Abstract
Background
Phytoplankton cultures are widely used in aquaculture for a variety of applications, especially as feed for fish larvae. Phytoplankton cultures are usually grown in outdoor tanks using natural seawater and contain probiotic or potentially pathogenic bacteria. Some Roseobacter clade isolates suppress growth of the fish pathogen Vibrio anguillarum. However, most published information concerns interactions between probiotic and pathogenic bacteria, and little information is available regarding the importance of phytoplankton in these interactions. The objectives of this study, therefore, were to identify probiotic Roseobacter clade members in phytoplankton cultures used for rearing fish larvae and to investigate their inhibitory activity towards bacterial fish pathogens in the presence of the phytoplankton Nannochloropsis oculata.
Methodology/Principal Findings
The fish pathogen V. anguillarum, was challenged with 6 Roseobacter clade isolates (Sulfitobacter sp. (2 strains), Thalassobius sp., Stappia sp., Rhodobacter sp., and Antarctobacter sp.) from phytoplankton cultures under 3 different nutritional conditions. In an organic nutrient-rich medium (VNSS), 6 Roseobacter clade isolates, as well as V. anguillarum, grew well (109 CFU/ml), even when cocultured. In contrast, in a phytoplankton culture medium (ESM) based on artificial seawater, coculture with the 6 isolates decreased the viability of V. anguillarum by approximately more than 10-fold. Excreted substances in media conditioned by growth of the phytoplankton N. oculata (NCF medium) resulted in the complete eradication of V. anguillarum when cocultured with the roseobacters. Autoclaved NCF had the same inhibitory effect. Furthermore, Sulfitobacter sp. much more efficiently incorporated 14C- photosynthetic metabolites (14C-EPM) excreted by N. oculata than did V. anguillarum.
Conclusion/Significance
Cocultures of a phytoplankton species and Roseobacter clade members exhibited a greater antibacterial effect against an important fish pathogen (V. anguillarum) than roseobacters alone. Thus, cooperation of N. oculata, and perhaps other phytoplankton species, with certain roseobacters might provide a powerful tool for eliminating fish pathogens from fish-rearing tanks.
Introduction
Nannochloropsis oculata, a marine eukaryotic unicellular phytoplankton, is extensively used in the aquaculture industry. It grows in a wide salinity range and has significant nutritional value because of its high content of protein and polyunsaturated fatty acids, especially eicosapentaenoic acid [1]. N. oculata belongs to the class Eustigmatophyceae; its cells are spherical or slightly ovoid in shape and measure approximately 2–4 µm in diameter.
Phytoplankton cultures are widely used in the aquaculture industry for a variety of purposes. These cultures are described as “green water” because they contain high levels of phytoplankton species such as Nannochloropsis sp. and Chlorella sp. “Green water” is added to fish larvae tanks because it enriches zooplankton and provides indirect and direct nutrition to fish larvae. Moreover, green water reduces the transparency of rearing water, minimizing larval exposure to light which acts as a stressor. Phytoplanktons also improve water quality by reducing ammonium ion concentrations and increasing dissolved oxygen concentrations by photosynthesis. Notably, they also produce antibacterial substances that can prevent disease outbreaks [2], [3], [4], [5], [6].
Most aquaculturists produce green water using untreated natural seawater in open air. Green water modifies the bacterial composition of water [7]. For example, when Nannochloropsis sp. are introduced into a culture, 46% of the total bacteria grow actively, with the population being dominated by Alphaproteobacteria and the Cytophaga-Flavobacterium cluster [8]. The dominance of both bacterial groups is highly associated with increased production of cultured fish larvae [9].
A number of Roseobacter clade members (Alphaproteobacteria) suppress the growth of the devastating fish pathogen Vibrio anguillarum [10], [11], [12], [13]. Tropodithietic acid (TDA) produced by some Phaeobacter and Ruegeria isolates inhibits growth of V. anguillarum [11], [13], [14], [15], [16]. In addition, Roseobacter clade bacteria abundance was highly correlated with phytoplankton blooms [17]. As informative as these studies are, they are few and have left a number of questions unanswered, such as whether other antibiotics are produced in cocultures. We were inspired, therefore, to extend these studies in order to fully exploit the potential aquacultural benefits of the synergism between Roseobacter clade members and phytoplankton like N. oculata.
Materials and Methods
Culture Conditions
N. oculata was obtained from the Susami Fish Nursery Center, Kinki University, Japan. Cultures (106 cells/ml) were started by transfer to freshly prepared phytoplankton culturing medium (ESM) containing artificial seawater (Nine Salt Solution, NSS) [18] and cultured for 7 days at 15°C. Each liter of ESM medium contained 12 mg NaNO3, 0.5 mg K2HPO4, 0.1 µg vitamin B12, 0.1 µg biotin, 10 µg thiamine HCl, 25.9 µg Fe-EDTA, 33.2 µg Mn-EDTA, 100 mg Tris (hydroxymethyl) aminomethane, and 2.5 ml of soil extract [19]. When the density (estimated daily using a Thoma blood-counting chamber, Hirschmann Techcolor) reached 108 cells/ml in late log-phase, the culture was filtered as described below in the “N. oculata culture filtrate (NCF) and media preparation” section.
Estimation of Roseobacter clade populations in N. oculata cultures
Ten-milliliter aliquots of N. oculata cultures were collected and fixed immediately with 20% paraformaldehyde-phosphate buffered saline (PBS) (pH 7.2) (final concentration, 2%). The fixed samples were filtered through a 0.2-µm polycarbonate filter for cell counts using the fluorochrome 4′, 6-diamidino-2-phehylindole (DAPI) [20]. Roseobacter numbers were estimated by fluorescence in situ hybridization (FISH) [21] using the 16S ribosomal RNA probe ROSEO536R [22]. Cells were counted using an epifluorescence microscope (BX-51; Olympus, Tokyo Japan) and UV (DAPI) and green (Cy3) excitation. FISH and DAPI double-positive bacteria were counted and designated as hybridized cells. Roseobacter abundance was expressed as the ratio of hybridized to total DAPI-stained cells. All experiments were performed at least in triplicate.
