Abstract
OBJECTIVES:
N-3 polyunsaturated fatty acids have been proposed as a novel treatment for preventing postoperative atrial fibrillation due to their potential anti-inflammatory and anti-arrhythmic effects. However, randomized studies have yielded conflicting results. The objective of this study is to review randomized trials of N-3 polyunsaturated fatty acid use for postoperative atrial fibrillation.
METHODS:
Using the CENTRAL, PUBMED, EMBASE, and LILACS databases, a literature search was conducted to identify all of the studies in human subjects that reported the effects of N-3 polyunsaturated fatty acids on the prevention of postoperative atrial fibrillation in cardiac surgery patients. The final search was performed on January 30, 2011. There was no language restriction, and the search strategy only involved terms for N-3 polyunsaturated fatty acids (or fish oil), atrial fibrillation, and cardiac surgery. To be included, the studies had to be randomized (open or blinded), and the enrolled patients had to be ≥18 years of age.
RESULTS:
Four randomized studies (three double-blind, one open-label) that enrolled 538 patients were identified. The patients were predominantly male, the mean age was 62.3 years, and most of the patients exhibited a normal left atrial size and ejection fraction. N-3 polyunsaturated fatty acid use was not associated with a reduction in postoperative atrial fibrillation. Similar results were observed when the open-label study was excluded.
CONCLUSIONS:
There is insufficient evidence to suggest that treatment with N-3 polyunsaturated fatty acids reduces postoperative atrial fibrillation. Therefore, their routine use in patients undergoing cardiac surgery is not recommended.
Keywords: Omega-3 fatty acids, Atrial fibrillation, Meta-analysis, Open Heart surgery, Prevention
INTRODUCTION
Atrial fibrillation (AF) is the most common arrhythmia following cardiac surgery. Its incidence ranges from 20 to 70%, depending on the patient age and the complexity of the surgical procedure.1-3 Although well tolerated in most cases, postoperative AF is associated with prolonged hospitalization, increased costs, morbidity, and mortality.4-6
The etiology of postoperative AF is multifactorial, and although several risk factors are associated with this condition, the underlying mechanisms are controversial. Furthermore, antiarrhythmic drugs have not been effective in preventing postoperative AF.2
Recent clinical and experimental studies have shown that N-3 polyunsaturated fatty acids (PUFAs) are effective in preventing AF,7,8 and PUFAs have been used to prevent AF in patients undergoing cardiac surgery. However, the studies supporting this treatment have reported conflicting results on its efficacy in preventing postoperative AF.9-13 The objective of this analysis is to estimate the effect of PUFA on preventing postoperative AF through a meta-analysis of randomized controlled clinical trials.
METHODS
Study Search
Using the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 3, 2008), PUBMED, EMBASE, and LILACS databases, a literature search was conducted to identify all of the studies that reported the effects of PUFAs on the prevention of postoperative AF in human cardiac surgery patients. The final literature search was performed on January 30th, 2011. To maximize the sensitivity, no language restriction was used, and the search strategy involved only terms for PUFA (or fish oil), AF, and cardiac surgery. All of the included studies were randomized, both open and blinded studies were considered, and the enrolled patients had to be ≥18 years of age. In addition, the reference lists from the included studies were scrutinized to identify additional citations and specialists in the field, and the authors of the included publications were contacted and asked for unpublished data to avoid publication bias.
Data Abstraction
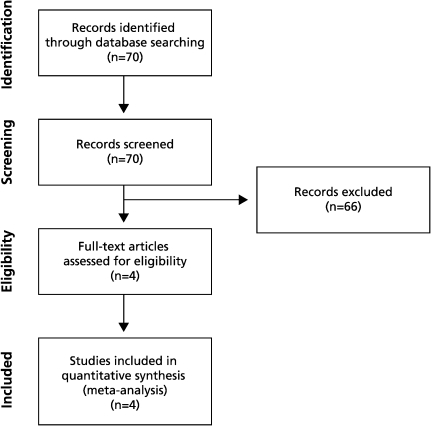
The outcome of interest was the occurrence of AF. Two reviewers (LA and JSH) independently screened, extracted data from, and performed quality assessment of the cohort trials identified by the literature search. Any disagreements between the examiners were settled by consensus. A funnel plot of the results was generated to detect any publication bias. Figure 1 demonstrates the study selection process according to the PRISMA flow diagram.
