Abstract
The suspension feeding bivalve Austrovenus stutchburyi is a key species on intertidal sandflats in New Zealand, affecting the appearance and functioning of these systems, but is susceptible to several environmental stressors including sedimentation. Previous studies into the effect of this species on ecosystem function have been restricted in space and time, limiting our ability to infer the effect of habitat change on functioning. We examined the effect of Austrovenus on benthic primary production and nutrient dynamics at two sites, one sandy, the other composed of muddy-sand to determine whether sedimentary environment alters this key species' role. At each site we established large (16 m2) plots of two types, Austrovenus addition and removal. In winter and summer we deployed light and dark benthic chambers to quantify oxygen and nutrient fluxes and measured sediment denitrification enzyme activity to assess denitrification potential. Rates of gross primary production (GPP) and ammonium uptake were significantly increased when Austrovenus was added, relative to removed, at the sandy site (GPP, 1.5 times greater in winter and summer; ammonium uptake, 8 times greater in summer; 3-factor analysis of variance (ANOVA), p<0.05). Denitrification potential was also elevated in Austrovenus addition plots at the sandy site in summer (by 1.6 times, p<0.1). In contrast, there was no effect of Austrovenus treatment on any of these variables at the muddy-sand site, and overall rates tended to be lower at the muddy-sand site, relative to the sandy site (e.g. GPP was 2.1 to 3.4 times lower in winter and summer, respectively, p<0.001). Our results suggest that the positive effects of Austrovenus on system productivity and denitrification potential is limited at a muddy-sand site compared to a sandy site, and reveal the importance of considering sedimentary environment when examining the effect of key species on ecosystem function.
Introduction
Estuaries are highly productive ecosystems that play a major role in biogeochemical cycles, but are subject to multiple stressors that will likely be exacerbated by climate change and expanding human habitation of coastal areas [1], [2], [3]. Although the effects of contaminants, invasive species, coastal alteration and development might be restricted to estuaries near large population centres, enhanced sedimentation rates threaten many estuaries, even when there have been only moderate levels of catchment development [1]. Deposition of large amounts of terrestrial sediments during storm events smother benthic communities, and elevated levels of suspended sediments reduce primary productivity and detrimentally affect suspension feeders (e.g. [4], [5], [6]). More pervasive and perhaps less obvious is the long term degradative change in the form of increasing muddiness that alters estuarine habitats and communities [7], [8].
If habitat change does lead to decreasing biodiversity, then that alone may cause shifts in ecosystem structure and function [9], [10], [11]. However, in many cases it has been shown in estuarine systems that certain key species, rather than biodiversity per se, can have a disproportionate effect on indicators of ecosystem functioning such as nutrient cycling and productivity (e.g. [12], [13], [14]). Although the loss of key species likely has important implications, many estuarine species exist across a range of sediment types [8]. Habitat change may not necessarily then cause species loss but might more subtly affect ecosystem function by alteration of a species' functional role. For example an estuarine bioturbating crab (Austrohelice crassa) displays functional plasticity, acting as a bioturbator in sandy sediments and as a bioirrigator in muddy cohesive sediments [15]. Thus, the influence of this species on biogeochemical exchange and microbial communities is likely to differ between habitat types [16]. However, most studies to date are restricted temporally and spatially making it difficult to understand the effects of habitat change on a key species' influence on ecosystem function. In this study we examined the effect of a suspension feeding bivalve on ecosystem function at two sites with contrasting sediment properties, in winter and in summer. As sedimentation alters estuarine habitats by increasing sediment mud content we used a site with muddy-sand sediments as a proxy for habitat change, to compare with a site comprising only sandy sediment.
Suspension feeding bivalves can act as key species in estuarine ecosystems by exerting top-down control on phytoplankton populations, affecting rates of nutrient regeneration, contributing to benthic-pelagic coupling, and providing an important food source for higher trophic levels (reviewed by [17]). Furthermore, accumulation of biodeposits and altered redox environments in sediments underlying bivalve beds may enhance sediment denitrification rates, the microbial reduction of NO3 − to N2 gas, which permanently removes fixed nitrogen from an ecosystem; thus, suspension feeding bivalves can also exert a bottom-up control on phytoplankton populations (e.g. [18]). Loss of suspension feeding bivalve populations has resulted in large shifts in ecosystem structure and function. For example, in Chesapeake Bay, USA, loss of eastern oyster (Crassostrea virginica) beds has substantially increased the incidence of phytoplankton blooms, sometimes resulting in the occurrence of deep-water hypoxia (e.g. [19], [20]). Conversely, invasion of aquatic systems by non-native suspension feeding bivalves, such as by the Asian clam (Potamocorbula amurensis) in San Francisco Bay and the zebra mussel (Dreissena polymorpha) in many freshwater systems in the USA, has resulted in reduced phytoplankton biomass (e.g. [21], [22]).
In New Zealand estuaries the dominant suspension feeding bivalve is the native clam Austrovenus stutchburyi (hereafter Austrovenus), which commonly exists in high density beds covering large areas of intertidal flats; typical bed densities average c. 1000 ind. m−2, although peak densities may be 2000–3000 ind. m−2 in some areas [23], [24]. Austrovenus is an infaunal species that bioturbates surficial sediments through vertical and horizontal movement, but has very short siphons and so lives close to the sediment surface (<5 cm). Austrovenus beds are found across a range of sediment types, although very high levels of sedimentation adversely affect abundance [8]. Austrovenus has been shown to be a key species influencing sediment stability, solute fluxes and macrofauna community structure as well as enhancing microphytobenthos productivity [12], [25]. However, populations are declining in some areas likely due to chronic sedimentation, pollution and over-harvesting [6], [26], [27].
In this study we manipulated the presence or absence of Austrovenus in situ at two estuarine sites, both with nearby high density Austrovenus beds, but with contrasting sediment properties. Our aim was to see if the role of this key species in ecosystem functioning was the same at a sandy site (a proxy for a habitat unimpacted by sedimentation) and at a muddy-sand site (a proxy for a habitat affected by a moderate level of sedimentation). In winter and summer, light and dark benthic chambers were used to quantify the effect of Austrovenus on O2 and nutrient (NH4 +, NO3 −, NO2 −, PO4 3−) fluxes, and to estimate gross primary production and nutrient uptake rates. Additionally, denitrification enzyme activity (DEA) assays were used to quantify the effect of Austrovenus on maximum sediment denitrification potential. Previously, high Austrovenus densities have been shown to enhance ammonium efflux which supported higher rates of microphytobenthos (MPB) production [25]. Additionally, we expect increased rates of primary production and nutrient cycling in summer compared to winter due to increases in macrofaunal, microbial and photosynthetic activity [8]. Greater retention of bivalve biodeposits was predicted for the more sheltered muddy-sand site. Microbial decomposition of biodeposits may result in enhanced nutrient regeneration and a stimulation of primary production [28]. Alternatively, biodeposit decomposition can elevate denitrification rates through coupled nitrification-denitrification, thus reducing primary production [18]. Our use of large experimental plots (16 m2) to reduce confounding edge effects (e.g. [12], [25]) will enhance our understanding of the relative importance of the dynamics of these different habitat types.
Methods
Ethics statement
This study complied with all existing legislation governing animal welfare and field-based experiments. Animal ethics approval/permits were not sought as benthic invertebrate fauna manipulated/sampled in this study are exempt from the Animal Welfare Act 1999. After consultation with the Bay of Plenty Regional Council permits were not required for the in situ faunal manipulations. The collection of benthic fauna was undertaken with a Ministry of Fisheries Special Permit (386) Client Number 8770024.
Study site and experimental set up
Tauranga Harbour is a large (200 km2) barrier-enclosed estuary on the north-eastern coast of New Zealand. We manipulated the presence or absence of Austrovenus at two sites with differing sedimentary characteristics on lower-mid intertidal flats in the harbour (Figure 1). The sandy site (37°27.77′S 175°57.90′E) was located near the northern harbour entrance and was composed of medium sands with no mud content (defined as the silt/clay fraction<63 µm grain size). The muddy-sand site (37°29.20′S 175°56.73′E) was located 3 km up the estuary in the entrance of a small inlet and was composed of fine sands with c. 13% mud content. Mean tidal currents at the sandy site were 13.2 cm s−1 (peak flow was 35 cm s−1), and at the muddy-sand site were 7.2 cm s−1 (peak flow was 18 cm s−1), as determined by deployment of a FSI current meter that included a spring and a neap tidal phase. Tides in the harbour are semi-diurnal and the mean immersion period at each site is 8 h. Water temperature in Tauranga Harbour typically fluctuates between 13°C in mid-winter (July/August) and 22°C in mid-summer (January/February) [29].
Figure 1. Location of sites (indicated by a star) in Tauranga Harbour, New Zealand.
Sd = Sandy site, Ms = Muddy-sand site.
