Abstract
The potential of anaflatoxin B1 (AnAFB1) conjugated to keyhole limpet hemocyanin (KLH) as a vaccine (AnAFB1-KLH) in controlling the carry over of the aflatoxin B1 (AFB1) metabolite aflatoxin M1 (AFM1) in cow milk is reported. AFB1 is the most carcinogenic compound in food and foodstuffs amongst aflatoxins (AFs). AnAFB1 is AFB1 chemically modified as AFB1-1(O-carboxymethyl) oxime. In comparison to AFB1, AnAFB1 has proven to be non-toxic in vitro to human hepatocarcinoma cells and non mutagenic to Salmonella typhimurium strains. AnAFB1-KLH was used for immunization of cows proving to induce a long lasting titer of anti-AFB1 IgG antibodies (Abs) which were cross reactive with AFB1, AFG1, and AFG2. The elicited anti-AFB1 Abs were able to hinder the secretion of AFM1 into the milk of cows continuously fed with AFB1. Vaccination of lactating animals with conjugated AnAFB1 may represent a solution to the public hazard constituted by milk and cheese contaminated with AFs.
Introduction
Mycotoxins are secondary metabolites produced by molds, classified among the most important risk factors in the food chain of humans and animals. The problem of mycotoxicoses is global and is particularly affecting the countries characterized by environmental and weather conditions favourable to contamination and growth of fungi both in field and storage of stocks. It has been estimated that 25% of the world's food crops is contaminated with mycotoxins, and more than 4.5 billion people and an undefined number of animals are chronically exposed to aflatoxins (AFs), the most relevant mycotoxins of medical interest [1], [2]. AFs (AFB1, AFB2, AFG1, AFG2), produced mainly by strains of Aspergillus flavus and A. parasiticus, may cause acute (hepatitis, oedema, hemorrhagic necrosis) or chronic (liver, lung, and kidney carcinomas and immunosuppression) toxic effects [2]. The main AF, AFB1, has a range of biological activities, including acute toxicity, teratogenicity, mutagenicity and carcinogenicity [3]. The International Agency for Research on Cancer (IARC) has classified AFB1 as the most important known carcinogenic compound (group 1), particularly related to hepatocarcinoma [4], [5].
In animal farming, AFs may cause reduced performance, increased susceptibility to infections, altered responsiveness to vaccinations [6], in addition to contamination of derived dietary products such as meat, eggs and milk [7], [8]. Following ingestion of contaminated feed, AFB1 is rapidly adsorbed and transported to the liver where it is partially metabolized into the hydroxy-derivate M1, which may be secreted in the milk of mammals, including dairy animals (carry over process) [9], [10]. AFM1, which is as toxic as AFB1, has been included in Group 2 (potentially carcinogenic for humans) by IARC [4]. By association with casein, AFM1 occurring in the milk concentrates during cheese making, thus increasing the risk potential of the milk/cheese production chain [11].
Industrialized countries have defined specific limits for AFM1 in the milk destined for human consumption (ranging from 0.05 µg AFM1/kg of the European Community to 0.5 µg/kg of USA), and for AFB1 in dairy animal feeds [12]. The best strategy to counter the AFs problem is the prevention of fungal contamination in the food chain. When outbreaks of AFs occur, some means of salvaging contaminated feeds involve physical or chemical detoxifying methods or inclusion in the diet of a variety of animals of sequestering agents, able to prevent AFs absorption in the gastro-intestinal tract [13]. However, none of these methods fulfill completely the efficacy, safety, and cost requisites of the task [14]. The production of a vaccine able to induce specific antibodies (Abs) neutralizing AFB1 and other AFs would be of great social, scientific and economic interest. Conventional vaccine approaches are not feasible due to the non immunogenicity of AFB1. Its conjugation to proteins, shown to be effective with other haptens, would be unlikely for human use and hardly proposable in animals, owing to the toxic properties of the molecule that might be released. A modified form of AFB1 (anaflatoxin B1, AnAFB1), devoid of toxicity and mutagenicity, still maintaining antigenicity when conjugated, would be a potential vaccine candidate. A method for the preparation and purification of AFB1-1(O-carboxymethyl) oxime from AFB1 has been described, and the derivative was shown to be nontoxic to chicken embryos [15]. Lower mortality and reduction of acute toxic effects in the liver was achieved in rabbits and rats immunized with AFB1-1(O-carboxymethyl) oxime conjugated to bovine serum albumin (BSA) or histone H1 and challenged with a single dose of AFB1 [16], [17], [18].
The aim of this study was to evaluate whether AnAFB1, represented by AFB1-1(O-carboxymethyl) oxime, verified in vitro to be nontoxic to human hepatocarcinoma cells and non mutagenic to Salmonella typhimurium strains, might constitute, following conjugation to a non-bovine protein (KLH), a potential vaccine to prevent the carry over of AFB1 as AFM1 in the milk of dairy cows receiving an AFB1-contaminated diet.
Results
Cytotoxicity assay of AFB1 and AnAFB1 on HepG2 cells
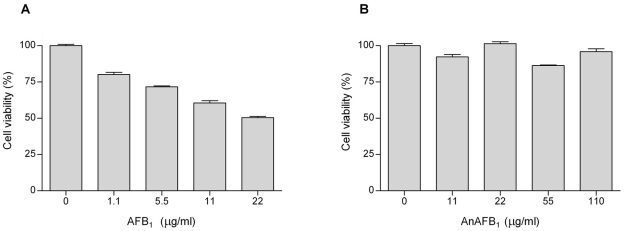
The effects of a range of concentrations of AFB1 and AnAFB1 on the viability of HepG2 hepatoblastoma cells were evaluated by a colorimetric assay. AFB1 caused a dose-dependent cytotoxicity, as survival of the cells inversely decreased with increases in the concentration of AFB1 from 1.1 to 22 µg/ml (Figure 1A). Cells exposed to AnAFB1 showed no significant decrease in cell viability at concentrations up to 110 µg/ml (Figure 1B).