Figure 1.
The PRISMA flow diagram.
Definition of Atrial Fibrillation
The definition of AF varied between the studies. Calo et al.9 defined postoperative AF as any electrocardiographically confirmed episode of AF of more than 5 minutes in duration or requiring intervention for angina or compromised hemodynamics during the hospitalization period. In the Heidt study,11 AF was detected by monitoring or 12-lead electrocardiography during the intensive care unit period and was defined as any confirmed episode of AF lasting ≥15 minutes. In the Heidarsdottir study,12 all of the patients underwent continuous electrocardiographic monitoring during hospitalization, and postoperative AF was defined as any episode lasting more than 5 minutes. The patients exited the study upon discharge from the hospital or two weeks after the surgery. Finally, Saravanan et al.13 defined AF as any episode lasting ≥30 seconds in the monitor recording (primary outcome). Clinically recognized AF (as documented in the patient's clinical records by the treating clinician) and the AF burden (defined as the percentage of time that a given patient had AF) were the secondary outcomes.
Statistical Analysis
The individual unit of analysis was the patient (not patient-years). Heterogeneity was evaluated using the Cochran Q and chi-squared tests. For the purpose of this analysis, we interpreted a chi-squared of ≥25 as indicative of significant heterogeneity. All of the analyses were performed according to the intention-to-treat principle. The overall treatment effects were reported via odds ratios with 95% confidence intervals. Random effects modeling with the Mantel-Haenszel test was performed to generate a pooled estimate of the effect of PUFA treatment on postoperative AF. To exclude ascertainment bias and any potential confounding due to concomitant treatments in open-label designs, we performed a sensitivity analysis that excluded the open-label trial. The statistical testing was two-tailed, and statistical significance was defined as p<0.05. All of the statistical analyses were conducted with the R Development Core Team software.
RESULTS
Search Results
Four clinical studies (538 patients) reporting the effects of PUFAs on the prevention of postoperative AF were identified and included in the final analyses of this study.9, Of the four studies, two12,13 reported no effects on postoperative AF, and two9,11 reported a significant reduction in the incidence of AF shortly after cardiac surgery.
Study Characteristics and Study Quality
The characteristics of the studies are summarized in Table 1. Most of the studies used encapsulated fish oil, but one11 examined the effects of intravenously administered n-3 fatty acids. Two studies12,13 had a placebo control, one11 administered saturated fatty acids as a control, and the other one9 enrolled patients who had not received PUFA as the control group. The Calo,9 Heidt,11 and Saravanan13 studies included patients who were >18 years of age, while Heidarsdottir12 included all consecutive patients who were >40 years old and scheduled for elective or urgent (not emergent) open heart surgery. The Calo9 and Heidt11 studies excluded patients with unstable hemodynamic conditions before surgery, and Saravanan13 included patients who underwent elective isolated coronary artery bypass graft surgery. Calo,9 Heidt,11 and Saravanan13 all excluded concomitant valvular surgery.
Table 1.