In June 2009, at both sites, six 4 m×4 m plots separated by 1 m were established in a line parallel with the channel. Austrovenus addition and removal treatments were alternated along the transect. The experimental plots were established on areas of sandflat where ambient Austrovenus densities were low (c. 300 ind. m−2), but were within 20 m of high density Austrovenus beds. Preliminary observations indicated that densities in the natural beds were c. 600–1200 ind. m−2 at the sandy site, and c. 2000–3000 ind. m−2 at the muddy-sand site. We noted however that Austrovenus individuals were larger at the sandy site (see results). We intended to raise the density in addition plots so that so that densities were comparable with natural densities for the sites, and so that biomass (and therefore first order excretory and respiration contribution to solute fluxes) was comparable between sites. Therefore, to create the addition treatments (+AS) we collected Austrovenus from the nearby natural beds and transplanted them to the plots during the same low tide to raise the density to c. 700 ind. m−2 at the sandy site and c. 2000 ind. m−2 at the muddy-sand site. Almost all the animals had buried into the sediment by the following day's low tide, and we observed no obvious Austrovenus mortality in the days and weeks following the transplants. To create the removal treatments (-AS) we manually removed all Austrovenus by finger plowing the sediment, minimising the impact of the manipulation on ambient macrofauna [12], which we repeated the following day to ensure almost total removal. Plastic mesh fences (15 cm in height) were buried 10 cm into the sediment around the perimeter of each plot to prevent the migration of adult Austrovenus. The large mesh size (1 cm) and short height (5 cm above sediment) of the fencing was used to minimise effects on water flow [30] and restrictions on the movement of smaller sized macrofauna. The Austrovenus manipulation was undertaken 6 weeks prior to the winter (August 2009) and summer (February-March 2010) benthic chamber incubations (see below) to allow the sediment and resident macrofauna to recover from the effects of the manipulation [12].
In situ chamber incubations
To measure the response of the soft-sediment systems to the Austrovenus manipulations, O2 and nutrient fluxes were measured in light and dark benthic chambers. One light and one dark chamber was deployed to each of the six plots per site on two consecutive days in both winter and summer. Chambers were placed at least 1 m inside each plot's fence to avoid edge artefacts (e.g. [31], [32]). The four incubations per plot (1 light plus 1 dark on 2 consecutive days) came from four distinct locations so that the same sediments were never resampled. Benthic chamber incubations took place during midday high tides when benthic algal activity was expected to be high.
The incubation chambers (square chambers with domed lids enclosing 0.25 m2 sediment and 35 L of mechanically-stirred overlying water) have been described previously [14]. Chamber bases were deployed during the low tide just prior to the incubation, and lids were attached during the incoming tide when water depth was c. 0.5 m. Measurements commenced 2 h before high water and continued for 4 h; Austrovenus exhibits a circatidal rhythm whereby feeding is limited to this period [33]. Initially, and once per hour during the incubation, a 60 mL water sample was carefully collected from each chamber using a Luer Lok syringe, without allowing any air bubbles to enter the syringe. O2 concentration was measured immediately with a hand held dissolved O2 probe (PreSens Fibox 3 PSt3) and the water was then filtered through a Whatman GF/C filter, stored on ice in the dark and frozen that day for later analysis of nutrients (NH4 +, NO3 −, NO2 −, PO4 3−) on a Thermo Scientific Aquakem 200 discrete analyzer. Water column effects on O2 and nutrient concentrations were found to be negligible based on incubation of ambient water in light and dark water bottles (1 L) for the same length of time and at the same depth as the chamber incubations. O2 and nutrient fluxes were calculated from the slope of the regression between concentration and incubation time, corrected for dilution of chamber water that occurred during each of the five 60 ml samplings. Additionally, HOBO® light meters and TidBit® temperature loggers were fitted to the outside of randomly selected chambers during the experiments.
After chamber deployment 16 surface sediment samples (1 cm depth) were taken from within each chamber footprint using a small syringe core (2.5 cm diameter). Samples were pooled and frozen for later analysis of pigments, grain size, organic matter, nitrogen and organic carbon content. One large core (13 cm diameter, 15 cm depth) was collected for macrofauna analysis, sieved on a 0.5 mm mesh and preserved in 70% isopropyl alcohol with Rose-Bengal stain. A second large core was collected for sediment denitrification and DEA assays (see below) and an additional estimate of Austrovenus density (sieved on a 1 mm mesh). For light chamber cores only, the surficial 5 cm of sediment was placed in airtight bags, kept cool and transported to the laboratory that evening for denitrification assays.
Sediment denitrification assays
Sediment denitrification rates were quantified within 24 h of collection using the chloramphenicol-amended acetylene (C2H2) inhibition technique [34], [35], [36]. Although this technique results in underestimation of actual denitrification rates due to blocking of nitrification by the C2H2, it has proven reliable for comparison of denitrification activity among treatments, sites and seasons as well as measuring nutrient limitation of denitrification [e.g. 37,38]. For each sediment sample (5 cm depth core from light chambers) we combined 30 mL of homogenized sediment with 25 mL unfiltered site water in preserve jars modified with n-butyl rubber septa in the lids (n = 6 per treatment, per site, per season). Chloramphenicol was added to the jars to suppress de novo enzyme production and the jars were purged with ultra-pure helium for 10 min to ensure anoxic conditions. Pure C2H2 was added to the jar headspace to prevent the conversion of N2O to N2 and gas samples were collected hourly beginning 10 mins after the addition of the C2H2 for 4 h. To maintain a constant pressure the headspace was replaced with a mixture of helium and C2H2 after each sample. The gas samples were analysed for N2O using a Varian CP 3800 gas chromatograph equipped with a HayeSep D column and electron capture detector. Denitrification rates were calculated from the linear increase in N2O concentration over time, normalized to the sediment surface area. To determine whether sediment denitrification was limited by nitrate or carbon we amended additional jars prepared identically to those above with additional nitrate (as potassium nitrate 10 mg N L−1), carbon (as glucose 12 mg C L−1) or both nitrate (10 mg N L−1) and carbon (12 mg C L−1). The DEA measurements were determined from the rates measured in the samples amended with nitrate and carbon (+N+C). DEA provides a measure of maximum denitrification potential by providing optimized conditions in anoxic, +N+C-amended slurries, valuable for making across-site comparisons [39], [40].
Laboratory analyses
Sediment chlorophyll a (chl a) and phaeopigment content were determined by extraction in 90% acetone and measurement of fluorescence before and after acidification on a Turner Designs 10-AU fluorometer [41]. Organic matter content (OM) was determined from dried (60°C for 24 h) and ashed (550°C for 4 h) sediment samples. Sediment grain size was measured on a Malvern Mastersizer-S after preparing the samples with 10% hydrogen peroxide to remove OM, removal of the >1 mm fraction, and addition of calgon to disperse the particles [42]. Organic carbon (OC) and total nitrogen (N) was measured on a LECO CHN analyser after removal of carbonate carbon from the samples by acidification with 1M hydrochloric acid [43]. Macrofauna samples were sorted into six broad taxonomic groups; Austrovenus, other bivalves, mudflat anemones (Anthopleura aureoradiata), annelids, crustaceans and gastropods counted and weighed (blotted wet weight). The weight of the bivalves included their shells. Austrovenus density and biomass in each chamber was estimated from the mean of the two large sediment cores.
Data analysis
To eliminate pseudo-replication, one representative value for each chamber type per plot was obtained prior to statistical analysis by averaging the data from the two light and two dark chambers deployed per plot. Sediment O2 and nutrient fluxes in the light and dark chambers were analysed separately. We defined the rate of net primary production (NPP) and sediment oxygen consumption (SOC) as the O2 flux in light and dark chambers respectively and estimated gross primary production (GPP) from NPP-SOC. GPP was standardised by the sediment chl a content to account for variations in microphytobenthos biomass. We estimated nutrient uptake rates (the difference between dark chamber flux and light chamber flux) to quantify usage by microbes and microphytes living in surficial sediments.
The response variables (NPP, SOC, GPP, nutrient fluxes and uptake, and sediment DEA) were analysed using 3-factor analysis of variance (ANOVA), with treatment (+AS, -AS), site (sandy, muddy-sand), and season (winter, summer) all considered as fixed factors. Any non-significant interaction terms of the highest order were removed and the analysis repeated. When the overall ANOVA was significant at α = 0.05, pairwise comparisons were performed using Tukey post-hoc tests. For sediment denitrification, 2-factor ANOVA by presence or absence of nitrate (N) or carbon (C) was used to identify the limiting nutrient. Single nutrient limitation by N or C is identified with a significant result for that treatment, and co-limitation is identified by a significant interaction term [44]. One-factor ANOVA were used to compare sediment properties, Austrovenus density and biomass between sites, seasons and treatments separately. In all tests, normality and homogeneity of variances were evaluated with Kolmogorov-Smironov tests and by plotting of residual versus predicted values. Variables were log or square root transformed where required. All statistical analyses were performed using Statistica (Version 8, Statsoft Inc., 2008).
Results
Macrofauna abundance and biomass
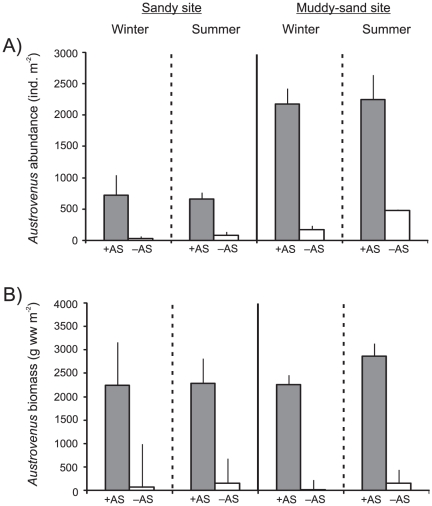
Austrovenus density in +AS plots ranged from c. 500 to 1000 ind. m−2 at the sandy site and from c. 1800 to 2500 ind. m−2 at the muddy-sand site (Figure 2). Small-scale spatial heterogeneity in Austrovenus density is characteristic of natural Austrovenus beds, as the adults tend to be aggregated rather than randomly or uniformly distributed (e.g. [24]). However, we expected the large size of our experimental plots (16 m2) to affect the sediment biogeochemical environment at a scale larger than the chamber footprints (0.25m2). Although we did not achieve total removal in -AS plots, Austrovenus density and biomass were at least an order of magnitude less than in the +AS plots.