Figure 1. In vitro effects of AFB1 (A) and AnAFB1 (B) on human hepatoblastoma cell viability.
In vitro mutagenicity assay of AFB1 and AnAFB1 in Salmonella typhimurium
The Ames test with strains TA 98 and TA 100 was carried out in order to elucidate whether AnAFB1 retained the mutagenic properties of AFB1. The results of the mutagenicity assay are shown in Table 1.
Table 1. Mutagenicity of AFB1 and AnAFB1 in S. typhimurium TA98 and TA100.
| dose | his + revertants* | ||
| (ng/plate) | TA 98 | TA 100 | |
| AFB1 | 0 | 6±2 | 25±22 |
| 100 | 175±31 | 133±21 | |
| 200 | 307±49 | 622±47 | |
| AnAFB1 | 0 | 6±2 | 25±22 |
| 100 | 2±1 | 26±14 | |
| 200 | 2±2 | 5±2 | |
| 500 | 4±2 | 59±25 | |
*mean of two independent, triplicate mutagenicity assays with SD.
AnAFB1, up to a concentration of 200 ng/plate, did not elicit a mutagenic response either in S. typhimurium TA98 or TA 100. At the concentration of 500 ng/plate, a positive, but very weak, mutagenic activity was observed only in S. typhimurium TA 100, while the positive control AFB1 induced a strong mutagenic response at concentrations of 100 and 200 ng/plate.
ELISA titration of anti-AFB1 Abs
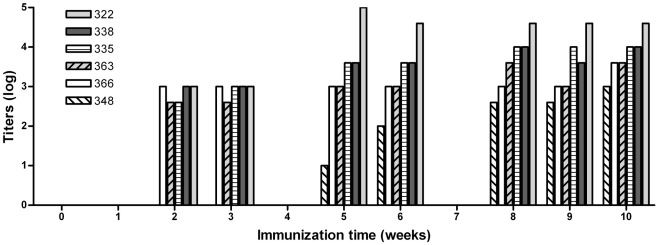
Production of Abs against AFB1 in vaccinated and control dairy cows was evaluated by ELISA. For all of the cows, dilutions of pre-immune control sera showed negligible binding to AFB1-BSA. When dilution series of cow sera were incubated with control BSA (unconjugated), only a low degree of nonspecific binding was detected (data not shown). Control cows (6 out of 6) did not produce, as expected, anti-AFB1 Abs (data not shown). Ab titers of vaccinated cows over a 10-week period are presented in Figure 2. Based on the anti-AFB1 Ab titer, at the 10th week it was possible to differentiate two groups among vaccinated cows. Animals producing the highest serum titers ranging from 10,000 to 40,000 were defined as “high responders” (cow number 322, 335, 338). The animals presenting titers ranging from 1,000 to 4,000 were defined as “low responders” (cow number 348, 363, 366). Following immunization none of the animals provided positive reactions to the intradermal tuberculin test as attested by the local competent authority.
Figure 2. Titers of anti-AFB1 Abs.
Cows numbered 322, 335, 338, 348, 363 and 366 were initially i.m. primed with 500 µg of AnAFB1-KLH conjugate and then boosted at week 3, 6 and 9 with the same amount of immunogen. Ab titers (presented in figure on a logarithmic scale) were determined by the method described in the text.
Cross-reactivity of anti-AFB1 Abs with other AFs
Sera from the 10th week bleeding were selected for cross-reactivity evaluation with AFs. The cross-reactivity of immune sera collected from each cow with AFB2, AFG1, and AFG2 was similar and averaged 17%, 31%, and 9%, respectively (Table 2). AFM1 displayed negligible cross-reactivity for all the immune sera, since 50% inhibition of binding to AFB1-BSA was not reached using concentrations up to 1000 ng/ml.
Table 2. Cross-reactivity of immune sera with AFs.
| Cow number | |||||||||||||
| 322 | 335 | 338 | 348 | 363 | 366 | ||||||||
| AF | IC50 * | % | IC50 | % | IC50 | % | IC50 | % | IC50 | % | IC50 | % | % average |
| AFB1 | 76.69 | 100 | 28.06 | 100 | 150.80 | 100 | 58.96 | 100 | 66.11 | 100 | 48.41 | 100 | 100 |
| AFB2 | 496.10 | 15 | 213.50 | 13 | 990.00 | 15 | 301.50 | 20 | 282.40 | 23 | 393.30 | 12 | 17 |
| AFG1 | 339.60 | 23 | 126.70 | 22 | 502.90 | 30 | 253.10 | 23 | 167.40 | 39 | 100.70 | 48 | 31 |
| AFG2 | 906.70 | 8 | 480.70 | 6 | 848.70 | 18 | 2611.00 | 2 | 730.50 | 9 | 405.70 | 12 | 9 |
*IC50 is the AF concentration (ng/ml) causing 50% inhibition of binding of the immune serum to the solid phase.
Quantification of AFM1 in milk samples
The efficacy of anti-AFB1 Abs in preventing or reducing the carry over of AFB1 as AFM1 into milk was evaluated by monitoring AFM1 concentrations in milk of the lactating dairy cows. An intermittent exposure regimen with two intoxication periods was designed to evaluate efficacy of anti-AFB1 Abs over time and in two different lactation (mid and late) stages.
