between THis57 and TSer195, the imidazole ring of THis57 is now rotated slightly and leads to a displacement of its Nε by 0.56 Å (Figure 2A). The newly formed hydrogen bond between TSer195 and VHis12 delocalize the electrons of TSer195 Oγ, making TSer195 a weak nucleophile and incapable of efficiently attacking the backbone C of the substrate. This explains the observed classical non-competitive inhibition for MH22 [17].
Figure 2. Thrombin catalytic triads in s-variegin-bound and hirugen-bound structures.
(A) Thrombin catalytic triad THis57, TAsp102 and TSer195 in thrombin:hirugen structure (green) and in thrombin:s-variegin structure (pink) are superimposed. The TSer195 Oγ in thrombin:s-variegin structure is displaced by 1.19 Å compared to thrombin:hirugen structure. The displacement of TSer195 Oγ in thrombin:s-variegin structure (pink) is due to interactions with VHis12 of s-variegin through hydrogen bond (dotted arrow), rendering TSer195 a weak nucleophile that is incapable of catalysis. The imidazole ring of THis57 also rotated, resulted in a displacement of its Nε by 0.56 Å. Overall, the distance between Nε of THis57 and Oγ of TSer195 increases to 3.60 Å (black arrow) from 2.79 Å (green arrow), disrupts the catalytic charge relay system. (B) The 2Fo-Fc electron density map of thrombin catalytic triad and VHis12 contoured at 1.0σ.
Interactions with prime subsites
In addition to the new hydrogen bond, the following interactions anchor s-variegin P2′ to P5′ residues V(12HKTA15) to the thrombin prime subsites (Figure 3A). Besides the catalytic residues, TLeu41, TCys42, TCys58, TTrp60D, TLys60F and TGlu192 are also in contact with VHis12. Two hydrogen bonds can be formed between VHis12 with TGlu39 and TGlu192 (Table S1). The P3′ (VLys13) interacts with TArg35, TGlu39, TTrp60D, TLys60F, TAsn143, TThr147, and TGlu192. The P4′ (VThr14) side chain is directed towards the base of the highly flexible autolysis-loop. The side chain occupies a surface lined by TLeu40, TTrp141, TGly142, TAsn143, TGln151 and TGly193. Interactions within this P3′ subsite are strengthened by two hydrogen bonds between VThr14 with TAsn143 and TGln151 (Table S1). The P5′ VAla is surrounded by TGln38, TGlu39, TArg73 and TGln151. Thus there are extensive interactions between the variegin peptide and thrombin prime subsites.
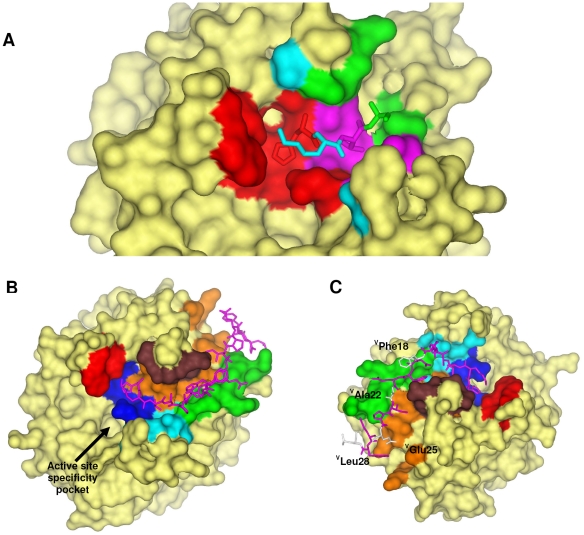
Figure 3. Interactions between thrombin and s-variegin.
(A) Prime subsites interactions between thrombin and s-variegin (residues P2′ to P5′) are shown. Density for s-variegin P1′ VMet11 cannot be traced in the structure. Thrombin S2′ subsite is colored in red, S3′ subsite in cyan, S4′ subsite in pink and S5′ subsite in green. (B) Thrombin residues that interfaced with s-variegin are colored according to their positions: catalytic pocket (blue): THis57, TCys58, TCys191, TGlu192, TGly193, TSer195; 60-loop (red): TTrp60D and TLys60F; autolysis loop (cyan): TTrp141, TGly142, TAsn143, TThr147 and TGln151; 34-loop (brown): TPhe34, TArg35, TGln38 and TGlu39; 70-loop (green): TArg73, TThr74, TArg75, TTyr76 and TArg77A; bottom of the cleft (orange): TMet32, TLeu40, TLeu41, TCys42, TLeu65, TArg67, TLys81, TIle82, TMet84 and TLys110. Sticks model of s-variegin is shown in pink. (C) All but four residues (VPhe18, VAla22, VGlu25 and VLeu28, white) on s-variegin have their side chains buried in the interface with thrombin.
Interactions with exosite-I
s-Variegin fits firmly into the canyon-like cleft extending from the thrombin active site to exosite-I. The walls of this hydrophobic cleft are formed by the 60- and autolysis- loops near the active site, and 34- and 70- loops at exosite-I, while many apolar residues in these loops line the bottom [2], [3]. s-Variegin is in close contact with multiple residues in exosite-I as depicted in Figure 3B. All but four residues of s-variegin (VPhe18, VAsp19, VAla22 and VGlu26) have their side chains buried in the interfaces with thrombin (Figure 3A).
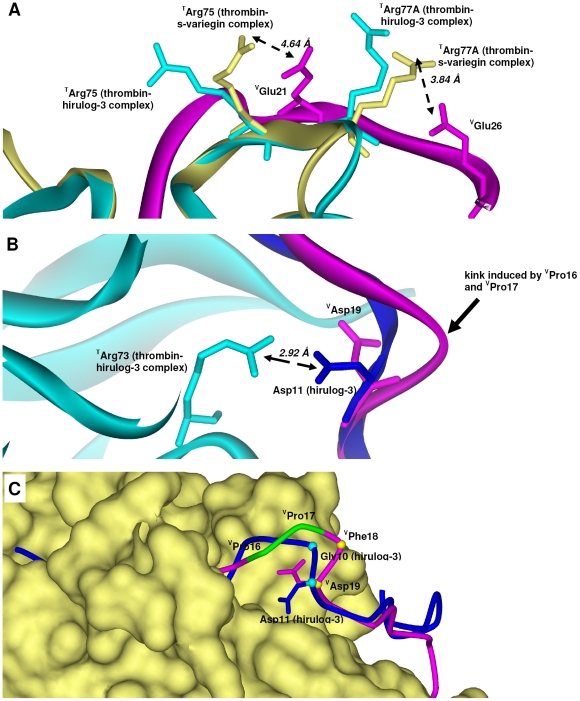
Interestingly, the high identity between C-terminus of s-variegin, hirulog-1/-3, hirugen and hirudin are not reflected in their respective salt bridges formation with exosite-I of thrombin. Despite the presence of multiple anionic residues in the s-variegin C-terminus and highly cationic exosite-I, only one strong salt bridge is formed (VGlu26∶TArg77A). This salt bridge (3.84 Å) is not observed in hirulog-1/-3, hirugen and hirudin structures as TArg77A adopts a different rotamer that points away from the inhibitor (Figure 4A and S2A). In addition, a weak salt bridge is also likely between VGlu21 and TArg75 (4.64 Å). In hirulog-1/-3, hirugen and hirudin structures the analogous Glu makes an ion pair with TArg75 of a 2 fold symmetry-related thrombin, although this interaction is suggested to occur within the same thrombin:inhibitor pair in solution [4], [22], [32]. In our structure, the TArg75 side chain is rotated by 80.5° about Cβ, compared to the thrombin:hirulog-3 complex (Figure 4A and S2A) facilitating this interation. In hirulog-1/-3 and hirugen structures, an ion pair between TArg73 and the Asp, analogous to VAsp19, can be observed. However, formation of this ion pair in the thrombin:s-variegin complex is not possible as the VAsp19 side chain points in an opposite direction into solvent. This difference is most likely due to the kink in the s-variegin backbone, induced by VPro16-VPro17 (see below) (Figure 4B–C and S2B).
Figure 4. Electrostatic interactions in thrombin:s-variegin structure.
(A) s-Variegin and hirulog-3 have distinct ion pairs formed with exosite-I of thrombin despite high sequence identity. A salt bridge (3.84 Å) between VGlu26 (pink) and TArg77A (yellow) is absent in hirulog-3 as TArg77A (cyan) points away from the inhibitor. Weak salt bridge (4.64 Å) is also likely between VGlu21 (pink) and TArg75 (yellow) rotated 90.5° about Cβ compared to TArg75 in hirulog-3 bound thrombin (cyan) to facilitate interaction with VGlu21 (pink). Electron density maps of residues involved are shown in Figure S2A. (B) The strong ion pair (Asp11∶TArg73, 2.92 Å) in thrombin:hirulog-3 structure is absent in thrombin:s-variegin structure since VAsp19 (pink) pointed to an opposite direction compared to the analogous hirulog-3 Asp11 (blue) due to a kink in s-variegin backbone (pink). Electron density maps of residues involved are shown in Figure S2B. (C) The presence of a VPro16-VPro17 (green) in s-variegin resulted in the kink. Superimposition of s-variegin (pink, only Cα positions traced) and hirulog-3 (blue, only Cα positions traced) based on their thrombin structures showed displacement of VPhe18 and VAsp19 from their corresponding residues Gly10 and Asp11 of hirulog-3 by 3.11 Å and 0.79 Å (measured by Cα positions), respectively. As a result, the distance between TArg73 and VAsp19 charges are 5.83 Å, rendering electrostatic interactions impossible.
The end of the canyon-like cleft is a relatively flatter surface and formed by TAsp63 to TIle68 and TLys81 to TLeu85. The residues VPro24 to VTyr27 are stacked loosely on top of this surface with one of the side chains (VGlu25) pointing towards solvent (Figure 3C). This s-variegin segment is in a different conformation when compared to hirulog-3/hirugen despite sequence similarity (Figure S1).
Design and characterization of variegin variants
Several new variegin variants were designed based on the thrombin:s-variegin structure, as well as background information available on thrombin:inhibitor interactions.
Optimization of the length of variegin. The lack of electron density for the last four C-terminal residues [V(29DDES32)] of s-variegin in the complex structure indicates flexibility and hence lack of close contacts with thrombin. As these residues are also not present in hirulogs or hirugen, two variants (EP21 and MH18) lacking the last four C-terminal residues were synthesized and characterized. IC50 and Ki values of EP21 and MH18 are essentially identical with their templates (EP25 and MH22, respectively) and indicate that the truncation of the four C-terminal residues does not alter the inhibitory activity (Table 2).