Figure 2. Austrovenus stutchburyi abundance and biomass.
Mean (+ 1 SD; n = 3) Austrovenus abundance (A) and biomass (B) in Austrovenus addition (+AS; grey fill) and removal (–AS; no fill) plots as a function of site and season.
Regardless of site or season, Austrovenus density and biomass were significantly greater in +AS compared to -AS plots (1-factor ANOVA, p<0.001). Densities in +AS plots were equivalent to planned densities, i.e. mean Austrovenus density in +AS plots was significantly lower at the sandy site (700 ind. m−2) compared to the muddy-sand site (2000 ind. m−2, p<0.001). Mean Austrovenus shell length (± SD) was significantly greater at the sandy site, 23.3 (±1.0) mm, compared to the muddy-sand site, 17.7 (±1.1) mm, (p<0.001). Thus, as expected, mean biomass in +AS plots (c. 2300 g ww m−2) was not significantly different between the two sites (p>0.05). There was no significant seasonal difference in Austrovenus density, size or biomass at either site (p>0.05).
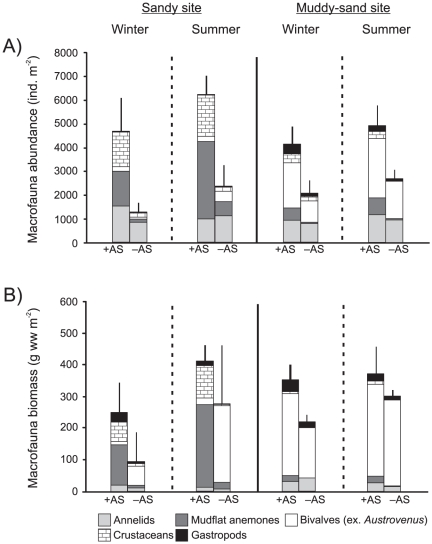
Abundance of other macrofaunal groups was dominated by annelids, Anthopleura aureoradiata (mudflat anemones, attached to the Austrovenus shells) and crustaceans (mostly barnacles, also attached to the Austrovenus shells) at the sandy site; annelids and other bivalves dominated at the muddy-sand site (Figure 3A). Austrovenus comprised c. 90% of the mean total macrofaunal biomass in the +AS plots. Other than Austrovenus the biggest contributors to macrofaunal biomass were Anthopleura in +AS plots at the sandy site, other bivalves in -AS plots at the sandy site, and other bivalves in both +AS and -AS plots at the muddy-sand site (Figure 3B).
Figure 3. Macrofauna (excluding Austrovenus stutchburyi) abundance and biomass.
Mean (+ 1 SD; n = 3) macrofauna abundance (A) and biomass (B) in Austrovenus addition (+AS) and removal (–AS) plots as a function of site and season.
Environmental variables
There were large differences in water temperature and photosynthetically active radiation (PAR) between winter and summer, with only small differences between sites within each of the seasons. Conversely, there were large differences in sediment properties between sites, but not between winter and summer (Table 1). Water temperature was greater in summer (c. 22°C) than in winter (c. 14°C). Levels of PAR were also much greater in summer (c. 1370 µmol photons m−2 s−1) than in winter (c. 80 µmol photons m−2 s−1). Regardless of season or Austrovenus treatment, median grain size was significantly lower at the muddy-sand site (c. 220 µm), compared to the sandy site (c. 420 µm, 1-factor ANOVA, p<0.001). Mud, OM, OC, N, chl a and phaeopigment content were all significantly greater at the muddy-sand site (p<0.001). We did not detect a significant effect (α = 0.05) of Austrovenus treatment on any sediment properties at the sandy site. However, at the muddy-sand site, in both winter and summer, grain size was greater (p<0.05) and mud content was lower (p<0.05) in +AS than -AS plots. Also at the muddy-sand site, OM content was lower in +AS plots than in -AS plots, although the effect was only marginally significant (p = 0.088 in winter, p = 0.075 in summer).
Table 1. Environmental variables as a function of site, season and treatment.
| Environmental variable | Treatment | Sandy Site | Muddy-sand site | ||
| Winter | Summer | Winter | Summer | ||
| Median grain size (µm) | +AS | 447 (38) | 393 (20) | 222 (8) | 262 (14) |
| –AS | 463 (50) | 389 (62) | 195 (15) | 221 (14) | |
| Silt/clay (%) | +AS | 0.0 (0.0) | 0.0 (0.0) | 10.8 (0.4) | 9.1 (1.2) |
| –AS | 0.0 (0.0) | 0.6 (1.0) | 17.0 (2.0) | 13.6 (2.1) | |
| Organic matter (%) | +AS | 1.1 (0.1) | 1.0 (0.1) | 3.7 (0.3) | 3.2 (0.1) |
| –AS | 1.2 (0.2) | 1.1 (0.3) | 4.2 (0.3) | 3.4 (0.1) | |
| Chlorophyll a (µg g dw−1) | +AS | 8.4 (0.5) | 8.5 (1.9) | 23.7 (1.3) | 17.7 (0.7) |
| –AS | 8.6 (0.6) | 8.2 (3.6) | 22.0 (1.6) | 14.5 (1.8) | |
| Phaeopigment (µg g dw−1) | +AS | 2.5 (0.2) | 1.4 (0.5) | 14.3 (0.4) | 7.3 (0.4) |
| –AS | 2.5 (0.2) | 1.6 (1.0) | 15.9 (1.9) | 6.0 (0.7) | |
| Organic carbon (%) | +AS | 0.15 (0.00) | 0.17 (0.01) | 0.37 (0.02) | 0.31(0.01) |
| –AS | 0.16 (0.01) | 0.18 (0.02) | 0.45 (0.07) | 0.34(0.04) | |
| Nitrogen (%) | +AS | 0.08 (0.00) | 0.09 (0.00) | 0.14 (0.01) | 0.12(0.01) |
| –AS | 0.08 (0.01) | 0.09 (0.01) | 0.14 (0.01) | 0.12(0.01) | |
| OC:N | +AS | 2.0 (0.1) | 2.0 (0.2) | 2.6 (0.2) | 2.7 (0.1) |
| –AS | 2.1 (0.2) | 2.1 (0.0) | 3.2 (0.4) | 2.8 (0.2) | |
| Water temperature (°C) | 13.9 (13.5 – 14.2) | 21.4 (21.0 – 21.6) | 14.7 (14.2 – 15.0) | 22.6 (22.0 – 23.1) | |
| PAR (µmol photons m−2 s−1) | 82 (58 – 105) | 1330 (560 – 2100) | 81 (68 – 93) | 1410 (1330 – 1490) | |
+AS = Austrovenus addition, –AS = Austrovenus removal, PAR = photosynthetically active radiation, OC:N = organic carbon to nitrogen ratio. For water temperature and PAR data represent mean and range in parentheses measured during chamber incubations. For sediment properties data represent mean (n = 3) with SD in parentheses.
O2 fluxes and GPP
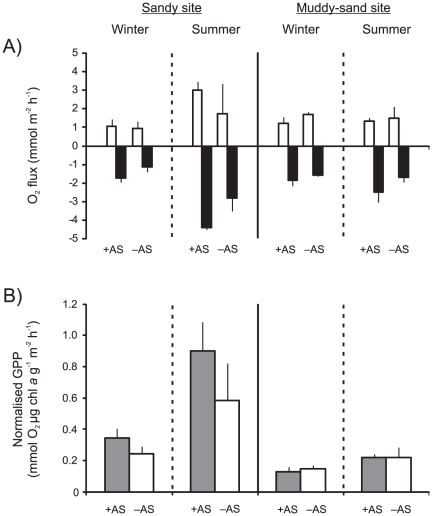
In dark chambers there was always an influx of O2 into the sediments, indicating sediment oxygen consumption (SOC), however, in light chambers there was always an efflux of O2 from the sediments which indicated that net primary production (NPP) was greater than zero (Figure 4A). There was a significant treatment effect on SOC which was 1.5× higher in +AS plots compared to -AS plots (3-factor ANOVA, p<0.001, Table 2). Post-hoc analysis of the site*season interaction (p<0.001) revealed that SOC was significantly greater (by 2.5×) in summer than in winter at the sandy site but that there was no significant difference between seasons at the muddy-sand site. Comparisons between sites within seasons demonstrated that SOC was significantly higher (by 1.7×) at the sandy than at the muddy-sand site in summer only (in winter there was no significant difference). For light chamber O2 fluxes, there was a marginally significant site*treatment interaction (p = 0.086). Closer examination suggested that NPP tended to be greater in +AS plots (compared to -AS plots) at the sandy site in summer. There was no indication of treatment effects on NPP in winter at the sandy site or at the muddy-sand site in either season. The site*season interaction was significant (p<0.05) with NPP greater (by 2.4×) in summer than in winter at the sandy site. There was no significant seasonal effect on NPP at the muddy-sand site and no significant difference between the sites in either season.
Figure 4. O2 fluxes and gross primary production (GPP).
(A) Mean (+ 1 SD; n = 3) O2 fluxes in light (no fill) and dark (black fill) chambers in Austrovenus addition (+AS) and removal (–AS) plots, as a function of site and season. Positive values represent an efflux out of the sediment, and negative values represent an influx into the sediment. (B) Mean (+ 1 SD; n = 3) normalised GPP (light minus dark chamber O2 flux) in +AS (grey fill) and –AS (no fill) plots, as a function of site and season.
