Abstract
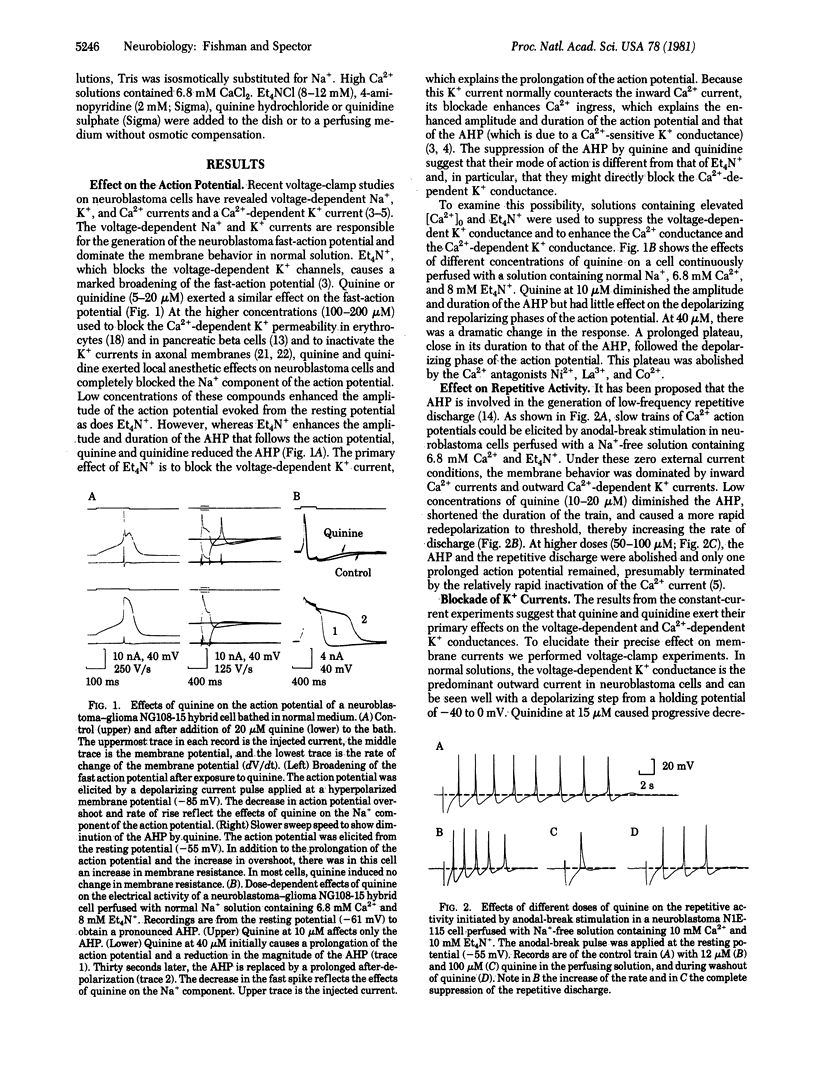
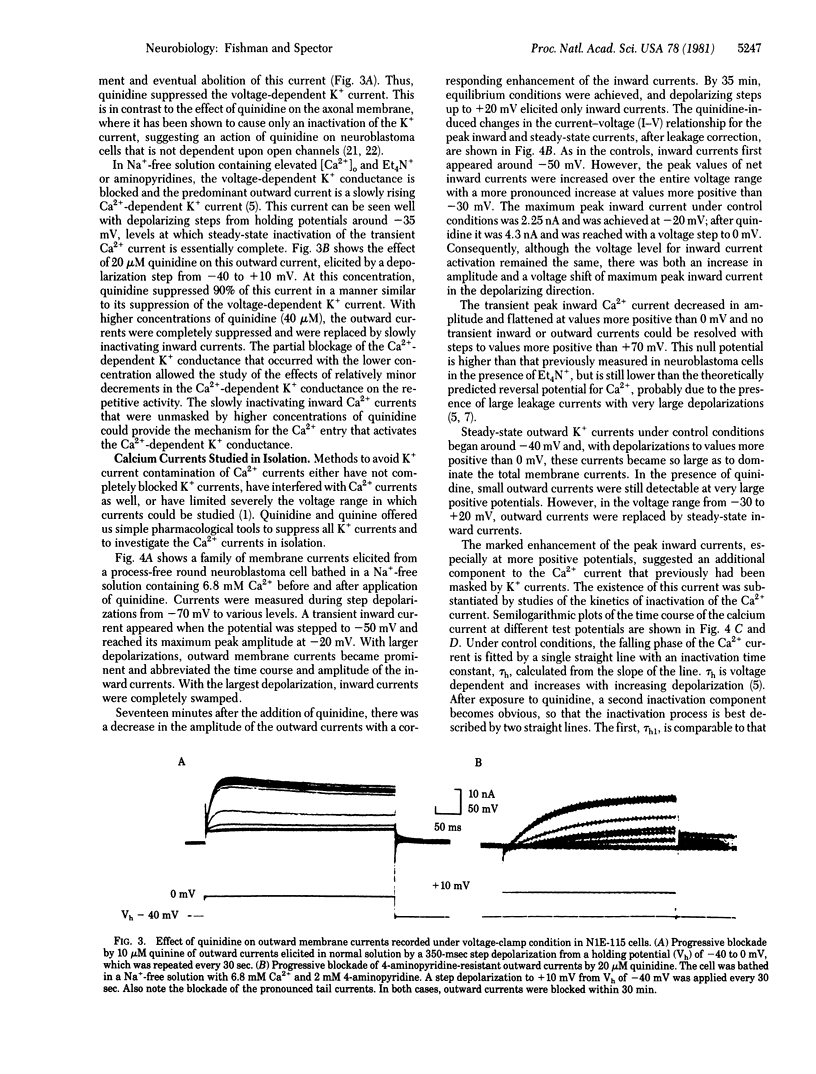
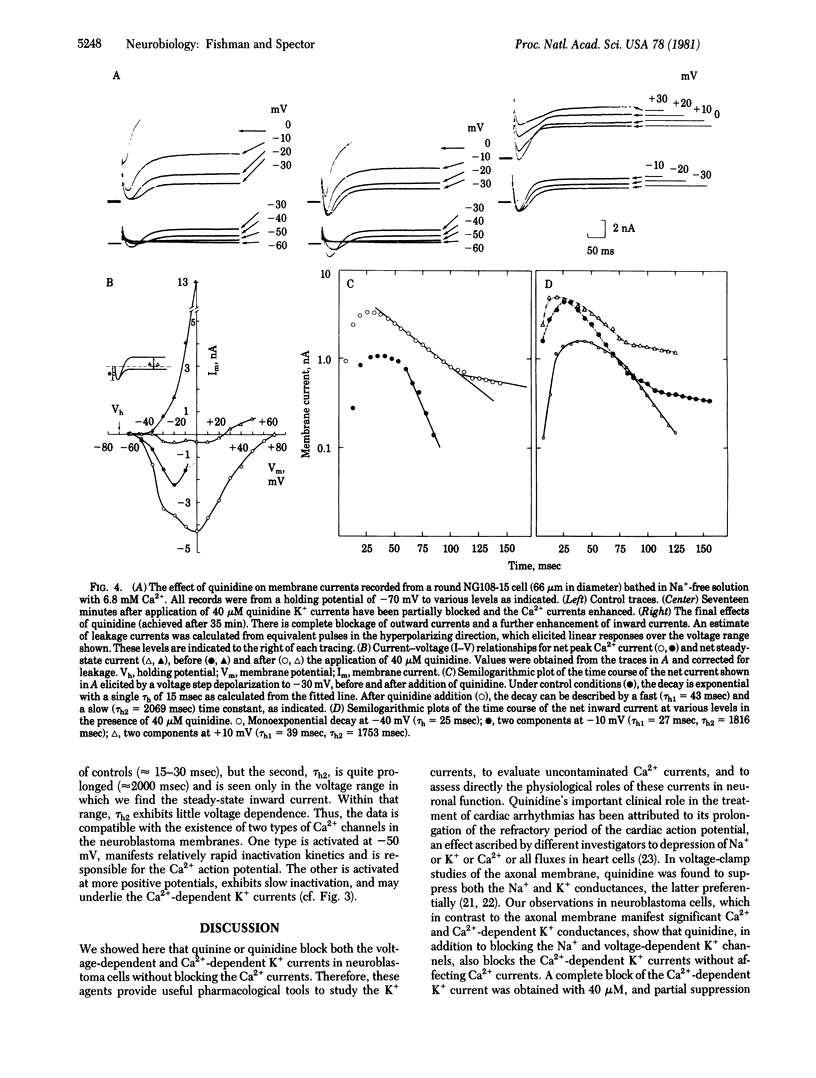
Quinine and quinidine have been evaluated with regard to their effects on the electrical activity of neuroblastoma cells. Under voltage-clamp conditions, we have found that quinine and quinidine block both the voltage-dependent and Ca2+-dependent K+ conductances. Blockage of the voltage-dependent K+ channel is manifest as an increase in the amplitude and in the duration of the action potential. Blockage of the Ca2+-dependent K+ channel in Na+-free (replaced by Tris) solutions containing 6.8 mM Ca2+ and tetraethylammonium ion or 4-aminopyridine (to block the voltage-dependent K+ current) is seen as a further prolongation of the Ca2+ action potential and diminution of the after-hyperpolarization. A critical role of the Ca2+-dependent K+ conductance in modulation of the rate and duration of trains of Ca2+ action potentials is shown by the use of low concentrations (5-40 microM) of quinine or quinidine, which diminish the Ca2+-dependent K+ conductance in a graded manner. After complete blockade of K+ currents, the peak Ca2+ currents are enhanced at all voltages, especially at values more positive than -30 mV, where a steady-state inward current appears as well. In this same voltage range, the decay of the Ca2+ current exhibits two time constants--that of the transient inward current, which is about 20 msec, and a much slower (approximately 2000 msec) component. It is suggested that neuroblastoma cells have two types of calcium channels--one which generates the Ca2+ action potential and a second, distinguished by activation at more depolarized levels and by a slow rate of inactivation, which underlies the calcium entry necessary to activate the Ca2+-dependent K+ conductance.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Smith S. J., Thompson S. H. Ionic currents in molluscan soma. Annu Rev Neurosci. 1980;3:141–167. doi: 10.1146/annurev.ne.03.030180.001041. [DOI] [PubMed] [Google Scholar]