Table 2. Sequence and activity of variegin and its variants.
| Name | Sequence | Pre-incubation tine (min) | IC50 (nM) | Ki (nM) | Mechanism | Plots shown in figure |
| s-variegin | SDQGDVAEPKMHKTAPPFDFEAIPEEYLDDES | 0 | 8.25±0.45 | 0.318±0.020 | Fast, tight-binding, competitive | Published [17] |
| 20 | 10.4±0.3 | |||||
| EP25 | SDQGDVAEPKMHKTAPPFDFEAIPEEYLDDES | 0 | 173±26 | 0.365±0.109 | Slow, tight-binding, competitive | S3 |
| 20 | 13.1±0.7 | |||||
| MH22 | SDQGDVAEPKMHKTAPPFDFEAIPEEYLDDES | 0 | 11.5±0.7 | 14.1±0.3 | Fast, tight-binding, noncompetitive | Published [17] |
| 20 | 12.3±1.9 | |||||
| Hirulog-1 | D FPRPGGGGNGDFEEIPEEYL | 0 | 72.6±3.9 | 2.94±0.12 | Fast, tight-binding, competitive | Published [17] |
| 10 | 102±13 | |||||
| EP21 | SDQGDVAEPKMHKTAPPFDFEAIPEEYLDDES | 0 | 177±7 | 0.315±0.024 | Slow, tight-binding, competitive | S4 |
| 20 | 16.2±2.9 | |||||
| MH18 | SDQGDVAEPKMHKTAPPFDFEAIPEEYLDDES | 0 | 10.9±1.2 | 14.9±3.5 | Fast, tight-binding, noncompetitive | S5 |
| 20 | 11.7±1.9 | |||||
| DV24 | SDQGDVAEPKMHKTAPPFDFEAIPEEYLDDES | 0 | 7.49±0.28 | 0.306±0.029 | Fast, tight-binding, competitive | S7 |
| 20 | 10.1±0.6 | |||||
| DV24H12A | SDQGDVAEPKMAKTAPPFDFEAIPEEYLDDES | 0 | 48.2±12.4 | 3.23±0.48 | Fast, tight-binding, competitive | S8 |
| 20 | 141±11 | |||||
| MH18H12A | SDQGDVAEPKMAKTAPPFDFEAIPEEYLDDES | 0 | 328±23 | 329±8 | Fast, tight-binding, noncompetitive | S9 |
| 20 | 343±46 | |||||
| DV24K10R | SDQGDVAEPRMHKTAPPFDFEAIPEEYLDDES | 0 | 6.98±0.76 | 0.259±0.015 | Fast, tight-binding, competitive | S10 |
| 20 | 12.0±0.4 | |||||
| DV23 | SDQGDVAEPKMHKTAPPFDFEAIPEEYLDDES | 0 | 45.4±1.6 | 2.19±0.23 | Fast, tight-binding, competitive | S11 |
| 20 | 77.8±6.1 | |||||
| DV23K10R | SDQGDVAEPRMHKTAPPFDFEAIPEEYLDDES | 0 | 12.9±1.0 | 0.600±0.010 | Fast, tight-binding, competitive | S12 |
| 20 | 102±1 | |||||
| EP25A22E | SDQGDVAEPKMHKTAPPFDFEEIPEEYLDDES | 0 | 124±23 | 0.311±0.070 | Slow, tight-binding, competitive | S13 |
| 20 | 13.5±2.1 | |||||
| MH22A22E | SDQGDVAEPKMHKTAPPFDFEEIPEEYLDDES | 0 | 13.6±0.5 | 15.1±1.0 | Fast, tight-binding, noncompetitive | S14 |
| 20 | 15.6±0.4 | |||||
| DV24Yphos | SDQGDVAEPKMHKTAPPFDFEAIPEEY¶LDDE | 0 | 8.67±0.45 | 0.327±0.032 | Fast, tight-binding, competitive | S15 |
| 20 | 12.4±1.2 | |||||
| DV24K10RYphos | SDQGDVAEPRMHKTAPPFDFEAIPEEY¶LDDES | 0 | 4.64±0.78 | 0.150±0.018 | Fast, tight-binding, competitive | S16 |
| 20 | 7.80±1.80 | |||||
| DV24Ysulf | SDQGDVAEPKMHKTAPPFDFEAIPEEY*LDDE | 0 | 1.66±0.18 | 0.0560±0.0180 | Fast, tight-binding, competitive | S17 |
| 20 | 2.02±0.29 | |||||
| DV24K10RYsulf | SDQGDVAEPRMHKTAPPFDFEAIPEEY*LDDE | 0 | 1.39±0.17 | 0.0420±0.0061 | Fast, tight-binding, competitive | S18 |
| 20 | 1.66±0.21 | |||||
| MH18Ysulf | SDQGDVAEPKMHKTAPPFDFEAIPEEY*LDDES | 0 | 1.26±0.18 | 1.25±0.18 | Fast, tight-binding, noncompetitive | S19 |
| 20 | 1.17±0.14 |
Y¶: phosphotyrosine; Y*: sulfotyrosine.
Previously, we have shown that the first seven N-terminal residues of variegin [V(1SDQGDVA7)] are responsible for its fast-binding kinetics, due to a possible steering effect towards the highly basic thrombin exosite-II [16]. EP25 and EP21, in which these residues are removed, act as slow-binding inhibitor. For complete inhibition, they required 20 min of pre-incubation with thrombin. Since exosite-II is located about 10 Å away from the active site [33], we extended EP21 by three residues at the N-terminal to include one of the two acidic residues, VAsp5, in DV24. The non-linear progress curves of thrombin inhibition by EP21 (characteristic of slow binding inhibitors), changed to linear progress curves of inhibition by DV24 (characteristic of fast binding inhibitor) (Figure S6). The IC50 and Ki values of DV24 are identical to those of s-variegin (Table 2). Like for s-variegin, a fast binding inhibitor, the IC50 of DV24 increases with pre-incubation due to the cleavage by thrombin. Thus, DV24 is eight residues shorter than s-variegin but retains fast-binding kinetics and potency.
Optimization of thrombin:variegin interactions. As observed in the thrombin:s-variegin structure, VHis12 binds to the prime subsite, with its side chain nitrogen forming hydrogen bond with TSer195 and disrupting the charge relay system of the thrombin catalytic triad. In order to verify the significance of VHis12, two variants were synthesized by replacing this residue with Ala. The two variants, DV24H12A and MH18H12A are based on the sequences of DV24 and MH18, repectively and represent the minimal interacting sequences of variegin and cleaved fragment. Both peptides lose their inhibitory potency significantly. IC50 (∼6 fold) and Ki (∼10 fold) values of DV24H12A increase when compared to DV24. Pre-incubation of DV24H12A with thrombin also causes a larger increase in IC50 (∼3 fold in 20 min) when compared to DV24 (<2 fold) (Table 2).
MH18H12A shows a more drastic increase in IC50 (∼30 fold) and Ki (∼22 fold) values, compared to MH18 (Table 2). Maximum inhibition by MH18H12A appears to saturate near 80% (highest concentration used is 30 µM), implying that the peptide is unable to completely inhibit thrombin. Thus, the single mutation of VHis12 to Ala significantly affects the inhibitory action of the peptide, ascertaining the importance of VHis12, as observed in the structure of thrombin:s-variegin complex. However, the activity is not completely abolished possibly due to other interactions in the prime subsites retained in these alanine mutants.
One striking difference between variegin and other thrombin substrates/inhibitors is the presence of Lys, instead of Arg, at P1. Typically, P1 Lys interacts with TAsp189 through a water molecule, resulting in reduced affinity and specificity [34], [35]. Therefore, using DV24 as a template sequence, the P1 residue (VLys10) was replaced by Arg in DV24K10R. The peptide has marginally improved IC50 and Ki values, when compared to DV24 (Table 2). Substitution of P1 Lys by Arg appears to accelerate the cleavage, as shown by the higher IC50 after 20 min pre-incubation, when compared to DV24 (Table 2).
As in hirulogs, hirugen and hirudin, the phenyl group of VPhe20 interacts with TPhe34 through π-stacking. In s-variegin, there are nine residues between this VPhe20 and the P1 residue, V(11MHKTAPPFD19), unlike in hirulog-1/-3, which has only eight residues (4PGGGGNGD11). VPro16 and VPro17 induce a kink in the s-variegin backbone, causing a slight bend upwards, away from thrombin (Figure 4B). This, in turn, causes displacement of VPhe18 and VAsp19 by about 3.11 Å and 0.79 Å (based on Cα positions), respectively against their analogs in hirulog-3 (Gly10 and Asp11). Crucially, Asp11 of hirulog-3 makes an ion pair with TArg73, while analogous VAsp19 does not (Figure 4B). In fact, the VAsp19 side chain points in the opposite direction (towards solvent), with a 5.83 Å distance between VAsp19 and TArg73 (Figure 4C). To remove the kink in the backbone, reposition VAsp19 and create the ionic interaction, VPro16 was deleted in variants DV23 and DV23K10R. However, DV23 shows an average of ∼7 fold reduction in IC50 when compared to DV24 (Table 2). The other variant, DV23K10R, is also less active when compared to DV24K10R, albeit to a lesser extent (Table 2). IC50 values of both DV23 and DV23K10R significantly increased upon pre-incubation, implies that the cleaved products no longer potently inhibits thrombin (Table 2). In addition, the peptide with Arg at P1 (DV23K10R) is hydrolyzed by thrombin at a faster rate than the peptide with Lys at P1 (DV23) judging from the more rapid increase of IC50 values with pre-incubation (Table 2). Thus, the deletion of VPro16 has an adverse effect on the activities of both the intact and cleaved peptides. This deletion probably compromises the interactions of P′ residues with the prime subsites, owing to their proximity to VPro16.
In hirudin, HGlu58 makes an ion-pair with TArg77A [3], [32]. However, in variegin, this Glu is replaced by VAla22 and its side chain is solvent exposed (Figure 3C). VAla22 was replaced by Glu in variants EP25A22E and MH22A22E. The four C-terminal residues were retained in these variants to maintain the original micro-environment near the C-terminus. IC50 and Ki values of EP25A22E and MH22A22E are similar to their templates (EP25 and MH22, respectively) (Table 2). Thus, the replacement of VAla22 by Glu does not enhance the activity of variegin.
Desulfation of Tyr63 in hirudin or hirugen is known to reduce their affinities to thrombin by about 10 fold [36]–[38]. Interestingly, the analogous residue in native variegin, VTyr27, is not sulfated. We postulated that modification of VTyr27 could also increase its binding affinity towards thrombin. Considering the similarity of phosphate and sulfate moieties (similar size and overall negative charge), phosphotyrosine and sulfotyrosine residues were incorporated to design new variants.
One phosphotyrosine residue was added to DV24 and DV24K10R to produce the variants DV24Yphos and DV24K10RYphos, respectively. DV24Yphos is marginally less active than DV24, whereas DV24K10RYphos has slightly improved activity (Table 2).
Similarly, a sulfotyrosine residue was incorporated in three new variants, DV24Ysulf, DV24K10RYsulf and MH18Ysulf. DV24Ysulf and DV24K10RYsulf show an average ∼5 fold increase in IC50 and Ki values when compared to the respective non-sulfated variants, DV24 and DV24K10R, respectively (Table 2). MH18Ysulf also has improved activity when compared to MH18 (Table 2). It is very likely that the presence of sulfo-Tyr27 and the truncation of extra residues in variegin variants cause a rearrangement of the C-terminal conformation to mimic the hirugen/hirudin C-termini. Strong affinities are obtained in these variants through optimization of C-terminal interactions.
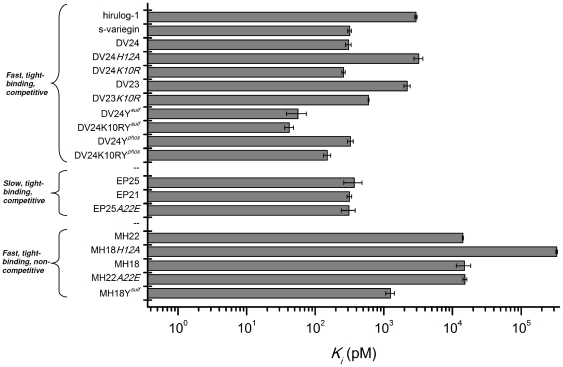
Figure 5 shows the plot of Ki values of all peptides (including hirulog-1/bivalirudin). Affinity of DV24K10RYsulf for thrombin (Ki = 42.0±6.1 pM) is ∼70 fold stronger than that of hirulog-1/bivalirudin (Ki = 2.94±0.12 nM). Based on the derived structure-activity relationships, we have designed a shorter (24-mer DV24K10RYsulf against 32-mer s-variegin) yet more potent thrombin inhibitor (Ki of 42 pM compared to 318 pM).