Studies characteristics.
| Results (POAF) | |||||||
| Study | N | Study design | Control | Inclusion criteria | Exclusion criteria | PUFA | Control |
| Calo | 160 | Open-label, prospective, randomized, controlled trial with parallel groups; not blinded | No PUFA | Age >18, NSR, stable hemodynamic conditions before surgery | Concomitant valvular surgery, a prior history of any SVT, the current use of an AAD other than BB, CCB, or digitalis | 15.2% | 33.3% |
| Heidt | 102 | Prospective, randomized double-blinded trial | IV saturated free fatty acids | Age >18, NSR, stable hemodynamic conditions before surgery, no angina at rest | Concomitant valvular surgery, a prior history of any SVT, the current use of an AAD other than BB or CCB | 17.3% | 30.6% |
| Heidarsdottir | 168 | Prospective, randomized, double-blinded, placebo-controlled trial | Placebo | All of the consecutive patients scheduled for elective or open heart surgery | Age <40, prior history of any SVT, the current use of amiodarone or sotalol, an emergent operation | 54.2% | 54.1% |
| Saravanan | 103 | Prospective, randomized, double-blinded, placebo-controlled trial | Placebo | Age >18, scheduled to undergo elective isolated CABG | A prior history of any atrial arrhythmia, the current use of a class-1 or class-3 AAD, fish oil use within 3 mos prior to surgery | 56% | 43% |
AAD, antiarrhythmic drugs; BB, beta-blockers; CABG, coronary artery bypass grafting; CCB, calcium channel blockers; IV, intravenous; NSR, normal sinus rhythm; POAF, postoperative atrial fibrillation; PUFA, polyunsaturated fatty acid; SVT, supraventricular tachycardia.
Baseline Patient Characteristics
The preoperative baseline characteristics are shown in Table 2. In general, the patients in both control and study groups tended to be male with a mean age of 62.3 years. Most of the patients in all of the studies had a normal left atrial size and ejection fraction. Systemic hypertension was more frequently observed in the Calo9 and Heidarsdottir12 populations. The concomitant use of beta-blockers was more common in the Heidarsdottir12 and Saravanan13 studies.
Table 2.
Baseline characteristics.
| Age | Male (%) | LA (mm) | EF (%) | HTN (%) | Beta-blocker (%) | |||||||
| Control | PUFA | Control | PUFA | Control | PUFA | Control | PUFA | Control | PUFA | Control | PUFA | |
| Calo | 64.9±9.1 | 66.2±8.0 | 84 | 86 | 39.7±5.2 | 39.7±5.1 | 55.3±11.4 | 56.3±12.1 | 81.5 | 78.5 | 56.8 | 58.2 |
| Heidt | NR | NR | 64 | 73 | 40.5±5.1 | 40.0±5.1 | 52.3±15.6 | 52.0±15.0 | NR | NR | NR | NR |
| Heidarsdottir | 67 (43, 82) | 67 (45, 82) | 76.9 | 81.9 | NR | NR | 60 (15, 77.5) | 60 (15, 70) | 64.7 | 61.4 | 74.1 | 78.3 |
| Saravanan | 68 (64, 73) | 64 (58, 71) | 82 | 77 | 6% hadLA≥2.3cm/m2 | 4% had LA≥2.3 cm/m2 | 8% had EF≤55% | 10% had EF≤55% | 29 | 35 | 82 | 88 |
The values are presented as the mean ± standard deviation or as the median (25th, 75th), unless otherwise indicated. EF, ejection fraction; HTN, hypertension; LA, left atrium; NR, not reported; PUFA, polyunsaturated fatty acid.
