Abstract
Background
Baylisascaris procyonis (Nematoda: Ascaridida), an intestinal nematode of raccoons, is emerging as an important helminthic zoonosis due to serious or fatal larval migrans in animals and humans. Despite its significant veterinary and public health impact, the epidemiology, molecular ecology and population genetics of this parasite remain largely unexplored. Mitochondrial (mt) genomes can provide a foundation for investigations in these areas and assist in the diagnosis and control of B. procyonis. In this study, the first complete mt genome sequence of B. procyonis was determined using a polymerase chain reaction (PCR)-based primer-walking strategy.
Methodology/Principal Findings
The circular mt genome (14781 bp) of B. procyonis contained 12 protein-coding, 22 transfer RNA and 2 ribosomal RNA genes congruent with other chromadorean nematodes. Interestingly, the B. procyonis mtDNA featured an extremely long AT-rich region (1375 bp) and a high number of intergenic spacers (17), making it unique compared with other secernentean nematodes characterized to date. Additionally, the entire genome displayed notable levels of AT skew and GC skew. Based on pairwise comparisons and sliding window analysis of mt genes among the available 11 Ascaridida mtDNAs, new primer pairs were designed to amplify specific short fragments of the genes cytb (548 bp fragment) and rrnL (200 bp fragment) in the B. procyonis mtDNA, and tested as possible alternatives to existing mt molecular beacons for Ascaridida. Finally, phylogenetic analysis of mtDNAs provided novel estimates of the interrelationships of Baylisasaris and Ascaridida.
Conclusions/Significance
The complete mt genome sequence of B. procyonis sequenced here should contribute to molecular diagnostic methods, epidemiological investigations and ecological studies of B. procyonis and other related ascaridoids. The information will be important in refining the phylogenetic relationships within the order Ascaridida and enriching the resource of markers for systematic, population genetic and evolutionary biological studies of parasitic nematodes of socio-economic importance.
Introduction
Baylisascaris procyonis, a ubiquitous helminth parasite of raccoons (Procyon lotor), is increasingly being recognized as an emerging public health concern in North America, Europe and parts of Asia [1], [2]. B. procyonis is the most common cause of clinical visceral (VLM), ocular (OLM) and neural larva (NLM) migrans in various species of birds and mammals, including humans [3]. Humans, as accidental intermediate hosts, become infected by the accidental consumption of infective B. procyonis eggs from the environment or articles contaminated with raccoon faeces [4]. Human infection with B. procyonis typically results in fatality or long-term neurological sequelae [5]–[10]. Clinical manifestations include eosinophilic encephalitis, ocular disease and eosinophilic cardiac pseudotumor. Fifteen recognized human cases of B. procyonis NLM, six of them fatal and predominantly involving children, were reported by Murray and Kazacos (2004). More than 12 additional unpublished cases of infection are also known (K.R. Kazacos, personal observation) [2], [11]. Epidemiological studies suggest that pica or geophagia and exposure to infected raccoons or environments contaminated with their faeces are the most important risk factors for human infection with B. procyonis. Current diagnosis of this parasitic infection is typically based on morphological examination. However, morphological characteristics can often be unrecognized even by experienced microscopists, and mistaken identification, particularly of helminth larvae, is not uncommon [12]. Moreover, the diagnosis becomes more difficult when identification and differentiation of eggs or larval are performed among a number of possible environmental cross-contaminating eggs of other parasites, including morphologically similar Baylisascaris spp. [2], and of possible contaminating nematode larvae, including those of Toxocara canis, Toxocara cati, Toxascaris leonina, Ascaris lumbricoides, and species of Angiostrongylus and Ancylostoma [12], [13]. Therefore, obtaining a more efficient and reliable way to identify and differentiate B. procyonis eggs or larvae has become crucial for clinical diagnosis, epidemiological investigation and laboratory tests, and achieving this goal is foreseeable only through utilization of molecular methodologies.
Recently, mitochondrial (mt) genomics have received increased attention, and mt DNA is regarded as an important and efficient source of genetic markers, being widely used for species-specific identification and differentiation of many zoonotic nematodes. Sequences of the mt cytochrome-oxydase I (cox1) and NADH dehydrogenase subunit 4 (nad4) genes have been used to identify and differentiate hookworm species [14] and other Strongylida [15], respectively. Additionally, the cytochrome-oxydase II (cox2) gene has also proven useful as a genetic marker for differentiation of species among T. canis, T. cati, T. leonina, Ascaris suum and B. procyonis [2], [16]. Surprisingly, based on the cox2 gene, B. procyonis and Baylisascaris columnaris cannot be distinguished between each other because of the high nucleotide sequence similarity [2]. Similar problems may be further exacerbated in attempts to perform species-specific differentiation between B. procyonis and other Baylisascaris spp., thus limiting the ability to accurately identify and to assess genetic variability in B. procyonis populations, which would be problematic for studies of its epidemiology, diagnosis and control. Compared to the use of partial mt genes, a complete mt genomic dataset would be especially powerful for displaying sufficient interspecies sequence variability and describing species specificity [15], [17]. Moreover, mt genomes contain useful genetic markers for studying the genetic structure within and among Baylisascaris spp., due to mutation rates which are proposed to be more rapid than nuclear genes, and presumed lack of recombination and maternal inheritance [15], [18]–[22]. However, no complete information on the mt genome of B. procyonis was previously available.
Herein, we first report the complete nucleotide sequence of the mt genome from a representative B. procyonis from China and compare the sequence and genome organization with the three other available complete mt genomes from the congeneric Baylisascaris schroederi, Baylisascaris ailuri and Baylisascaris transfuga [23], as well as the sequences from related nematodes in the same order. Based on comparative mitogenomics, whether mt gene fragments currently utilized as genetic markers (such as cox2) offer the best regions for characterization or species identification and recognition is discussed. Additionally, new PCR primer pairs designed to amplify short fragments of mtDNA for B. procyonis were developed with the aim of providing the ability to differentiate between B. procyonis and other species of ascaridoids, including morphologically similar Baylisascaris spp. Finally, the phylogenetic relationships of the species B. procyonis within the genus Baylisasaris and of the genus Baylisascaris within the order Ascaridida were also investigated by the construction of phylogenetic trees (NJ, MP and ML) using the protein-coding amino acid sequence dataset.