Isolation and identification of Roseobacter clade members
Aliquots of N. oculata cultures were serially diluted 10-fold (up to 10−6) and spread onto VNSS agar (0.5 g yeast, 1 g trypticase peptone, 0.5 g glucose, 0.01 g FeSO4·7H2O, 0.01 g Na2HPO4·H2O, and 15 g Bacto Agar in 1 L artificial seawater) [18] and incubated in the dark at 20°C for 2–5 days. Beige, brown, or pink colonies were selected, because these colors are highly characteristic of the roseobacters [23]. Colonies isolated by 2 rounds of streaking were sub-cultured in VNSS. The Roseobacter were first identified by FISH, as indicated above, and by PCR.
The 16S rRNA gene was amplified by PCR using a universal primer, forward primer 27F (5′-AGAGTTTGATCMTGGCTCAG-3′), and reverse primer 1492R (5′-TACGGYTACCTTGTTACGACTT-3′). All PCR products were purified by ExoSAP-IT (USB Corporation) and sequenced by Solgent Co. Ltd. with the 16S rRNA gene primers 27F, 1492R, 518R (5′-CCAGCAGCCGCGGTAATACG-3′) and 785F (5′-GGATTAGATACCCTGGTA-3′). Sequences were tested with the BLAST program for matches to sequences deposited in GenBank, National Center for Biotechnology Information, USA (http://www.ncbi.nlm.nih.gov/genbank/index.html). Multiple sequences were simultaneously aligned using ClustalW and phylogenetic trees were generated using the neighbor-joining (NJ) method [24] with MEGA4 software [25]. The nucleotide sequences of the 16S rRNA genes determined in this study have been deposited in the DDBJ (DNA Data Bank of Japan) database under accession numbers AB607861 to AB607885.
Bacterial culture conditions
We studied Roseobacter isolates that we obtained here from N. oculata cocultures and Vibrio anguillarum strain psh-9019 (Gammaproteobacteria), originally isolated from a diseased fish, Plecoglossus altivelis (Salmoniforms) [26], [27]. V. anguillarum strain psh-9019 (serotype J-O-1) causes vibriosis in freshwater and seawater fish [28]. Bacteria were cultured for at least 16 h with shaking (120 rpm) in VNSS liquid medium. After incubation, 1 ml of each bacterial culture was centrifuged at 8,000×g for 5 min and washed twice with sterile 3% NaCl.
N. oculata culture filtrate (NCF) and media preparation
Three different types of media were used to compare the growth of roseobacters and V. anguillarum as follows: 1) VNSS, an organic nutrient-rich medium, for heterotrophic marine bacteria; 2) ESM, a phytoplankton culturing medium used for cultivating N. oculata; and 3) NCF, a medium containing substances excreted by N. oculata grown in ESM. To prepare NCF medium, N. oculata was cultured for 7 days until late log-phase and then centrifuged at 5,000×g for 15 min. The supernatant was passed through a GF/C filter (Whatman) and then sterilized by filtration through a 0.1 µm pore polypropylene syringe filter (Iwaki) and kept at 4°C. All experiments were carried out within 14 hours after filtration. Both ESM and VNSS media were sterilized by autoclaving at 121°C for 20 min.
Effects of Roseobacter clade strains on the viability of V. anguillarum
Roseobacter strains RO3, RO7, R11, R16, R18, or R27, were each inoculated together with V. anguillarum strain psh-9019 into VNSS, ESM and NCF media at a final concentration of 104 cells/ml in quadruplicate. Individual quadruplicate cultures of each strain in these same media served as controls. Aliquots (1 ml) were taken once daily for 7 days and serially diluted 10-fold. Ten microliters of these dilutions were added (5 drops per dilution) to VNSS agar plates using the drop plate method [29], incubated in the dark at 20°C for 3 days, and colonies were counted after incubation using a stereoscopic optical magnifier (Nikon model: 232063, 2× objective). Roseobacter and V. anguillarum colonies could be easily differentiated due to the differences in colony colors: cream-brown and white-gray, respectively.
Uptake of compounds excreted by N. oculata
To determine whether Roseobacter clade strain RO3 and V. anguillarum strain psh-9019 were able to take up metabolites excreted by N. oculata, we followed the method of Berman [30] as modified by Kamjunke et al. [31]. Axenic N. oculata NIES-2145 (Microbial Culture Collection, National Institute for Environmental Studies, Japan) was cultured for 7 days in ESM medium (200 ml) until late log-phase. One milliliter of the undiluted axenic culture was spread onto ESM and VNSS agar media to check for bacterial contamination. NaH14CO3 (50 mCi/mmol; PerkinElmer Life & Analytical Sciences) was added (final concentration, 1 µCi/ml) to the N. oculata culture, which was then incubated for 14 h under fluorescent light (50 µmol photons/m2/s). The culture was then passed through a GF/C filter, and the filtrate (14C-EPM: 14C- labeled excreted photosynthetic metabolites) (10 ml) collected in sterile 15-ml tubes.