Figure 5. Ki values of all peptides (including hirulog-1/bivalirudin).
Peptides are grouped according to their mechanism of actions. All competitive inhibitors (fast or slow) have higher affinities to thrombin compared to hirulog-1/bivalirudin. The most potent variant DV24K10RYsulf is about 70-fold stronger. Even their cleavage products (non-competitive inhibitors) are potent inhibitor, with one of them, MH18Ysulf, binds to thrombin approximately 2-fold tighter than hirulog-1/bivalirudin.
In vivo antithrombotic effects of the peptides
Five inhibitors were selected as representatives to test for their antithrombotic effects in vivo using the zebrafish larvae venous thrombosis model: (1) s-variegin, the full-length variegin, a fast and tight-binding competitive inhibitor; (2) EP25, without seven N-terminal residues, has similar affinity for thrombin, but is a slow and tight-binding competitive inhibitor; (3) MH22, the cleaved product that is a fast and tight-binding, noncompetitive inhibitor; (4) DV24K10RYsulf, the most potent in vitro inhibitor that is a fast and tight-binding competitive inhibitor and (5) hirulog-1/bivalirudin, a fast, tight-binding, competitive inhibitor currently in clinical use. Hirulog-1/bivalirudin was used as a positive control.
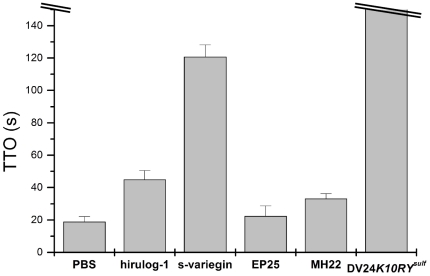
All five peptides were injected into the zebrafish larvae circulation through the posterior (caudal) cardinal vein at a single dose (500 µM, 10 nl). The antithrombotic effects of the peptides were measured as the delay in time-to-occlusion (TTO) of the caudal vein after laser ablation (Figure 6). Overall, other than EP25, the antithrombotic effects of the peptides correlated well with their affinities for thrombin. Thus, the slow-binding inhibitory mode (EP25) is not desirable for in vivo efficacy while both fast, competitive (s-variegin, DV24K10RYsulf and hirulog-1/bivalirudin) and fast, noncompetitive (MH22) inhibitors are effective. Our results are consistent with similar observations reported earlier about the importance of rapid thrombin inhibition for efficacious antithrombotic agents [39].
Figure 6. In vivo antithrombotic effects of peptides.
Zebrafish 4 days post-fertilization larvae were injected with 10 nl of different peptides at 500 µM or 10 nl of PBS as control. TTO for larvae injected with PBS, hirulog-1/bivalirudin, s-variegin, EP25 and MH22 are 19.0±3.2 s, 45.0±5.5 s, 120.8±7.4 s, 22.5±6.2 s and 33.3±2.9 s, respectively. Within 150 s, no thrombus was formed in larvae injected with DV24K10RYsulf. With the exception of the slow binding inhibitor EP25, the abilities of the peptides to prolong TTO correlate with their in vitro Ki (n = 4, error bars represent S.D.).
Discussion
Variegin belongs to a unique class of thrombin inhibitors that have potential as antithrombotic agents [16], [17]. We solved the structure of thrombin:s-variegin complex at 2.4 Å resolution. Despite the use of full-length s-variegin for co-crystallization, only the density of its C-terminal fragment was observed. The cleaved fragment (equivalent to MH22) stays bound tightly to the prime subsites and exosite-I, exhibiting prolonged inhibitory action (>18 h) [17].
Active site inhibitors of thrombin typically target the non-prime subsites, hindering the access of substrates (including the chromogenic substrate S2238 used in this study) to the catalytic residues [29], [31], [40]. The full-length variegin also acts in a similar way and competitively inhibits the thrombin active site [16]. The present thrombin:s-variegin structure reveals two exciting features: (1) the novel mechanism of thrombin inhibition through disruption of the charge relay system, and (2) the binding with thrombin prime subsites. The cleaved product (MH22) still retains inhibition through this novel mechanism, as observed in the structure. The lack of overlaps in MH22 and S2238 binding sites (non-prime and S1′ subsites), demonstrates the feasibility of simultaneous binding of S2238 and MH22 to thrombin with no changes in affinities of either (Figure S20). The binding of substrate (S2238) to the thrombin active site becomes non-productive due to the disruption of the charge relay system by MH22 (Figure 2). We also speculate that the binding of variegin/MH22 to the prime subsites induces minor structural changes that may interfere with the entry of S2238 or exit of products from the active site without affecting the strength of substrate binding. Thus the structure explains the classical noncompetitive inhibition by MH22.
The interaction between the variegin and thrombin prime subsites is equally interesting. The S′ subsites' interactions between the inhibitors and protease are important for binding [41]. A systematic probing of thrombin S′ subsites with ‘methyl scan’ also has demonstrated the potential for targeting prime subsites in the design of inhibitors [42]. However, unlike variegin, other naturally occurring thrombin inhibitors, including hirudin [31], rhodniin [43], ornithodorin [44] and boophilin [45], are not inserted into the canyon-like cleft (the prime subsites) connecting the active site and exosite-I. Similarly, the previous structures of thrombin, in complex with other macromolecular substrates [46]–[50], typically lack complete details in the interactions within this region. Thus, when compared to the non-prime subsites, understanding of the binding preferences for the thrombin prime subsites (especially S2′ and beyond) is much less complete [1]. Recently, the structure of a PAR1 fragment in complex with an inactive thrombin mutant TSer195A was published [51]. This structure provided new details on the interaction within the prime subsites. However, there is considerable disorderliness in the PAR1 molecule binding to this region. As a result, it was suggested that the prime subsites binding segment of PAR1 snaps away from thrombin upon cleavage [51]. In the case of substrates, such a mechanism is probably advantageous to ensure a faster turnover. However, inhibitors are developed to bind to thrombin as tight and long as possible. In this specific case, variegin has evolved to bind to the thrombin prime subsites and thus the structure of the thrombin:s-variegin complex provides a unique opportunity to understand the interaction of this inhibitor with the thrombin prime subsites which is of extreme importance to the design of more effective inhibitors.
In hirulog-1/bivalirudin, the glycyl linkers connect the active site and exosite-I binding moieties without displaying specific interactions with the thrombin prime subsites. As a result, this segment is disordered in the crystal structure [4] and the activity is rapidly lost after cleavage by thrombin [17], [52]. When anchored by a non-hydrolyzable active site binding moiety and an exosite-I binding segment, the non-specific linker is forced to fit into the canyon-like cleft in the prime subsites, as seen in hirulog-3 [22] and P498 [40]. In these cases, the S′ subsite interactions are sub-optimal due to the lack of specific side chain interactions. As a result, extensive and lengthy optimizations, through synthetic chemistry using multiple and unnatural amino acids, were necessary to produce inhibitors with enhanced binding to the prime subsites [42], [53], [54]. In contrast, tight binding of s-variegin to the prime subsites is achieved through specific interactions involving the side chains of natural amino acids. Thus the thrombin-variegin complex provides important and detailed structural information for inhibition of the thrombin prime subsites. This structural observation for the prime subsites binding in variegin is also consistent with the structure-function data presented here and elsewhere [16], [17]. The firm insertion of variegin peptides into the cleft in an extended conformation is probably the simplest structure needed to achieve strongest and instantaneous binding to the thrombin catalytic pocket, prime subsites and exosite-I. This ‘minimalistic’ approach in nature (ticks) confers an advantage of minimum energy expenditure (protein synthesis) for maximum outcome (potent inhibition of coagulation enzymes to facilitate blood-feeding).
Based on the thrombin:s-variegin structure and other available information, we performed targeted structure-function relationship studies on variegin. Substitution of P1 Lys by Arg is reported to increase affinity for thrombin by ∼10 fold through a better fit to the S1 subsite [2], [35]. However, our results show that the improvement in affinity by this substitution varies. In DV24, DV24Ysulf and DV24Yphos, the effects of substitution are less than 2 fold. It is likely that the extensive interactions between the s-variegin P′ residues and thrombin S′ subsite compensate for the loose fitting of P1 Lys. In contrast, DV23/DV23K10R-thrombin interactions around the prime subsites are likely to be disturbed due to the deletion of Pro16 (DV23 Ki is ∼7 fold higher than DV24). In this situation, P1 Arg facilitates stronger binding of the V(5DVAEPR10) sequence to the active site compared to P1 Lys, which is reflected by the higher gain in affinity (∼4 fold decrease in Ki). These results further support the importance of prime subsite interactions.
We have also shown that addition of three residues at the N-terminus of the slow-binding inhibitor EP21 changes it to a fast-binding inhibitor. Thus, the prime subsites anchoring effect (discussed above) mainly drives affinity for the thrombin active site, while the N-terminal steering effect is needed for proper pre-orientation of this segment. Hence, the P1 to P3 residues of s-variegin are inserted rapidly into the thrombin active site, assisted by prime subsites targeting and the N-terminal negative charge. Once in the acidic S1 pocket, P1 Lys/Arg interacts with TAsp189 with only a minimal overall loss of binding strength. This suggests a less stringent requirement for the P1 residue that can be exploited for the design of new specific thrombin inhibitors. In addition to the prime subsite interaction, VHis12 draws TSer192 out of position and affects the geometry of the crucial catalytic residue as well as interfering in the charge relay system. The result is decreased catalytic efficiency. Such a design may be exploited in the development of inhibitors for other serine proteases.
The new variegin variants cover a big range of potency, speed of onset and kinetic parameters, showing the potential to ‘tune’ variegin to provide different therapeutic properties. The unique ability of variegin to potently inhibit thrombin (initially competitive, subsequently noncompetitive) for a long duration represents a new approach to anticoagulation when compared to other direct thrombin inhibitors on the market. Variegin and some of its variants inhibit thrombin potently with low Ki (between 0.04 to 0.4 nM). Their affinities for thrombin are stronger than hirulog-1/bivalirudin (Ki = 2.3 nM) [55], argatroban (Ki = 3.2 nM) [56] and dabigatran (Ki = 4.5 nM) [57] but weaker than hirudin (Ki = 0.2 pM) [36]. While weaker affinities may translate to lower efficacy and increased probability of side effects, the almost irreversible binding of hirudin to thrombin (Ki = 0.2 pM) may be responsible for an increased risk of major bleeding when compared to unfractionated heparin [58]. Thus, in terms of affinity for thrombin, variegin may represent a good balance between potency and safety. The prolonged action of variegin might also allow single dose administration instead of continuous infusion (as in the case of hirulog-1/bivalirudin) [11] especially in short procedures such as percutaneous coronary intervention. We also have preliminary data showing the possibility of using protamine as antidote for variegin. Overall, variegin (with its variants) represents a fine balance between hirudin and hirulog-1/bivalirudin for most of their properties (Ki, size, duration of action etc.). At this point, the strong in vivo antithrombotic effects support the continual development of variegin and its variants as potential and improved anticoagulants.
Supporting Information
Conformation of s-variegin C-terminus. s-Variegin C-terminus (pink) has a vastly different conformation compared to hirulog-1, hirulog-3, hirugen and sulfo-hirudin: (A) Residues PEEYL in hirulog-1 (red) are disordered and missing from the structure. (B) Residues PEEYL in hirulog-3 (blue) form a 310 helix turn. (C) These residues in hirugen (green), with sulfated tyrosine, also form a 310 helix turn. (D) Other than Tyr-sulfation, sulfo-hirudin (cyan) C-terminus has an extra Gln, forms a full α-helical turn.