Results and Discussion
Main features of the mt genome of B. procyonis
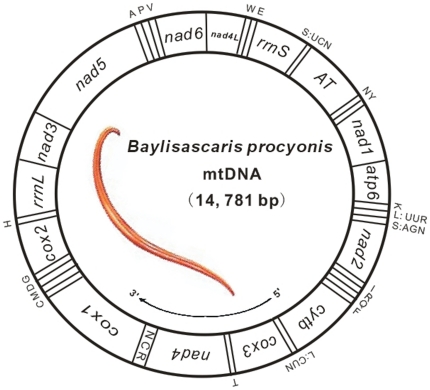
The complete mt genome of B. procyonis was 14781 bp in size (GenBank accession No. JF951366) and encoded 36 genes, including 12 protein-coding genes (1 subunit of the ATP synthase, atp6; 3 subunits of cytochrome c oxidase, cox1-3; 1 subunit of cytochrome c-ubiquinol oxidoreductase, cytb; and 7 subunits of NADH dehydrogenase, nad1-6 and nad4L), 22 transfer RNA (trn) genes (two coding for leucine and two coding for serine) and the small and large subunit ribosomal RNAs (rrnS and rrnL) (Figure 1; Table S1). As with other chromadorean nematode mtDNAs sequenced thus far, the B. procyonis mt genome also lacked the gene encoding atp8. All genes were distributed on the same strand and transcribed in the same direction (5′ to 3′), typical for other nematodes reported to date (except for Trichinella spiralis and Xiphinema americanum) (Figure 1) [24], [25]. Gene order for B. procyonis mtDNA followed the GA7 arrangement [26], with the exception of the relative positions of the AT-rich region and the number of non-coding regions (NCRs). A similar gene arrangement was described previously for members of the orders Ascaridida and Strongylida as well as for the free-living nematode Caenorhabditis elegans [23], [27]–[33]. Only one long unassigned region was present between the genes rrnS and nad1, flanked at the 5′ end by the gene trnS (UCN) and at the 3′ end by the genes trnN and trnY, and it was deemed homologous to the AT-rich region (also known as the control region) by analysis of positional homology, general structure and base content. In addition, the B. procyonis mtDNA contained 17 intergenic spacers ranging in length from 1 to 118 bp (211 bp in total) including the NCR region (Table S1), which was the highest number of intergenic spacers identified in a nematode mt genome thus far [23]. There were only two overlaps found between the genes, with one (1 bp) between cox1 and trnC and another (3 bp) between trnF and cytb (see Table S1).
Figure 1. Graphical representation of B. procyonis mt genome.
Gene abbreviations are as follows: atp6, ATP synthase subunits 6; cox1-3, cytochrome oxidase c subunits 1–3; cytb, cytochrome b; nad1-nad6 and nad4L, NADH dehydrogenase subunits 1–6 and 4L; rrnL and rrnS, ribosomal RNAs. Transfer RNA genes are indicated by one letter symbol according to the IPUC-IUB single-letter amino acid codes. The two leucine and the two serine tRNA genes are differentiated by their respective anti-codons. AT denotes the AT-rich region. The direction of transcription is indicated by an arrow (5′ to 3′).
Base composition and codon usage
The overall base composition (coding strand) for the mt genome sequence of B. procyonis was as follows: A = 22.0%, C = 8.1%, G = 21.4%, T = 48.5%. The A+T bases comprised 68.6% of the protein-coding genes, 72.0% of the rRNAs, 69.6% of the tRNAs and 82.8% of the AT-rich region, giving a total A+T content of 70.5% in this mt genome. This figure was slightly higher than that in the congeneric species B. ailuri (69.5%), B. transfuga (69.4%) and B. schroederi (68.6%), and it was well within the range of the AT contents reported for other nematode species in the same order (68.3–72.0%) (Table 1). In general, the AT and GC skews on the two complementary DNA strands for each mtDNA are regarded as a measure of the compositional asymmetry [34]. For the entire B. procyonis mtDNA, the AT and GC skews for the coding strand were −0.376 and 0.448, respectively, which were significant compared with those of other nematodes characterized to date (AT skews ranging from −0.384 to −0.353, and GC skews from 0.320 to 0.457). A similar trend was also observed in the protein-coding genes. The nucleotide frequency of the protein-coding genes was observed to be in the order T > G > A > C, which excessively favored T (50.8%) and was skewed against C (8.31%). This nucleotide bias would have an appreciable effect on both the codon usage pattern and the relative synonymous codon usage (RSCU). Indeed, the protein-coding genes of B. procyonis were biased towards codons with many T residues [e.g., 13.9% were TTT (phenylalanine)] over those with many C residues [e.g., <0.1% were TCC (serine) or CTC (leucine)] (see Table S2). This phenomenon could be explained by synonymous codon usage bias. Generally codon bias is proposed to be highest in gene regions of functional significance and believed to be important for maximizing translation efficiency [35], [36]. Interestingly, similar nucleotide bias was also reflected in the choices of initiation and termination codons. The most frequently used start codon for B. procyonis was TTG (6 of 12 protein-coding genes; cox1-2, nad1, nad3-4 and nad6) followed by GTG (three genes; nad2, cytb and cox3), and ATT (nad4L and nad5) and ATA (atp6) were also used as initiation codons (Table S1). Ten of the 12 protein-coding genes were predicted to use TAG (atp6, cytb, cox1-3, nad1, nad4 and nad6) or TAA (nad3 and nad4L) as the termination codons, while the remaining genes (nad2 and nad5) were deduced to end with an incomplete codon T.
Table 1. Size and nucleotide composition of different genomic regions in 11 ascarids reported within Ascaridida.
| Species | mtDNA | PCGsb | rRNAs | tRNAs | AT-region | References | |||||
| Size a | AT% | Size a | AT% | Size a | AT% | Size a | AT% | Size a | AT% | ||
| Anisakis simplex | 13916 | 71.2 | 10274 | 69.5 | 1656 | 74.3 | 1208 | 72.4 | 515 | 87.2 | Kim et al. (2006) |
| Ascaris suum | 14284 | 72.0 | 10397 | 70.5 | 1661 | 74.7 | 1252 | 71.0 | 886 | 84.6 | Okimoto et al. (1992) |
| Baylisascaris ailuri | 14657 | 69.5 | 10287 | 67.9 | 1657 | 69.5 | 1241 | 67.0 | 1282 | 82.0 | Xie et al. (2011) |
| Baylisascaris procyonis | 14781 | 70.5 | 10289 | 68.6 | 1664 | 72.0 | 1246 | 69.6 | 1375 | 82.8 | This study |
| Baylisascaris schroederi | 14778 | 68.6 | 10290 | 67.1 | 1657 | 69.8 | 1241 | 67.3 | 1406 | 78.9 | Xie et al. (2011) |
| Baylisascaris transfuga | 14898 | 69.4 | 10290 | 67.6 | 1658 | 69.7 | 1244 | 67.5 | 1516 | 82.3 | Xie et al. (2011) |
| Contracaecum rudolphii B | 14022 | 70.4 | 10281 | 69.0 | 1650 | 72.3 | 1256 | 70.6 | 588 | 89.1 | Unpublished |
| Toxocara canis | 14163 | 68.3 | 10294 | 67.3 | 1617 | 69.6 | 1222 | 69.3 | 828 | 78.1 | Jex et al. (2008) |
| Toxocara canis | 14322 | 68.6 | 10308 | 67.2 | 1655 | 69.8 | 1251 | 68.5 | 975 | 79.5 | Li et al. (2008) |
| Toxocara cati | 14029 | 69.9 | 10284 | 68.8 | 1651 | 71.5 | 1248 | 70.2 | 711 | 81.3 | Li et al. (2008) |
| Toxocara malaysiensis | 14266 | 68.9 | 10297 | 67.8 | 1651 | 68.5 | 1252 | 70.3 | 936 | 78.4 | Li et al. (2008) |
In base pairs.
All protein-coding genes were taken into account.