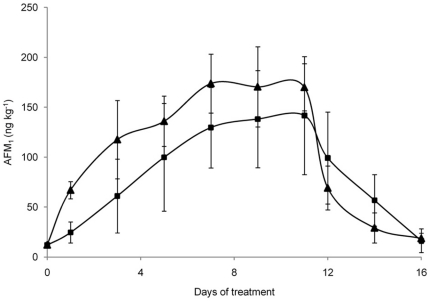
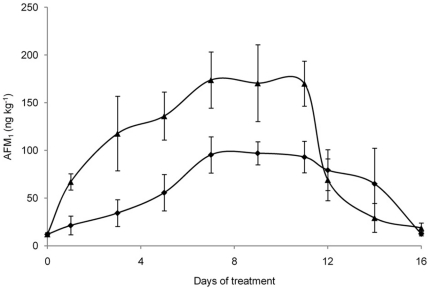
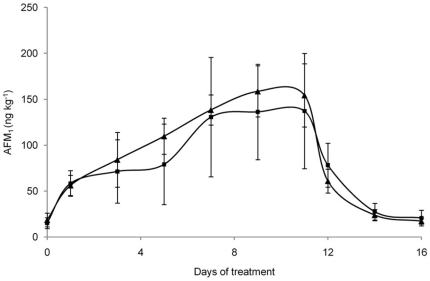
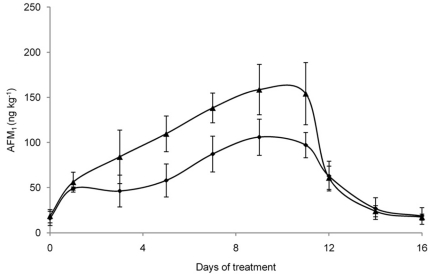
Basal diet AFB1 level contributed to a milk AFM1 contamination of 12.1±1.3 and 16.8±6.6 ng/kg in the first and second period, respectively (as calculated on day 0). Results of AFM1 quantification in the milk collected during the first intoxication period (144 µg AFB1 per cow per day) are shown in Figure 3. At day 1, the milk sampled from the control cows had an AFM1 concentration higher than the tolerable level allowed by the EC (0.05 µg/kg) [19]. On the contrary, the milk samples collected from vaccinated cows had an AFM1 concentration lower than control milk and below the EC maximum allowed level. However, the AFM1 concentration in milk increased at every milking and reached a steady-state condition from day 7 of intoxication period for both groups. On day 11, when AFB1 administration was stopped, the mean AFM1 concentration decreased quickly to return at the base line on day 16. During the experimental period, the milk of vaccinated cows consuming contaminated feed showed AFM1 levels always lower (even non statistically significant) than the milk of control animals. In particular, at the steady state condition, the average AFM1 concentration in milk collected from vaccinated cows was 20% lower than in milk of control animals (137 ng/kg vs 171 ng/kg). A negative correlation between serum AFB1 specific Ab titers and the average concentration of AFM1 detected in the milk at the steady state condition during the first experimental period (r = −0.72, p<0.05) was observed in vaccinated cows. Importantly, the “high responder” cows, showing the highest titers of anti-AFB1 Abs, produced an average milk AFM1 concentration at the steady state about 46% lower (p<0.05) than control cows (95 ng/kg vs 177 ng/kg; Figure 4). During the second experimental period, AFM1 in milk from vaccinated cows consuming the contaminated feed (145 µg AFB1 per cow per day) showed the same trend as in first experimental period, with AFM1 levels always lower (even non statistically significant) than that of control cows consuming AFB1. In particular, at the steady state condition, average AFM1 concentration in milk collected from vaccinated cows was 11% lower than in milk of control animals (134 vs 154 ng/kg, respectively) (Figure 5). Similarly to first exposure period, the average concentration of AFM1 in milk at the steady state condition was correlated (r = −0.68, p<0.05) with anti-AFB1 Ab titers in the serum of vaccinated cows, with average AFM1 concentration in milk of “high responder” cows 37% lower (p<0.05) of that observed in control cows (97 vs 154 ng/kg, respectively; Figure 6).
Figure 3. Average AFM1 concentration in milk collected during the first experimental period.
Six vaccinated (▪) and six control (▴) cows were fed 144 µg of AFB1/day from day 1 to day 11. Data are presented as mean ± SD.
Figure 4. Average AFM1 concentrations in milk collected during the first experimental period from high responder cows.
Three high responder vaccinated cows (♦) and six control cows (▴) were fed 144 µg of AFB1/day from day 1 to day 11. Data are presented as mean ± SD.
Figure 5. Average AFM1 concentrations in milk collected during the second experimental period.
Six vaccinated (▪) and six control (▴) cows were fed 145 µg of AFB1/day from day 1 to day 11. Data are presented as mean ± SD.
Figure 6. Average AFM1 concentrations in milk collected during the second experimental period from high responder cows.
Three high responder vaccinated cows (♦) and six control cows (▴) were fed 145 µg of AFB1/day from day 1 to day 11. Data are presented as mean ± SD.
Discussion
The occurrence of AFM1 in milk and its derivatives is a serious problem of food safety, as milk is a primary source of human nutrition, in particular for infants and children. To reduce human and animal exposure, industrialized countries have defined specific limits for AFM1 in the milk and for AFB1 in the feed of dairy animals [12]. Currently, the best strategy in order to avoid exceeding of the maximum limit of AFM1 in the milk destined for human consumption is the prevention of AF contamination of feeds, but in spite of the control measures taken, production of AFM1-free milk is not always achieved. In industrialized countries the milk having higher levels of contamination than the current legal limits is destroyed, although exposure to low doses of AFM1, which could be present beneath that limit, is not prevented, with potential health effects due to accumulation. Moreover, in developing countries, where food availability has often to be considered before food safety, there is a lack of legislation of acceptable limits for AFs and populations are undoubtedly exposed to high amounts of AFM1 in milk.