(TIF)
Electrostatic interactions in thrombin:s-variegin structure. (A) Figure shows the electron density map (2Fo-Fc, 0.9σ) of residues described in Figure 4A in the main manuscript. Thrombin is colored yellow and s-variegin is colored pink. Map for thrombin colored in light cyan and map for s-variegin colored in gray. Residues involved in forming salt bridges are labeled. (B) Figure shows the electron density map (2Fo-Fc, 0.9σ) of residues described in Figure 4B in the main manuscript. Thrombin is colored yellow and s-variegin is colored pink. Map for thrombin colored in light cyan and map for s-variegin colored in gray.
(TIF)
Variegin variant EP25 (slow binding, competitive inhibitor). (A) Dose response curves of thrombin (1.65 nM) inhibited by EP25 (0.1 nM, 0.3 nM, 1 nM, 3 nM, 10 nM, 30 nM, 100 nM, 300 nM, 1000 nM, 3000 nM) in S2238 (100 µM) showed a left shift with increased pre-incubation time due to slow binding. IC50 are 173±26 nM without pre-incubation (▪ solid line) and 13.1±0.7 nM with 20 min pre-incubation (○ dotted line) (n = 3, error bars represent S.D.). (B) Progress curves (not shown) of thrombin (0.8 nM) inhibited by EP25 (9.4 nM, 12.5 nM, 18.8 nM, 25 nM, 37.5 nM, 50 nM, 75 nM and 100 nM) in S2238 (100 µM) were fitted to equation (6) describing a slow binding inhibitor to obtain a k for each concentrations of EP25. Plot of k against EP25 concentrations (▪ solid line) is hyperbolic and fitted to equation (7) producing Ki′ of 0.882±0.128 nM, representing the dissociation constant of initial collision complex EI (scheme 1). Ki calculated from equation (8) is 0.365±0.109 nM (n = 3, error bars represent S.D.).
(TIF)
Variegin variant EP21 (slow binding, competitive inhibitor). (A) Dose response curves of thrombin (1.65 nM) inhibited by EP21 (0.3 nM, 1 nM, 3 nM, 10 nM, 30 nM, 100 nM, 300 nM, 1000 nM, 3000 nM and 10000 nM) in S2238 (100 µM) showed a left shift with increased pre-incubation time due to slow binding. IC50 are 177±7 nM without pre-incubation (▪ solid line) and 16.2±2.9 nM with 20 min pre-incubation (○ dotted line) (n = 3, error bars represent S.D.). (B) Progress curves (Figure S4) of thrombin (0.8 nM) inhibited by EP21 (18.8 nM, 25 nM, 37.5 nM, 50 nM, 75 nM, 100 nM and 150 nM) in S2238 (100 µM) were fitted to equation (6) describing a slow binding inhibitor to obtain a k for each concentrations of EP21. Plot of k against EP21 concentrations (▪ solid line) is hyperbolic and fitted to equation (7) producing Ki′ of 1.66±0.36 nM, representing the dissociation constant of initial collision complex EI (scheme 1). Ki calculated from equation (8) is 0.315±0.024 nM (n = 3, error bars represent S.D.).
(TIF)
Variegin variant MH18 (fast, tight-binding, noncompetitive inhibitor). (A) Dose response curves of thrombin inhibition (1.65 nM) by MH18 (0.1 nM, 0.3 nM, 1 nM, 3 nM, 10 nM, 30 nM, 100 nM, 300 nM, 1000 nM, 3000 nM and 10000 nM) in S2238 (100 µM) are independent of pre-incubation time. IC50 are 10.9±1.2 nM without pre-incubation (▪ solid line) and 11.7±1.9 nM with 20 min pre-incubation (○ dotted line) (n = 3, error bars represent S.D.). (B) Thrombin (1.65 nM) inhibition was tested with MH18 (0.39 nM, 0.78 nM, 1.56 nM, 3.13 nM, 6.25 nM, 12.5 nM, 25 nM, 50 nM, 100 nM and 200 nM) in S2238 (100 µM) (▪ solid line). Apparent inhibition constant Ki′ obtained by fitting data to equation (2), describing fast and tight-binding inhibitor, is 14.9±3.5 nM. Ki calculated from equations (4) and (5), describing noncompetitive inhibitors, is 14.9±3.5 nM (n = 3, error bars represent S.D.).
(TIF)
Progress curves of thrombin inhibitied by EP21 and DV24. (A) Progress curves of thrombin (0.8 nM) inhibited by different concentrations of EP21 using S2238 (100 µM) as substrate, without pre-incubation of thrombin and EP21. The non-linear behavior of the curves at the beginning of the reactions and an improved IC50 with pre-incubation (Figure S4) suggested equilibrium of inhibition was achieved slowly, characteristic of slow-binding inhibitors. (B) Progress curves of thrombin (1.65 nM) inhibited by different concentrations of DV24: 0 nM (▪), 0.1 nM (□), 0.3 nM (•), 1 nM (○), 3 nM (▴), 10 nM (▵), 30 nM (▾), 100 nM (▿), 300 nM (⧫) and 1000 nM (⋄) using S2238 (100 µM) as substrate, without pre-incubation of thrombin and DV24. The linear curves indicate the equilibrium of inhibition was achieved upon mixing of thrombin and DV24, characteristic of fast-binding inhibitors.
(TIF)
Variegin variant DV24 (fast, tight-binding, competitive inhibitor). (A) Dose-response curves of thrombin (1.65 nM) inhibited by DV24 (0.1 nM, 0.3 nM, 1 nM, 3 nM, 10 nM, 30 nM, 100 nM, 300 nM, 1000 nM and 3000 nM) in S2238 (100 µM) showed a right shift with increased pre-incubation time due to cleavage. IC50 are 7.49±0.28 nM without pre-incubation (▪ solid line) and 10.1±0.6 nM with 20 min pre-incubation (○ dotted line) (n = 3, error bars represent S.D.). (B) Thrombin (1.65 nM) inhibition was tested with DV24 (0.39 nM, 0.78 nM, 1.56 nM, 3.13 nM, 6.25 nM, 12.5 nM, 25 nM, 50 nM, 100 nM and 200 nM) in S2238 (100 µM) (▪ solid line). Apparent inhibition constant Ki′ obtained by fitting data to equation (2), describing fast and tight-binding inhibitor, is 9.74±0.91 nM. Ki calculated from equation (3), describing competitive inhibitors, is 0.306±0.029 nM (n = 3, error bars represent S.D.).
(TIF)
Variegin variant DV24 H12A (fast, tight-binding, competitive inhibitor). (A) Dose-response curves of thrombin (1.65 nM) inhibited by DV24H12A (0.001 µM, 0.003 µM, 0.01 µM, 0.03 µM, 0.3 µM, 1 µM, 3 µM, 10 µM and 30 µM) in S2238 (100 µM) showed a right shift with increased pre-incubation time due to cleavage. IC50 are 48.2±12.4 nM without pre-incubation (▪ solid line) and 141±11 nM with 20 min pre-incubation (○ dotted line) (n = 3, error bars represent S.D.). (B) Thrombin (1.65 nM) inhibition was tested with DV24H12A (1.95 nM, 3.91 nM, 7.81 nM, 15.6 nM, 31.3 nM, 62.5 nM, 125 nM, 250 nM, 500 nM and 1000 nM) in S2238 (100 µM) (▪ solid line). Apparent inhibition constant Ki′ obtained by fitting data to equation (2), describing fast and tight-binding inhibitor, is 103±15 nM. Ki calculated from equation (3), describing competitive inhibitors, is 3.23±0.48 nM (n = 3, error bars represent S.D.).
(TIF)
Variegin variant MH18 H12A (fast, noncompetitive inhibitor). (A) Dose-response curves of thrombin (1.65 nM) inhibition by MH18H12A (0.001 µM, 0.003 µM, 0.01 µM, 0.03 µM, 0.3 µM, 1 µM, 3 µM, 10 µM and 30 µM) in S2238 (100 µM) are independent of pre-incubation time. IC50 are 328±23 nM without pre-incubation (▪ solid line) and 343±46 nM with 20 min pre-incubation (n = 3, error bars represent S.D.). (B) Thrombin (1.65 nM) inhibition was tested with 1 µM MH18H12A (○ dotted line) in S2238 (4.69 µM, 9.34 µM, 18.8 µM, 37.5 µM, 75 µM, 150 µM and 300 µM) and without the inhibitor (▪ solid line) in S2238 (3.13 µM, 6.25 µM, 12.5 µM, 25 µM, 50 µM, 100 µM, 200 µM). MH18H12A is unable to inhibit thrombin at equimolar concentration, hence is not considered as tight-binding inhibitor. The double-reciprocal plot showed noncompetitive inhibition and Ki is 329±8 nM (n = 3, error bars represent S.D.).
(TIF)
Variegin variant DV24 K10R (fast, tight-binding, competitive inhibitor). (A) Dose-response curves of thrombin (1.65 nM) inhibited by DV24K10R (0.1 nM, 0.3 nM, 1 nM, 3 nM, 10 nM, 30 nM, 100 nM, 300 nM, 1000 nM and 3000 nM) in S2238 (100 µM) showed a right shift with increased pre-incubation time due to cleavage. IC50 are 6.98±0.76 nM without pre-incubation (▪ solid line) and 12.0±0.4 nM with 20 min pre-incubation (○ dotted line) (n = 3, error bars represent S.D.). (B) Thrombin (1.65 nM) inhibition was tested with DV24K10R (0.39 nM, 0.78 nM, 1.56 nM, 3.13 nM, 6.25 nM, 12.5 nM, 25 nM, 50 nM, 100 nM and 200 nM) in S2238 (100 µM) (▪ solid line). Apparent inhibition constant Ki′ obtained by fitting data to equation (2), describing fast and tight-binding inhibitor, is 8.27±0.85 nM. Ki calculated from equation (3), describing competitive inhibitors, is 0.259±0.015 nM (n = 3, error bars represent S.D.).
(TIF)
Variegin variant DV23 (fast, tight-binding, competitive inhibitor). (A) Dose-response curves of thrombin (1.65 nM) inhibited by DV23 (0.1 nM, 0.3 nM, 1 nM, 3 nM, 10 nM, 30 nM, 100 nM, 300 nM, 1000 nM and 3000 nM) in S2238 (100 µM) showed a right shift with increased pre-incubation time due to cleavage. IC50 are 45.4±1.6 nM without pre-incubation (▪ solid line) and 77.8±6.1 nM with 20 min pre-incubation (○ dotted line) (n = 3, error bars represent S.D.). (B) Thrombin (1.65 nM) inhibition was tested with DV23 (3.91 nM, 7.81 nM, 15.6 nM, 31.3 nM, 62.5 nM, 125 nM, 250 nM and 500 nM) S2238 (100 µM) (▪ solid line). Apparent inhibition constant Ki′ obtained by fitting data to equation (2), describing fast and tight-binding inhibitor, is 69.6±7.8 nM. Ki calculated from equation (3), describing competitive inhibitors, is 2.19±0.23 nM (n = 3, error bars represent S.D.).
(TIF)
Variegin variant DV23 K10R (fast, tight-binding, competitive inhibitor). (A) Dose-response curves of thrombin (1.65 nM) inhibited by DV23K10R (0.1 nM, 0.3 nM, 1 nM, 3 nM, 10 nM, 30 nM, 100 nM, 300 nM, 1000 nM and 3000 nM) in S2238 (100 µM) showed a strong right shift with increased pre-incubation time due to cleavage. IC50 are 12.9±1.0 nM without pre-incubation (▪ solid line) and 102±1 nM with 20 min pre-incubation (○ dotted line) (n = 3, error bars represent S.D.). (B) Thrombin (1.65 nM) inhibition was tested with DV23K10R (3.91 nM, 7.81 nM, 15.6 nM, 31.3 nM, 62.5 nM, 125 nM, 250 nM and 500 nM) in S2238 (100 µM) (▪ solid line). Apparent inhibition constant Ki′ obtained by fitting data to equation (2), describing fast and tight-binding inhibitor, is 19.1±1.9 nM. Ki calculated from equation (3), describing competitive inhibitors, is 0.600±0.010 nM (n = 3, error bar represents S.D.).