Roseobacter clade strain RO3 and V. anguillarum strain psh-9019 were grown and pre-cultured as described above in “Bacterial culture conditions.” Heat-killed (70°C for 45 min) Roseobacter clade strain RO3 and V. anguillarum strain psh-9019 served as controls. Both viable and control heat-killed organisms were added individually to 10 ml of N. oculata filtrate containing 14C-EPM. After 0 h,1 h, 3 h, and 6 h, 0.5 ml of each subsample was passed through 0.22-µm nitrocellulose membrane filters (Millipore), which were washed with 1 ml of 1 M HCl and dried in a ventilated chamber overnight to remove the remaining inorganic 14C. After drying, filters were inserted into 4-ml scintillation vials containing 3 ml Optiphase HiSafe 3 scintillator (PerkinElmer Life & Analytical Sciences), and radioactivity was counted in a liquid scintillation counter (LSC-5100, Aloka). Experiments were performed in quadruplicate.
Heat stability of the EPM
N. oculata filtrates (NCF) were prepared as described in the “N. oculata culture filtrate (NCF) and media preparation” section above. After filtration, NCF were autoclaved at 121 °C for 20 minutes. The ability of Roseobacter clade strain RO3 to inhibit the growth of V. anguillarum in the presence of autoclaved NCF medium was determined as described above.
Statistical analysis
The Shapiro-Wilk tests were used to test the null hypothesis that samples were acquired from a normally distributed population. Significant differences between control and individual samples of each medium were analyzed with independent samples using the Student t-test. The Mann-Whitney U test were used to evaluate data that were not normally distributed. Statistical analyses were done using StatPlus:mac 2009 (AnalystSoft Inc., USA).
Results
Estimation of Roseobacter clade populations in N. oculata cultures
N. oculata cell densities in late log-phase “green water” ranged from 2.3 to 6.3×106 cells/ml (3 trials), and total bacterial counts were 1.3 to 3.8×106 cells/ml (Table 1). Roseobacter clade bacteria estimated by FISH in N. oculata late-log cultures represented 11.4% to 13.2% of the total bacteria (Table 1).
Table 1. Estimation of Roseobacter clade populations in N. oculata cultures.
| N. oculata density (×106 cells/ml) | Total bacteria count (×106 cells/ml) | Proportion of Roseobacter clade bacteria (%) | |
| Trial 1 | 6.3±1.6 | 3.8±0.6 | 11.4±1.9 |
| Trial 2 | 6.1±0.6 | 3.1±2.9 | 12.8±1.3 |
| Trial 3 | 2.3±1.9 | 1.3±3.2 | 13.2±1.6 |
± indicated SD (n = 3 for each trial).
Isolation and identification of Roseobacter clade species
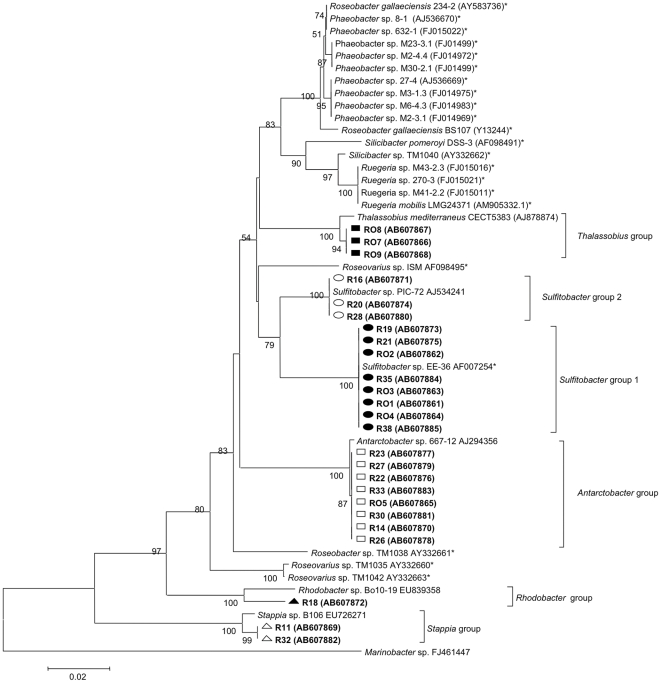
Two-hundred forty-two bacterial strains were isolated from the N. oculata culture by the serial dilution method described above (Trial 3 in Table 1). Twenty-five strains were identified by FISH as Roseobacter clade bacteria. Their 16S rRNA genes were sequenced and shown to be related to the following Roseobacter clade genera: Sulfitobacter sp., Antarctobacter sp., Thalassobius sp., Stappia sp., and Rhodobacter sp. (Fig. 1). All 25 isolates could be assigned to the Roseobacter clade [32] and could be divided into 6 groups representing 5 genera (2 Sulfitobacter groups) (Fig. 1). Isolates RO3, RO7, R11, R16, R18, and R27 representing each group were tested for their abilities to inhibit V. anguillarum (Fig. 1 and Table 2).
Figure 1. Phylogenetic tree of Roseobacter clade members isolated from Nannochloropsis oculata compared with potential probiotic species (*).
Closed squares: Thalassobius group, open circles: Sulfitobacter group 2, closed circles: Sulfitobacter group 1, open squares: Antarctobacter group, closed triangles: Rhodobacter group, and open triangles: Stappia group. Numbers by the branches are bootstrap values from 1000 replicates (>50%). The scale bar indicates the number of base pair substitutions per nucleotide position. Marinobacter sp. was used as the outgroup. Roseobacter isolated in this study are in bold type.
Table 2. Phylogenetic sequences of culturable Roseobacter clade isolated from N. oculata culture.
| Isolate | aAccession no. | Closest relative | Similarity (%) | Source of closest relative |
| RO3 | AB607863 | Sulfitobacter sp. | 100 | deep sea sediment |
| RO7 | AB607866 | Thalassobius sp. | 98.1 | seawater |
| R11 | AB607869 | Stappia sp. | 98.8 | deep seawater |
| R16 | AB607871 | Sulfitobacter sp. | 100 | seawater |
| R18 | AB607872 | Rhodobacter sp. | 96.4 | marine cyanobacteria |
| R27 | AB607879 | Antarctobacter sp. | 99.6 | dinoflagellates, Alexandrium spp. |
Accession number for sequences obtained in this study.