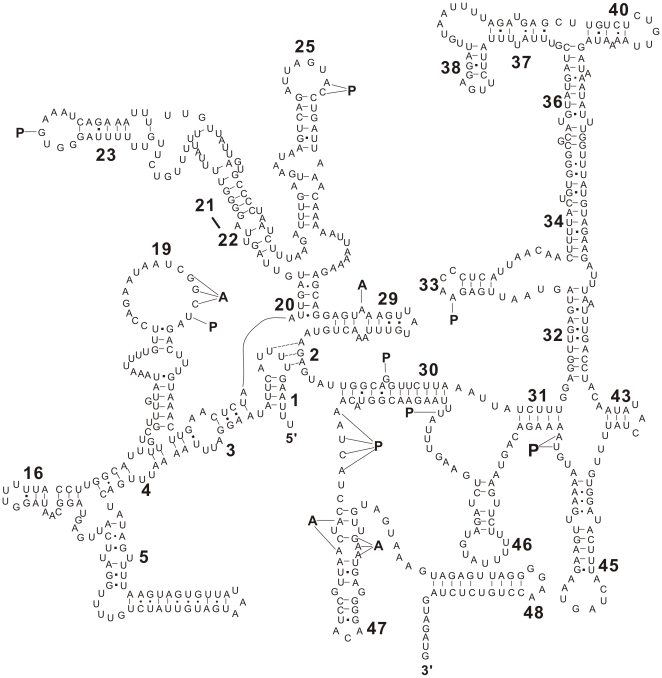
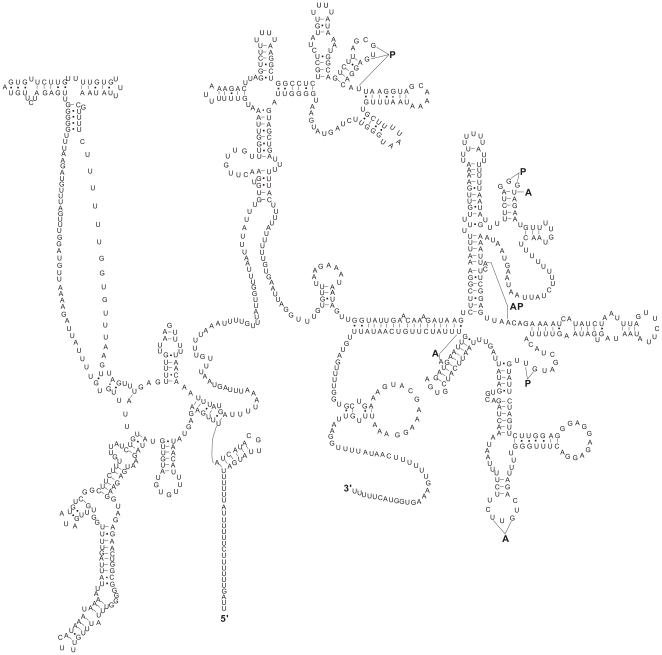
Twenty-two tRNAs were predicted in B. procyonis mtDNA, ranging from 51 to 62 bp in size, and all anti-codon sequences were the same as in other nematodes examined [23], [28] (see Table S1). Their secondary structures were similar to those of all other secernentean nematodes studied to date [23], [27]–[33], [37]–[39], but they were distinctly different from the conventional cloverleaf-like structures described in other metazoan mtDNAs (Figure S1). The lengths of B. procyonis rrnS and rrnL were 700 bp and 964 bp, respectively, and the corresponding secondary structures are shown in Figure 2 (rrnS) and Figure 3 (rrnL). However, it seemed that the relatively high AT content displayed in B. procyonis mtDNA was conspicuous for the tRNAs and rRNAs genes, compared with those of the congeneric species. The AT-contents of the rRNAs sequence were 2.5%, 2.2% and 2.3% greater than that of B. ailuri (69.5%), B. schroederi (69.7%) and B. transfuga (69.8%), respectively. Likewise, the AT contents of the tRNAs sequence were 2.6%, 2.3% and 2.1% more than those found in B. ailuri (67.0%), B. schroederi (67.3%) and B. transfuga (67.5%), respectively (Table 1) [23]. Interestingly, this level of AT content of the B. procyonis mt tRNAs and rRNAs genes does not influence their secondary structures. As shown in Figures S1, 2 and 3, the secondary structures of tRNAs and rRNAs in B. procyonis were similar with those exhibited in the congeneric B. ailuri, B. transfuga and B. schroederi as well as other nematodes described in Ascaridida to date [23], [27], [28], [30], [33].
Figure 2. Predicted secondary structure for the small ribosomal RNA gene subunit (rrnS) in B. procyonis mtDNA.
Base-pairing is indicated as follows: Watson-Crick pairs by lines, wobble GU pairs by large dots and other non-canonical pairs by small dots. Conserved secondary structure elements are denoted by bold numbers (1–48) [29]. Binding sites for the amino-acyl trn (A) or peptidyl-transferase (P) [54] are indicated by lines.
Figure 3. Predicted secondary structure for the large ribosomal RNA gene subunit (rrnL) in B. procyonis mtDNA.
Symbols for base-pairings are as in Figure 2. Binding sites for the amino-acyl trn (A), peptidyl-transferase (P) or both (AP) [54] are indicated by lines.
The length of the AT-rich region was 1375 bp in the B. procyonis mtDNA (Table S1 or Figure 1), and its predicted complex stem-loop structures are shown in Figure S2. This region along with its counterpart in B. schroederi (1406 bp), B. ailuri (1282 bp) and B. transfuga (1516 bp) represented the longest studied thus far among most other secernentean nematodes [23]. In secondary structure analysis, it appeared that there was an additional stem-loop structure at the end of the AT-rich region in B. procyonis compared with that of B. ailuri, while some stems or loops were missing compared with that of B. transfuga and B. schroederi (not shown). These differences may relate to the AT-rich region as being the most variable portion of the genome both in terms of length and nucleotide sequence. However, many similar stem-loop structures found in these four Baylisascaris species implied that they may be conserved and function in regulation of transcription and control of DNA replication [40]. Additionally, the AT-rich region of the B. procyonis mtDNA contained 31 regions with varying numbers of the dinucleotide (TA) repeat (n = 3 to 21) within a total of 344 bp. Similar multiple TA repeats have been described in the AT-rich region of the mt genomes for other Ascaridida and Strongylida species [23], [27]–[33]. Currently the function or role of these AT repeats remains unclear. Other repetitive elements, such as CR1-CR6 identified in the C. elegans AT-rich region [27] were not found in B. procyonis.