Atrial Fibrillation and Clinical Outcomes
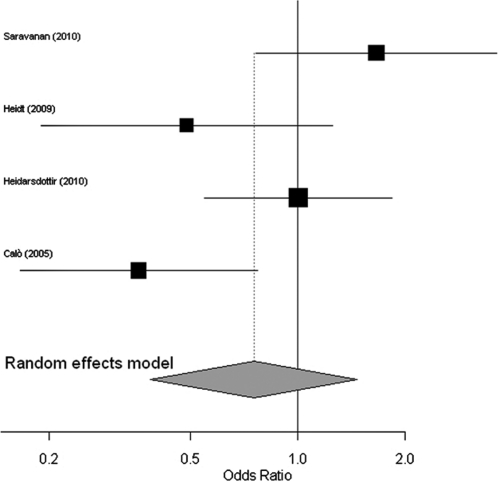
Figure 2 shows a forest plot comparing the total mortality of the patients that received PUFAs to those that did not receive PUFAs. The use of PUFAs was not associated with a reduction in the occurrence of postoperative AF in the patients undergoing cardiac surgery compared to the untreated patients (odds ratio, 0.79; 95% confidence interval, 0.56 - 1.13; p = 0.195). Similar results were observed when the open-label study was excluded from the analyses (odds ratio, 0.99; 95% confidence interval, 0.65 - 1.49; p = 0.963). Heidarsdottir et al.12 reported no differences in the postoperative outcomes (such as blood transfusion, the re-operation frequency, and major bleeding) between the groups. Two patients in the PUFA group died. One patient died due to a massive myocardial infarction and heart failure three days after surgery, and another patient died from an ascending aortic rupture two days after surgery. One patient in the PUFA arm and three patients in the placebo group suffered cerebrovascular accidents. Gastrointestinal discomfort was more common in the PUFA group and resulted in the discontinuation of two patients. In the Calo9 study, no significant adverse reactions were observed in the PUFA patients, except for a single case of an allergic reaction, and there were no differences in the incidence of non-fatal complications or death between the groups. In both this study and in the study by Heidt et al.,11 PUFA treatment was associated with a shorter hospital stay. In contrast, Saravanan et al.13 found no difference in the length of the hospital stay between the groups. No drug-related complications were reported by Saravanan et al.13
Figure 2.
A meta-analysis of the randomized studies examining the incidence of postoperative atrial fibrillation.
DISCUSSION
This analysis showed no benefit of PUFAs for reducing AF following cardiac surgery.
Although several strategies involving PUFAs have been developed for preventing postoperative AF, none have proven its efficacy or justify its routine use in this setting. Clinical and experimental studies have shown that the administration of PUFAs reduces major cardiac events, including sudden cardiac death, ventricular fibrillation, and AF.14-16 In a cohort of 4815 patients with 12 years of follow-up, Mozaffarian et al.17,18 demonstrated that fish intake is associated with a lower incidence of AF.
Several studies have demonstrated the protective effect of PUFAs against ventricular arrhythmias. Hyperpolarization of the resting membrane potential and an increase in the phase-4 refractory period have been postulated to be the main antiarrhythmic effects of PUFAs.7,19 Moreover, PUFAs have been shown to reduce the asynchronous contractile activity of myocytes,20 increase electrical stability by prolonging the inactivation of fast outward sodium channels, and modulate calcium release as a result of their direct interaction with the sarcoplasmic reticulum. However, other studies have demonstrated that PUFAs shorten the effective refractory period and action potential,21-23 which favors the initiation and maintenance of AF. In fact, a few experimental studies have demonstrated a pro-arrhythmic effect of PUFAs.24,25 In terms of adverse side effects, the studies reported different outcomes and results, but in general, PUFAs do not seem to be directly associated with any bleeding or ischemic complications.
Clinical studies have reported several benefits of PUFA treatment for chronic and acute inflammation.26-28 EPA and DHA can inhibit the conversion of arachidonic acid to prostaglandin E2 and leukotriene B4, and they also decrease the synthesis of tumor necrosis factor alpha and interleukin-1 beta.29 The trauma caused by surgery initiates both local and systemic inflammatory responses, reflected by increased C-reactive protein levels. Moreover, it is possible that local atrial inflammation may produce atrial conduction abnormalities and consequent reentrant arrhythmias. In a study conducted by Halonen et al.,30 the administration of intravenous hydrocortisone reduced the incidence of AF after cardiac surgery, suggesting that an exaggerated inflammatory response could be an etiological factor for the development of postoperative AF. A study by Mariscalco et al.10 showed higher white blood cell counts after surgery in patients affected by early AF and lower levels of postoperative C-reactive protein levels in the PUFA-treated group, suggesting that PUFAs have anti-inflammatory properties. This was contradicted by a study by Saravanan,13 which showed that C-reactive protein levels were not affected by PUFAs. This discrepancy might be due to the low doses of PUFAs used in this study, which may have been below the threshold required to exert any anti-inflammatory effects. Interestingly, patients in the Calo et al. study population were more likely to have a history of myocardial infarction and/or off-pump surgery. This might also explain the better response to PUFA therapy in this study compared to other studies.