Effect of Roseobacter clade strains on the viability of V. anguillarum
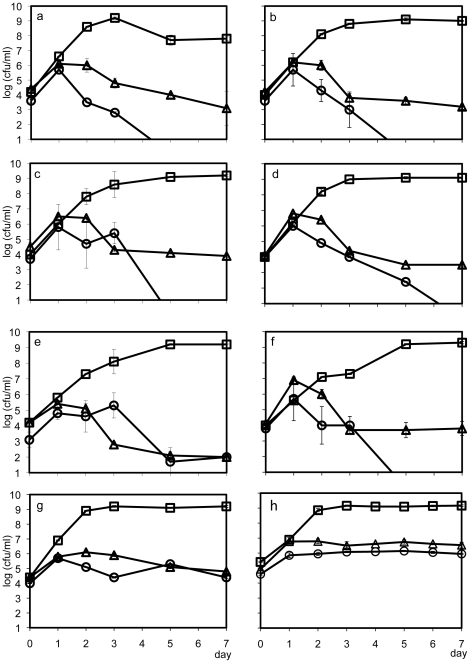
In VNSS medium, which is rich in organic nutrients, the control V. anguillarum culture grew to 1.6×109 CFU/ml (squares in Fig. 2g). Similar results were obtained in mixed cultures of V. anguillarum and Roseobacter clade bacteria (squares in Fig. 2b–f), except for Sulfitobacter sp. RO3 (squares in Fig. 2a) (P<0.05). In the presence of Sulfitobacter sp. RO3, V. anguillarum counts reached 1.6×109 CFU/ml and then decreased 10-fold (squares in Fig. 2a). In VNSS medium, Sulfitobacter sp. RO3 grew to 2.3×109 CFU/ml regardless of the presence of V. anguillarum (squares in Fig. 2h). All other roseobacters grew similarly to Sulfitobacter sp. RO3 in VNSS (data not shown).
Figure 2. Cell densities of Vibrio anguillarum (a–g) and Sulfitobacter sp. RO3 (h) under 3 different nutritional conditions.
V. anguillarum viable cell counts in the presence of a) Sulfitobacter sp. RO3, b) Sulfitobacter sp. R16, c)Thalassobius sp. RO7, d) Antarctobacter sp. R27, e) Stappia sp. R11, f) Rhodobacter sp. R18, and g) control (V. anguillarum only). Squares: VNSS (an organic nutrient-rich medium), triangles: ESM (phytoplankton culturing medium), and circles: NCF (medium containing excreted substances by N. oculata). (Error bars = standard deviation, SD).
In phytoplankton culturing medium, ESM, V. anguillarum cell counts in mixed cultures with 5 of 6 Roseobacter clade strains increased after 2 days from 1.3–3.2×104 CFU/ml to 1.0–2.5×106 CFU/ml, similar to controls (triangles in Fig. 2a–d and 2f). In the case of Stappia sp. R11, V. anguillarum counts only increased to 5.4×105 CFU/ml (triangles in Fig. 2e). At the end of the experiment, the V. anguillarum control count in ESM was about 104 CFU/ml (triangles in Fig. 2g). In stationary phase in ESM, V. anguillarum cell counts in mixed cultures were significantly lower than those in the controls, decreasing to 103 CFU/ml (triangles in Fig. 2a–d and 2f) (P<0.05). V. anguillarum and Stappia sp. R11 counts were more than 10-fold lower than those of V. anguillarum cultured with the other roseobacters (∼1.0×102 CFU/ml) (triangles in Fig. 2e) (P<0.05). Even though the coexistence of Roseobacter clade strains significantly decreased V. anguillarum numbers (in the stationary phase) in ESM medium, viable V. anguillarum persisted in mixed cultures for the entire experiment.
In NCF medium containing the substances excreted by N. oculata, control V. anguillarum CFUs were similar to those observed in ESM medium (circles in Fig. 2g). In contrast, when exposed to Roseobacter clade bacterial strains combined with substances excreted by N. oculata, all V. anguillarum were eradicated after 5 days of coculture (circles in Fig. 2a–d and 2f), except for Stappia sp. R11 (circles in Fig. 2e). When V. anguillarum was cocultured with Stappia sp. R11 in NCF, its colony counts were 1.0×102 CFU/ml after 5 days and remained so until the end of the experiment (circles in Fig. 2e). In ESM and NCF (triangles and circles in Fig. 2h), Sulfitobacter sp. RO3 grew to 5.5×105 and 6. 7×106 CFU/ml, respectively, even in the presence of V. anguillarum. Other roseobacters showed the same growth patterns as Sulfitobacter sp. RO3 (triangles and circles in Fig. 2h) in ESM and NCF media when cocultured with V. anguillarum (data not shown).
Uptake of compounds excreted by N. oculata
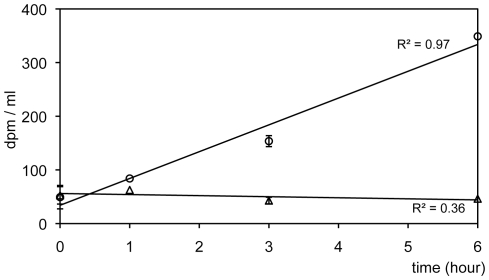
14C-EPM uptake by Sulfitobacter sp. was significantly higher than that of V. anguillarum (circles in Fig. 3). There was a direct relation between time and nutrient uptake values for 14C-EPM by Sulfitobacter sp. (r2 = 0.97) for the 6 h of incubation. In contrast, there was no significant 14C-EPM uptake by V. anguillarum (triangles in Fig. 3). Furthermore, there was no significant difference in bacterial size and counts for both bacterial species during the incubation (data not shown). These findings provide a basis for establishing a potential inhibitory mechanism that operates in these mixed cultures.