Table 2. 3-factor ANOVA (analysis of variance) results for sediment oxygen consumption (SOC; dark chamber O2 flux), net primary production (NPP; light chamber O2 flux) and gross primary production normalised by sediment chl a content (GPP/chl a).
| Variable | Source | d.f. | MS | F | p | Significant Tukey post-hoc test | ||
| Site | Season | Treatment | ||||||
| SQRT SOC | Site | 1 | 0.170 | 12.9 | 0.002 | |||
| Season | 1 | 1.02 | 78.2 | < 0.001 | ||||
| Treatment | 1 | 0.422 | 32.2 | < 0.001 | +AS>–AS | |||
| Site*Season | 1 | 0.482 | 36.8 | < 0.001 | Su: Sd>Ms | Sd: Wi<Su | ||
| Site* Treatment | 1 | 0.0287 | 2.19 | 0.157 | ||||
| Season* Treatment | 1 | 0.0455 | 3.47 | 0.080 | ||||
| Error | 17 | 0.0131 | ||||||
| NPP | Site | 1 | 0.364 | 0.800 | 0.383 | |||
| Season | 1 | 2.65 | 5.83 | 0.027 | ||||
| Treatment | 1 | 0.205 | 0.451 | 0.511 | ||||
| Site*Season | 1 | 2.96 | 6.51 | 0.021 | Sd: Wi<Su | |||
| Site* Treatment | 1 | 1.51 | 3.32 | 0.086 | ||||
| Season* Treatment | 1 | 0.917 | 2.02 | 0.174 | ||||
| Error | 17 | 0.455 | ||||||
| SQRT GPP/chl a | Site | 1 | 0.457 | 86.3 | < 0.001 | |||
| Season | 1 | 0.246 | 46.4 | < 0.001 | ||||
| Treatment | 1 | 0.024 | 4.61 | 0.047 | ||||
| Site*Season | 1 | 0.071 | 13.5 | 0.002 | Su & Wi: Sd>Ms | Sd: Wi<Su | ||
| Site* Treatment | 1 | 0.035 | 6.60 | 0.020 | +AS & –AS: Sd>Ms | Sd: +AS>–AS | ||
| Season* Treatment | 1 | 0.006 | 1.17 | 0.295 | ||||
| Error | 17 | 0.005 | ||||||
Factors are site (Sd = Sandy, Ms = Muddy-sand), season (Wi = Winter, Su = Summer) and treatment (+AS = Austrovenus addition, –AS = Austrovenus removal). Values in bold are significant at p<0.05. Tukey post-hoc tests for significant differences between site, season and treatment are shown at α = 0.05. SOC and GPP/chl a were square root (SQRT) transformed prior to analysis.
Mean GPP ranged from 2.1 to 7.4 mmol O2 m−2 h−1 at the sandy site, but the range was much smaller at the muddy-sand site (3.1 to 3.8 mmol O2 m−2 h−1). When normalised by sediment chl a content (a proxy for primary producer biomass), GPP at the sandy site was consistently greater (0.24 to 0.90 mmol O2 µg chl a g−1 m−2 h−1) than at the muddy-sand site (0.13 to 0.22 mmol O2 µg chl a g−1 m−2 h−1, Figure 4B). There were significant site*season and site*treatment interaction effects on normalised GPP (3-factor ANOVA, p<0.05, Table 2). Post-hoc analysis showed that normalised GPP was higher in +AS plots compared to –AS plots at the sandy site, (by 1.4× in winter and by 1.5× in summer), but there was no significant difference between the treatments at the muddy-sand site in either season. Between sites within season comparisons demonstrated that normalised GPP was greater at the sandy site in both winter (by 2.1×) and summer (by 3.4×). Comparison between seasons within sites demonstrated that normalised GPP was greater at the sandy site in summer compared to winter (by 2.6×), but there was no significant difference between winter and summer at the muddy-sand site.
Nutrient fluxes and uptake
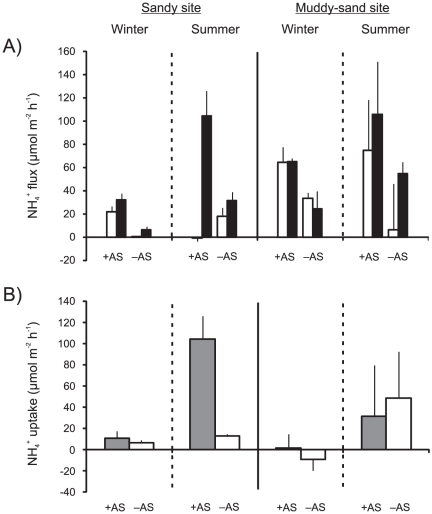
In dark and light chambers there was nearly always a net efflux of ammonium (NH4 +) from the sediment, the only exception being some light chambers in the +AS plots at the sandy site in summer, when there was a small influx (Figure 5A). There was a significant treatment effect on dark chamber NH4 + flux which was 2.6× greater in +AS plots compared with –AS plots (3-factor ANOVA, p<0.001, Table 3). Post-hoc analysis of the site*season interaction (p<0.05) showed that dark chamber NH4 + flux was greater in summer than in winter at both sites (by 1.8× at the muddy-sand site, and by 3.6× at the sandy site). Comparisons between sites within seasons demonstrated that dark chamber NH4 + flux was greater (by 2.3×) at the muddy-sand site than at the sandy site in winter only (in summer there was no significant difference). The effect of Austrovenus treatment on light chamber NH4 + flux was not consistent across sites and seasons (3-factor ANOVA, p<0.05, Table 3). Post-hoc analysis of the site*season*treatment interaction revealed that light chamber NH4 + flux was significantly greater (by 11.8×) in +AS compared to -AS plots at the muddy-sand site in summer, but there was no significant difference between treatments in winter or at the sandy site in either season. Comparison between sites within seasons and treatments revealed that in +AS plots in summer light chamber NH4 + flux was significantly greater at the muddy-sand site; at the sandy site NH4 + flux was negative (−0.52 µmol m−2 h−1) indicating a small influx into the sediment, but at the muddy-sand site NH4 + flux was positive (74.6 µmol m−2 h−1) indicating a large efflux out of the sediment. In contrast, in +AS plots in winter and in -AS plots in both seasons, there was no significant difference between the sites.
Figure 5. NH4 + fluxes and uptake.
(A) Mean (+ 1 SD; n = 3) NH4 + fluxes in light (no fill) and dark (black fill) chambers in Austrovenus addition (+AS) and removal (–AS) plots, as a function of site and season. Positive values represent an efflux out of the sediment, and negative values represent an influx into the sediment. (B) Mean (+ 1 SD; n = 3) NH4 + uptake (dark minus light chamber NH4 + flux) in +AS (grey fill) and –AS (no fill) plots, as a function of site and season.
Table 3. 3-factor ANOVA (analysis of variance) results for dark and light chamber NH4 + flux and NH4 + uptake.
| Variable | Source | d.f. | MS | F | p | Significant Tukey post-hoc test | ||
| Site | Season | Treatment | ||||||
| Log10 Dark NH4 + | Site | 1 | 0.464 | 16.4 | < 0.001 | |||
| Season | 1 | 1.24 | 44.0 | < 0.001 | ||||
| Treatment | 1 | 1.52 | 53.7 | < 0.001 | +AS>–AS | |||
| Site*Season | 1 | 0.161 | 5.70 | 0.029 | Wi: Sd<Ms | Sd & Ms: Wi<Su | ||
| Site* Treatment | 1 | 0.105 | 3.72 | 0.071 | ||||
| Season* Treatment | 1 | 0.078 | 2.75 | 0.116 | ||||
| Error | 17 | 0.028 | ||||||
| Light NH4 + | Site | 1 | 7190 | 15.3 | 0.001 | |||
| Season | 1 | 172 | 0.366 | 0.554 | ||||
| Treatment | 1 | 3850 | 8.19 | 0.011 | ||||
| Site*Season | 1 | 58.7 | 0.125 | 0.728 | ||||
| Site* Treatment | 1 | 3470 | 7.39 | 0.015 | ||||
| Season* Treatment | 1 | 2.55 | 0.005 | 0.942 | ||||
| Site*Season*Treatment | 1 | 2290 | 4.88 | 0.042 | +AS Su: Sd<Ms | Ms Su: +AS>–AS | ||
| Error | 16 | 470 | ||||||
| NH4 + uptake | Site | 1 | 1430 | 2.28 | 0.151 | |||
| Season | 1 | 13400 | 21.4 | < 0.001 | ||||
| Treatment | 1 | 2950 | 4.69 | 0.046 | ||||
| Site*Season | 1 | 66.0 | 0.110 | 0.749 | ||||
| Site* Treatment | 1 | 3980 | 6.32 | 0.023 | ||||
| Season* Treatment | 1 | 1320 | 2.09 | 0.168 | ||||
| Site*Season* Treatment | 1 | 4990 | 7.92 | 0.012 | +AS Su: Sd>Ms | +AS Sd: Wi<Su | Sd Su: +AS>–AS | |
| Error | 16 | 629 | ||||||
Factors are site (Sd = Sandy, Ms = Muddy-sand), season (Wi = Winter, Su = Summer) and treatment (+AS = Austrovenus addition, –AS = Austrovenus removal). Values in bold are significant at p<0.05. Tukey post-hoc tests for significant differences between site, season and treatment are shown at α = 0.05.
Dark NH4 + flux was log10 transformed prior to analysis.