Calo et al.9 first demonstrated that PUFA administration during hospitalization significantly reduced the occurrence of postoperative AF in patients undergoing coronary artery bypass graft surgery (18.1% absolute risk reduction [RR]; 54.4% relative RR) and was associated with a shorter hospital stay (7.3±2.1 days vs. 8.2±2.6 days for PUFA patients and control patients, respectively; p = 0.017). In the multivariable analysis, age and the use of PUFAs were significant independent predictors of postoperative AF. However, this study was not blinded or placebo-controlled, and it excluded patients undergoing valvular surgery and those with a history of AF, which are factors that increase the risk for postoperative AF. Another limitation of this study was that the patients in the PUFA group were hospitalized for fewer days and may have had unidentified asymptomatic AF after discharge.
The results of this study are congruent with those found by Heidt.11 Only patients undergoing coronary artery bypass graft procedures were included in that study. The patients treated with PUFAs developed significantly less AF than control patients (17.3% vs. 30.6%, respectively; p<0.05). More recently, Mariscalco et al. demonstrated that preoperative PUFA therapy is associated with a decreased incidence of early AF after cardiac surgery but does not prevent late AF. PUFA administration was independently associated with a 46% relative reduction in the risk of early AF development (OR, 0.54; 95% CI, 0.31–0.92). This study included coronary artery bypass surgery procedures and valve repair, combined surgeries, and other types of cardiac surgery. Independent predictors of early AF were age, the left atrial area, reoperation, and the use of an intra-aortic balloon pump. However, this study was limited by its lack of randomization; therefore, a causal relationship between PUFAs and postoperative AF cannot be accurately assessed.
Contrary to previous studies, two recent, randomized, double-blind, and placebo-controlled studies failed to demonstrate any beneficial effects of PUFA administration.12,13 In addition, the recently published Efficacy and Safety of Prescription Omega-3 Acid Ethyl Esters (P-OM3) for the Prevention of Recurrent Symptomatic Atrial Fibrillation trial showed similar results. This was a prospective, randomized, and double-blinded trial of 663 AF patients (average age, 61 years; 56% male) enrolled at 96 sites in which the use of a fish oil-derived product (4 grams/day) was compared to the use of a placebo.31 The two main findings of this study were that the paroxysmal AF patients who received omega-3 did not differ significantly from the patients of the placebo group in terms of the time to the first recurrence of symptoms, and there was no significant difference in persistent AF between the treated and placebo groups.
Differences in the patient profiles, the type of surgery, the definition of arrhythmia, and the method of arrhythmia surveillance could explain the contradictory results of these studies. In particular, the method used to evaluate the occurrence of AF and its burden had the most significant effect. Calo et al.9 and Heidarsdottir et al.12 defined AF as any episode lasting more than 5 minutes, Heidt et al.11 used a cutoff of 15 minutes and Saravanan et al.13 used a cutoff of 30 seconds. This difference likely explains the higher overall incidence of AF in the latter study.
The method of rhythm surveillance (continuous monitoring vs. electrocardiogram monitoring only if symptoms are present) might also affect the detection of AF. Unsurprisingly, the occurrence of AF was higher in the studies that used continuous electrocardiographic monitoring, which was observed in the Saravanan sample.
Another important issue that must be considered is the concomitant use of beta-blockers and statins. In Calo et al.,9 for example, the percentage of patients taking both drugs was much lower than in Saravanan et al. (57.5% on beta-blockers and 56.9% on statins compared to 85% and 98%, respectively). This may have offset the beneficial effects of PUFAs in the Saravanan population (Table 3).