Figure 3. Uptake by Sulfitobacter sp. RO3 and Vibrio anguillarum of 14C-excreted photosynthetic metabolites (EPM) produced by N. oculata cultures.
Circles: Sulfitobacter sp. and triangles: V. anguillarum (Error bars = SD).
Heat stability of the EPM
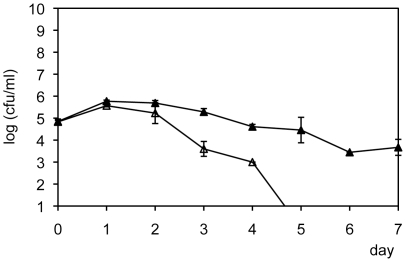
To gain some insight into the properties of the excreted by N. oculata, we tested the ability of autoclaved NCF medium to mediate the growth inhibition. This treatment had no detectable effect (Fig. 4).
Figure 4. Cell densities of V. anguillarum in autoclaved NCF medium.
Closed triangles: V. anguillarum only (control), and open triangles: V. anguillarum in the mix culture of Sulfitobacter sp. (Error bars = SD).
Discussion
Of all the bacterial genera present in marine environments, the Roseobacter clade is one of the most abundant. Here, we found that Roseobacter clade bacteria composed approximately 11.4–13.2% of the bacteria in indoor N. oculata cultures that were designed to simulate outdoor cultures at actual aquaculture production sites (Table 1). These values are comparable to Roseobacter levels in coastal seawater, (<1–25%) [17], [33], [34]. Among the culturable bacteria in the Roseobacter clade, Phaeobacter spp., Silicibacter sp. TM1040, Sulfitobacter sp. EE36, Roseobacter sp. TM1038, and Roseovarius spp. have been reported to possess potentially probiotic properties [11], [13], [15], [17] (Fig. 1 of the present study). Fig. 1 (in bold) shows that none of the Roseobacter clade species isolated from N. oculata cultures studied here represented known, potentially probiotic bacteria, except for Sulfitobacter sp. Our new isolates represent 5 (Sulfitobacter sp., Antarctobacter sp., Thalassobius sp., Stappia sp., and Rhodobacter sp.) of the 52 Roseobacter clade genera (Fig. 1). They might be useful as probiotics for treating fish infected with pathogenic bacteria such as V. anguillarum.
Adding phytoplankton to fish larvae tanks increases larval survival [3], [4], [5], [9] by inhibiting growth of pathogenic bacteria. This process could be mediated by at least 2 possible mechanisms. One involves either nutrient uptake preferences or nutrient competition, or both, by bacteria. The second, and by far more complex, entails direct interaction among microbes such as phytoplankton and pathogenic bacteria, probiotic bacteria and pathogenic bacteria, and phytoplankton-probiotic bacteria and pathogenic bacteria.
Regarding competition for nutrient uptake, Roseobacter clade abundance in coastal seawater correlates with the release of organic substances from natural phytoplankton blooms such as dimethylsulfoniopropionate (DMSP) [35], [36] and amino acids [37]. N. oculata may also excrete some substances like DMSP or amino acids that more optimally support the growth of Roseobacter clade bacteria. Our metabolic studies revealed that V. anguillarum did not appreciably incorporate 14C-labeled excreted metabolites by the phytoplankton, in contrast to Sulfitobacter sp. (Fig. 3). Furthermore, plate counts of V. anguillarum in NCF medium at the end of the experiment were similar compared to those in ESM medium (Fig. 2g). In the case of Roseobacter clade bacteria, however, their plate counts in NCF were 10-fold higher than that in ESM (triangles and circles in Fig. 2h). Alonso et al. [38] reported that in a low nutrient environment, Roseobacter clade bacteria were the main glucose consumers followed by Gammaproteobacteria. In other experiments not presented here, we observed a similar nutrient uptake pattern of 14C-labeled glucose by Sulfitobacter sp. and V. anguillarum. This indicates that Roseobacter clade bacteria more efficiently scavenge small amounts of organic nutrients under the oligotrophic conditions mimicked by ESM and NCF media. ESM medium contains soil extract and vitamins as the only supplemental organic nutrients. Thus, due to their more efficient nutrient-scavenging system, it is likely that Roseobacter clade bacteria will win the competition for nutrients over V. anguillarum. This would account for the poorer survival of V. anguillarum after reaching stationary phase in ESM medium (triangles in Fig. 2a–f). In contrast, V. anguillarum coexisted with Roseobacter clade bacteria in VNSS medium did not show such a decrease in CFU (squares in Fig. 2a–f). This might have occurred because there was either no apparent competition for nutrients or specific nutrient selectivity.
Regarding the second mechanism described above involving complex intercellular interactions, there was no direct inhibition of fish pathogens by phytoplankton, in contrast to other findings [39], [40]. As there was no difference in the viabilities of V. anguillarum between ESM and NCF (Fig. 2g), we concluded that N. oculata did not directly inhibit V. anguillarum's viability. In contrast, the diatom Skeletonema costatum and the macroalga Ulva clathrata produce organic compounds that inhibit V. anguillarum [39], [40].