NH4 + uptake exhibited a far greater range at the sandy (6 to 105 µmol NH4 + m−2 h−1) compared to the muddy-sand site (−9 to 49 µmol NH4 + m−2 h−1; Figure 5B). The effect of Austrovenus treatment on NH4 + uptake was inconsistent across sites and seasons (3-factor ANOVA, p<0.05, Table 3). Post-hoc analysis of the site*season*treatment interaction demonstrated that NH4 + uptake was significantly increased (by 8×) in +AS compared to -AS plots at the sandy site in summer, but there was no significant difference between treatments in winter. At the muddy-sand site there was no treatment effect in either season. Comparison between seasons within treatments and sites revealed that in +AS plots at the sandy site NH4 + uptake was significantly greater (by 10×) in summer compared to winter, but there was no significant difference between the seasons in -AS plots. At the muddy-sand site there was no significant difference between the seasons in +AS or -AS plots. Comparison between sites within treatments and seasons revealed that NH4 + uptake was significantly greater (by 3.4×) at the sandy site in +AS plots in summer, but there was no significant difference between the sites in +AS plots in winter. There was also no significant difference between the sites in -AS plots in either season.
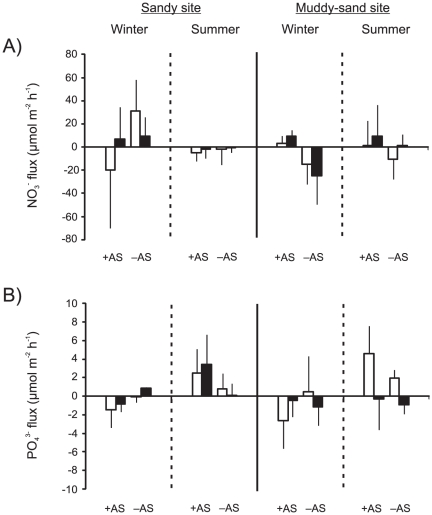
NH4 + is the form of dissolved inorganic nitrogen (DIN) most readily taken up by microphytobenthos (MPB) and, as is typically the case in New Zealand estuaries, comprised the majority (c. 80%) of DIN in our samples [12], [25], [45]. We measured high variation in NO3 − and PO4 3− fluxes (Figure 6). Additionally, chamber nutrient concentrations were often near instrument detection limits, particularly for NO2 − (0.005 µmol L−1), and this led to uncertainty in flux estimates (mean r2<0.3). There were no obvious treatment effects and no further analyses were conducted for NO3 −, NO2 − or PO4 3− fluxes.
Figure 6. NO3 − and PO4 3 − fluxes.
Mean (+ 1 SD; n = 3) NO3 − (A) and PO4 3− (B) fluxes in light (no fill) and dark (black fill) chambers in Austrovenus addition (+AS) and removal (–AS) plots, as a function of site and season. Positive values represent an efflux out of the sediment, and negative values represent an influx into the sediment.
Sediment denitrification rates
Non-amended denitrification rates (0 to 30 µmol N m−2 h−1) were lower than the sediment denitrification potential (38 to 164 µmol N m−2 h−1), which was determined from samples amended with nitrate and carbon (DEA). Two-way ANOVA revealed a significant effect of nitrate addition at both sites in winter and in summer, indicating that denitrification was always N limited, regardless of site or season (Table 4).
Table 4. 2-factor ANOVA (analysis of variance) results determining whether nitrogen, carbon or both nutrients are limiting dentrification rates.
| Site/Season | Source | d.f. | MS | F | p | Significant Tukey post-hoc test | |
| Nitrogen | Carbon | ||||||
| Sandy site in winter | Nitrogen | 1 | 189 | 193 | < 0.001 | ||
| Carbon | 1 | 8.05 | 8.23 | 0.009 | |||
| Nitrogen*Carbon | 1 | 6.74 | 6.89 | 0.016 | +C & –C: +N>–N | –N: –C>+C | |
| Error | 20 | 0.980 | |||||
| Sandy site in summer | Nitrogen | 1 | 92000 | 97.8 | < 0.001 | +N>–N | |
| Carbon | 1 | 548 | 0.582 | 0.454 | |||
| Nitrogen*Carbon | 1 | 548 | 0.582 | 0.454 | |||
| Error | 20 | 942 | |||||
| Muddy-sand site in winter | Nitrogen | 1 | 256 | 447 | < 0.001 | +N>–N | |
| Carbon | 1 | 5.61 | 9.77 | 0.005 | –C>+C | ||
| Nitrogen*Carbon | 1 | 0.226 | 0.394 | 0.537 | |||
| Error | 20 | 0.574 | |||||
| Muddy-sand site in summer | Nitrogen | 1 | 8640 | 291 | < 0.001 | +N>–N | |
| Carbon | 1 | 56.5 | 1.90 | 0.183 | |||
| Nitrogen*Carbon | 1 | 56.5 | 1.90 | 0.183 | |||
| Error | 20 | 29.7 | |||||
Factors are nitrogen (N) and carbon (C). Values in bold are significant at p<0.05. Tukey post-hoc tests for significant differences between presence/absence (+/–) of nitrogen and carbon are shown at α = 0.05.
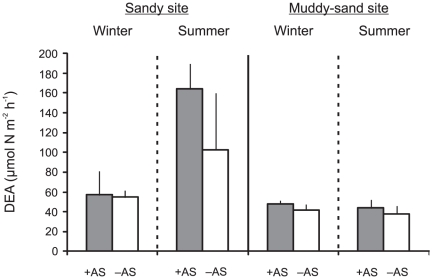
As with GPP and NH4 + uptake, the range in sediment denitrification potential was greater at the sandy site (55 to 164 µmol N m−2 h−1) compared to the muddy-sand site (38 to 48 µmol N m−2 h−1; Figure 7). Denitrification potential did trend towards an increase in +AS compared to -AS plots at the sandy site, especially in summer, although the treatment effect was only marginally significant (3-factor ANOVA, p = 0.078, Table 5). There was a significant site*season interaction (p<0.001) and post-hoc analysis demonstrated that denitrification potential was significantly greater (by 2.4×) in summer compared to winter at the sandy site, but there was no significant seasonal effect at the muddy-sand site. Also, denitrification potential was significantly greater (by 3×) at the sandy site than at the muddy-sand site in summer, but there was no significant difference between the sites in winter.
Figure 7. Sediment DEA (denitrification enzyme activity; i.e. denitrification potential).
Mean (+ 1 SD; n = 3) DEA in Austrovenus addition (+AS; grey fill) and removal (–AS; no fill) plots as a function of site and season.
Table 5. 3-factor ANOVA (analysis of variance) results for log10 transformed DEA (denitrification enzyme activity; i.e. sediment denitrification potential).
| Variable | Source | d.f. | MS | F | p | Significant Tukey post-hoc test | ||
| Site | Season | Treatment | ||||||
| Log10 DEA | Site | 1 | 0.486 | 34.8 | < 0.001 | |||
| Season | 1 | 0.145 | 10.4 | 0.005 | ||||
| Treatment | 1 | 0.049 | 3.52 | 0.078 | ||||
| Site*Season | 1 | 0.242 | 17.3 | < 0.001 | Su: Sd>Ms | Sd: Wi<Su | ||
| Site* Treatment | 1 | 0.004 | 0.310 | 0.585 | ||||
| Season* Treatment | 1 | 0.023 | 1.62 | 0.220 | ||||
| Error | 17 | 0.014 | ||||||
Factors are site (Sd = Sandy, Ms = Muddy-sand), season (Wi = Winter, Su = Summer) and treatment (+AS = Austrovenus addition, –AS = Austrovenus removal). Values in bold are significant at p<0.05. Tukey post-hoc tests for significant differences between site, season and treatment are shown at α = 0.05.
Discussion
At the sandy site, there were significant increases in many response variables (i.e. SOC, NPP, GPP, NH4 + uptake and denitrification potential) in summer, compared to winter. In contrast, for the same variables at the muddy-sand site, there was no significant difference between winter and summer measurements. Similarly, the effect of Austrovenus treatment on response variables was inconsistent between sites and seasons. Although both SOC and dark chamber NH4 + fluxes increased significantly in +AS plots regardless of site and season, GPP (and NPP to a lesser extent) were increased in +AS plots only at the sandy site. An increase in GPP indicates increased MPB productivity as water column primary production was negligible. Our results suggest that increased availability of NH4 + drives this increase in MPB productivity as NH4 + uptake was higher in +AS plots at the sandy site, especially in summer. There was also a trend for greater denitrification potential in +AS sandy site plots in summer. At the muddy-sand site there was no significant effect of Austrovenus on GPP, NH4 + uptake or denitrification potential. Furthermore, GPP and denitrification potential were both significantly lower than at the sandy site.
As for other suspension feeding bivalves in coastal systems worldwide, resuspended MPB are an important component of Austrovenus' diet, especially as water column primary productivity is typically low in New Zealand estuaries [46], [47], [48]. Previous research with Austrovenus and other large bioturbating macrofauna has also observed an increase in MPB productivity even though MPB are often a major food source for the animals [12], [14], [25]. However, this study suggests that the positive effect of Austrovenus on MPB productivity is not consistent across habitat types, and that there can be substantial temporal variability in GPP. At both sites, the lower rates of GPP in winter are likely to be caused by limited MPB and bivalve activity. MPB photosynthetic activity was likely limited by wintertime water temperatures and reduced levels of PAR, while the reduced dark chamber NH4 + fluxes in the wintertime Austrovenus addition treatments provided evidence of reduced metabolic rates (i.e. less NH4 + excretion during the colder winter period). More surprising are the low rates of GPP in summer, and lack of an effect of Austrovenus on GPP, at the muddy-sand site. As dark chamber NH4 + fluxes in +AS plots were similar between the two sites it seems unlikely that the reason for low MPB productivity at the muddy-sand site was nutrient limitation.