Table 3.
The PUFA type and dose, the definition of AF, and the rhythm surveillance method.
| Study | Type/dose of PUFA | Definition of AF | Rhythm surveillance |
| Calo | Daily doses of 850–882 mg EPA and DHA in an average ratio of 1:2 EPA:DHA, initiated at least 5 days before surgery and continued until hospital discharge | Any episode lasting more than 5 min or requiring intervention | Continuous rhythm monitoring for the first 4–5 days, followed by daily ECG until hospital discharge |
| Heidt | Infusion pump (100 mg soya oil/kg body weight/day) started at least 12 hours before surgery and continued until transfer to the ward | Any episode lasting more than 15 min | Monitoring or 12-lead ECG during the ICU period |
| Heidarsdottir | Two capsules twice daily for a daily dose of 1240 mg EPA, initiated 5–7 days before surgery and continued until hospital discharge or a maximum of 2 weeks after surgery | Any episode lasting more than 5 min | Continuous ECG monitoring during hospitalization |
| Saravanan | Daily doses of 2 mg of a commercially available n-3 PUFA preparation, providing 85–88% EPA+DHA as ethyl esters in a ratio of 1∶2∶1 and initiated at least 5 days before CABG and continued until the day of discharge | Any episode lasting more than 30 sec | Heart rhythm monitoring (Holter) from the immediate post-op period to 5 days after surgery; if the patient was hospitalized for longer than 5 days, ECG was performed daily |
CABG, coronary artery bypass grafting; DHA, docosahexaenoic acid; ECG, electrocardiograph; EPA, eicosapentaenoic acid; ICU, intensive care unit; PUFA, polyunsaturated fatty acid.
CLINICAL IMPLICATIONS
Atrial fibrillation is the most common arrhythmia following cardiac surgery, and inflammation plays a role in its genesis. PUFAs have some anti-inflammatory properties and antiarrhythmic effects. However, this meta-analysis showed that PUFA treatment is not associated with a reduction in the incidence of AF following cardiac surgery. Thus, the routine use of PUFAs in patients undergoing cardiac surgery for the prevention of postoperative AF is not recommended.
LIMITATIONS
Our study has some limitations. The major limitation is the clinical and statistical heterogeneity of the included studies. To overcome this degree of heterogeneity, it would be necessary to perform a large and adequately powered randomized study, which would be difficult to be conducted and expensive. Therefore, the lack of statistical power and the heterogeneity among the studies could, in part, have explained our findings. We only included randomized studies because they are the gold standard for assessing treatment effects. However, the population involved in randomized clinical trials is usually strictly selected and might not reflect the true profiles of the patients treated in clinical practice. Thus, caution should be taken when extrapolating our results to this setting. Furthermore, we could not systematically assess the adverse events associated with PUFA treatment because these outcomes were not systematically and consistently reported in the included studies. Various fatty acids and dosages were used in the studies, and we were unable to investigate the influence of the duration and dosage of preoperative PUFA treatment and its effects on the incidence of postoperative AF. The definition of AF also varied among the studies, which could have affected our findings. Finally, the overall incidence of postoperative AF depended on the type and duration of the monitoring techniques, which varied among the studies.