An alternative management of the problem, therefore, could rely on a preventive approach such as a safe and reliable vaccine for dairy cattle which should be effective in avoiding AFB1 carry over in the milk.
We report here the proof-of-concept that AnAFB1, composed of AFB1-1(O-carboxymethyl) oxime, which proved to be non toxic and non mutagenic in controlled experimental conditions, may fulfill with the purpose. AnAFB1 conjugated to a carrier protein (KLH) and with Freund's adjuvant elicited in cows cross-reactive anti-AFs long lasting Ig, mostly pertaining to IgG, although other Ig classes could contribute to observed results, since the Abs used to detect anti-AFB1 Abs were not gamma chain specific. Anti-AFs Abs proved to substantially reduce AFB1 carry over as AFM1 in the milk for a prolonged period of ingestion of contaminated feed. No adverse effect on animal health was observed in AFB1 exposed cows, consistently with previous works adopting similar contamination levels [20], [21]. It is notable that a single administration of the vaccine in complete Freund's adjuvant did not induce delayed hypersensitivity in any of the vaccinated cows, as demonstrated by negative intradermal tuberculin test. This finding should exclude any misleading evaluation of livestock health.
According to Ab titer specific for AFB1, it was possible to recognize, among the 6 vaccinated cows, 3 high responder and 3 low responder animals. The titer of Abs specific to AFB1 in vaccinated cows correlated well with the prevention of carry over in the milk, following exposure of the cows to feed contaminated with AFB1. Cows of the high responder group (titers ranging from 10,000 to 40,000) presented, at the steady state, significantly lower concentrations of AFM1 in the milk than cows of the low responder group (titers ranging from 1,000 to 4,000). Moreover, the anti-AFB1 Abs elicited in the high responder cows appeared to reduce the excretion of AFM1 in the milk following intoxication either in the mid or late lactation stages, thus conferring protection over the whole production cycle, before the drying off period.
The reasons why healthy, immunocompetent animals may be differently susceptible to immunization are not well understood, but it has been reported that cows could be phenotypically classified as low or high responders, based on the magnitude and kinetics of the Ab response to injection of various antigens [22], [23], [24]. Recognized factors of variation in cows' responsiveness to immunization include, among others, the energy balance derived from feeding regimen, peripartum stress and lactation stage [22], [23], [24]. In addition to these non-genetic effects, there is growing evidence that the individual's genotype may predetermine immunological responses to infection and vaccination [23], [24], [25]. The Ab response to immunization also appears to be often dependent on the adjuvant adopted. Currently, the exact mechanism of action of many adjuvants is still unknown, and research continues to strive to identify the best adjuvant or combination of adjuvants to elicit the correct immune response for a given antigen [26]. Furthermore, selection of carrier proteins used in haptenic vaccines has proven to be greatly important in eliciting potent anti-hapten Ab because it exerts a clear influence on the immune response [27]. It is therefore possible that appropriate vaccine formulations (e.g. different adjuvants or carriers) could stimulate the immunity and surmount deficiencies in low responder cows.
Reduction of AFM1 in milk obtained in high responder vaccinated cows was comparable with reductions obtained adding to animal diet the best AFs sequestering agents, which are able to reduce AFM1 transfer in the milk up to 50%, depending on the extent of contamination [28], [29]. The results obtained by vaccination, moreover, could be cumulated with the ones eventually achieved by adopting alternative treatments.
Easy detection of anti-AFB1 Abs could allow monitoring the immunological status of milk animals in order to determine the protective titre and evaluate the need for booster injections.
Although the schedule of immunization, the nature of adjuvant and carrier, the requirement for boosters and some other factors, such as the fate of AFB1 captured by antibodies, should be furtherly investigated, conjugated AnAFB1 may represent a possible solution to a global and serious health problem.
Materials and Methods
Ethics statement
The research protocol and animal care were in accordance with the EC Council Directive guidelines for animals used for experimental and other scientific purposes [30].
The study has been approved by the local health autority “Azienda Unità Sanitaria Locale di Piacenza” (protocol number 24567) and by the National Ministry of Health according to legislative decree 116/92.
Preparation of AnAFB1
AFB1 (Sigma-Aldrich, Inc., St. Louis, MO, USA) was converted to AFB1-1(O-carboxymethyl) oxime using a method previously described [15]. Briefly, carboxymethylhydroxylamine•HCl (10 mg, 0.046 mmol) was added to a solution of AFB1 (10 mg, 0.032 mmol) in methanol/water/pyridine (4∶1∶1) and the mixture was refluxed at 60°C for 3 h. After maintenance overnight at room temperature, the solution was concentrated under vacuum. The product was purified by flash chromatography (CHCl3∶MeOH = 7∶3), and confirmed to be AFB1-1(O-carboxymethyl) oxime by UPLC/MS (Acquity, Waters, Milford, MA, USA). The UPLC separation was performed on a reversed-phase C-18 column (UPLC BEH Aquity, Waters; 17 µm×21 mm), eluted at a flow rate of 300 µl/min. Mobile phase consisted of 0.2% formic acid in H2O (solvent A) and 0.2% formic acid in acetonitrile (solvent B).