(TIF)
Variegin variant EP25 A22E (slow binding, competitive inhibitor). (A) Dose-response curves of thrombin (1.65 nM) inhibited by EP25A22E (0.1 nM, 0.3 nM, 1 nM, 3 nM, 10 nM, 30 nM, 100 nM, 300 nM, 1000 nM and 3000 nM) in S2238 (100 µM) showed a left shift due to slow binding. IC50 are 124±23 nM without pre-incubation (▪ solid line) and 13.5±2.1 nM with 20 min pre-incubation (○ dotted line) (n = 3, error bars represent S.D.). (B) Progress curves (not shown) of thrombin (0.8 nM) inhibited by EP25A22E (9.38 nM, 12.5 nM, 18.8 nM, 25 nM, 37.5 nM, 50 nM, 75 nM, 100 nM, 150 nM, 200 nM and 300 nM) in S2238 (100 µM) were fitted to equation (6) describing a slow binding inhibitor to obtain a k for each concentrations of EP25A22E. Plot of k against EP25A22E concentrations (▪ solid line) is hyperbolic and was fitted to equation (7) producing Ki′ of 1.02±0.060 nM, representing the dissociation constant of initial collision complex EI (scheme 1). Ki calculated from equation (8) is 0.311±0.070 nM (n = 3, error bars represent S.D.).
(TIF)
Variegin variant MH22 A22E (fast, tight-binding, noncompetitive inhibitor). (A) Dose-response curves of thrombin (1.65 nM) inhibited by MH22A22E (0.1 nM, 0.3 nM, 1 nM, 3 nM, 10 nM, 30 nM, 100 nM, 300 nM, 1000 nM and 3000 nM) in S2238 (100 µM) are independent of pre-incubation time. IC50 are 13.62±0.45 nM without pre-incubation (▪ solid line) and 15.6±0.4 nM with 20 min pre-incubation (○ dotted line) (n = 3, error bars represent S.D.). (B) Thrombin (1.65 nM) inhibition was tested with MH22A22E (0.39 nM, 0.78 nM, 1.56 nM, 3.13 nM, 6.25 nM, 12.5 nM, 25 nM, 50 nM, 100 nM and 200 nM) in S2238 (100 µM) (▪ solid line). Apparent inhibition constant Ki′ obtained by fitting data to equation (2), describing fast and tight-binding inhibitor, is 15.1±1.0 nM. Ki calculated from equations (4) and (5), describing noncompetitive inhibitors, is 15.1±1.0 nM (n = 3, error bars represent S.D.).
(TIF)
Variegin variant DV24 Yphos (fast, tight-binding, competitive inhibitor). (A) Dose-response curves of thrombin (1.65 nM) inhibited by DV24Yphos (0.03 nM, 0.1 nM, 0.3 nM, 1 nM, 3 nM, 10 nM, 30 nM, 100 nM, 300 nM and 1000 nM) in S2238 (100 µM) showed a right shift with increased pre-incubation time due to cleavage. IC50 are 8.67±0.45 nM without pre-incubation (▪ solid line) and 12.4±1.2 nM with 20 min pre-incubation (○ dotted line) (n = 3, error bars represent S.D.). (B) Thrombin (1.65 nM) inhibition was tested with DV24Yphos (0.39 nM, 0.78 nM, 1.56 nM, 3.13 nM, 6.25 nM, 12.5 nM, 25 nM, 50 nM, 100 nM and 200 nM) in S2238 (100 µM) (▪ solid line). Apparent inhibition constant Ki′ obtained by fitting data to equation (2), describing fast and tight-binding inhibitors, is 10.4±1.0 nM. Ki calculated from equation (3), describing competitive inhibitors, the inhibition constant is 0.327±0.032 nM (n = 3, error bars represent S.D.).
(TIF)
Variegin variant DV24 K10RYphos (fast, tight-binding, competitive inhibitor). (A) Dose-response curves of thrombin (1.65 nM) inhibited by DV24K10RYphos (0.03 nM, 0.1 nM, 0.3 nM, 1 nM, 3 nM, 10 nM, 30 nM, 100 nM, 300 nM and 1000 nM) in S2238 (100 µM) showed a right shift with increased pre-incubation time due to cleavage. IC50 are 4.64±0.78 nM without pre-incubation (▪ solid line) and 7.80±1.80 nM with 20 min pre-incubation (○ dotted line) (n = 3, error bars represent S.D.). (B) Thrombin (1.65 nM) inhibition was tested with DV24K10RYphos (0.39 nM, 0.78 nM, 1.56 nM, 3.13 nM, 6.25 nM, 12.5 nM, 25 nM, 50 nM, 100 nM and 200 nM) in S2238 (100 µM) (▪ solid line). Apparent inhibition constant Ki′ obtained by fitting data to equation (2), describing fast and tight-binding inhibitors, is 4.78±0.57 nM. Ki calculated from equation (3), describing competitive inhibitors, is 0.150±0.018 nM (n = 3, error bars represent S.D.).
(TIF)
Variegin variant DV24 Ysulf (fast, tight-binding, competitive inhibitor). (A) Dose-response curves of thrombin (1.65 nM) inhibited by DV24Ysulf (0.05 nM, 0.15 nM, 0.45 nM, 1.5 nM, 4.5 nM, 15 nM, 45 nM, 150 nM, 450 nM and 1500 nM) in S2238 (100 µM) showed a right shift with increased pre-incubation time due to cleavage. IC50 are 1.66±0.18 nM without pre-incubation (▪ solid line) and 2.02±0.29 nM with 20 min pre-incubation (○ dotted line) (n = 3, error bars represent S.D.). (B) Thrombin (1.65 nM) inhibition was tested with DV24Ysulf (0.20 nM, 0.39 nM, 0.78 nM, 1.56 nM, 3.13 nM, 6.25 nM, 12.5 nM, 25 nM, 50 nM and 100 nM) in S2238 (100 µM) (▪ solid line). Apparent inhibition constant Ki′ obtained by fitting data to equation (2), describing fast and tight-binding inhibitor, is 1.78±0.47 nM. Ki calculated from equation (3), describing competitive inhibitors, is 0.056±0.015 nM (n = 3, error bars represent S.D.).
(TIF)
Variegin variant DV24 K10RYsulf (fast, tight-binding, competitive inhibitor). (A) Dose-response curves of thrombin (1.65 nM) inhibited by DV24K10RYsulf (0.05 nM, 0.15 nM, 0.45 nM, 1.5 nM, 4.5 nM, 15 nM, 45 nM, 150 nM, 450 nM and 1500 nM) in S2238 (100 µM) showed a right shift with increased pre-incubation time due to cleavage. IC50 are 1.39±0.17 nM without pre-incubation (▪ solid line) and 1.66±0.21 nM with 20 min pre-incubation (○ dotted line) (n = 3, error bars represent S.D.). (B) Thrombin (1.65 nM) inhibition was tested with DV24K10RYsulf (0.20 nM, 0.39 nM, 0.78 nM, 1.56 nM, 3.13 nM, 6.25 nM, 12.5 nM, 25 nM, 50 nM and 100 nM) in S2238 (100 µM) (▪ solid line). Apparent inhibition constant Ki′ obtained by fitting data to equation (2), describing fast and tight-binding inhibitors, is 1.33±0.19 nM. Ki calculated from equation (3), describing competitive inhibitors, is 0.0420±0.0061 nM (n = 3, error bars represent S.D.).
(TIF)
Variegin variant MH18 Ysulf (fast, tight-binding, noncompetitive inhibitor). (A) Dose-response curves of thrombin (1.65 nM) inhibited by MH18Ysulf (0.03 nM, 0.1 nM, 0.3 nM, 1 nM, 3 nM, 10 nM, 30 nM, 100 nM, 300 nM and 1000 nM) in S2238 (100 µM) are independent of pre-incubation time. IC50 are 1.26±0.18 nM without pre-incubation (▪ solid line) and 1.17±0.14 nM with 20 min pre-incubation (○ dotted line) (n = 3, error bar represents S.D.). (B) Thrombin (1.65 nM) inhibition was tested with MH18Ysulf (0.20 nM, 0.39 nM, 0.78 nM, 1.56 nM, 3.13 nM, 6.25 nM, 12.5 nM, 25 nM, 50 nM and 100 nM) in S2238 (100 µM) (▪ solid line). Apparent inhibition constant Ki′ obtained by fitting data to equation (2), describing fast and tight-binding inhibitors, is 1.25±0.18 nM. Ki calculated from equations (4) and (5), describing noncompetitive inhibitors, is 1.25±0.18 nM (n = 3, error bar represents S.D.).
(TIF)
Noncompetitive inhibition of thrombin by MH22. s-Variegin binds to both the non-prime and prime subsites of thrombin active site and is cleaved between Lys-Met. After cleavage, the fragment C-terminal to the scissile bond (MH22) noncompetitively inhibits thrombin. The chromogenic substrate S2238 binds mainly to the non-prime subsites and is cleaved between Arg and para-nitroaniline (pNA). The overlaps between s-variegin and S2238 binding sites resulted in the observed competitive inhibition. In contrast, the noncompetitive inhibition observed for MH22 showed the lack of overlaps between MH22 and S2238 even in the S1′ subsite (red box). Indeed, no density was observed for P1′ Met in the present structure, most likely reflects the lack of contact with thrombin and hence leaves a free S1′ site for the binding of pNA moiety when MH22 is bound to thrombin.
(TIF)
A list of possible direct hydrogen bonds between s-variegin and thrombin calculated based on the online server PISA [28].
(TIF)
A detailed account for the selection and use of equations to fit the data of thrombin inhibitions is available in Materials and Methods S1.