- Akaike N., Lee K. S., Brown A. M. The calcium current of Helix neuron. J Gen Physiol. 1978 May;71(5):509–531. doi: 10.1085/jgp.71.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwater I., Dawson C. M., Ribalet B., Rojas E. Potassium permeability activated by intracellular calcium ion concentration in the pancreatic beta-cell. J Physiol. 1979 Mar;288:575–588. [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Barret J. N. Separation of two voltage-sensitive potassium currents, and demonstration of a tetrodotoxin-resistant calcium current in frog motoneurones. J Physiol. 1976 Mar;255(3):737–774. doi: 10.1113/jphysiol.1976.sp011306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A. Calcium current in molluscan neurones: measurement under conditions which maximize its visibility. J Physiol. 1979 Jan;286:41–60. doi: 10.1113/jphysiol.1979.sp012606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Lux H. D. A voltage-sensitive persistent calcium conductance in neuronal somata of Helix. J Physiol. 1976 Jan;254(1):129–151. doi: 10.1113/jphysiol.1976.sp011225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Thomas M. V. Intracellular calcium accumulation during depolarization in a molluscan neurone. J Physiol. 1980 Nov;308:259–285. doi: 10.1113/jphysiol.1980.sp013471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Fukuda J., Eaton D. C. Membrane currents carried by Ca, Sr, and Ba in barnacle muscle fiber during voltage clamp. J Gen Physiol. 1974 May;63(5):564–578. doi: 10.1085/jgp.63.5.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hencek M., Zachar J. Calcium currents and conductances in the msucle membrane of the crayfish. J Physiol. 1977 Jun;268(1):51–71. doi: 10.1113/jphysiol.1977.sp011846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Rojas E., Taylor R. E., Vergara J. Calcium and potassium systems of a giant barnacle muscle fibre under membrane potential control. J Physiol. 