Cytotoxicity assay of AnAFB1 on HepG2
Human hepatoblastoma (HepG2) cell line (BS-TCL-79, ATCC, Rockville, MD, USA) was maintained in tissue culture flasks with Dulbecco's modified Eagle's medium (DMEM) (Sigma-Aldrich) containing 10% fetal calf serum, 1% antibiotics (10,000 U/ml penicillin, 10,000 U/ml streptomycin sulfate), 1% glutamine, and 1 mM sodium pyruvate (Life Technologies, Gaithersburg, MD, USA). Cells were cultured in a humidified incubator under 5% CO2 at 37°C and split twice a week. Cell viability was assessed by spectrophotometrically measuring alamarBlue (AB) (Biosource International Inc., Camarillo, CA, USA) reduction by mitochondrial enzyme activity [31]. The day before treatment HepG2 were trypsinized and seeded at a density of 5×104 cells/well in flat bottom 96-wells plates (Costar, Corning Inc., Corning, NY, USA). After 24 h incubation under 5% CO2 at 37°C, the medium in each well was discarded and replaced by fresh medium containing increasing dilutions of AnAFB1 or AFB1, obtained from stock solutions prepared in dimethylsulfoxide (DMSO). All experiments included both untreated and solvent control cultures. After 24 h of treatment, the medium was discarded and cells were washed twice with PBS. Cells were then incubated for 2 h at 37°C under 5% CO2 in 100 µl of incubation medium along with 10% (v/v) AB. Absorbance values were measured at 570/595 nm with a Sunrise Absorbance Reader (Tecan Italia Srl., Milan, Italy). The values were corrected for background of negative controls containing medium without cells. Treated cell viability was calculated in comparison to untreated cultures, assumed to be 100%.
In vitro mutagenicity assay of AnAFB1 in Salmonella typhimurium
Mutagenicity of AnAFB1, in comparison with AFB1, was assessed in the reverse mutation assay by the standard plate incorporation method developed by Maron and Ames [32]. The compounds were tested on His− S. typhimurium TA 98 and TA 100 (kindly donated by Dott. Cassoni, ARPA, Parma, Italy) with in vitro extracellular microsomal activation (S9 from Aroclor 1254-induced rats, Moltox Inc., Boone, NC, USA). Overnight cultures of the test strains were grown for 8–16 h in Nutrient Broth n.2 (Fluka Chemika, Buchs, Switzerland) to a cell density of approximately 109 CFU/ml. Three concentrations (100, 200 and 500 ng/plate) for AnAFB1 and two concentrations (100 and 200 ng/plate) for AFB1 were tested, with three replicate plates at each dose. The compounds were dissolved in DMSO and diluted in distilled water immediately prior to testing. 0.1 ml each of the test sample and bacterial suspension with 0.5 ml PBS (pH 7.4) or 0.5 ml of the metabolic system S9 were added to 2 ml of molten top agar (45°C) supplemented with 0.05 mM L-histidine and 0.05 mM d-biotin and subsequently poured onto minimal glucose agar plates (15 ml/plate). After 48 h at 37°C the colonies (His+ revertants) in each plate were counted. Solvent alone served as negative control (spontaneous mutation frequency) while AFB1 was included as a positive control [33]. A mutagenic potential was assumed, if a two-fold or greater increase was seen in the number of revertant colonies of the treated cultures in comparison to negative controls.
Conjugation of AnAFB1 to KLH
AnAFB1 was conjugated to KLH (Sigma-Aldrich) to be used as immunogen. The coupling reaction was carried out using a method previously described [34]. N-hydroxysuccinimide (1.1 mg) and N,N′-diisopropylcarbodiimide (1.45 µl), dissolved in 600 µl of dimethylformamide (DMF), were added to AnAFB1 (2 mg) in 600 µl of dry dichloromethane at 0°C, followed by 4-(dimethylamino)pyridine (1 mg). Then, the active ester was slowly added to a pre-cooled aqueous buffered solution (31 mM Na2HPO4, pH 9.1) containing 20 mg KLH and not more than 10% (v/v) DMF. The mixture was kept at 4°C overnight, and then the conjugate was separated from unreacted reagents and byproducts, desalted and extensively dialyzed against PBS by using a 10 kDa cut-off centrifugal filter tube (Microcon YM-10, Millipore Corporation, Bedford, MA, USA). The protein concentration was determined by using the Bradford method, using BSA (Sigma-Aldrich) as external standard. In order to estimate the AFB1 loading on KLH, UV absorbance was measured at 363 nm, assuming that the absorbance of the conjugated AFB1 was the same as the unmodified toxin (ε = 21,800 L·mol−1·cm−1). The ratio between the concentration of the bound toxin and that of the protein gave the loading degree of the conjugate.
Animal immunization
Two groups of six multiparous lactating Holstein Friesian dairy cows were used in the present study. Cows were housed in a free stall barn (CERZOO research and experimental centre, San Bonico, Italy) and had free access to water. The diet was formulated according to the nutrient requirements of dairy cattle for an average cow weighting 650 kg, 140 days in milk (DIM) and a 35 kg milk yield (3.8% fat and 3.35% protein) [35]. The diet, composed both by forages and concentrate (total mixed ration), was fed ad libitum. Cows were milked twice a day and individual milk yield was recorded at every milking (Afimilk system, S.A.E. Afikim, Kibbutz Afikim, Israel). The animals were regularly inspected by the local competent authority; official intradermal tuberculin test was carried out and interpreted according to EC Commission Regulations [36]. Each cow was immunized by intramuscular (i.m.) neck injection with an 1 ml emulsion of 500 µg of either AnAFB1-KLH (vaccinated animals) or KLH (control animals) in complete Freund's adjuvant (Sigma-Aldrich) in a 1∶1 (v/v) ratio. Priming injection was done at 85±21 DIM and was followed by three similar dose booster injections in incomplete Freund's adjuvant at three week intervals. Animals were bled via the jugular artery prior to immunization and weekly thereafter. The blood was allowed to clot 60 min at 37°C, and the serum was obtained by centrifugation (1500 g, 10 min) and stored at −20°C until assay.