We believe that direct growth inhibition of bacterial fish pathogens by Roseobacter clade bacteria [10], [11], [12], [13], [41] may explain our findings (Fig. 2a–f). Roseobacter clade bacteria benefit scallop [42] and turbot larvae [43] propagation by eliminating bacterial fish pathogens. Many studies have shown that cell densities of 106–109 CFU/ml Roseobacter clade bacteria were necessary to reduce the population of bacterial fish pathogens by approximately 10-fold [11], [12], [13], [16]. In the present study, in ESM medium, probiotic bacteria that grew to the highest density of 105 CFU/ml directly inhibited V. anguillarum (triangles in Fig. 2a–f). This cell density, 105 CFU/ml, is lower than those reported by others [11], [12], [13], [16].
Biofilm formation under static culture conditions enabled the bacteria Phaeobacter spp., Silicibacter sp. TM1040, Sulfitobacter sp. EE36, Roseobacter sp. TM1038, Roseovarius spp., and Pseudoalteromonas spp., to produce antibacterials against V. anguillarum [10], [13], [15], [44]. Tropodithietic acid (TDA) was reported to be the antibacterial compound produced by Phaeobacter spp., Silicibacter sp. and Ruegeria sp. [13], [15]. Static conditions and brown pigments were among the characteristics that correlate with TDA production [13], [15]. Roseobacter isolates in this study may produce antibacterial compounds other than TDA as the isolates were cultured in shaking conditions and did not produce brown pigment, although they exhibited antibacterial activity towards V. anguillarum. Shaking cultures more closely approximate actual conditions in an aquaculture facility.
Many studies have only focused on probiotic bacterial interactions with bacterial fish pathogens including V. anguillarum [10], [11], [12], [13], [14], [15], [16]. D′ Alvise et al. [16] observed eradication action of V. anguillarum by the probiotic bacteria, Ruegeria M43-2.3 (static culture) and Phaeobacter M23-3.1 (shaken and static cultures). Interestingly, our study demonstrated that shaken Roseobacter cultures were capable of killing V. anguillarum completely only in the presence of substances excreted from phytoplankton (circles in Fig. 2a–e), and none of them belonged to the Phaeobacter sp. group as observed by others [16] (Fig. 1).
The inhibitory activities of Roseobacter clade members Sulfitobacter sp., Thalassobius sp., Rhodobacter sp., and Antarctobacter sp. (but not Stappia sp.) against V. anguillarum were markedly affected by heat-stable substances excreted by N. oculata (circles in Fig. 2a–d and 2f) (Fig. 4). N. oculata, N. granulata, N. oceanica, and N. salina produce putrescine, a heat stable polyamine [45], [46]. Moreover, N. oculata CCMP525 produce low molecular weight signaling molecules similar to acyl-homoserine lactones, which are produced by bacteria for cell-to-cell communication systems that regulate gene expression [47]. Acyl-homoserine lactone analogs are heat stable [48]. These heat stable compounds may have been excreted by N. oculata in this study and could have acted as signaling molecules for communicating with Sulfitobacter sp. RO3 resulting in inhibition of V. anguillarum's growth. These results showed that phytoplankton cultures used as “green water” for producing fish larvae likely play an important role in enhancing the inhibitory effect of Roseobacter clade bacteria against V. anguillarum. We also observed similar inhibitory effects of other marine microalgae used for aquaculture, such as a marine Chlorella sp. filtrate (data not shown).
In our recent studies, a microcosm experiment employing N. oculata cultures showed that both culturable and non-culturable Vibrio species pathogenic for fish, decreased when N. oculata started to grow exponentially [49]. Furthermore, an in vivo experiment we proved that a high abundance of Alphaproteobacteria in N. oculata cultures is the key factor for high survival and growth of fish larvae [9]. To our knowledge, the present study is the first to report that Sulfitobacter sp., Thalassobius sp., Stappia sp., Rhodobacter sp., and Antarctobacter sp. isolated from phytoplankton cultures could interfere with the growth of V. anguillarum and that Roseobacter clade bacteria in concert with a phytoplankton exhibited an enhanced antibacterial effect. Roseobacter clade bacteria were better at competing for nutrients or as antibacterial agents. Thus, our research provides compelling evidence that phytoplankton cooperating with certain roseobacters provides aquaculturists with a powerful tool for controlling bacterial fish pathogens.