Levels of variability and informativeness within and between Ascaridida mtDNAs
The comparison of protein-coding and rRNA genes in B. procyonis mtDNA with those of ten other published Ascaridida nematodes [B. ailuri, B. transfuga, B. schroederi and A. suum (Ascarididae family); Anisakis simplex and Contracaecum rudolphii B (Anisakidae family); T. canis, T. cati and Toxocara malaysiensis (Toxocaridae family)] is shown in Table S3. The deduced length of the 12 protein-coding genes were consistent with those of the congeneric B. ailuri, B. transfuga and B. schroederi, except for the cox1 (which was one amino acid longer than that of B. ailuri) and nad2 (which was one nucleotide shorter than that of B. transfuga and B. schroederi) genes, and along with two rRNA genes were in the size range of those of the other seven nematode mtDNAs. The nucleotide and amino acid sequences similarities for each of the 12 mt proteins of B. procyonis ranged from 85.9–92.2% and 83.9–98.3%, respectively, between B. procyonis and the congeneric B. ailuri, B. transfuga and B. schroederi; and from 82.5–92.1% and 82.3–97.9%, respectively, between B. procyonis and A. suum. The nucleotide and amino acid sequences similarities between B. procyonis and each species of Toxocaridae were 77.6–89.1% and 74.0–95.4%, respectively; and 73.8–85.9% and 71.5–93.1%, respectively, between B. procyonis and each species of Anisakidae. Based on the sequence similarities, the most conserved protein-coding genes among the 11 species (including B. procyonis) were cox1 and cox2, while the least conserved were cytb and nad4. For the genes rrnS and rrnL, the highest nucleotide similarities were observed between those of B. procyonis and the congeneric B. ailuri, B. transfuga, B. schroederi and A. suum, with the percent identities being above 91.0% and 85.9%, respectively, followed by the similarities between B. procyonis and individual species representing the Toxocaridae and Anisakidae families, with the percent identities ranging from 78.1–81.9% and 75.9–79.2%, respectively (Table S3). In addition, the nucleotide sequence of the AT-region in the B. procyonis mtDNA appeared to share low similarity (all values <60%) with that of the ten described Ascaridida species, including the congeneric B. ailuri, B. transfuga and B. schroederi (not shown). Combined, these results from pairwise comparisons of nucleotide and amino acid sequences from the protein-coding genes as well as the nucleotide sequences of the rRNA genes suggested that B. procyonis mtDNA most closely resemble those of members of the Ascarididae family, followed by members of the Toxocaridae and Anisakidae families.
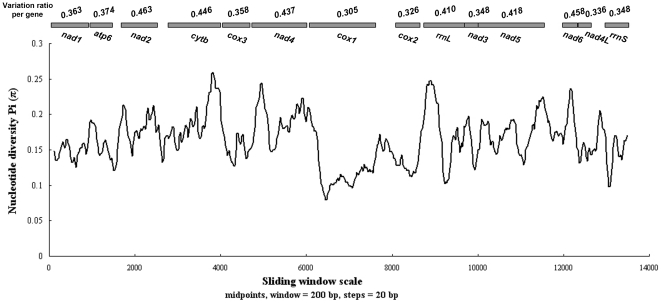
Sliding window analysis of the complete nucleotide alignment of 11 available Ascaridida mtDNAs provided an indication of nucleotide diversity Pi (π) within and between mt genes (see Figure 4). In the curve, the nucleotide variation within and between mt genes among the aligned Ascaridida genomes was intuitively displayed for any given window of 200 bp and steps of 20 bp, with the Pi (π) ranging from 0.075 to 0.262. Coupled with computation of the number of variable positions per unit length of gene, the sliding window showed that the genes with low sequence variability included cox1 (0.305), cox2 (0.326), nad4L (0.336), nad3 (0.348) and rrnS (0.348), while the genes with high sequence variability included nad2 (0.463), nad6 (0.458), cytb (0.446), nad4 (0.437), nad5 (0.418) and rrnL (0.410). Interestingly, amongst the genes with high sequence variability, the genes with pronounced peaks and troughs of Pi (π) appeared to possess higher sequence variability than others, such as nad2, cytb, nad4, nad5 and rrnL (see Figure 4). Based on these results, it seemed that cox1 and cox2 were still the most conserved protein-coding genes, and cytb and nad4 were still within the least conserved ones. These observations were remarkably consistent with the findings from pairwise comparisons made among the nucleotide and amino acid sequences from the protein-coding genes in B. procyonis mtDNA with those of the other ten published Ascaridida nematodes. These results further suggested that there are still a considerable number of alternative genes (aside from the cox2 gene [2]) to be determined as new genetic markers for phylogenetics, population genetics and diagnostics. Current mt genes used as molecular targets for PCR assays based approaches for detection/diagnostics in the order Ascaridida include cox2 and cytb [2], [41], and cox2 is also targeted for development of a probe-based (using a molecular beacon) real-time PCR for diagnosis [16]. Although relatively easy to amplify routinely, based on pairwise comparison and sliding window analysis of mt genes among the available 11 Ascaridida mtDNAs, cox2 is among the slowest evolving and least variable genes available in B. procyonis mtDNA. Therefore, more reliable, or at least more informative, markers should be considered for future work, especially for diagnostics/detection involving cross contamination or other Baylisascaris species. From the analysis in the present study, compared with the cox2 gene, it seemed that cytb and nad4 may be more suitable as molecular genetic markers for diagnosis and identification between B. procyonis and other related ascaridoids because of their higher variability. As shown in sliding window analysis (Figure 4), both the cytb and nad4 genes were found to possess more variable positions per unit length of gene than cox2. Perhaps these markers can be further validated when additional Ascaridida mt genomes become available, especially from the genus Baylisascaris.
Figure 4. Sliding window analysis of the alignment of complete mtDNAs of 11 species of Ascaridida.
The black line shows the value of nucleotide diversity Pi (π) in a sliding window analysis of window size 200 bp with step size 20, and the value is inserted at its mid-point. Gene boundaries are indicated with a variation ratio per gene.
Prediction of novel mt fragments for PCR specific identification within Ascaridida
Considering the level of nucleotide variability and nucleotide and/or amino acid sequence similarity within and between mt genes among the available Ascaridida mtDNAs, the genes rrnL and cytb were selected as potentially new mt fragments for PCR specific identification within the Ascaridida species. Subsequently, PCR primer pairs were designed targeting a 200 bp fragment in rrnL and a 548 bp fragment in cytb after manual inspection of the pre-aligned rrnL and cytb sequences. The primer pairs were: A (rrnL) forward 5′-GAGAACTGGCGGGG-3′, reverse 5′-CTCACACTGACTTACACACC-3′; B (cytb) forward 5′-TCCTTAGTAATGAGTATTGCGT-3′, reverse 5′-TATAACGACATTTGAAAAACACC-3′. The specificities of the two primer pairs designed herein were tested by PCR of the mt DNA from B. procyonis, T. canis, T. leonina, A. suum and the four congeners (B. schroederi, B. ailuri, B. transfuga and B. columnaris). As expected, the two targeted PCR amplification bands (200 bp rrnL and 548 bp cytb) were observed only in B. procyonis (data not shown). The PCR results demonstrated that the two fragments could be readily used to differentiate B. procyonis from other related ascaridoids, including the morphologically similar Baylisascaris spp. This finding would not only fill the technical gap by providing new mt fragments for effectively and specifically distinguishing between B. procyonis and B. columnaris [2], but it would also be useful for improving the efficiency and accuracy of environmental investigations for B. procyonis. Samples of B. procyonis eggs collected in nature are often cross-contaminated with eggs of other congeners, such as B. transfuga (from bears), B. columnaris (from skunks) and B. melis (from badgers), due to the presence of various infected hosts in an area. Such conditions could make it time-consuming or difficult for accurate identification of the parasitic species based on either the current methods of morphological examination [11] or existing molecular beacons (e.g., cox2) [2], [16]. Herein, we postulated that PCR amplification of the pair of the newly identified mt fragments (200 bp rrnL and 548 bp cytb) using primers developed in this study could rapidly and accurately identify and discriminate between B. procyonis and B. transfuga, B. columnaris and B. melis. However, other faint bands were also detected in our PCR assays, such as a 300bp band in B. procyonis and multiple bands in B. transfuga (not shown), which may relate to the specificity of primer pair A. These results suggested that the limited number of available mt genomes in the genus Baylisascaris is still a major barrier for screening effective mt fragments for PCR specific amplification used for identification and differentiation between B. procyonis and other congeners.