1973 Mar;229(2):409–455. doi: 10.1113/jphysiol.1973.sp010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M., Kandel E. R. Mechanism of calcium current modulation underlying presynaptic facilitation and behavioral sensitization in Aplysia. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6912–6916. doi: 10.1073/pnas.77.11.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A., Shakhovalov Y. A. Separation of sodium and calcium currents in the somatic membrane of mollusc neurones. J Physiol. 1977 Sep;270(3):545–568. doi: 10.1113/jphysiol.1977.sp011968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W. Calcium-dependent potassium activation in nervous tissues. Annu Rev Biophys Bioeng. 1978;7:1–18. doi: 10.1146/annurev.bb.07.060178.000245. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Spector I. Ionic currents in cultured mouse neuroblastoma cells under voltage-clamp conditions. J Physiol. 1978 May;278:265–286. doi: 10.1113/jphysiol.1978.sp012303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar W. H., Spector I. The calcium action potential and a prolonged calcium dependent after-hyperpolarization in mouse neuroblastoma cells. J Physiol. 1979 Jul;292:297–306. doi: 10.1113/jphysiol.1979.sp012851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar W. H., Spector I. The calcium current and the activation of a slow potassium conductance in voltage-clamped mouse neuroblastoma cells. J Physiol. 1979 Jul;292:307–323. doi: 10.1113/jphysiol.1979.sp012852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C., Lecar H. Voltage oscillations in the barnacle giant muscle fiber. Biophys J. 1981 Jul;35(1):193–213. doi: 10.1016/S0006-3495(81)84782-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrath H. Action potential, membrane currents and force of contraction in mammalian heart muscle fibers treated with quinidine. J Pharmacol Exp Ther. 1981 Jan;216(1):176–182. [PubMed] [Google Scholar]