Acknowledgments
We would like to thank Taniguchi of Kinki University, for his helpful comments and support.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by the Grant-in-Aid for Scientific Research (B) (21380127) of the Japan Society for the Promotion of Science and by the Global COE program (International Education and Research Center for Aquaculture Science of Bluefin Tuna and Other Cultured Fish) of the Ministry of Education, Culture, Sports, Science and Technology of Japan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Tonon T, Harvey D, Larson TR, Graham IA. Long chain polyunsaturated fatty acid production and partitioning to triacylglycerols in four microalgae. Phytochemistry. 2002;61:15–24. doi: 10.1016/s0031-9422(02)00201-7. [DOI] [PubMed] [Google Scholar]
- 2.Huervana FH, De La Cruz JJY, Caipang CMA. Inhibition of luminous Vibrio harveyi by “green water” obtained from tank culture of Tilapia, Oreochromis mossambicus. Acta Ichthyol Piscat. 2006;36:17–23. [Google Scholar]
- 3.Muller-Feuga A. The role of microalgae in aquaculture: situation and trends. J Appl Phycol. 2000;12:527–534. [Google Scholar]
- 4.Øie G, Makridis P, Reitan KI, Olsen Y. Protein and carbon utilization of rotifers (Brachionus plicatilis) in first feeding of turbot larvae (Scophthalmus maximus L.). Aquaculture. 1997;153:103–122. [Google Scholar]
- 5.Palmer PJ, Burke MJ, Palmer CJ, Burke JB. Developments in controlled green-water larval culture technologies for estuarine fishes in Queensland, Australia and elsewhere. Aquaculture. 2007;272:1–21. [Google Scholar]
- 6.Tendencia EA, de la Peňa MR, Fermin AC, Lio-Po G, Choresca CH, Jr, et al. Antibacterial activity of Tilapia hornorum against Vibrio harveyi. Aquaculture. 2004;232:145–152. [Google Scholar]
- 7.Salvesen I, Reitan KI, Skjermo J, Øie G. Microbial environments in marine larviculture: Impacts of algal growth rates on the bacterial load in six microalgae. Aquacult Int. 2000;8:275–287. [Google Scholar]
- 8.Nakase G, Eguchi M. Analysis of bacterial communities in Nannochloropsis sp. cultures used for larval fish production. Fish Sci. 2007;73:543–549. [Google Scholar]
- 9.Nakase G, Nakagawa Y, Miyashita S, Nasu T, Senoo S, et al. Association between bacterial community structures and mortality of fish larvae in intensive rearing systems. Fish Sci. 2007;73:784–791. [Google Scholar]
- 10.Hjelm M, Riaza A, Formoso F, Melchiorsen J, Gram L. Seasonal incidence of autochthonous antagonistic Roseobacter spp. and Vibrionaceae strains in turbot larva (Scophthalmus maximus) rearing system. Appl Environ Microbiol. 2004;70:7288–7294. doi: 10.1128/AEM.70.12.7288-7294.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porsby CH, Nielsen KF, Gram L. Phaeobacter and Ruegeria species of the Roseobacter clade colonize separate niches in a Danish turbot (Scophthalmus maximus)-rearing farm and antagonize Vibrio anguillarum under different growth conditions. Appl Environ Microbiol. 2008;74:7356–7364. doi: 10.1128/AEM.01738-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prado S, Montes J, Romalde JL, Barja JL. Inhibitory activity of Phaeobacter strains against aquaculture pathogenic bacteria. Int Microbiol. 2009;12:107–114. [PubMed] [Google Scholar]
- 13.Bruhn JB, Gram L, Belas R. Production of antibacterial compounds and biofilm formation by Roseobacter species are influenced by culture conditions. Appl Environ Microbiol. 2007;73:442–450. doi: 10.1128/AEM.02238-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruhn JB, Haagensen JAJ, Bagge-Ravn D, Gram L. Culture conditions of Roseobacter strain 27-4 affects its attachment and biofilm formation as quantified by real-time PCR. Appl Environ Microbiol. 2006;72:3011–3015. doi: 10.1128/AEM.72.4.3011-3015.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruhn JB, Nielsen KF, Hjelm M, Hansen M, Bresciani J, et al. Ecology, inhibitory activity and morphogenesis of a marine antagonistic bacterium belonging to the Roseobacter clade. Appl Environ Microbiol. 2005;71:7263–7270. doi: 10.1128/AEM.71.11.7263-7270.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D'Alvise PW, Melchiorsen J, Porsby CH, Nielsen KF, Gram L. Inactivation of Vibrio anguillarum by attached and planktonic Roseobacter cells. Appl Environ Microbiol. 2010;76:2366–2370. doi: 10.1128/AEM.02717-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buchan A, González JM, Moran MA. Overview of the marine Roseobacter lineage. Appl Environ Microbiol. 2005;71:5665–5677. doi: 10.1128/AEM.71.10.5665-5677.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eguchi M, Nishikawa T, MacDonald K, Cavicchioli R, Gottschal JC, et al. Responses to stress and nutrient availability by marine ultramicrobacterium Sphingomonas sp. strain RB2256. Appl Environ Microbiol. 1996;62:1287–1294. doi: 10.1128/aem.62.4.1287-1294.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Provasoli L, McLaughlin JJA, Droop MR. The development of artificial media for marine algae. Arch Mikrobiol. 1957;25:392–428. doi: 10.1007/BF00446694. [DOI] [PubMed] [Google Scholar]
- 20.Porter GK, Feig YS. The use of DAPI for identifying and counting aquatic microflora. Limnol and Oceanogr. 1980;25:943–948. [Google Scholar]
- 21.Glöckner FO, Fuchs BM, Amann R. Bacterioplankton composition of lakes and oceans: a first comparison based on fluorescence in situ hybridization. Appl Environ Microbiol. 1999;65:3721–3726. doi: 10.1128/aem.65.8.3721-3726.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brinkmeyer R, Rappé M, Gallacher S, Medlin L. Development of clade- (Roseobacter and Alteromonas) and taxon-specific oligonucleotide probes to study interactions between toxic dinoflagellates and their associated bacteria. Eur J Phycol. 2000;35:315–329. [Google Scholar]
- 23.Garrity GM, Bell JA, Liburn T. Family I. Rhodobacteraceae fam. nov. In: Brenner DJ, Krieg NR, Staley JT, Garrity GM, editors. Bergey's Manual of Systematic Bacteriology, 2nd Ed., vol. 2, The Proteobacteria, part C, The Alpha-, Beta-, Delta-, and Epsilon proteobacteria. New York: Springer; 2005. pp. 161–229. [Google Scholar]
- 24.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic tress. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 25.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 26.Miyamoto N, Eguchi M. Development of monoclonal antibodies that specifically react with a fish pathogen, Vibrio anguillarum serotype J-O-1. Fish Sci. 1996;62:710–714. [Google Scholar]
- 27.Miyamoto N, Eguchi M. Direct detection of a fish pathogen, Vibrio anguillarum serotype J-O-1 in freshwater by fluorescent antibody technique. Fish Sci. 1997;63:253–257. [Google Scholar]
- 28.Tajima K, Ezura Y, Kimura T. Studies on the taxonomy and serology of causative organisms of fish vibriosis. Fish Pathol. 1985;20:131–142. [Google Scholar]
- 29.Herigstad B, Hamilton M, Heersink J. How to optimize the drop plate method for enumerating bacteria. J Microbiol Met. 2001;44:121–129. doi: 10.1016/s0167-7012(00)00241-4. [DOI] [PubMed] [Google Scholar]
- 30.Berman T. Size fractionation of natural aquatic populations associated with autotrophic and heterotrophic carbon uptake. Mar Biol. 1975;33:215–220. [Google Scholar]
- 31.Kamjunke N, Köhler B, Wannicke N, Tittel J. Algae as competitors for glucose with heterotrophic bacteria. J Phycol. 2008;44:616–623. doi: 10.1111/j.1529-8817.2008.00520.x. [DOI] [PubMed] [Google Scholar]
- 32.Garrity GM, Lilburn TG, Cole JR, Harrison SH, Euzeby J, et al. The taxonomic outline of Bacteria and Archaea. 2007. TOBA Release 7.7. Michigan State University Board of Trustees, MI, USA.