Despite the heterogeneity of the studies included in our analysis, we demonstrated that there is currently insufficient evidence to suggest that PUFA treatment reduces AF following cardiac surgery. In addition, little information on the adverse events associated with PUFA treatment is available. A large, multicenter, randomized trial with well-defined clinical outcomes and a consistent method of detection of postoperative AF is still needed to assess the potential effect of PUFAs on postoperative AF and its potential adverse outcomes.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Lauer MS, Eagle KA, Buckley MJ, DeSanctis RW. Atrial fibrillation following coronary artery bypass surgery. Prog Cardiovasc Dis. 1989;31:367–78. doi: 10.1016/0033-0620(89)90031-5. 10.1016/0033-0620(89)90031-5 [DOI] [PubMed] [Google Scholar]
- 2.Kaireviciute D, Aidietis A, Lip GY. Atrial fibrillation following cardiac surgery: Clinical features and preventative strategies. Eur Heart J. 2009;30:410–25. doi: 10.1093/eurheartj/ehn609. 10.1093/eurheartj/ehn609 [DOI] [PubMed] [Google Scholar]
- 3.Mathew JP, Fontes ML, Tudor IC, Ramsay J, Duke P, Mazer CD, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291:1720–9. doi: 10.1001/jama.291.14.1720. 10.1001/jama.291.14.1720 [DOI] [PubMed] [Google Scholar]
- 4.Ommen SR, Odell JA, Stanton MS. Atrial arrhythmias after cardiothoracic surgery. N Engl J Med. 1997;336:1429–34. doi: 10.1056/NEJM199705153362006. 10.1056/NEJM199705153362006 [DOI] [PubMed] [Google Scholar]
- 5.Mariscalco G, Klersy C, Zanobini M, Banach M, Ferrarese S, Borsani P, et al. Atrial fibrillation after isolated coronary surgery affects late survival. Circulation. 2008;118:1612–8. doi: 10.1161/CIRCULATIONAHA.108.777789. 10.1161/CIRCULATIONAHA.108.777789 [DOI] [PubMed] [Google Scholar]
- 6.Villareal RP, Hariharan R, Liu BC, Kar B, Lee VV, Elayda M, et al. Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. J Am Coll Cardiol. 2004;43:742–8. doi: 10.1016/j.jacc.2003.11.023. 10.1016/j.jacc.2003.11.023 [DOI] [PubMed] [Google Scholar]
- 7.Kang JX, Leaf A. Antiarrhythmic effects of polyunsaturated fatty acids. Recent studies. Circulation. 1996;94:1774–80. doi: 10.1161/01.cir.94.7.1774. [DOI] [PubMed] [Google Scholar]
- 8.Kang JX, Leaf A. Protective effects of free polyunsaturated fatty acids on arrhythmias induced by lysophosphatidylcholine or palmitoylcarnitine in neonatal rat cardiac myocytes. Eur J Pharmacol. 1996;297:97–106. doi: 10.1016/0014-2999(95)00701-6. 10.1016/0014-2999(95)00701-6 [DOI] [PubMed] [Google Scholar]
- 9.Calo L, Bianconi L, Colivicchi F, Lamberti F, Loricchio ML, de Ruvo E, et al. N-3 fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: A randomized, controlled trial. J Am Coll Cardiol. 2005;45:1723–8. doi: 10.1016/j.jacc.2005.02.079. 10.1016/j.jacc.2005.02.079 [DOI] [PubMed] [Google Scholar]
- 10.Mariscalco G, Sarzi Braga S, Banach M, Borsani P, Bruno VD, Napoleone M, et al. Preoperative n-3 polyunsatured fatty acids are associated with a decrease in the incidence of early atrial fibrillation following cardiac surgery. Angiology. 2010;61:643–50. doi: 10.1177/0003319710370962. 10.1177/0003319710370962 [DOI] [PubMed] [Google Scholar]
- 11.Heidt MC, Vician M, Stracke SK, Stadlbauer T, Grebe MT, Boening A, et al. Beneficial effects of intravenously administered n-3 fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: A prospective randomized study. Thorac Cardiovasc Surg. 2009;57:276–80. doi: 10.1055/s-0029-1185301. 10.1055/s-0029-1185301 [DOI] [PubMed] [Google Scholar]