Phylogeny
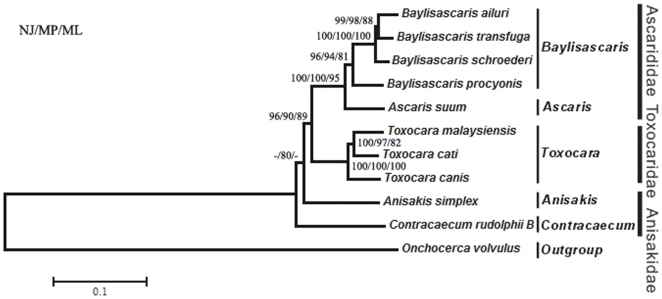
The availability of the B. procyonis mt genome provided us with another opportunity to probe the phylogenetic positions of the species B. procyonis within the genus Baylisasaris and of the genus Baylisascaris within the order Ascaridida. As expected from and congruent with previous phylogenetic analyses [23], [28], [29], [31], [38], phylogenies in this study inferred from the concatenated amino acid sequence dataset derived from 12 protein-coding mitochondrial genes. After the final alignment, the concatenated amino acid sequences (containing 3423 residues, including 2151 variable and 662 parsimony-informative) of 12 protein-coding genes for the 11 taxas (B. procyonis B. schroederi, B. ailuri, B. transfuga, A. suum, T. canis, T. cati, T. malaysiensis, A. simplex, C. rudolphii B and Onchocerca volvulus) were used to reconstruct the phylogenetic relationships based on maximum parsimony (MP), neighbor-joining (NJ) and maximum likelihood (ML) methods (Figure 5). All three phylogenetic analyses (NJ/MP/ML) conducted clearly supported the distinct classification positions of the genera Baylisascaris and Ascaris (family Ascarididae), Toxocara (family Toxocaridae), Anisakis (family Anisakidae) in the order Ascaridida, each as a monophyletic group with high statistical support (all bootstrap values >80) (Figure 5). Among the genus Baylisascaris, the phylogenetic analysis indicated a closer relationship between B. procyonis and B. schroederi than between B. procyonis and B. ailuri or B. transfuga. This finding was congruent with the results of a previous study using partial sequences of nuclear internal transcribed spacers (ITS) as genetic markers [42] and further confirmed the phylogenetic position of B. procyonis within the genus Baylisascaris. For the interrelationships of B. ailuri, B. transfuga and B. schroederi in the genus Baylisascaris and T. canis, T. cati and T. malaysiensis in genus Toxocara, their phylogenetic topologies were consistent with previously proposed molecular phylogeny based on mt data [23], [28].
Figure 5. Phylogenetic relationships of ten Ascaridida species for which complete mtDNAs are available, inferred from NJ, MP and ML analysis for amino acid sequence data derived from 12 protein-coding genes, utilizing one filarioid species (O. volvulus) as the outgroup.
Numbers above the branches represent bootstrap values derived from different analyses in the order: NJ/MP/ML. The scale indicates an estimate of substitutions per site, using the optimized model setting. The branches that were not universally supported with values of ≤50% are indicated with “-” in each supporting values of the node.
In addition, the genus Baylisascaris was determined to be more closely related to Ascaris than to Toxocara, Anisakis and Contracaecum in the order Ascaridida in our phylogenetic analysis (Figure 5), which was consistent with results of previous morphological and molecular studies [23], [42]–[44]. But relationships between any of the Baylisascaris spp. (B. procyonis B. schroederi, B. ailuri and B. transfuga), Toxocara spp. (T. canis, T. cati and T. malaysiensis), Ascaris spp. (A. suum) and Anisakis spp. (A. simplex) and Contracaecum spp. (C. rudolphii B) were poorly inferred in the NJ and ML analyses (Figure 5). Therefore, a larger study of the evolutionary relationships among taxa within the order Ascaridida using mt data is warranted. The recent validation of the high-throughput sequencing technique for the sequencing of mt genomes provides a platform for an in-depth phylogenetic analysis of the order Ascaridida [32].
In conclusion, the complete mt genome of B. procyonis involved in 98–99% of Baylisascaris NLM and OLM cases in humans or other animals [11] was reported in our study. B. procyonis mtDNA showed a typical chromadorean mitogenome structure, but a long AT-rich region and high number of intergenic spacers made it unique compared with other nematodes characterized to date. In addition, the entire genome displayed notable levels of AT and GC skewing. Based on pairwise comparison and sliding window analysis within and between mt genes among the available 11 Ascaridida mtDNAs (including B. procyonis mtDNA), two new mt fragments (200 bp rrnL and 548 bp cytb) proved to be suitable as molecular targets for PCR based diagnosis and identification of B. procyonis. Finally the analysis of amino acids deduced from mtDNAs provided substantial support for the phylogenetic relationships of Ascaridida species; B. procyonis was more closely related to B. schroederi than to B. ailuri and B. transfuga in the genus Baylisascaris, and the genus Baylisascaris was more closely related to Ascaris than to Toxocara, Anisakis and Contracaecum in the order Ascaridida. The complete mitogenome of B. procyonis sequenced here is expected to render implications for molecular diagnostic methods, epidemiological investigations and ecological studies of B. procyonis and other related ascaridoids. The findings are important in the refinement of the phylogenetic relationships within the order Ascaridida and in accumulating valid markers for systematic, population genetic and evolutionary biological studies of parasitic nematodes of socio-economic importance.
Materials and Methods
An adult female specimen of B. procyonis was obtained from an infected raccoon housed in the Chengdu Zoological Garden, Sichuan Province of China, after treatment with pyrantel pamoate. After washing in physiological saline, the morphological identification of the worm was performed based on the taxonomic key of Hartwich (1962). Total genomic DNA was isolated from a small portion (1 cm) of the specimen using the Universal Genomic DNA Extraction Kit Ver. 3.0 (TaKaRa, Japan). In order to further verify the identity of the specimen, the ITS1 and ITS2 sequences of nuclear ribosomal DNA (rDNA) were amplified by the PCR and compared with those previously reported for B. procyonis (Accession numbers: AB053230 and AB051231) [45].