- 33.Bano N, Hollibaugh JT. Phylogenetic composition of bacterioplankton assemblages from the Arctic Ocean. Appl Environ Microbiol. 2002;68:505–518. doi: 10.1128/AEM.68.2.505-518.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brinkhoff T, Giebel HA, Simon M. Diversity, ecology, and genomics of the Roseobacter clade: a short overview. Arch Microbiol. 2008;189:531–539. doi: 10.1007/s00203-008-0353-y. [DOI] [PubMed] [Google Scholar]
- 35.González JM, Simó R, Massana R, Covert JS, Casamayor EO, et al. Bacterial community structure associated with a dimethylsulfoniopropionate-producing North Atlantic algal bloom. Appl Environ Microbiol. 2000;66:4237–4246. doi: 10.1128/aem.66.10.4237-4246.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zubkov MV, Fuchs BM, Archer SD, Kiene RP, Amann R, et al. Linking the composition of bacterioplankton to rapid turnover of dissolved dimethylsulphoniopropionate in an algal bloom in the North Sea. Environ Microbiol. 2001;3:304–311. doi: 10.1046/j.1462-2920.2001.00196.x. [DOI] [PubMed] [Google Scholar]
- 37.Moran MA, Belas R, Schell MA, González JM, Sun F, et al. Ecological genomics of marine roseobacters. Appl Environ Microbiol. 2007;73:4559–4569. doi: 10.1128/AEM.02580-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alonso C, Pernthaler J. Roseobacter and SAR11 dominate microbial glucose uptake in coastal North Sea waters. Environ Microbiol. 2006;8:2022–2030. doi: 10.1111/j.1462-2920.2006.01082.x. [DOI] [PubMed] [Google Scholar]
- 39.Lu K, Lin W, Liu J. The characteristics of nutrient removal and inhibitory effect of Ulva clathrata on Vibrio anguillarum 65. J Appl Phycol. 2008;20:1061–1068. [Google Scholar]
- 41.Brinkhoff T, Bach G, Heidorn T, Liang L, Schlingloff A, et al. Antibiotic production by a Roseobacter clade-affiliated species from the German Wadden Sea and its antagonistic effects on indigenous isolates. Appl Environ Microbiol. 2004;70:2560–2565. doi: 10.1128/AEM.70.4.2560-2565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruiz-Ponte C, Samain JF, Sánchez JL, Nicolas JL. The benefit of a Roseobacter species on the survival of scallop larvae. Mar Biotechnol. 1999;1:52–59. doi: 10.1007/pl00011751. [DOI] [PubMed] [Google Scholar]
- 43.Planas M, Peréz-Lorenzo M, Hjelm M, Gram L, Fiksdal IU, et al. Probiotic effect in vivo of Roseobacter strain 27-4 against Vibrio (Listonella) anguillarum infections in turbot (Scophthalmus maximus L.) larvae. Aquaculture. 2006;255:323–333. [Google Scholar]
- 44.Holmström C, Kjelleberg S. Marine Pseudoalteromonas species are associated with higher organisms and produce biologically active extracellular agents. FEMS Microbiol Ecol. 1999;30:285–293. doi: 10.1111/j.1574-6941.1999.tb00656.x. [DOI] [PubMed] [Google Scholar]
- 45.Hamana K, Sakamoto A, Nishina M, Niitsu M. Cellular polyamine profile of the phyla Dinophyta, Apicomplexa, Ciliophora, Euglenozoa, Cercozoa and Heterokonta. J Gen Appl Microbiol. 2004;50:297–303. doi: 10.2323/jgam.50.297. [DOI] [PubMed] [Google Scholar]
- 46.Hamana K. Cellular polyamines of phototrophs and heterotrophs belonging to the lower eukaryotic phyla Cercoza, Euglenozoa, Heterokonta and Metamonada. J Gen Appl Microbiol. 2008;54:135–140. doi: 10.2323/jgam.54.135. [DOI] [PubMed] [Google Scholar]
- 47.Natrah FMI, Kenmegne MM, Wiyoto W, Sorgeloos P, Bossier P, et al. Effects of micro-algae commonly used in aquaculture on acyl-homoserine lactones quorum sensing. Aquaculture. 2011;317:53–57. [Google Scholar]
- 48.Thorne SH, Williams HD. Cell density-dependent starvation survival of Rhizobium leguminosarum bv. phaseoli: identification of the role of an N-acyl homoserine lactone in adaptation to stationary-phase survival. J Bacteriol. 1999;181:981–990. doi: 10.1128/jb.181.3.981-990.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taniguchi A, Sharifah NE, Eguchi M. Possible role of microalga Nannochloropsis oculata in controlling Vibrio species in fish larva rearing water. Aqua Sci. 2011 (in press) [Google Scholar]