Abstract
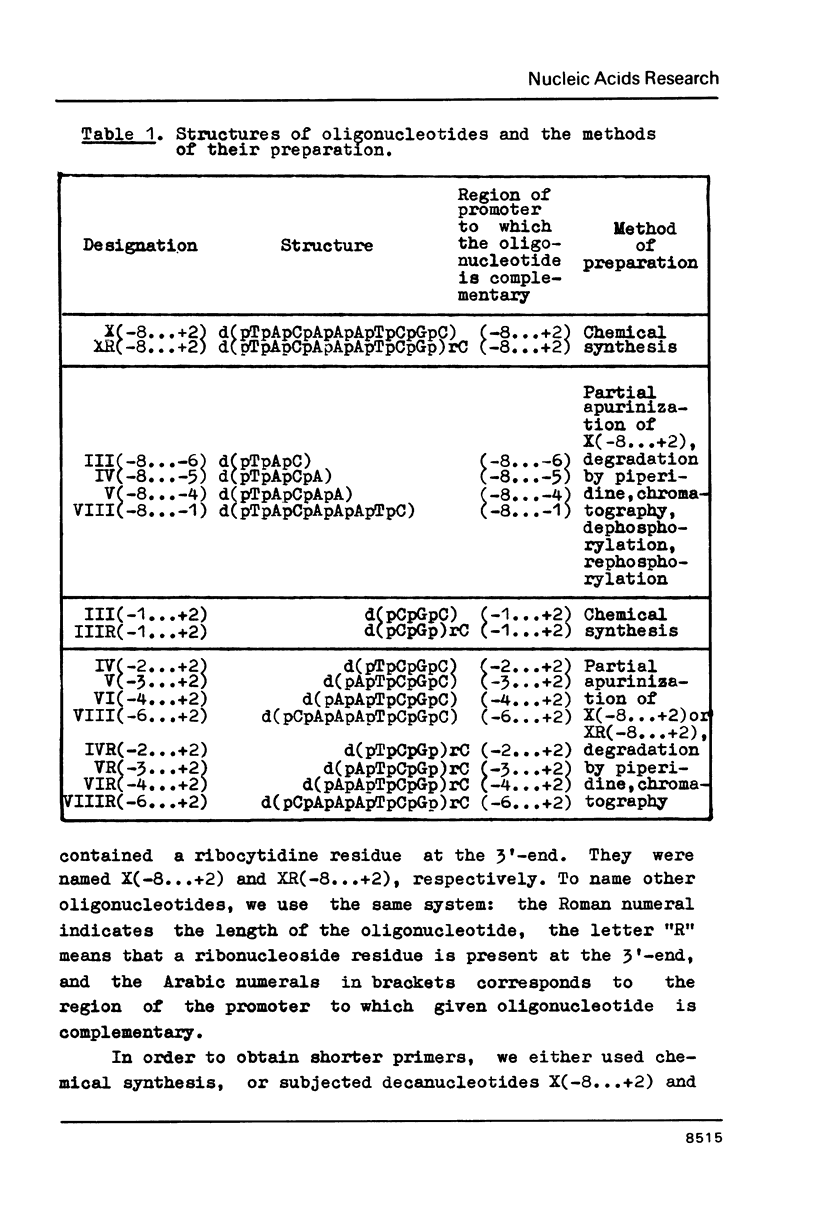
Primer-dependent transcription by E. coli RNA polymerase on T7 promoter A2 has been studied. Synthetic deoxyribonucleotides complementary to the promoter over the region -8...+2 were taken as primers. A ribonucleoside residue was present at the 3'-end of some of these oligonucleotides. The octanucleotide complementary to the region -8...-1 appeared to be an active primer. Oligonucleotides having lengths from 3 to 6 nucleotide residues complementary to the promoter over the region -4...+2 also exhibited primer activity. The latter was some 5-10 times greater in the case of oligonucleotides having a ribonucleoside residue at the 3'-end. Oligonucleotides which on complementary binding do not reach the center of phosphodiester bond synthesis, as well as the decanucleotides (-8...+2) and octanucleotides (-6...+2) of both the ribo- and deoxyribo-series were inactive as primers.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dausse J. P., Sentenac A., Fromageot P. Interaction of RNA polymerase from Escherichia coli with DNA. Analysis of T7 DNA early-promoter sites. Eur J Biochem. 1975 Sep 15;57(2):569–578. doi: 10.1111/j.1432-1033.1975.tb02332.x. [DOI] [PubMed] [Google Scholar]
- Duckworth M. L., Gait M. J., Goelet P., Hong G. F., Singh M., Titmas R. C. Rapid synthesis of oligodeoxyribonucleotides VI. Efficient, mechanised synthesis of heptadecadeoxyribonucleotides by an improved solid phase phosphotriester route. Nucleic Acids Res. 1981 Apr 10;9(7):1691–1706. doi: 10.1093/nar/9.7.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez N., Wiggs J., Chamberlin M. J. A simple procedure for resolution of Escherichia coli RNA polymerase holoenzyme from core polymerase. Arch Biochem Biophys. 1977 Aug;182(2):404–408. doi: 10.1016/0003-9861(77)90521-5. [DOI] [PubMed] [Google Scholar]
- Grachev M. A., Zaychikov E. F. Initiation by Escherichia coli RNA-polymerase: transformation of abortive to productive complex. FEBS Lett. 1980 Jun 16;115(1):23–26. doi: 10.1016/0014-5793(80)80718-6. [DOI] [PubMed] [Google Scholar]
- Hansen U. M., McClure W. R. Role of the sigma subunit of Escherichia coli RNA polymerase in initiation. II. Release of sigma from ternary complexes. J Biol Chem. 1980 Oct 25;255(20):9564–9570. [PubMed] [Google Scholar]
- Hoffman D. J., Niyogi S. K. RNA initiation with dinucleoside monophosphates during transcription of bacteriophage T4 DNA with RNA polymerase of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Feb;70(2):574–578. doi: 10.1073/pnas.70.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribnow D. Bacteriophage T7 early promoters: nucleotide sequences of two RNA polymerase binding sites. J Mol Biol. 1975 Dec 15;99(3):419–443. doi: 10.1016/s0022-2836(75)80136-7. [DOI] [PubMed] [Google Scholar]
- Siebenlist U. RNA polymerase unwinds an 11-base pair segment of a phage T7 promoter. Nature. 1979 Jun 14;279(5714):651–652. doi: 10.1038/279651a0. [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Simpson R. B., Gilbert W. E. coli RNA polymerase interacts homologously with two different promoters. Cell. 1980 Jun;20(2):269–281. doi: 10.1016/0092-8674(80)90613-3. [DOI] [PubMed] [Google Scholar]
- Smagowicz W. J., Scheit K. H. Primed abortive initiation of RNA synthesis by E. coli RNA polymerase on T7 DNA. Steady state kinetic studies. Nucleic Acids Res. 1978 Jun;5(6):1919–1932. doi: 10.1093/nar/5.6.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C. P., Jay E., Bahl C. P., Wu R. A reliable mapping method for sequence determination of oligodeoxyribonucleotides by mobility shift analysis. Anal Biochem. 1976 Jul;74(1):73–93. doi: 10.1016/0003-2697(76)90311-0. [DOI] [PubMed] [Google Scholar]