The entire mt genome was amplified in ten overlapping segments (ranging in length from 873 bp to 2.47 kb) by PCR with Ex Taq Polymerase (TaKaRa, Japan), using ∼15 ng of total genomic DNA from the sample as template. The PCR primers were designed based on the alignments of the relatively conserved regions of congeneric B. schroederi, B. ailuri and B. transfuga and A. suum mt genome sequences. The names and corresponding primer sequences are shown in Table S4. All PCR reactions were carried out in a final reaction volume of 25 µl containing 1.5 µl of genomic DNA extract, 1 U Ex Taq Polymerase, 10× Ex Taq buffer, 0.2 mM of each dNTP, 10 pmol of each primer and ddH2O. PCR cycling conditions carried out in a Mastercycler Gradient 5331 thermocycler (Ependorf, Germany) were 5 min denaturation at 95°C, followed by 35 cycles of 30 s at 95°C, 30 s at 55°C and 2 to 5 min at 68°C according to the product length, with a final extension at 68°C for 10 min. Each PCR yielded a single band, detected in a 1% (W/V) agarose gel stained with ethidium bromide (not shown). Each amplicon was then purified using the TIANgel Midi Purification Kit (TiangenBiotech, China) and was subjected to automated sequencing, either directly or following sub-cloning into the pMD19-T vector (TaKaRa, Japan). To ensure maximum accuracy, each amplicon was sequenced twice independently, and in case of discrepancies a third PCR product was sequenced. Sequencing was performed using terminator-based cycle sequencing with BigDye chemistry (Applied Biosystems, Foster City, CA, USA) on an ABI 3730 DNA sequencer (Applied Biosystems), utilizing a primer-walking strategy (in both directions). The consensus sequences were assembled manually in a single contig and aligned with the published complete mt genome sequences of B. transfuga and A. suum [23], [27] using the Clustal X program, and the circular map was drawn using the program MacVector v. 9.5 (http://www.macvector.com/index.html). Genome annotation, and the comparisons with congeneric B. schroederi, B. ailuri and B. transfuga as well as other nematodes in the same order were performed using DNAMAN version 3.0 (Lynnon Biosoft, Quebec, Canada) and on-line blast tools available through the NCBI website [46]. Base composition and codon usage were calculated in DNAStar software (DNAStar, USA). Secondary structures of tRNA and rRNA were predicted using standard approaches [29]. The complete nucleotide sequences of mtDNAs for 11 Ascaridata species (including B. procyonis) were aligned using the MEGA 3.1 [47]. Subsequently, the complete alignment was used to accomplish sliding window analyses with the DnaSP ver.5.10 software package (http://www.ub.es/dnasp) [48]. A sliding window of 200 bp and steps of 20 bp were used to estimate nucleotide diversity Pi (π) for the complete alignment. Nucleotide diversity for the complete alignment was plotted against midpoint positions of each window, and gene boundaries were indicated. Based on Pi (π) values and nucleotide and/or amino acid sequence similarities within and between mt genes among the available Ascaridida mtDNAs, new mt fragments for PCR specific identification were selected, and corresponding PCR primer pairs for amplification were developed with either Primer Premier version 5.0 (Premier Biosoft International, Palo Alto, CA) or an on-line program PRIMER3 (www.genome.wi.mit.edu/cgi-bin/primer/primer3www.cgi), with the parameters modified as follows: melting temperature = 45.0∼55.0°C, minimum number of 3′-end matches = 3, optimal primer length interval = (14, 24 bp), optimal PCR product length interval = (200, 400, 600 bp), minimum product length = 150 bp. The specificities of the primer pairs designed were tested by PCR. All PCR reactions containing 10∼20 ng of the genomic DNA were performed in 50 µl volumes with 10 pmol of each primer, 250 µM of each dNTP, 2.0 mM MgCl2, and 2 U Taq polymerase under the following conditions: an initial denaturation at 94°C for 4 min followed by 35 cycles of 94°C for 30 sec, 50∼55°C for 30 sec, 72°C for 45 sec, and a final step at 72°C for 10 min.
For the phylogenetic analysis, in addition to B. procyonis mt genome sequenced in this study [GenBank: JF951366], the following mtDNAs from Nematoda were retrieved from GenBank: B. ailuri [GenBank: HQ671080], B. transfuga [GenBank: HQ671079], B. schroederi [GenBank: HQ671081], A. suum [GenBank: NC_001327], T. canis [GenBank: NC_010690], T. cati [GenBank: NC_010773], T. malaysiensis [GenBank: NC_010527], A. simplex [GenBank: NC_007934], C. rudolphii B [GenBank: NC_014870] and O. volvulus [GenBank: NC_001861]. Phylogenetic analyses were performed using the ten ascaridoid species (B. procyonis, B. schroederi, B. ailuri, B. transfuga, T. canis, T. cati, T. malaysiensis, A. suum, A. simplex and C. rudolphii B) as ingroups, and one filarioid species (O. volvulus) serving as outgroup. Twelve mitochondrial protein sequences were inferred using the Invertebrate Mitochondrial Code (Table five GenBank; http://www.ncbi.nlm.nih.gov/Taxonomy/Utils/wprintgc.cgi?mode=c#SG5). The predicted amino acid sequences were aligned using T-COFFEE 7.81 [49], the ambiguous regions within these alignments filtered with GBLOCKS 0.91 b [50], and then the filtered individual sequences were concatenated for subsequent phylogenetic analysis. The Dayhoff matrix model determined by ProtTest 2.0 [51] was employed in the NJ analysis using MEGA 3.1 [47]. MP phylogenetic reconstructions were conducted in PAUP* 4.0b10 [52], using heuristic searches with a tree-bisection-reconnection (TBR) branch-swapping algorithm and 1000 random-addition sequence replicates with ten trees held at each step, and finally the optimal topology was obtained using Kishino-Hasegawa. The ML computations were performed using PHYML 3.0 [53] under the LG +C4 + F + I model of amino acid substitution selected with ProtTest program. Branch supports were evaluated by bootstrapping analysis of 1000 replicates for NJ and MP trees, and 100 replicates for the ML tree.
Supporting Information
Predicted secondary structure of 22 tRNAs genes in the mt genome of B. procyonis .
(TIF)
Secondary structure predicted for the AT-rich region in the B. procyonis mt genome.
(TIF)
Mitochondrial genome profiles of B. procyonis .
(DOC)
Nucleotide codon usage for 12 protein-coding genes of the mitochondrial genome of B. procyonis . Total number of codons is 3429. a Total number in all open reading frames. *** Stop codon. RSCU, relative synonymous codon usage.
(DOC)
Comparison of mitochondrial protein and rRNA genes with those of other nematodes sequenced in Ascaridida. a: From Jex et al. (2008). GenBank accession no. EU730761. b: From Li et al. (2008). GenBank accession no. NC_010690. Bp: B. procyonis, Bs: B. schroederi, Ba: B. ailuri, Bt: B. transfuga, Asu: A. suum, Asi: A. simplex, Cr: C. rudolphii B, Tcan: T. canis, Tcat: T. cati, Tmal: T. malaysiensis.
(DOC)
List of the ten primer pairs for PCR amplification and their positions in the mt genome of B. procyonis.