(DOC)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The work is supported by the Academic Research Grants from National University of Singapore. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Huntington JA. Molecular recognition mechanisms of thrombin. J Thromb Haemost. 2005;3:1861–1872. doi: 10.1111/j.1538-7836.2005.01363.x. [DOI] [PubMed] [Google Scholar]
- 2.Bode W, Turk D, Karshikov A. The refined 1.9-A X-ray crystal structure of D-Phe-Pro-Arg chloromethylketone-inhibited human alpha-thrombin: structure analysis, overall structure, electrostatic properties, detailed active-site geometry, and structure-function relationships. Protein Sci. 1992;1:426–471. doi: 10.1002/pro.5560010402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rydel TJ, Tulinsky A, Bode W, Huber R. Refined structure of the hirudin-thrombin complex. J Mol Biol. 1991;221:583–601. doi: 10.1016/0022-2836(91)80074-5. [DOI] [PubMed] [Google Scholar]
- 4.Skrzypczak-Jankun E, Carperos VE, Ravichandran KG, Tulinsky A, Westbrook M, et al. Structure of the hirugen and hirulog 1 complexes of alpha-thrombin. J Mol Biol. 1991;221:1379–1393. [PubMed] [Google Scholar]
- 5.Huntington JA. How Na+ activates thrombin–a review of the functional and structural data. Biol Chem. 2008;389:1025–1035. doi: 10.1515/BC.2008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lane DA, Philippou H, Huntington JA. Directing thrombin. Blood. 2005;106:2605–2612. doi: 10.1182/blood-2005-04-1710. [DOI] [PubMed] [Google Scholar]
- 7.Koh CY, Kini RM. Anticoagulants from hematophagous animals. Expert Rev Hematol. 2008;1:135–139. doi: 10.1586/17474086.1.2.135. [DOI] [PubMed] [Google Scholar]
- 8.Ajjan R, Grant PJ. Coagulation and atherothrombotic disease. Atherosclerosis. 2006;186:240–259. doi: 10.1016/j.atherosclerosis.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 9.Gross PL, Weitz JI. New anticoagulants for treatment of venous thromboembolism. Arterioscler Thromb Vasc Biol. 2008;28:380–386. doi: 10.1161/ATVBAHA.108.162677. [DOI] [PubMed] [Google Scholar]
- 10.Greinacher A, Warkentin TE. The direct thrombin inhibitor hirudin. Thromb Haemost. 2008;99:819–829. doi: 10.1160/TH07-11-0693. [DOI] [PubMed] [Google Scholar]
- 11.Warkentin TE, Greinacher A, Koster A. Bivalirudin. Thromb Haemost. 2008;99:830–839. doi: 10.1160/TH07-10-0644. [DOI] [PubMed] [Google Scholar]
- 12.Yeh RW, Jang IK. Argatroban: update. Am Heart J. 2006;151:1131–1138. doi: 10.1016/j.ahj.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Eriksson BI, Smith H, Yasothan U, Kirkpatrick P. Dabigatran etexilate. Nat Rev Drug Discov. 2008;7:557–558. doi: 10.1038/nrd2622. [DOI] [PubMed] [Google Scholar]
- 14.Champagne DE. Antihemostatic strategies of blood-feeding arthropods. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4:375–396. doi: 10.2174/1568006043335862. [DOI] [PubMed] [Google Scholar]
- 15.Koh CY, Kini RM. Molecular diversity of anticoagulants from haematophagous animals. Thromb Haemost. 2009;102:437–453. doi: 10.1160/TH09-04-0221. [DOI] [PubMed] [Google Scholar]
- 16.Koh CY, Kazimirova M, Trimnell A, Takac P, Labuda M, et al. Variegin, a novel fast and tight binding thrombin inhibitor from the tropical bont tick. J Biol Chem. 2007;282:29101–29113. doi: 10.1074/jbc.M705600200. [DOI] [PubMed] [Google Scholar]
- 17.Koh CY, Kazimirova M, Nuttall PA, Kini RM. Noncompetitive inhibitor of thrombin. Chembiochem. 2009;10:2155–2158. doi: 10.1002/cbic.200900371. [DOI] [PubMed] [Google Scholar]
- 18.Yonemura H, Imamura T, Soejima K, Nakahara Y, Morikawa W, et al. Preparation of recombinant alpha-thrombin: high-level expression of recombinant human prethrombin-2 and its activation by recombinant ecarin. J Biochem (Tokyo) 2004;135:577–582. doi: 10.1093/jb/mvh070. [DOI] [PubMed] [Google Scholar]
- 19.Soejima K, Mimura N, Yonemura H, Nakatake H, Imamura T, et al. An efficient refolding method for the preparation of recombinant human prethrombin-2 and characterization of the recombinant-derived alpha-thrombin. J Biochem (Tokyo) 2001;130:269–277. doi: 10.1093/oxfordjournals.jbchem.a002982. [DOI] [PubMed] [Google Scholar]
- 20.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods in Enzymology. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 21.McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, et al. Phaser crystallographic software. J Appl Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiu X, Padmanabhan KP, Carperos VE, Tulinsky A, Kline T, et al. Structure of the hirulog 3-thrombin complex and nature of the S′ subsites of substrates and inhibitors. Biochemistry. 1992;31:11689–11697. doi: 10.1021/bi00162a004. [DOI] [PubMed] [Google Scholar]
- 23.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 24.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 25.Winn MD, Isupov MN, Murshudov GN. Use of TLS parameters to model anisotropic displacements in macromolecular refinement. Acta Crystallogr D Biol Crystallogr. 2001;57:122–133. doi: 10.1107/s0907444900014736. [DOI] [PubMed] [Google Scholar]
- 26.Laskowsi RA, MacArthur MW, Moss DS, Thornton JM. PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr. 1993;26:283–291. [Google Scholar]
- 27.Davis IW, Leaver-Fay A, Chen VB, Block JN, Kapral GJ, et al. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 2007;35:W375–W383. doi: 10.1093/nar/gkm216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol Biol. 2007;372:774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 29.Bode W, Mayr I, Baumann U, Huber R, Stone SR, et al. The refined 1.9 A crystal structure of human alpha-thrombin: interaction with D-Phe-Pro-Arg chloromethylketone and significance of the Tyr-Pro-Pro-Trp insertion segment. EMBO J. 1989;8:3467–3475. doi: 10.1002/j.1460-2075.1989.tb08511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jagadeeswaran P, Paris R, Rao P. Laser-induced thrombosis in zebrafish larvae: a novel genetic screening method for thrombosis. Methods Mol Med. 2006;129:187–195. doi: 10.1385/1-59745-213-0:187. [DOI] [PubMed] [Google Scholar]
- 31.Liu CC, Brustad E, Liu W, Schultz PG. Crystal structure of a biosynthetic sulfo-hirudin complexed to thrombin. J Am Chem Soc. 2007;129:10648–10649. doi: 10.1021/ja0735002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rydel TJ, Ravichandran KG, Tulinsky A, Bode W, Huber R, et al. The structure of a complex of recombinant hirudin and human alpha-thrombin. Science. 1990;249:277–280. doi: 10.1126/science.2374926. [DOI] [PubMed] [Google Scholar]
- 33.Page MJ, Macgillivray RT, Di Cera E. Determinants of specificity in coagulation proteases. J Thromb Haemost. 2005;3:2401–2408. doi: 10.1111/j.1538-7836.2005.01456.x. [DOI] [PubMed] [Google Scholar]
- 34.Perona JJ, Craik CS. Structural basis of substrate specificity in the serine proteases. Protein Sci. 1995;4:337–360. doi: 10.1002/pro.5560040301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vindigni A, Dang QD, Di Cera E. Site-specific dissection of substrate recognition by thrombin. Nat Biotechnol. 1997;15:891–895. doi: 10.1038/nbt0997-891. [DOI] [PubMed] [Google Scholar]
- 36.Stone SR, Hofsteenge J. Kinetics of the inhibition of thrombin by hirudin. Biochemistry. 1986;25:4622–4628. doi: 10.1021/bi00364a025. [DOI] [PubMed] [Google Scholar]
- 37.Dodt J, Kohler S, Baici A. Interaction of site specific hirudin variants with alpha-thrombin. FEBS Lett. 1988;229:87–90. doi: 10.1016/0014-5793(88)80803-2. [DOI] [PubMed] [Google Scholar]
- 38.Braun PJ, Dennis S, Hofsteenge J, Stone SR. Use of site-directed mutagenesis to investigate the basis for the specificity of hirudin. Biochemistry. 1988;27:6517–6522. doi: 10.1021/bi00417a048. [DOI] [PubMed] [Google Scholar]
- 39.Stone SR, Tapparelli C. Thrombin inhibitors as antithrombotic agents: the importance of rapid inhibition. J Enzyme Inhib. 1995;9:3–15. doi: 10.3109/14756369509040677. [DOI] [PubMed] [Google Scholar]
- 40.Fethiere J, Tsuda Y, Coulombe R, Konishi Y, Cygler M. Crystal structure of two new bifunctional nonsubstrate type thrombin inhibitors complexed with human alpha-thrombin. Protein Sci. 1996;5:1174–1183. doi: 10.1002/pro.5560050620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laskowski M, Jr, Kato I. Protein inhibitors of proteinases. Annu Rev Biochem. 1980;49:593–626. doi: 10.1146/annurev.bi.49.070180.003113. [DOI] [PubMed] [Google Scholar]
- 42.Slon-Usakiewicz JJ, Purisima E, Tsuda Y, Sulea T, Pedyczak A, et al. Nonpolar interactions of thrombin S′ subsites with its bivalent inhibitor: methyl scan of the inhibitor linker. Biochemistry. 1997;36:13494–13502. doi: 10.1021/bi970857h. [DOI] [PubMed] [Google Scholar]
- 43.van de Locht A, Lamba D, Bauer M, Huber R, et al. Two heads are better than one: crystal structure of the insect derived double domain Kazal inhibitor rhodniin in complex with thrombin. EMBO J. 1995;14:5149–5157. doi: 10.1002/j.1460-2075.1995.tb00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van de Locht A, Stubbs MT, Bode W, Friedrich T, Bollschweiler C, et al. The ornithodorin-thrombin crystal structure, a key to the TAP enigma? EMBO J. 1996;15:6011–6017. [PMC free article] [PubMed] [Google Scholar]
- 45.Macedo-Ribeiro S, Almeida C, Calisto BM, Friedrich T, Mentele R, et al. Isolation, cloning and structural characterisation of boophilin, a multifunctional Kunitz-type proteinase inhibitor from the cattle tick. PLoS ONE. 2008;3:e1624. doi: 10.1371/journal.pone.0001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bah A, Chen Z, Bush-Pelc LA, Mathews FS, Di Cera E. Crystal structures of murine thrombin in complex with the extracellular fragments of murine protease-activated receptors PAR3 and PAR4. Proc Natl Acad Sci U S A. 2007;104:11603–11608. doi: 10.1073/pnas.0704409104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gandhi PS, Chen Z, Mathews FS, Di Cera E. Structural identification of the pathway of long-range communication in an allosteric enzyme. Proc Natl Acad Sci U S A. 2008;105:1832–1837. doi: 10.1073/pnas.0710894105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin PD, Robertson W, Turk D, Huber R, Bode W, et al. The structure of residues 7–16 of the A alpha-chain of human fibrinogen bound to bovine thrombin at 2.3-A resolution. J Biol Chem. 1992;267:7911–7920. [PubMed] [Google Scholar]
- 49.Martin PD, Malkowski MG, DiMaio J, Konishi Y, Ni F, et al. Bovine thrombin complexed with an uncleavable analog of residues 7–19 of fibrinogen A alpha: geometry of the catalytic triad and interactions of the P1′, P2′, and P3′ substrate residues. Biochemistry. 1996;35:13030–13039. doi: 10.1021/bi960656y. [DOI] [PubMed] [Google Scholar]
- 50.Stubbs MT, Oschkinat H, Mayr I, Huber R, Angliker H, et al. The interaction of thrombin with fibrinogen. A structural basis for its specificity. Eur J Biochem. 1992;206:187–195. doi: 10.1111/j.1432-1033.1992.tb16916.x. [DOI] [PubMed] [Google Scholar]
- 51.Gandhi PS, Chen Z, Di Cera E. Crystal structure of thrombin bound to the uncleaved extracellular fragment of PAR1. J Biol Chem. 2010;285:15393–15398. doi: 10.1074/jbc.M110.115337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Witting JI, Bourdon P, Brezniak DV, Maraganore JM, Fenton JW. Thrombin-specific inhibition by and slow cleavage of hirulog-1. Biochem J. 1992;283(Pt 3):737–743. doi: 10.1042/bj2830737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matthews JH, Krishnan R, Costanzo MJ, Maryanoff BE, Tulinsky A. Crystal structures of thrombin with thiazole-containing inhibitors: probes of the S1′ binding site. Biophys J. 1996;71:2830–2839. doi: 10.1016/S0006-3495(96)79479-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slon-Usakiewicz JJ, Sivaraman J, Li Y, Cygler M, Konishi Y. Design of P1′ and P3′ residues of trivalent thrombin inhibitors and their crystal structures. Biochemistry. 2000;39:2384–2391. [PubMed] [Google Scholar]
- 55.Maraganore JM, Bourdon P, Jablonski J, Ramachandran KL, Fenton JW. Design and characterization of hirulogs: a novel class of bivalent peptide inhibitors of thrombin. Biochemistry. 1990;29:7095–7101. doi: 10.1021/bi00482a021. [DOI] [PubMed] [Google Scholar]
- 56.Fareed J, Jeske WP. Small-molecule direct antithrombins: argatroban. Best Pract Res Clin Haematol. 2004;17:127–138. doi: 10.1016/j.beha.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 57.Wienen W, Stassen JM, Priepke H, Ries UJ, Hauel N. In-vitro profile and ex-vivo anticoagulant activity of the direct thrombin inhibitor dabigatran and its orally active prodrug, dabigatran etexilate. Thromb Haemost. 2007;98:155–162. [PubMed] [Google Scholar]
- 58.White CM. Thrombin-directed inhibitors: pharmacology and clinical use. Am Heart J. 2005;149:S54–S60. doi: 10.1016/j.ahj.2004.10.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Conformation of s-variegin C-terminus. s-Variegin C-terminus (pink) has a vastly different conformation compared to hirulog-1, hirulog-3, hirugen and sulfo-hirudin: (A) Residues PEEYL in hirulog-1 (red) are disordered and missing from the structure. (B) Residues PEEYL in hirulog-3 (blue) form a 310 helix turn. (C) These residues in hirugen (green), with sulfated tyrosine, also form a 310 helix turn. (D) Other than Tyr-sulfation, sulfo-hirudin (cyan) C-terminus has an extra Gln, forms a full α-helical turn.