Enzyme-linked immunosorbent assay (ELISA)
For titration of specific antibody, wells of polystyrene microtiter plates (Costar) were coated overnight (4°C) with 50 µl of AFB1-BSA conjugate (Sigma-Aldrich) or BSA control protein, 20 µg/ml, in 0.05 M sodium carbonate-bicarbonate buffer (pH 9.6). Plates were washed at this point and after each incubation step with 100 µl of PBS containing 0.05% Tween 20. Wells were blocked for 60 min at 37°C with 100 µl of 1% (w/v) gelatin from porcine skin (Type A; Sigma-Aldrich) in PBS. To each well, 50 µl of serially diluted immune serum or control (pre-immune serum) was added, gently mixed, and incubated at 37°C for 90 min. 50 µl of rabbit anti-bovine IgG (whole molecule) peroxidase conjugate Abs (Sigma-Aldrich, product number A7414) diluted 1∶25,000 in PBS were added to each well. After 60 min incubation at 37°C, 50 µl of freshly prepared cromogen/substrate solution, constituted by 1 mg of 3,3′, 5,5′-tetramethylbenzidine dihydrochloride and 2 µl of 30% H2O2 in 10 ml of citric buffer (51 mM Na2HPO4, 24 mM citric acid, pH 5.0), were added. The reaction was stopped after ten minutes with 25 µl of 0.5 M H2SO4. The optical density (OD) at 450 nm was read by using a Multiskan Ascent (Labsystems, Helsinki, Finland) and the titer of each immune serum was defined as the inverse of the highest dilution that gave 0.1 OD above the pre-immune serum at the same dilution. To compensate for between-plate variability, individual plates were normalized to the mean of the appropriate positive control.
A competitive indirect ELISA (ci-ELISA) was used to assess the cross-reactivity of anti-AFB1 Abs in cow's sera with other AFs. Microtiter plates were coated with AFB1-BSA and blocked as described above. Increasing concentrations of AFB1, AFB2, AFG1, AFG2, or AFM1 in 25 µl PBS were mixed with a 25 µl volume of immune serum (diluted 1∶400 in PBS) in the wells of the microtiter plates and incubated for 1 h at 37°C. Bound Abs were determined by the addition of anti-bovine IgG peroxidase conjugate as described above. The absorbance in the assay without free AFs was assumed as the maximal value. AF concentration causing 50% inhibition (IC50) of binding of the immune serum to AFB1-BSA was calculated by variable slope nonlinear regression analysis of curves obtained by plotting the percent absorbance values versus log AF concentration using GraphPhad Prism 4.01 software (San Diego, CA, USA). The relative cross-reactivity of the immune serum with different AFs was calculated as (IC50 of AFB1/IC50 of other AF)×100.
Quantification of AFB1 in feeds
Ten grams of dried feed was suspended in 100 ml acetone∶water solution (85∶15), shaken at 150 r.p.m. for 45 min (Universal table Shaker 709, ASAL srl, Milan, Italy), filtered (Schleicher & Schuell 595 ½ filter paper, Dassel, Germany), and 5 ml was loaded on an immunoaffinity column (Aflatoxin Easy-extract, Rhone Diagnostics Technologies, Glasgow, UK) and the column washed with 45 ml bidistilled water. The column was further washed with 5 ml water and bound AFB1 eluted with 2.5 ml of methanol. The extract was dried under nitrogen, redissolved in 1 ml acetonitrile∶water (25∶75) solution and filtered (Millipore Corporation; HV 0.45 µm). HPLC analysis was performed with a Perkin Elmer LC (Perkin Elmer, Norwalk, CT, USA) equipped with an LC-200 pump and a Jasco FP-1520 fluorescence detector (Jasco, Tokyo, Japan) set at 365 nm (excitation) and 440 nm (emission). Calibration was performed as a function of standard AFB1 (Sigma-Aldrich) concentrations. Standard solutions were prepared and checked according to the AOAC method 970.44 [37]. AFB1 was separated with a reverse-phase C18 Superspher column (Merck, Darmstadt, Germany; 4 µm particle size, 125×4 mm i.d.) at room temperature, with water∶acetonitrile∶methanol (59∶15∶26) mobile phase at a flow rate of 1 ml/min.
Treatment of dairy cows with AFB1
The study consisted of two successive intoxication periods: the first, starting 1 week after the last booster injection, carried out at mid lactation stage (155±21 DIM, average milk yield of 32.1±5.6 kg/day per cow), and the second at late lactation stage (246±21 DIM, average milk production of 28.8±3.1 kg/day per cow).
The basal diet had an AFB1 content of 0.50 µg kg−1 in the first and 0.55 µg kg−1 in the second experimental period, corresponding to about 11.5–12.7 µg per cow per day based on an average ingestion of 23 kg dry matter per cow per day. Corn meal naturally hypercontaminated was diluted in about 300 g of AFB1-free soy bean meal to obtain a bolus, which gave a calculated daily AFB1 total ingestion of 144 µg and 145 µg, respectively.
Experimental periods lasted 16 days, consisting of 11 days of intoxication and 5 days of clearance (no AFB1 in the diet). Individual milk samples were collected at day 0, 1, 3, 5, 7, 9, 11, 12, 14, and 16. A representative sample for each day of milking was then obtained and stored at −18°C for subsequent analysis. Basal diet samples were collected on days 0 and 11 of each experimental period, dried at 55°C in a ventilated oven to constant weight, and then ground with a 1 mm sieve (Thomas-Wiley Laboratory Mill, Arthur H. Thomas Co., Philadelphia, PA, USA) and frozen until analysed for AFs.