(DOC)
Acknowledgments
We thank HJY from Veterinary Department at Chengdu Zoological Garden of China for the sampling contribution; YL and ZGC from Sichuan Agricultural University of China, and MWL from Guangdong Ocean University of China, for their technical assistance and discussion.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by grant from the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT) (grant no. IRT0848), the Research Fund (Project No. CPF2010-08) for the Chengdu Research of Giant Panda Breeding and the Special Funds for the Training of PhD Students from the Ministry of Education, China (grant no. 20095103110007). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Blizzard EL, Yabsley MJ, Beck MF, Harsch S. Geographic expansion of Baylisascaris procyonis roundworms, Florida, USA. Emerg Infect Dis. 2011;16:1803–1804. doi: 10.3201/eid1611.100549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dangoudoubiyam S, Vemulapalli R, Kazacos KR. PCR assays for detection of Baylisascaris procyonis eggs and larvae. J Parasitol. 2009;95:571–577. doi: 10.1645/GE-1905.1. [DOI] [PubMed] [Google Scholar]
- 3.Kazacos KR, Boyce WM. Baylisascaris larva migrans. J Am Vet Med Assoc. 1989;195:894–903. [PubMed] [Google Scholar]
- 4.Kazacos KR. Protecting children from helminthic zoonoses. Contemp Pediatr. 2000;17:1–24. [Google Scholar]
- 5.Park SY, Glaser C, Murray WJ, Kazacos KR, Rowley HA, et al. Raccoon roundworm (Baylisascaris procyonis) encephalitis: case report and field investigation. Pediatrics. 2000;106:E56. doi: 10.1542/peds.106.4.e56. [DOI] [PubMed] [Google Scholar]
- 6.Gavin PJ, Shulman ST, Kazacos KR, Davis AT, Mets MB, et al. American Society of Microbiology Conference Abstract No. C-443; 2001. Neural larva migrans caused by the raccoon roundworm Baylisascaris procyonis: Two pediatric cases from Chicago, Illinois. [Google Scholar]
- 7.Boschetti A, Kasznica J. Visceral larva migrans induced eosinophilic cardiac pseudotumor: a cause of sudden death in a child. J Forensic Sci. 1995;40:1097–1099. [PubMed] [Google Scholar]
- 8.Cunningham CK, Kazacos KR, McMillan JA, Lucas JA, McAuley JB, et al. Diagnosis and management of Baylisascaris procyonis infection in an infant with nonfatal meningoencephalitis. Clin Infect Dis. 1994;18:868–872. doi: 10.1093/clinids/18.6.868. [DOI] [PubMed] [Google Scholar]
- 9.Huff DS, Neafie RC, Binder MJ, De Leon GA, Brown LW, et al. Case 4. The first fatal Baylisascaris infection in humans: an infant with eosinophilic meningoencephalitis. Pediatr Pathol. 1984;2:345–352. doi: 10.3109/15513818409022268. [DOI] [PubMed] [Google Scholar]
- 10.Gavin PJ, Kazacos KR, Shulman ST. Baylisascariasis. Clin Microbiol Rev. 2005;18:703–718. doi: 10.1128/CMR.18.4.703-718.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kazacos KR. Baylisascaris procyonis and related species. In: Samuel WM, Pybus MJ, Kocan AA, editors. Parasitic diseases of wild mammals. Ames: Iowa State University Press; 2001. pp. 301–341. (2nd) [Google Scholar]
- 12.Orihel T, Ash LR. Chicago: American Society of Clinical Pathologists; 1995. Parasites in human tissue. [Google Scholar]
- 13.Beaver PC, Jung RC, Cupp EW. Philadelphia: Lea & Febiger Press; 1984. Clinical parasitology.825 [Google Scholar]
- 14.Zhan B, Li T, Xiao S, Zheng F, Hawdon JM. Species-specific identification of human hookworms by PCR of the mitochondrial cytochrome oxidase I gene. J Parasitol. 2001;87:1227–1229. doi: 10.1645/0022-3395(2001)087[1227:SSIOHH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 15.Hu M, Chilton NB, Gasser RB. The mitochondrial genomics of parasitic nematodes of socio-economic importance: recent progress, and implications for population genetics and systematics. Adv Parasitol. 2004;56:133–212. doi: 10.1016/s0065-308x(03)56003-1. [DOI] [PubMed] [Google Scholar]
- 16.Gatcombe RR, Jothikumar N, Dangoudoubiyam S, Kazacos KR, Hill VR. Evaluation of a molecular beacon real-time PCR assay for detection of Baylisascaris procyonis in different soil types and water samples. Parasitol Res. 2010;106:499–504. doi: 10.1007/s00436-009-1692-6. [DOI] [PubMed] [Google Scholar]
- 17.Gasser RB, Monti JR, Bao ZQ, Polderman AM, Nansen P, Chilton NB. A mutation scanning approach for the identification of hookworm species and analysis of population variation. Mol Biochem Parasitol. 1998;92:303–312. doi: 10.1016/s0166-6851(98)00008-5. [DOI] [PubMed] [Google Scholar]
- 18.Anderson TC, Blouin MS, Beech RN. Population biology of parasitic nematodes: applications of genetic markers. Adv Parasitol. 1998;41:219–283. doi: 10.1016/s0065-308x(08)60425-x. [DOI] [PubMed] [Google Scholar]
- 19.Blouin MS. Mitochondrial DNA diversity in nematodes. J Helminthol. 1998;72:285–289. doi: 10.1017/s0022149x00016618. [DOI] [PubMed] [Google Scholar]
- 20.Blouin MS. Molecular prospecting for cryptic species of nematodes: mitochondrial DNA versus internal transcribed spacer. Int J Parasitol. 2002;32:527–531. doi: 10.1016/s0020-7519(01)00357-5. [DOI] [PubMed] [Google Scholar]
- 21.Gasser RB. Molecular tools - advances, opportunities and prospects. Vet Parasitol. 2006;136:69–89. doi: 10.1016/j.vetpar.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Hu M, Gasser RB. Mitochondrial genomes of parasitic nematodes--progress and perspectives. Trends Parasitol. 2006;22:78–84. doi: 10.1016/j.pt.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Xie Y, Zhang ZH, Wang CD, Lan JC, Li Y, et al. Complete mitochondrial genomes of Baylisascaris schroederi, Baylisascaris ailuri and Baylisascaris transfuga from Giant Panda, Red Panda and Polar Bear. Gene. 2011;482:59–67. doi: 10.1016/j.gene.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Lavrov DV, Brown WM. Trichinella spiralis mtDNA: a nematode mitochondrial genome that encodes a putative ATP8 and normally structured tRNAS and has a gene arrangement relatable to those of coelomate metazoans. Genetics. 2001;157:621–637. doi: 10.1093/genetics/157.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He Y, Jones J, Armstrong M, Lamberti F, Moens M. The mitochondrial genome of Xiphinema americanum sensu stricto (Nematoda: Enoplea): considerable economization in the length and structural features of encoded genes. J Mol Evol. 2005;61:819–833. doi: 10.1007/s00239-005-0102-7. [DOI] [PubMed] [Google Scholar]
- 26.Yatawara L, Wickramasinghe S, Rajapakse RP, Agatsuma T. The complete mitochondrial genome of Setaria digitata (Nematoda: Filarioidea): Mitochondrial gene content, arrangement and composition compared with other nematodes. Mol Biochem Parasitol. 2010;173:32–38. doi: 10.1016/j.molbiopara.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Okimoto R, Macfarlane JL, Clary DO, Wolstenholme DR. The mitochondrial genomes of two nematodes, Caenorhabditis elegans and Ascaris suum. Genetics. 1992;130:471–498. doi: 10.1093/genetics/130.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li MW, Lin RQ, Song HQ, Wu XY, Zhu XQ. The complete mitochondrial genomes for three Toxocara species of human and animal health significance. BMC Genomics. 2008;9:224. doi: 10.1186/1471-2164-9-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu M, Chilton NB, Gasser RB. The mitochondrial genomes of the human hookworms, Ancylostoma duodenale and Necator americanus (Nematoda: Secernentea). Int J Parasitol. 2002;32:145–158. doi: 10.1016/s0020-7519(01)00316-2. [DOI] [PubMed] [Google Scholar]