Muddy sediments, despite often having higher microalgal biomass, can be less productive (in terms of rates of photosynthesis and oxygen evolution) than sandy sediments [49]. Resuspension of fine sediments, causing light limitation at the benthos, is more likely in muddy sediments, but we did not observe higher levels of turbidity at our muddier site on the days that we sampled. However, productivity can be enhanced in sandy sediments because light can penetrate further into the sediment column (as there is greater interstitial space between sediment grains). This increased sediment permeability can enhance solute flux (by permitting pore-water advection), and more frequent resuspension can cause a higher turnover of algal biomass [49], [50], [51]. Furthermore, sediment grain size can affect microbial composition and activity, and thus organic matter remineralisation, nutrient availability and MPB productivity [51]. In fact, we found normalised GPP was significantly increased at the sandy site compared to the muddy-sand site even in -AS plots, although the effect was enhanced in +AS plots.
At the sandy site other macrofaunal abundance and biomass in +AS plots was dominated by mudflat anemones (Anthopleura aureoradiata). Previous work has described a mutualistic relationship between Austrovenus and A. aureoradiata whereby the anemones use the living bivalves as hard substrate for attachment and the bivalves gain protection from parasitic infection [52]. The anemones may also benefit from greater NH4 + availability in Austrovenus beds as endosymbiotic zooxanthellae can uptake NH4 + from surrounding water [53]. It is probable that mudflat anemones significantly contribute to, and complicate, nutrient recycling and productivity at the sandy site, by both excretion and uptake of NH4 +, but further work is needed to determine whether this species is a net source or sink of NH4 +, and its effect on system productivity. Barnacles were also supported on Austrovenus shells at the sandy site. It is therefore possible that the positive effect on productivity measured in +AS plots at the sandy site is not attributable to Austrovenus alone, but to the combination of Austrovenus and the macrofaunal communities they support.
In contrast, at the muddy-sand site other macrofaunal abundance and biomass was dominated by other bivalves (mostly the deposit feeders Nucula hartvigiana and Macomona liliana). We expected OM content to increase in +AS plots at this site, due to retention of biodeposits in the lower energy environment, but instead found the reverse to be true. Deposit-feeder abundance and biomass was higher in +AS plots and they may have utilised the increased supply of OM. Alternatively, decreased mud content and increased grain size in +AS plots suggests that Austrovenus bioturbation enhanced fine sediment and OM transport by destabilising the sediment [54], [55]. Furthermore, there was no difference in sediment C, N and C:N ratio between +AS and -AS plots. Typically, these parameters are found to increase under epifaunal bivalve beds, particularly so under longline mussel farms [34], [56], [57]. Although biodeposition rates would almost certainly be lower for an infaunal bivalve bed than for a three dimensional epifaunal bed/longline our results suggest that Austrovenus biodeposits do not accumulate at either site. It is probable that OM is dispersed by currents at the sandy site, and quickly utilised by deposit feeders and/or resuspended by bivalve bioturbation at the muddy-sand site.
Unamended sediment denitrification rates were nil or low, likely because sediment nitrification may be a major source of NO3 - for denitrification and coupled nitrification-denitrification is inhibited by our method (e.g. [58]). This is further reinforced by the low measured NO3 - fluxes into the sediment. Our expectation was that increased N from Austrovenus biodeposits (at the more sheltered muddy-sand site especially) would fuel coupled nitrification-denitrification but we found that OM content was not increased in +AS plots, and denitrification remained N limited regardless of site, season or addition/removal of Austrovenus. However, sediment denitrification potential (as measured with excess nitrate and carbon) did trend towards an increase in +AS plots at the sandy site in summer. Although Austrovenus biodeposits may not accumulate at the sandy site, bivalve bioturbation and excretion may have enhanced NH4 + availability, thus providing a source of nitrogen for nitrification [59], [60]. NH4 + uptake was significantly increased in +AS plots at the sandy site in summer, which may have been partly due to increased nitrification. Without measuring sediment nitrification rates, however, it is not possible to separate uptake by nitrifiers from that by MPB (and perhaps anemones also). Nitrifiers are known to be poor competitors for nitrogen [61], [62], but oxygen production by benthic photosynthesis may enhance rates of coupled nitrification-denitrification when NH4 + is not limiting [63]. Our results suggest that increased availability of NH4 + at the sandy site in summer as a result of Austrovenus activity likely increases both MPB productivity and sediment denitrification, though concurrent measurements of GPP, nitrification and denitrification would be needed to confirm this.
A possible confounding factor influencing the interpretation of our results is the difference in Austrovenus size between the two sites. Individuals were significantly larger at the sandy site (c. 23 mm shell length) than at the muddy-sand site (c. 18 mm shell length). Previous research has indicated that Austrovenus condition is enhanced in sandy compared to muddier sediments [64], and the bivalves in our experimental plots had been transplanted from nearby beds at each site so represented a natural size for the habitat type. As biomass was comparable between our sites we would not expect first order excretion rates to be substantially different between sites. However, the size difference might affect the degree to which Austrovenus bioturbation alters sediment chemistry. Bioturbation by macrofauna that mix surficial sediments, such as Austrovenus, can facilitate the release of solutes from sediment porewater [45]. Previous experiments have shown that Austrovenus tend to be retained in unfenced high-density plots, i.e. individual bivalves display minimal horizontal movement through surface sediments when in high-density beds [23], [25]. The main effect of Austrovenus bioturbation in bivalve beds is therefore likely to be small-scale (< 2–3 cm) vertical movement as the bivalves move to the sediment-water interface to feed around high tide, and thereafter retreat to just below the sediment surface. The larger bivalves at our sandy site may have reworked sediment to a greater depth than the smaller individuals at our muddy-sand site. However, solute gradients are likely to be steeper in sediments at the muddy-sand site, potentially offsetting the size difference, and making it difficult to speculate on size-specific bioturbation effects on solute fluxes.
It is well documented that denitrification is often highly variable over small spatial and temporal scales in estuaries, due to variable O2 profiles, nitrate and OM availability in the sediment [40], [65]. This is caused by a variety of processes such as frequent wetting/drying due to the tides or macrofauna activity (especially bioturbation and burrow building) which create anoxic denitrification microsites and make collection of a large number of replicates crucial [65]. More sophisticated (but more expensive) techniques, such as isotope-pairing techniques using Membrane Inlet Mass Spectrometry, can quantify denitrification rates without blocking nitrification, which may help to resolve the complicated interactions among macrofauna, such as Austrovenus, MPB and microbial communities (e.g. [66], [67]). Our work shows that these interactions are likely to be further complicated by context (i.e. spatial and temporal variability), so future studies should be mindful of this.
There is typically a trade-off between the size of experimental plots and the number of replicates that can be established. We recognise the low levels of replication (n = 3 per treatment) inherent in our experiments, but our efforts were focused on using relatively large plots as the estuarine intertidal is dynamic and subject to substantial bedload transport and sediment reworking rates (e.g. [68]); consequently results from experiments using smaller-scale manipulations may be dominated by edge effects [69], [70]. Furthermore, modifications of sediment stability associated with the addition or removal of macrofauna are often scale and/or density dependent [71], [72], [73]. We recognise also that there are limitations associated with using benthic chambers to measure solute fluxes, such as stirring-induced pressure gradients that affect rates of porewater exchange [74], or altered boundary layer dynamics [75]. However, in sediments colonised by large bioturbating or bioirrigating macrofauna and by patchy MPB communities (as in this study) there is considerable small-scale spatial and temporal heterogeneity in solute distribution. Benthic chambers have the advantage of integrating fluxes over a large sediment surface area, and in this study our intention was to identify any differences in relative fluxes between our sites, seasons and treatments, rather than quantifying absolute fluxes.
Conclusions
Austrovenus enhanced primary productivity and sediment denitrification potential at the sandy site, whereas there was no effect of Austrovenus on these variables at the muddy-sand site, leading us to hypothesise that increasing estuarine mud content may limit the influence of this key species on ecosystem function. However, there is a need to sample across a gradient of increasing muddiness to further explore these relationships. Similarly, there is a need for more comprehensive sampling to better resolve temporal variability. Previous research has established that high levels of sedimentation are likely to reduce Austrovenus populations [8], but our results indicate that moderate levels of sedimentation may reduce the positive effect of this species on system productivity even when they persist. Furthermore, our results suggest that denitrification potential is lower in muddy-sand compared to sandy sediments so moderate levels of sedimentation may also limit the system's ability to counteract the effects of eutrophication. The study reveals that it is important to consider context, i.e. the range of conditions inhabited by a particular species, in order to assess the effect of key species on ecosystem function. It appears that it is not just the loss of key species, but alteration of those species' habitats (even without substantial changes in biomass), that has the potential to alter ecosystem function.