Quantification of AFM1 in milk samples
Milk samples (50 ml) were defatted by centrifugation (7,000 r.p.m. for 10 min at 4°C) and filtered with Schleicher & Schuell 595 ½ filter paper (Dassel). Then, 20 ml was passed through the immunoaffinity column (Aflatoxin Easy-extract) and AFM1 was quantified by HPLC, following separation with a reverse-phase C18 LiChrosper 100 column (Merck, Darmstadt, Germany; 5 µm particle size, 125×4 mm i.d.) at room temperature, with water∶acetonitrile (75∶25) mobile phase at a flow rate of 1 ml/min. AFM1 (Sigma-Aldrich) was used as calibration standard, as previously described for AFB1 quantification.
Statistical analyses
All data are presented as means ± standard deviations (SD). Differences between immunization groups and the results of the cytotoxicity and mutagenicity assays were analysed using the Student's t test. Concerning the excretion pattern of AFM1 into milk, the steady-state condition was determined in agreement to Littell et al. [38]. AFM1 milk concentration at the plateau condition was subjected to Analysis of Variance (ANOVA). The statistical model included fixed effects of treatment, time of measurement and the treatment×time of measurement interactions, with cow as the random variable. Differences between means were accepted as significant if p<0.05.
Footnotes
Competing Interests: The authors have read the journal's policy and declare that LP and GP have the following conflicts: Patent application which the authors may benefit from. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Funding: This work was supported by funds from “Fondazione Cassa di Risparmio di Parma e Piacenza” http://www.fondazionecrp.it/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Boutrif E. FAO programmes for prevention, regulation, and control of mycotoxins in food. Nat Toxins. 1995;3:322–326; discussion 341. doi: 10.1002/nt.2620030430. [DOI] [PubMed] [Google Scholar]
- 2.Williams JH, Phillips TD, Jolly PE, Stiles JK, Jolly CM, et al. Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. Am J Clin Nutr. 2004;80:1106–1122. doi: 10.1093/ajcn/80.5.1106. [DOI] [PubMed] [Google Scholar]
- 3.McLean M, Dutton MF. Cellular interactions and metabolism of aflatoxin: an update. Pharmacol Ther. 1995;65:163–192. doi: 10.1016/0163-7258(94)00054-7. [DOI] [PubMed] [Google Scholar]
- 4.IARC. Some naturally occurring substances - food items and constituents, heterocyclic aromatic amines and mycotoxins. IARC Monogr Eval Carcinog Risks Hum. Lyon, France: World Health Organization, International Agency for Research on Cancer; 1993. pp. 245–391. [Google Scholar]
- 5.Henry SH, Bosch FX, Troxell TC, Bolger PM. Policy forum: public health. Reducing liver cancer–global control of aflatoxin. Science. 1999;286:2453–2454. doi: 10.1126/science.286.5449.2453. [DOI] [PubMed] [Google Scholar]
- 6.Bondy GS, Pestka JJ. Immunomodulation by fungal toxins. J Toxicol Environ Health B Crit Rev. 2000;3:109–143. doi: 10.1080/109374000281113. [DOI] [PubMed] [Google Scholar]
- 7.Herzallah SM. Determination of aflatoxins in eggs, milk, meat and meat products using HPLC fluorescent and UV detectors. Food Chem. 2009;114:1141–1146. [Google Scholar]
- 8.U.S. Department of Health and Human Services PHS, National Toxicology Program. Report on Carcinogens, Eleventh Edition. 2005.
- 9.Eaton DL, Ramsdell HS, Neal GE. Biotransformation of Aflatoxins. Toxicology of Aflatoxins∶Human Health, Veterinary, and Agricultural Significance: Eaton DL and Groopman JD. San Diego: Academic Press, Inc; 1994. pp. 45–71. [Google Scholar]
- 10.Gallo A, Moschini M, Masoero F. Aflatoxin absorption in the gastro-intestinal tract and in the vaginal mucosa in lactating dairy cows. Ital J Anim Sci. 2008;7:53–63. [Google Scholar]
- 11.Yousef AE, Marth EH. Stability and degradation of aflatoxin M1. In: van Egmond HP, editor. Mycotoxins in Dairy Products. London: Elsevier Applied Science; 1989. pp. 127–161. [Google Scholar]
- 12.FAO. Worldwide regulations for mycotoxins in food and feed in 2003. 2004. FAO Food and Nutrition Paper 81: Food and Agriculture Organization of the United Nations.