- 30.Jex AR, Waeschenbach A, Littlewood DT, Hu M, Gasser RB. The mitochondrial genome of Toxocara canis. PLoS Negl Trop Dis. 2008;2:e273. doi: 10.1371/journal.pntd.0000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jex AR, Waeschenbach A, Hu M, van Wyk JA, Beveridge I, et al. The mitochondrial genomes of Ancylostoma caninum and Bunostomum phlebotomum--two hookworms of animal health and zoonotic importance. BMC Genomics. 2009;10:79. doi: 10.1186/1471-2164-10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jex AR, Hu M, Littlewood DT, Waeschenbach A, Gasser RB. Using 454 technology for long-PCR based sequencing of the complete mitochondrial genome from single Haemonchus contortus (Nematoda). BMC Genomics. 2008;9:11. doi: 10.1186/1471-2164-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wickramasinghe S, Yatawara L, Rajapakse RP, Agatsuma T. Toxocara vitulorum (Ascaridida: Nematoda): mitochondrial gene content, arrangement and composition compared with other Toxocara species. Mol Biochem Parasitol. 2009;166:89–92. doi: 10.1016/j.molbiopara.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 34.Saccone C, Gissi C, Lanave C, Larizza A, Pesole G, et al. Evolution of the mitochondrial genetic system: an overview. Gene. 2000;261:153–159. doi: 10.1016/s0378-1119(00)00484-4. [DOI] [PubMed] [Google Scholar]
- 35.Sharp PM, Matassi G. Codon usage and genome evolution. Curr Opin Genet Dev. 1994;4:851–860. doi: 10.1016/0959-437x(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 36.Duret L, Mouchiroud D. Expression pattern and, surprisingly, gene length shape codon usage in Caenorhabditis, Drosophila, and Arabidopsis. Proc Natl Acad Sci U S A. 1999;96:4482–4487. doi: 10.1073/pnas.96.8.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu M, Chilton NB, Gasser RB. The mitochondrial genome of Strongyloides stercoralis (Nematoda) - idiosyncratic gene order and evolutionary implications. Int J Parasitol. 2003;33:1393–1408. doi: 10.1016/s0020-7519(03)00130-9. [DOI] [PubMed] [Google Scholar]
- 38.Hu M, Gasser RB, Abs El-Osta YG, Chilton NB. Structure and organization of the mitochondrial genome of the canine heartworm, Dirofilaria immitis. Parasitology. 2003;127:37–51. doi: 10.1017/s0031182003003275. [DOI] [PubMed] [Google Scholar]
- 39.Keddie EM, Higazi T, Unnasch TR. The mitochondrial genome of Onchocerca volvulus: sequence, structure and phylogenetic analysis. Mol Biochem Parasitol. 1998;95:111–127. doi: 10.1016/s0166-6851(98)00102-9. [DOI] [PubMed] [Google Scholar]
- 40.Clayton DA. Replication and transcription of vertebrate mitochondrial DNA. Annu Rev Cell Biol. 1991;7:453–478. doi: 10.1146/annurev.cb.07.110191.002321. [DOI] [PubMed] [Google Scholar]
- 41.Leles D, Araújo A, Vicente AC, Iñiguez AM. Molecular diagnosis of ascariasis from human feces and description of a new Ascaris sp. genotype in Brazil. Vet Parasitol. 2009;163:167–170. doi: 10.1016/j.vetpar.2009.03.050. [DOI] [PubMed] [Google Scholar]
- 42.He GZ, Niu LL, Yang GY, Deng JB, Wang S, et al. Sequence analysis of ITS-2 rDNA of roundworms from Ailuropoda melanoleuca and rare wild animals. Chin J Vet Sci. 2008;38:933–938. [Google Scholar]
- 43.Nadler SA, Hudspeth DS. Ribosomal DNA and phylogeny of the Ascaridoidea (Nemata: Secernentea): implications for morphological evolution and classification. Mol Phylogenet Evol. 1998;10:221–236. doi: 10.1006/mpev.1998.0514. [DOI] [PubMed] [Google Scholar]
- 44.Hartwich G. Keys to genera of the Ascaridoidea. In: Anderson RC, Chabaud AG, Willmott S, editors. CIH keys to the nematode parasites of vertebrates, Volume 2. Commonwealth Agricultural Bureaux, Farnham Royal; 1974. pp. 1–14. [Google Scholar]
- 45.Blizzard EL, Davis CD, Henke S, Long DB, Hall CA, et al. Distribution, prevalence, and genetic characterization of Baylisascaris procyonis in selected areas of Georgia. J Parasitol. 2010;96:1128–1133. doi: 10.1645/GE-2518.1. [DOI] [PubMed] [Google Scholar]
- 46.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar S, Tamura K, Nei M. MEGA3: Integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief Bioinform. 2004;5:150–163. doi: 10.1093/bib/5.2.150. [DOI] [PubMed] [Google Scholar]
- 48.Rozas J, Sanchez-DelBarrio JC, Messeguer X, Rozas R. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics. 2003;19:2496–2497. doi: 10.1093/bioinformatics/btg359. [DOI] [PubMed] [Google Scholar]
- 49.Notredame C, Higgins DG, Heringa J. T-Coffee: A novel method for fast and accurate multiple sequence alignment. J Mol Biol. 2000;302:205–217. doi: 10.1006/jmbi.2000.4042. [DOI] [PubMed] [Google Scholar]
- 50.Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol. 2000;17:540–552. doi: 10.1093/oxfordjournals.molbev.a026334. [DOI] [PubMed] [Google Scholar]
- 51.Abascal F, Zardoya R, Posada D. ProtTest: selection of best-fit models of protein evolution. Bioinformatics. 2005;21:2104–2105. doi: 10.1093/bioinformatics/bti263. [DOI] [PubMed] [Google Scholar]
- 52.Swofford DL. Sunderland, MA, Sinauer Associates; 2002. PAUP*: Phylogenetic analysis using parsimony (and other methods). [Google Scholar]
- 53.Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- 54.Okimoto R, Macfarlane JL, Wolstenholme DR. The Mitochondrial Ribosomal RNA Genes of the Nematodes Caenorhabditis elegans and Ascaris suum: Consensus Secondary-Structure Models and Conserved Nucleotide Sets for Phylogenetic Analysis. J Mol Evol. 1994;39:598–613. doi: 10.1007/BF00160405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Predicted secondary structure of 22 tRNAs genes in the mt genome of B. procyonis .
(TIF)
Secondary structure predicted for the AT-rich region in the B. procyonis mt genome.
(TIF)
Mitochondrial genome profiles of B. procyonis .
(DOC)
Nucleotide codon usage for 12 protein-coding genes of the mitochondrial genome of B. procyonis . Total number of codons is 3429. a Total number in all open reading frames. *** Stop codon. RSCU, relative synonymous codon usage.
(DOC)
Comparison of mitochondrial protein and rRNA genes with those of other nematodes sequenced in Ascaridida. a: From Jex et al. (2008). GenBank accession no. EU730761. b: From Li et al. (2008). GenBank accession no. NC_010690. Bp: B. procyonis, Bs: B. schroederi, Ba: B. ailuri, Bt: B. transfuga, Asu: A. suum, Asi: A. simplex, Cr: C. rudolphii B, Tcan: T. canis, Tcat: T. cati, Tmal: T. malaysiensis.
(DOC)
List of the ten primer pairs for PCR amplification and their positions in the mt genome of B. procyonis.
(DOC)