Acknowledgments
We thank A. Jones, C. Huisemann, C. Satake, C. Taylor, C. Niemand, D. Kohlmeier, D. Bell, E. Coleman, H. Needham, J. Blair, L. McCartain, M. Knox, N. Pereira, N. Volkenborn, P. Ross, R. Bell, R. Gladstone-Gallagher, and W. Powrie for help in the field. We also thank two anonymous reviewers for their constructive comments that greatly improved this manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was supported by a University of Waikato Doctoral Scholarship to HJ. DB was supported by the New Zealand Foundation for Research, Science and Technology (FRST Contract No. UOWX0505). AL was supported by the New Zealand Foundation for Research Science & Technology (FRST Contract No. C01X0501). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kennish MJ. Environmental threats and environmental future of estuaries. Environmental Conservation. 2002;29:78–107. [Google Scholar]
- 2.Gray JS. Marine biodiversity: Patterns, threats and conservation needs. Biodiversity and Conservation. 1997;6:153–175. [Google Scholar]
- 3.Levin LA, Boesch DF, Covich A, Dahm C, Erseus C, et al. The function of marine critical transition zones and the importance of sediment biodiversity. Ecosystems. 2001;4:430–451. [Google Scholar]
- 4.Ellis J, Cummings V, Hewitt J, Thrush S, Norkko A. Determining effects of suspended sediment on condition of a suspension feeding bivalve (Atrina zelandica): results of a survey, a laboratory experiment and a field transplant experiment. Journal of Experimental Marine Biology and Ecology. 2002;267:147–174. [Google Scholar]
- 5.Norkko A, Thrush SF, Hewitt JE, Cummings VJ, Norkko J, et al. Smothering of estuarine sandflats by terrigenous clay: the role of wind-wave disturbance and bioturbation in site-dependent macrofaunal recovery. Marine Ecology Progress Series. 2002;234:23–41. [Google Scholar]
- 6.Norkko J, Hewitt JE, Thrush S. Effects of increased sedimentation on the physiology of two estuarine soft-sediment bivalves, Austrovenus stutchburyi and Paphies australis. Journal of Experimental Marine Biology and Ecology. 2006;333:12–26. [Google Scholar]
- 7.Thrush SF, Hewitt JE, Cummings V, Ellis JI, Hatton C, et al. Muddy waters: elevating sediment input to coastal and estuarine habitats. Frontiers in Ecology and the Environment. 2004;2:299–306. [Google Scholar]
- 8.Thrush SF, Hewitt JE, Norkko A, Nicholls PE, Funnell GA, et al. Habitat change in estuaries: predicting broad-scale responses of intertidal macrofauna to sediment mud content. Marine Ecology Progress Series. 2003;263:101–112. [Google Scholar]
- 9.Hooper DU, Chapin FS, Ewel JJ, Hector A, Inchausti P, et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecological Monographs. 2005;75:3–35. [Google Scholar]
- 10.Loreau M, Naeem S, Inchausti P, Bengtsson J, Grime JP, et al. Biodiversity and ecosystem functioning: Current knowledge and future challenges. Science. 2001;294:804–808. doi: 10.1126/science.1064088. [DOI] [PubMed] [Google Scholar]
- 11.Naeem S. Ecosystem consequences of biodiversity loss: The evolution of a paradigm. Ecology. 2002;83:1537–1552. [Google Scholar]
- 12.Thrush SF, Hewitt JE, Gibbs M, Lundquist C, Norkko A. Functional role of large organisms in intertidal communities: Community effects and ecosystem function. Ecosystems. 2006;9:1029–1040. [Google Scholar]
- 13.Widdicombe S, Austen MC. Experimental evidence for the role of Brissopsis lyrifera (Forbes, 1841) as a critical species in the maintenance of benthic diversity and the modification of sediment chemistry. Journal of Experimental Marine Biology and Ecology. 1998;228:241–255. [Google Scholar]
- 14.Lohrer AM, Thrush SF, Gibbs M. Bioturbators enhance ecosystem function through complex biogeochemical interactions. Nature. 2004;431:1092–1095. doi: 10.1038/nature03042. [DOI] [PubMed] [Google Scholar]
- 15.Needham HR, Pilditch CA, Lohrer AM, Thrush SF. Habitat dependence in the functional traits of Austrohelice crassa, a key bioturbating species. Marine Ecology Progress Series. 2010;414:179–193. [Google Scholar]
- 16.Needham HR, Pilditch CA, Lohrer AM, Thrush SF. Context specific bioturbation mediates changes to ecosystem functioning. Ecosystems. 2011. in press. DOI: 10.1007/s10021-10011-19468-10020.
- 17.Newell RIE. Ecosystem influences of natural and cultivated populations of suspension-feeding bivalve molluscs: a review. Journal of Shellfish Research. 2004;23:51–61. [Google Scholar]
- 18.Newell RIE, Cornwell JC, Owens MS. Influence of simulated bivalve biodeposition and microphytobenthos on sediment nitrogen dynamics: a laboratory study. Limnology and Oceanography. 2002;47:1367–1379. [Google Scholar]
- 19.Jackson JBC, Kirby MX, Berger WH, Bjorndal KA, Botsford LW, et al. Historical overfishing and the recent collapse of coastal ecosystems. Science. 2001;293:629–638. doi: 10.1126/science.1059199. [DOI] [PubMed] [Google Scholar]
- 20.Kemp WM, Boynton WR, Adolf JE, Boesch DF, Boicourt WC, et al. Eutrophication of Chesapeake Bay: historical trends and ecological interactions. Marine Ecology Progress Series. 2005;303:1–29. [Google Scholar]
- 21.Alpine AE, Cloern JE. Trophic interactions and direct physical effects control phytoplankton biomass and production in an estuary. Limnology and Oceanography. 1992;37:946–955. [Google Scholar]
- 22.Barbiero RP, Rockwell DC, Warren GJ, Tuchman ML. Changes in spring phytoplankton communities and nutrient dynamics in the eastern basin of Lake Erie since the invasion of Dreissena spp. Canadian Journal of Fisheries and Aquatic Sciences. 2006;63:1549–1563. [Google Scholar]
- 23.Whitlatch RB, Hines AH, Thrush SF, Hewitt JE, Cummings V. Benthic faunal responses to variations in patch density and patch size of a suspension-feeding bivalve. Journal of Experimental Marine Biology and Ecology. 1997;216:171–189. [Google Scholar]
- 24.Hewitt JE, Thrush SF, Cummings VJ, Pridmore RD. Matching patterns with processes: Predicting the effect of size and mobility on the spatial distributions of the bivalves Macomona liliana and Austrovenus stutchburyi. Marine Ecology Progress Series. 1996;135:57–67. [Google Scholar]
- 25.Sandwell DR, Pilditch CA, Lohrer AM. Density dependent effects of an infaunal suspension-feeding bivalve (Austrovenus stutchburyi) on sandflat nutrient fluxes and microphytobenthic productivity. Journal of Experimental Marine Biology and Ecology. 2009;373:16–25. [Google Scholar]
- 26.Cummings V, Hewitt J, Halliday J, Mackay G. Optimizing the success of Austrovenus stutchburyi restoration: Preliminary investigations in a New Zealand estuary. Journal of Shellfish Research. 2007;26:89–100. [Google Scholar]
- 27.De Luca-Abbott S. Biomarkers of sub-lethal stress in the soft-sediment bivalve Austrovenus stutchburyi exposed in-situ to contaminated sediment in an urban New Zealand harbour. Marine Pollution Bulletin. 2001;42:817–825. doi: 10.1016/s0025-326x(00)00226-5. [DOI] [PubMed] [Google Scholar]
- 28.Giles H, Pilditch CA. Effects of mussel (Perna canaliculus) biodeposit decomposition on benthic respiration and nutrient fluxes. Marine Biology. 2006;150:261–271. [Google Scholar]
- 29.Greig MJ, Ridgway NM, Shakespeare BS. Sea surface temperature variations at coastal sites around New Zealand. New Zealand Journal of Marine and Freshwater Research. 1988;22:391–400. [Google Scholar]
- 30.Miller LP, Gaylord B. Barriers to flow: The effects of experimental cage structures on water velocities in high-energy subtidal and intertidal environments. Journal of Experimental Marine Biology and Ecology. 2007;344:215–228. [Google Scholar]
- 31.Hall SJ, Raffaelli D, Turrell WR. Predator-caging experiments in marine systems: A re-examination of their value. American Naturalist. 1990;136:657–672. [Google Scholar]
- 32.Hulberg LW, Oliver JS. Caging manipulations in marine soft-bottom communities: Importance of animal interactions or sedimentary habitat modifications. Canadian Journal of Fisheries and Aquatic Sciences. 1980;37:1130–1139. [Google Scholar]
- 33.Beentjes MP, Williams BG. Endogenous circatidal rhythmicity in the New Zealand cockle Chione stutchburyi (Bivalvia, Veneridae). Marine Behaviour and Physiology. 1986;12:171–180. [Google Scholar]
- 34.Bruesewitz DA, Tank JL, Bernot MJ, Richardson WB, Strauss EA. Seasonal effects of the zebra mussel (Dreissena polymorpha) on sediment denitrification rates in Pool 8 of the Upper Mississippi River. Canadian Journal of Fisheries and Aquatic Sciences. 2006;63:957–969. [Google Scholar]
- 35.Inwood SE, Tank JL, Bernot MJ. Patterns of denitrification associated with land use in 9 midwestern headwater streams. Journal of the North American Benthological Society. 2005;24:227–245. [Google Scholar]
- 36.Knowles R. Acetylene inhibition technique: development, advantages, and potential problems. In: Revsbech NP, Sorensen J, editors. Denitrification in Soil and Sediment. New York: Plenum Press; 1990. pp. 151–166. [Google Scholar]