(TIF)
Electrostatic interactions in thrombin:s-variegin structure. (A) Figure shows the electron density map (2Fo-Fc, 0.9σ) of residues described in Figure 4A in the main manuscript. Thrombin is colored yellow and s-variegin is colored pink. Map for thrombin colored in light cyan and map for s-variegin colored in gray. Residues involved in forming salt bridges are labeled. (B) Figure shows the electron density map (2Fo-Fc, 0.9σ) of residues described in Figure 4B in the main manuscript. Thrombin is colored yellow and s-variegin is colored pink. Map for thrombin colored in light cyan and map for s-variegin colored in gray.
(TIF)
Variegin variant EP25 (slow binding, competitive inhibitor). (A) Dose response curves of thrombin (1.65 nM) inhibited by EP25 (0.1 nM, 0.3 nM, 1 nM, 3 nM, 10 nM, 30 nM, 100 nM, 300 nM, 1000 nM, 3000 nM) in S2238 (100 µM) showed a left shift with increased pre-incubation time due to slow binding. IC50 are 173±26 nM without pre-incubation (▪ solid line) and 13.1±0.7 nM with 20 min pre-incubation (○ dotted line) (n = 3, error bars represent S.D.). (B) Progress curves (not shown) of thrombin (0.8 nM) inhibited by EP25 (9.4 nM, 12.5 nM, 18.8 nM, 25 nM, 37.5 nM, 50 nM, 75 nM and 100 nM) in S2238 (100 µM) were fitted to equation (6) describing a slow binding inhibitor to obtain a k for each concentrations of EP25. Plot of k against EP25 concentrations (▪ solid line) is hyperbolic and fitted to equation (7) producing Ki′ of 0.882±0.128 nM, representing the dissociation constant of initial collision complex EI (scheme 1). Ki calculated from equation (8) is 0.365±0.109 nM (n = 3, error bars represent S.D.).
(TIF)
Variegin variant EP21 (slow binding, competitive inhibitor). (A) Dose response curves of thrombin (1.65 nM) inhibited by EP21 (0.3 nM, 1 nM, 3 nM, 10 nM, 30 nM, 100 nM, 300 nM, 1000 nM, 3000 nM and 10000 nM) in S2238 (100 µM) showed a left shift with increased pre-incubation time due to slow binding. IC50 are 177±7 nM without pre-incubation (▪ solid line) and 16.2±2.9 nM with 20 min pre-incubation (○ dotted line) (n = 3, error bars represent S.D.). (B) Progress curves (Figure S4) of thrombin (0.8 nM) inhibited by EP21 (18.8 nM, 25 nM, 37.5 nM, 50 nM, 75 nM, 100 nM and 150 nM) in S2238 (100 µM) were fitted to equation (6) describing a slow binding inhibitor to obtain a k for each concentrations of EP21. Plot of k against EP21 concentrations (▪ solid line) is hyperbolic and fitted to equation (7) producing Ki′ of 1.66±0.36 nM, representing the dissociation constant of initial collision complex EI (scheme 1). Ki calculated from equation (8) is 0.315±0.024 nM (n = 3, error bars represent S.D.).
(TIF)
Variegin variant MH18 (fast, tight-binding, noncompetitive inhibitor). (A) Dose response curves of thrombin inhibition (1.65 nM) by MH18 (0.1 nM, 0.3 nM, 1 nM, 3 nM, 10 nM, 30 nM, 100 nM, 300 nM, 1000 nM, 3000 nM and 10000 nM) in S2238 (100 µM) are independent of pre-incubation time. IC50 are 10.9±1.2 nM without pre-incubation (▪ solid line) and 11.7±1.9 nM with 20 min pre-incubation (○ dotted line) (n = 3, error bars represent S.D.). (B) Thrombin (1.65 nM) inhibition was tested with MH18 (0.39 nM, 0.78 nM, 1.56 nM, 3.13 nM, 6.25 nM, 12.5 nM, 25 nM, 50 nM, 100 nM and 200 nM) in S2238 (100 µM) (▪ solid line). Apparent inhibition constant Ki′ obtained by fitting data to equation (2), describing fast and tight-binding inhibitor, is 14.9±3.5 nM. Ki calculated from equations (4) and (5), describing noncompetitive inhibitors, is 14.9±3.5 nM (n = 3, error bars represent S.D.).
(TIF)
Progress curves of thrombin inhibitied by EP21 and DV24. (A) Progress curves of thrombin (0.8 nM) inhibited by different concentrations of EP21 using S2238 (100 µM) as substrate, without pre-incubation of thrombin and EP21. The non-linear behavior of the curves at the beginning of the reactions and an improved IC50 with pre-incubation (Figure S4) suggested equilibrium of inhibition was achieved slowly, characteristic of slow-binding inhibitors. (B) Progress curves of thrombin (1.65 nM) inhibited by different concentrations of DV24: 0 nM (▪), 0.1 nM (□), 0.3 nM (•), 1 nM (○), 3 nM (▴), 10 nM (▵), 30 nM (▾), 100 nM (▿), 300 nM (⧫) and 1000 nM (⋄) using S2238 (100 µM) as substrate, without pre-incubation of thrombin and DV24. The linear curves indicate the equilibrium of inhibition was achieved upon mixing of thrombin and DV24, characteristic of fast-binding inhibitors.
(TIF)
Variegin variant DV24 (fast, tight-binding, competitive inhibitor). (A) Dose-response curves of thrombin (1.65 nM) inhibited by DV24 (0.1 nM, 0.3 nM, 1 nM, 3 nM, 10 nM, 30 nM, 100 nM, 300 nM, 1000 nM and 3000 nM) in S2238 (100 µM) showed a right shift with increased pre-incubation time due to cleavage. IC50 are 7.49±0.28 nM without pre-incubation (▪ solid line) and 10.1±0.6 nM with 20 min pre-incubation (○ dotted line) (n = 3, error bars represent S.D.). (B) Thrombin (1.65 nM) inhibition was tested with DV24 (0.39 nM, 0.78 nM, 1.56 nM, 3.13 nM, 6.25 nM, 12.5 nM, 25 nM, 50 nM, 100 nM and 200 nM) in S2238 (100 µM) (▪ solid line). Apparent inhibition constant Ki′ obtained by fitting data to equation (2), describing fast and tight-binding inhibitor, is 9.74±0.91 nM. Ki calculated from equation (3), describing competitive inhibitors, is 0.306±0.029 nM (n = 3, error bars represent S.D.).
(TIF)
Variegin variant DV24 H12A (fast, tight-binding, competitive inhibitor). (A) Dose-response curves of thrombin (1.65 nM) inhibited by DV24H12A (0.001 µM, 0.003 µM, 0.01 µM, 0.03 µM, 0.3 µM, 1 µM, 3 µM, 10 µM and 30 µM) in S2238 (100 µM) showed a right shift with increased pre-incubation time due to cleavage. IC50 are 48.2±12.4 nM without pre-incubation (▪ solid line) and 141±11 nM with 20 min pre-incubation (○ dotted line) (n = 3, error bars represent S.D.). (B) Thrombin (1.65 nM) inhibition was tested with DV24H12A (1.95 nM, 3.91 nM, 7.81 nM, 15.6 nM, 31.3 nM, 62.5 nM, 125 nM, 250 nM, 500 nM and 1000 nM) in S2238 (100 µM) (▪ solid line). Apparent inhibition constant Ki′ obtained by fitting data to equation (2), describing fast and tight-binding inhibitor, is 103±15 nM. Ki calculated from equation (3), describing competitive inhibitors, is 3.23±0.48 nM (n = 3, error bars represent S.D.).
(TIF)
Variegin variant MH18 H12A (fast, noncompetitive inhibitor). (A) Dose-response curves of thrombin (1.65 nM) inhibition by MH18H12A (0.001 µM, 0.003 µM, 0.01 µM, 0.03 µM, 0.3 µM, 1 µM, 3 µM, 10 µM and 30 µM) in S2238 (100 µM) are independent of pre-incubation time. IC50 are 328±23 nM without pre-incubation (▪ solid line) and 343±46 nM with 20 min pre-incubation (n = 3, error bars represent S.D.). (B) Thrombin (1.65 nM) inhibition was tested with 1 µM MH18H12A (○ dotted line) in S2238 (4.69 µM, 9.34 µM, 18.8 µM, 37.5 µM, 75 µM, 150 µM and 300 µM) and without the inhibitor (▪ solid line) in S2238 (3.13 µM, 6.25 µM, 12.5 µM, 25 µM, 50 µM, 100 µM, 200 µM). MH18H12A is unable to inhibit thrombin at equimolar concentration, hence is not considered as tight-binding inhibitor. The double-reciprocal plot showed noncompetitive inhibition and Ki is 329±8 nM (n = 3, error bars represent S.D.).
(TIF)
Variegin variant DV24 K10R (fast, tight-binding, competitive inhibitor). (A) Dose-response curves of thrombin (1.65 nM) inhibited by DV24K10R (0.1 nM, 0.3 nM, 1 nM, 3 nM, 10 nM, 30 nM, 100 nM, 300 nM, 1000 nM and 3000 nM) in S2238 (100 µM) showed a right shift with increased pre-incubation time due to cleavage. IC50 are 6.98±0.76 nM without pre-incubation (▪ solid line) and 12.0±0.4 nM with 20 min pre-incubation (○ dotted line) (n = 3, error bars represent S.D.). (B) Thrombin (1.65 nM) inhibition was tested with DV24K10R (0.39 nM, 0.78 nM, 1.56 nM, 3.13 nM, 6.25 nM, 12.5 nM, 25 nM, 50 nM, 100 nM and 200 nM) in S2238 (100 µM) (▪ solid line). Apparent inhibition constant Ki′ obtained by fitting data to equation (2), describing fast and tight-binding inhibitor, is 8.27±0.85 nM. Ki calculated from equation (3), describing competitive inhibitors, is 0.259±0.015 nM (n = 3, error bars represent S.D.).