- Nelson P. G., Henkart M. P. Oscillatory membrane potential changes in cells of mesenchymal origin: the role of an intracellular calcium regulating system. J Exp Biol. 1979 Aug;81:49–61. doi: 10.1242/jeb.81.1.49. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Takahashi K., Yamashita N. Ionic currents through the membrane of the mammalian oocyte and their comparison with those in the tunicate and sea urchin. J Physiol. 1977 May;267(2):465–495. doi: 10.1113/jphysiol.1977.sp011822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Takahashi K., Yoshii M. Membrane currents of the tunicate egg under the voltage-clamp condition. J Physiol. 1976 Jan;254(3):607–638. doi: 10.1113/jphysiol.1976.sp011249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro E., Castellucci V. F., Kandel E. R. Presynaptic membrane potential affects transmitter release in an identified neuron in Aplysia by modulating the Ca2+ and K+ currents. Proc Natl Acad Sci U S A. 1980 Jan;77(1):629–633. doi: 10.1073/pnas.77.1.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelbaum S. A., Tsien R. W. Calcium-activated transient outward current in calf cardiac Purkinje fibres. J Physiol. 1980 Feb;299:485–506. doi: 10.1113/jphysiol.1980.sp013138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Study R. E., Breakefield X. O., Bartfai T., Greengard P. Voltage-sensitive calcium channels regulate guanosine 3',5'-cyclic monophosphate levels in neuroblastoma cells. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6295–6299. doi: 10.1073/pnas.75.12.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. H. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977 Feb;265(2):465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong B. S. Quinidine interactions with Myxicola giant axons. Mol Pharmacol. 1981 Jul;20(1):98–106. [PubMed] [Google Scholar]
- Yeh J. Z., Narahashi T. Mechanism of action of quinidine on squid axon membranes. J Pharmacol Exp Ther. 1976 Jan;196(1):62–70. [PubMed] [Google Scholar]