- 13.CAST. Mycotoxins: Risk in plant, animal and human system. In: Richard JL, Payne GA, editors. Task Force Report No 139. Ames,, Iowa, USA: Council for Agricultural Science and Technology (CAST); 2003. [Google Scholar]
- 14.Piva G, Galvano A, Pierti A, Piva A. Detoxification methods of aflatoxins. A review. Nutr Res. 1995;5:689–715. [Google Scholar]
- 15.Chu FS, Hsia MT, Sun PS. Preparation and characterization of aflatoxin B1-1-(O-carboxymethyl) oxime. J Assoc Off Anal Chem. 1977;60:791–794. [PubMed] [Google Scholar]
- 16.Ueno I, Chu FS. Modification of hepatotoxic effects of aflatoxin B1 in rabbits by immunization. Experientia. 1978;34:85–86. doi: 10.1007/BF01921918. [DOI] [PubMed] [Google Scholar]
- 17.Odunola OA, Uwaifo AO. Binding reaction of aflatoxin B1 with immunoglobulin G against aflatoxin B1-bovine serum albumin complex. Afr J Med Med Sci. 1998;27:1–4. [PubMed] [Google Scholar]
- 18.Odunola OA, Uwalfo AO. Immune response to aflatoxin B1-histone H1 complex. Afr J Med Med Sci. 2000;29:105–110. [PubMed] [Google Scholar]
- 19.European Commission. Commission Regulation 2001/466/EC of 8 March 2001 setting maximum levels for certain contaminants in foodstuffs (Text with EEA relevance). Off J Eur Commun. 2001;L77:1–13. [Google Scholar]
- 20.Diaz DE, Hagler WM, Jr, Blackwelder JT, Eve JA, Hopkins BA, et al. Aflatoxin binders II: reduction of aflatoxin M1 in milk by sequestering agents of cows consuming aflatoxin in feed. Mycopathologia. 2004;157:233–241. doi: 10.1023/b:myco.0000020587.93872.59. [DOI] [PubMed] [Google Scholar]
- 21.Masoero F, Gallo A, Moschini M, Piva G, Diaz D. Carryover of aflatoxin from feed to milk in dairy cows with low or high somatic cell counts. Animal. 2007;1:1344–1350. doi: 10.1017/S1751731107000663. [DOI] [PubMed] [Google Scholar]
- 22.Wagter LC, Mallard BA, Wilkie BN, Leslie KE, Boettcher PJ, et al. A Quantitative Approach to Classifying Holstein Cows Based on Antibody Responsiveness and Its Relationship to Peripartum Mastitis Occurrence. J Dairy Sci. 2000;83:488–498. doi: 10.3168/jds.S0022-0302(00)74908-3. [DOI] [PubMed] [Google Scholar]
- 23.Nino-Soto MI, Heriazon A, Quinton M, Miglior F, Thompson K, et al. Differential gene expression of high and low immune responder Canadian Holstein dairy cows. Dev Biol (Basel) 2008;132:315–320. doi: 10.1159/000317277. [DOI] [PubMed] [Google Scholar]
- 24.Hernandez A, Karrow N, Mallard BA. Evaluation of immune responses of cattle as a means to identify high or low responders and use of a human microarray to differentiate gene expression. Genet Sel Evol. 2003;35:S67–81. doi: 10.1186/1297-9686-35-S1-S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newman MJ, Truax RE, French DD, Dietrich MA, Franke D, et al. Evidence for genetic control of vaccine-induced antibody responses in cattle. Vet Immunol Immunopathol. 1996;50:43–54. doi: 10.1016/0165-2427(95)05483-9. [DOI] [PubMed] [Google Scholar]
- 26.Lambrecht BN, Kool M, Willart MA, Hammad H. Mechanism of action of clinically approved adjuvants. Curr Opin Immunol. 2009;21:23–29. doi: 10.1016/j.coi.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Xu Q-H, Zhao X-N, Cheng J-P, Wei C-H, Zhang Q-H, et al. Influence of Carrier Proteins on the Immunologic Response to Haptenic Antitetrodotoxin Vaccine. Bioconjugate Chem. 2006;17:1508–1513. doi: 10.1021/bc060083u. [DOI] [PubMed] [Google Scholar]
- 28.Kutz RE, Sampson JD, Pompeu LB, Ledoux DR, Spain JN, et al. Efficacy of Solis, NovasilPlus, and MTB-100 to reduce aflatoxin M1 levels in milk of early to mid lactation dairy cows fed aflatoxin B1. J Dairy Sci. 2009;92:3959–3963. doi: 10.3168/jds.2009-2031. [DOI] [PubMed] [Google Scholar]
- 29.Diaz D, Hagler W, Hopkins B, Whitlow L. Aflatoxin Binders I: In vitro binding assay for aflatoxin B1 by several potential sequestering agents. Mycopathologia. 2003;156:223–226. doi: 10.1023/a:1023388321713. [DOI] [PubMed] [Google Scholar]
- 30.European Community. 1986/609/EC. Council Directive 86/609/EEC of 24 November 1986 on the approximation of laws, regulations and administrative provisions of the Member States regarding the protection of animals used for experimental and other scientific purposes. Official Journal L. 1986;358:1–28. [Google Scholar]
- 31.Nociari M, Shalev A, Benias P, Russo C. A novel one-step, highly sensitive fluorometric assay to evaluate cell-mediated cytotoxicity. J Immunol Methods. 1998;213:157–167. doi: 10.1016/s0022-1759(98)00028-3. [DOI] [PubMed] [Google Scholar]
- 32.Maron DM, Ames BN. Revised methods for the Salmonella mutagenicity test. Mutat Res. 1983;113:173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- 33.Wong JJ, Hsieh DP. Mutagenicity of aflatoxins related to their metabolism and carcinogenic potential. Proc Natl Acad Sci U S A. 1976;73:2241–2244. doi: 10.1073/pnas.73.7.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee N, McAdam D, Skerritt J. Hapten synthesis and development of ELISAs for detection of endosulfan in water and soil. J Agric Food Chem. 1995;43:1730–1739. [Google Scholar]
- 35.National Research Council. Nutrient requirements of dairy cattle, seventh revised edition. National Academy of Science, Washington, D.C; 2001. [Google Scholar]
- 36.European Commission. Commission Regulation 2002/1226/EC of 8 July 2002 amending annexes A and B of the consolidated Council Directive 64/432/EEC. Off J Euro Commun. 2002;L179:13–18. [Google Scholar]
- 37.AOAC. Preparation of standards for mycotoxins. In: Helrich KC, editor. Official methods of analysis of the AOAC. Arlington, USA: Association of Official Analytical Chemists (AOAC); 1995. [Google Scholar]
- 38.Littell RC, Henry PR, Ammerman CB. Statistical analysis of repeated measures data using SAS procedures. J Anim Sci. 1998;76:1216–1231. doi: 10.2527/1998.7641216x. [DOI] [PubMed] [Google Scholar]