(TIF)
Variegin variant DV23 (fast, tight-binding, competitive inhibitor). (A) Dose-response curves of thrombin (1.65 nM) inhibited by DV23 (0.1 nM, 0.3 nM, 1 nM, 3 nM, 10 nM, 30 nM, 100 nM, 300 nM, 1000 nM and 3000 nM) in S2238 (100 µM) showed a right shift with increased pre-incubation time due to cleavage. IC50 are 45.4±1.6 nM without pre-incubation (▪ solid line) and 77.8±6.1 nM with 20 min pre-incubation (○ dotted line) (n = 3, error bars represent S.D.). (B) Thrombin (1.65 nM) inhibition was tested with DV23 (3.91 nM, 7.81 nM, 15.6 nM, 31.3 nM, 62.5 nM, 125 nM, 250 nM and 500 nM) S2238 (100 µM) (▪ solid line). Apparent inhibition constant Ki′ obtained by fitting data to equation (2), describing fast and tight-binding inhibitor, is 69.6±7.8 nM. Ki calculated from equation (3), describing competitive inhibitors, is 2.19±0.23 nM (n = 3, error bars represent S.D.).
(TIF)
Variegin variant DV23 K10R (fast, tight-binding, competitive inhibitor). (A) Dose-response curves of thrombin (1.65 nM) inhibited by DV23K10R (0.1 nM, 0.3 nM, 1 nM, 3 nM, 10 nM, 30 nM, 100 nM, 300 nM, 1000 nM and 3000 nM) in S2238 (100 µM) showed a strong right shift with increased pre-incubation time due to cleavage. IC50 are 12.9±1.0 nM without pre-incubation (▪ solid line) and 102±1 nM with 20 min pre-incubation (○ dotted line) (n = 3, error bars represent S.D.). (B) Thrombin (1.65 nM) inhibition was tested with DV23K10R (3.91 nM, 7.81 nM, 15.6 nM, 31.3 nM, 62.5 nM, 125 nM, 250 nM and 500 nM) in S2238 (100 µM) (▪ solid line). Apparent inhibition constant Ki′ obtained by fitting data to equation (2), describing fast and tight-binding inhibitor, is 19.1±1.9 nM. Ki calculated from equation (3), describing competitive inhibitors, is 0.600±0.010 nM (n = 3, error bar represents S.D.).
(TIF)
Variegin variant EP25 A22E (slow binding, competitive inhibitor). (A) Dose-response curves of thrombin (1.65 nM) inhibited by EP25A22E (0.1 nM, 0.3 nM, 1 nM, 3 nM, 10 nM, 30 nM, 100 nM, 300 nM, 1000 nM and 3000 nM) in S2238 (100 µM) showed a left shift due to slow binding. IC50 are 124±23 nM without pre-incubation (▪ solid line) and 13.5±2.1 nM with 20 min pre-incubation (○ dotted line) (n = 3, error bars represent S.D.). (B) Progress curves (not shown) of thrombin (0.8 nM) inhibited by EP25A22E (9.38 nM, 12.5 nM, 18.8 nM, 25 nM, 37.5 nM, 50 nM, 75 nM, 100 nM, 150 nM, 200 nM and 300 nM) in S2238 (100 µM) were fitted to equation (6) describing a slow binding inhibitor to obtain a k for each concentrations of EP25A22E. Plot of k against EP25A22E concentrations (▪ solid line) is hyperbolic and was fitted to equation (7) producing Ki′ of 1.02±0.060 nM, representing the dissociation constant of initial collision complex EI (scheme 1). Ki calculated from equation (8) is 0.311±0.070 nM (n = 3, error bars represent S.D.).
(TIF)
Variegin variant MH22 A22E (fast, tight-binding, noncompetitive inhibitor). (A) Dose-response curves of thrombin (1.65 nM) inhibited by MH22A22E (0.1 nM, 0.3 nM, 1 nM, 3 nM, 10 nM, 30 nM, 100 nM, 300 nM, 1000 nM and 3000 nM) in S2238 (100 µM) are independent of pre-incubation time. IC50 are 13.62±0.45 nM without pre-incubation (▪ solid line) and 15.6±0.4 nM with 20 min pre-incubation (○ dotted line) (n = 3, error bars represent S.D.). (B) Thrombin (1.65 nM) inhibition was tested with MH22A22E (0.39 nM, 0.78 nM, 1.56 nM, 3.13 nM, 6.25 nM, 12.5 nM, 25 nM, 50 nM, 100 nM and 200 nM) in S2238 (100 µM) (▪ solid line). Apparent inhibition constant Ki′ obtained by fitting data to equation (2), describing fast and tight-binding inhibitor, is 15.1±1.0 nM. Ki calculated from equations (4) and (5), describing noncompetitive inhibitors, is 15.1±1.0 nM (n = 3, error bars represent S.D.).
(TIF)
Variegin variant DV24 Yphos (fast, tight-binding, competitive inhibitor). (A) Dose-response curves of thrombin (1.65 nM) inhibited by DV24Yphos (0.03 nM, 0.1 nM, 0.3 nM, 1 nM, 3 nM, 10 nM, 30 nM, 100 nM, 300 nM and 1000 nM) in S2238 (100 µM) showed a right shift with increased pre-incubation time due to cleavage. IC50 are 8.67±0.45 nM without pre-incubation (▪ solid line) and 12.4±1.2 nM with 20 min pre-incubation (○ dotted line) (n = 3, error bars represent S.D.). (B) Thrombin (1.65 nM) inhibition was tested with DV24Yphos (0.39 nM, 0.78 nM, 1.56 nM, 3.13 nM, 6.25 nM, 12.5 nM, 25 nM, 50 nM, 100 nM and 200 nM) in S2238 (100 µM) (▪ solid line). Apparent inhibition constant Ki′ obtained by fitting data to equation (2), describing fast and tight-binding inhibitors, is 10.4±1.0 nM. Ki calculated from equation (3), describing competitive inhibitors, the inhibition constant is 0.327±0.032 nM (n = 3, error bars represent S.D.).
(TIF)
Variegin variant DV24 K10RYphos (fast, tight-binding, competitive inhibitor). (A) Dose-response curves of thrombin (1.65 nM) inhibited by DV24K10RYphos (0.03 nM, 0.1 nM, 0.3 nM, 1 nM, 3 nM, 10 nM, 30 nM, 100 nM, 300 nM and 1000 nM) in S2238 (100 µM) showed a right shift with increased pre-incubation time due to cleavage. IC50 are 4.64±0.78 nM without pre-incubation (▪ solid line) and 7.80±1.80 nM with 20 min pre-incubation (○ dotted line) (n = 3, error bars represent S.D.). (B) Thrombin (1.65 nM) inhibition was tested with DV24K10RYphos (0.39 nM, 0.78 nM, 1.56 nM, 3.13 nM, 6.25 nM, 12.5 nM, 25 nM, 50 nM, 100 nM and 200 nM) in S2238 (100 µM) (▪ solid line). Apparent inhibition constant Ki′ obtained by fitting data to equation (2), describing fast and tight-binding inhibitors, is 4.78±0.57 nM. Ki calculated from equation (3), describing competitive inhibitors, is 0.150±0.018 nM (n = 3, error bars represent S.D.).
(TIF)
Variegin variant DV24 Ysulf (fast, tight-binding, competitive inhibitor). (A) Dose-response curves of thrombin (1.65 nM) inhibited by DV24Ysulf (0.05 nM, 0.15 nM, 0.45 nM, 1.5 nM, 4.5 nM, 15 nM, 45 nM, 150 nM, 450 nM and 1500 nM) in S2238 (100 µM) showed a right shift with increased pre-incubation time due to cleavage. IC50 are 1.66±0.18 nM without pre-incubation (▪ solid line) and 2.02±0.29 nM with 20 min pre-incubation (○ dotted line) (n = 3, error bars represent S.D.). (B) Thrombin (1.65 nM) inhibition was tested with DV24Ysulf (0.20 nM, 0.39 nM, 0.78 nM, 1.56 nM, 3.13 nM, 6.25 nM, 12.5 nM, 25 nM, 50 nM and 100 nM) in S2238 (100 µM) (▪ solid line). Apparent inhibition constant Ki′ obtained by fitting data to equation (2), describing fast and tight-binding inhibitor, is 1.78±0.47 nM. Ki calculated from equation (3), describing competitive inhibitors, is 0.056±0.015 nM (n = 3, error bars represent S.D.).
(TIF)
Variegin variant DV24 K10RYsulf (fast, tight-binding, competitive inhibitor). (A) Dose-response curves of thrombin (1.65 nM) inhibited by DV24K10RYsulf (0.05 nM, 0.15 nM, 0.45 nM, 1.5 nM, 4.5 nM, 15 nM, 45 nM, 150 nM, 450 nM and 1500 nM) in S2238 (100 µM) showed a right shift with increased pre-incubation time due to cleavage. IC50 are 1.39±0.17 nM without pre-incubation (▪ solid line) and 1.66±0.21 nM with 20 min pre-incubation (○ dotted line) (n = 3, error bars represent S.D.). (B) Thrombin (1.65 nM) inhibition was tested with DV24K10RYsulf (0.20 nM, 0.39 nM, 0.78 nM, 1.56 nM, 3.13 nM, 6.25 nM, 12.5 nM, 25 nM, 50 nM and 100 nM) in S2238 (100 µM) (▪ solid line). Apparent inhibition constant Ki′ obtained by fitting data to equation (2), describing fast and tight-binding inhibitors, is 1.33±0.19 nM. Ki calculated from equation (3), describing competitive inhibitors, is 0.0420±0.0061 nM (n = 3, error bars represent S.D.).
(TIF)
Variegin variant MH18 Ysulf (fast, tight-binding, noncompetitive inhibitor). (A) Dose-response curves of thrombin (1.65 nM) inhibited by MH18Ysulf (0.03 nM, 0.1 nM, 0.3 nM, 1 nM, 3 nM, 10 nM, 30 nM, 100 nM, 300 nM and 1000 nM) in S2238 (100 µM) are independent of pre-incubation time. IC50 are 1.26±0.18 nM without pre-incubation (▪ solid line) and 1.17±0.14 nM with 20 min pre-incubation (○ dotted line) (n = 3, error bar represents S.D.). (B) Thrombin (1.65 nM) inhibition was tested with MH18Ysulf (0.20 nM, 0.39 nM, 0.78 nM, 1.56 nM, 3.13 nM, 6.25 nM, 12.5 nM, 25 nM, 50 nM and 100 nM) in S2238 (100 µM) (▪ solid line). Apparent inhibition constant Ki′ obtained by fitting data to equation (2), describing fast and tight-binding inhibitors, is 1.25±0.18 nM. Ki calculated from equations (4) and (5), describing noncompetitive inhibitors, is 1.25±0.18 nM (n = 3, error bar represents S.D.).
(TIF)
Noncompetitive inhibition of thrombin by MH22. s-Variegin binds to both the non-prime and prime subsites of thrombin active site and is cleaved between Lys-Met. After cleavage, the fragment C-terminal to the scissile bond (MH22) noncompetitively inhibits thrombin. The chromogenic substrate S2238 binds mainly to the non-prime subsites and is cleaved between Arg and para-nitroaniline (pNA). The overlaps between s-variegin and S2238 binding sites resulted in the observed competitive inhibition. In contrast, the noncompetitive inhibition observed for MH22 showed the lack of overlaps between MH22 and S2238 even in the S1′ subsite (red box). Indeed, no density was observed for P1′ Met in the present structure, most likely reflects the lack of contact with thrombin and hence leaves a free S1′ site for the binding of pNA moiety when MH22 is bound to thrombin.
(TIF)
A list of possible direct hydrogen bonds between s-variegin and thrombin calculated based on the online server PISA [28].
(TIF)
A detailed account for the selection and use of equations to fit the data of thrombin inhibitions is available in Materials and Methods S1.
(DOC)