- 37.Bernot MJ, Dodds WK, Gardner WS, McCarthy MJ, Sobolev D, et al. Comparing denitrification estimates for a Texas estuary by using acetylene inhibition and membrane inlet mass spectrometry. Applied and Environmental Microbiology. 2003;69:5950–5956. doi: 10.1128/AEM.69.10.5950-5956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruesewitz DA, Tank JL, Hamilton SK. Seasonal effects of zebra mussels on littoral nitrogen transformation rates in Gull Lake, Michigan, USA. Freshwater Biology. 2009;54:1427–1443. [Google Scholar]
- 39.Bruesewitz DA, Hamilton DP, Schipper LA. Denitrification potential in lake sediment increases across a gradient of catchment agriculture. Ecosystems. 2011;14:341–352. [Google Scholar]
- 40.Groffman PM, Altabet MA, Bohlke JK, Butterbach-Bahl K, David MB, et al. Methods for measuring denitrification: Diverse approaches to a difficult problem. Ecological Applications. 2006;16:2091–2122. doi: 10.1890/1051-0761(2006)016[2091:mfmdda]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 41.Arar EJ, Collins GB. Cincinnati: National Exposure Research Laboratory, U.S. Environmental Protection Agency; 1997. In vitro determination of chlorophyll a and phaeophytin a in marine and freshwater algae by fluorescence. [Google Scholar]
- 42.Singer JK, Anderson JB, Ledbetter MT, McCave IN, Jones KPN, et al. An assessment of analytical techniques for the size analysis of fine-grained sediments. Journal of Sedimentary Petrology. 1988;58:534–543. [Google Scholar]
- 43.Ryba SA, Burgess RM. Effects of sample preparation on the measurement of organic carbon, hydrogen, nitrogen, sulfur, and oxygen concentrations in marine sediments. Chemosphere. 2002;48:139–147. doi: 10.1016/s0045-6535(02)00027-9. [DOI] [PubMed] [Google Scholar]
- 44.Tank JL, Dodds WK. Nutrient limitation of epilithic and epixylic biofilms in ten North American streams. Freshwater Biology. 2003;48:1031–1049. [Google Scholar]
- 45.Lohrer AM, Halliday NJ, Thrush SF, Hewitt JE, Rodil IF. Ecosystem functioning in a disturbance-recovery context: Contribution of macrofauna to primary production and nutrient release on intertidal sandflats. Journal of Experimental Marine Biology and Ecology. 2010;390:6–13. [Google Scholar]
- 46.Kang CK, Sauriau PG, Richard P, Blanchard GF. Food sources of the infaunal suspension-feeding bivalve Cerastoderma edule in a muddy sandflat of Marennes-Oléron Bay, as determined by analyses of carbon and nitrogen stable isotopes. Marine Ecology Progress Series. 1999;187:147–158. [Google Scholar]
- 47.Safi KA. Microalgal populations of three New Zealand coastal locations: forcing functions and benthic-pelagic links. Marine Ecology Progress Series. 2003;259:67–78. [Google Scholar]
- 48.Kang CK, Lee YW, Choy EJ, Shin JK, Seo IS, et al. Microphytobenthos seasonality determines growth and reproduction in intertidal bivalves. Marine Ecology Progress Series. 2006;315:113–127. [Google Scholar]
- 49.Billerbeck M, Roy H, Bosselmann K, Huettel M. Benthic photosynthesis in submerged Wadden Sea intertidal flats. Estuarine, Coastal and Shelf Science. 2007;71:704–716. [Google Scholar]
- 50.Blanchard GF, Guarini JM, Orvain F, Sauriau PG. Dynamic behaviour of benthic microalgal biomass in intertidal mudflats. Journal of Experimental Marine Biology and Ecology. 2001;264:85–100. [Google Scholar]
- 51.Middelburg JJ, Barranguet C, Boschker HTS, Herman PMJ, Moens T, et al. The fate of intertidal microphytobenthos carbon: An in situ C-13-labeling study. Limnology and Oceanography. 2000;45:1224–1234. [Google Scholar]
- 52.Mouritsen KN, Poulin R. The mud flat anemone-cockle association: mutualism in the intertidal zone? Oecologia. 2003;135:131–137. doi: 10.1007/s00442-003-1183-x. [DOI] [PubMed] [Google Scholar]
- 53.Morar SR, Bury SJ, Wilkinson SP, Davy SK. Sedimentary nitrogen uptake and assimilation in the temperate zooxanthellate sea anemone Anthopleura aureoradiata. Journal of Experimental Marine Biology and Ecology. 2011;399:110–119. [Google Scholar]
- 54.Davis WR. The role of bioturbation in sediment resuspension and its interaction with physical shearing. Journal of Experimental Marine Biology and Ecology. 1993;171:187–200. [Google Scholar]
- 55.Ciutat A, Widdows J, Pope ND. Effect of Cerastoderma edule density on near-bed hydrodynamics and stability of cohesive muddy sediments. Journal of Experimental Marine Biology and Ecology. 2007;346:114–126. [Google Scholar]
- 56.Giles H, Pilditch CA, Bell DG. Sedimentation from mussel (Perna canaliculus) culture in the Firth of Thames, New Zealand: Impacts on sediment oxygen and nutrient fluxes. Aquaculture. 2006;261:125–140. [Google Scholar]
- 57.Stenton-Dozey J, Probyn T, Busby A. Impact of mussel (Mytilus galloprovincialis) raft-culture on benthic macrofauna, in situ oxygen uptake, and nutrient fluxes in Saldanha Bay, South Africa. Canadian Journal of Fisheries and Aquatic Sciences. 2001;58:1021–1031. [Google Scholar]
- 58.Seitzinger SP. Denitrification in freshwater and coastal marine ecosystems: ecological and geochemical significance. Limnology and Oceanography. 1988;33:702–724. [Google Scholar]
- 59.Bruesewitz DA, Tank JL, Bernot MJ. Delineating the effects of zebra mussels (Dreissena polymorpha) on N transformation rates using laboratory mesocosms. Journal of the North American Benthological Society. 2008;27:236–251. [Google Scholar]
- 60.Gardner WS, Yang LY, Cotner JB, Johengen TH, Lavrentyev PJ. Nitrogen dynamics in sandy freshwater sediments (Saginaw Bay, Lake Huron). Journal of Great Lakes Research. 2001;27:84–97. [Google Scholar]
- 61.Risgaard-Petersen N. Coupled nitrification-denitrification in autotrophic and heterotrophic estuarine sediments: On the influence of benthic microalgae. Limnology and Oceanography. 2003;48:93–105. [Google Scholar]
- 62.Sundback K, Miles A, Goransson E. Nitrogen fluxes, denitrification and the role of microphytobenthos in microtidal shallow-water sediments: an annual study. Marine Ecology Progress Series. 2000;200:59–76. [Google Scholar]
- 63.An S, Joye SB. Enhancement of coupled nitrification-denitrification by benthic photosynthesis in shallow estuarine sediments. Limnology and Oceanography. 2001;46:62–74. [Google Scholar]
- 64.Norkko J, Thrush SF. Ecophysiology in environmental impact assessment: implications of spatial differences in seasonal variability of bivalve condition. Marine Ecology Progress Series. 2006;326:175–186. [Google Scholar]
- 65.Seitzinger S, Harrison JA, Bohlke JK, Bouwman AF, Lowrance R, et al. Denitrification across landscapes and waterscapes: A synthesis. Ecological Applications. 2006;16:2064–2090. doi: 10.1890/1051-0761(2006)016[2064:dalawa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 66.An SM, Gardner WS, Kana T. Simultaneous measurement of denitrification and nitrogen fixation using isotope pairing with membrane inlet mass spectrometry analysis. Applied and Environmental Microbiology. 2001;67:1171–1178. doi: 10.1128/AEM.67.3.1171-1178.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kana TM, Sullivan MB, Cornwell JC, Groszkowski KM. Denitrification in estuarine sediments determined by membrane inlet mass spectrometry. Limnology and Oceanography. 1998;43:334–339. [Google Scholar]
- 68.Grant J, Turner SJ, Legendre P, Hume TM, Bell RG. Patterns of sediment reworking and transport over small spatial scales on an intertidal sandflat, Manukau Harbour, New Zealand. Journal of Experimental Marine Biology and Ecology. 1997;216:33–50. [Google Scholar]
- 69.Englund G, Cooper SD. Scale effects and extrapolation in ecological experiments. Advances in Ecological Research. 2003;33:161–213. [Google Scholar]
- 70.Hewitt JE, Pridmore RD, Thrush SF, Cummings VJ. Assessing the short-term stability of spatial patterns of macrobenthos in a dynamic estuarine system. Limnology and Oceanography. 1997;42:282–288. [Google Scholar]
- 71.Coco G, Thrush SF, Green MO, Hewitt JE. Feedbacks between bivalve density, flow and suspended sediment concentration on patch stable states. Ecology. 2006;87:2862–2870. doi: 10.1890/0012-9658(2006)87[2862:fbbdfa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 72.Friedrichs M, Graf G, Springer B. Skimming flow induced over a simulated polychaete tube lawn at low population densities. Marine Ecology Progress Series. 2000;192:219–228. [Google Scholar]
- 73.Thrush SF, Whitlatch RB, Pridmore RD, Hewitt JE, Cummings VJ, et al. Scale-dependent recolonization: The role of sediment stability in a dynamic sandflat habitat. Ecology. 1996;77:2472–2487. [Google Scholar]
- 74.Glud RN, Forster S, Huettel M. Influence of radial pressure gradients on solute exchange in stirred benthic chambers. Marine Ecology Progress Series. 1996;141:303–311. [Google Scholar]
- 75.Tengberg A, Stahl H, Gust G, Muller V, Arning U, et al. Intercalibration of benthic flux chambers I. Accuracy of flux measurements and influence of chamber hydrodynamics. Progress in Oceanography. 2004;60:1–28. [Google Scholar]