- 12.Heidarsdottir R, Arnar DO, Skuladottir GV, Torfason B, Edvardsson V, Gottskalksson G, et al. Does treatment with n-3 polyunsaturated fatty acids prevent atrial fibrillation after open heart surgery. Europace. 2010;12:356–63. doi: 10.1093/europace/eup429. 10.1093/europace/eup429 [DOI] [PubMed] [Google Scholar]
- 13.Saravanan P, Bridgewater B, West AL, O'Neill SC, Calder PC, Davidson NC. Omega-3 fatty acid supplementation does not reduce risk of atrial fibrillation after coronary artery bypass surgery: A randomized, double-blind, placebo-controlled clinical trial. Circ Arrhythm Electrophysiol. 2010;3:46–53. doi: 10.1161/CIRCEP.109.899633. 10.1161/CIRCEP.109.899633 [DOI] [PubMed] [Google Scholar]
- 14.de Lorgeril M, Renaud S, Mamelle N, Salen P, Martin JL, Monjaud I, et al. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet. 1994;343:1454–9. doi: 10.1016/s0140-6736(94)92580-1. 10.1016/S0140-6736(94)92580-1 [DOI] [PubMed] [Google Scholar]
- 15.Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, et al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: Diet and reinfarction trial (dart) Lancet. 1989;2:757–61. doi: 10.1016/s0140-6736(89)90828-3. 10.1016/S0140-6736(89)90828-3 [DOI] [PubMed] [Google Scholar]
- 16.Siscovick DS, Raghunathan TE, King I, Weinmann S, Wicklund KG, Albright J, et al. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. JAMA. 1995;274:1363–7. doi: 10.1001/jama.1995.03530170043030. 10.1001/jama.274.17.1363 [DOI] [PubMed] [Google Scholar]
- 17.Mozaffarian D, Psaty BM, Rimm EB, Lemaitre RN, Burke GL, Lyles MF, et al. Fish intake and risk of incident atrial fibrillation. Circulation. 2004;110:368–73. doi: 10.1161/01.CIR.0000138154.00779.A5. 10.1161/01.CIR.0000138154.00779.A5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLennan PL, Bridle TM, Abeywardena MY, Charnock JS. Dietary lipid modulation of ventricular fibrillation threshold in the marmoset monkey. Am Heart J. 1992;123:1555–61. doi: 10.1016/0002-8703(92)90809-a. 10.1016/0002-8703(92)90809-A [DOI] [PubMed] [Google Scholar]
- 19.Fynn SP, Todd DM, Hobbs WJ, Armstrong KL, Garratt CJ. Role of dispersion of atrial refractoriness in the recurrence of clinical atrial fibrillation; a manifestation of atrial electrical remodelling in humans. Eur Heart J. 2001;22:1822–34. doi: 10.1053/euhj.2001.2607. 10.1053/euhj.2001.2607 [DOI] [PubMed] [Google Scholar]
- 20.Nicod L, Rodriguez S, Letang JM, Viollon-Abadie C, Jacqueson A, Berthelot A, et al. Antioxidant status, lipid peroxidation, mixed function oxidase and udp-glucuronyl transferase activities in livers from control and doca-salt hypertensive male sprague dawley rats. Mol Cell Biochem. 2000;203:33–9. doi: 10.1023/a:1007041532523. 10.1023/A:1007041532523 [DOI] [PubMed] [Google Scholar]
- 21.Verkerk AO, van Ginneken AC, Berecki G, den Ruijter HM, Schumacher CA, Veldkamp MW, et al. Incorporated sarcolemmal fish oil fatty acids shorten pig ventricular action potentials. Cardiovasc Res. 2006;70:509–20. doi: 10.1016/j.cardiores.2006.02.022. 10.1016/j.cardiores.2006.02.022 [DOI] [PubMed] [Google Scholar]
- 22.Den Ruijter HM, Berecki G, Opthof T, Verkerk AO, Zock PL, Coronel R. Pro- and antiarrhythmic properties of a diet rich in fish oil. Cardiovasc Res. 2007;73:316–25. doi: 10.1016/j.cardiores.2006.06.014. 10.1016/j.cardiores.2006.06.014 [DOI] [PubMed] [Google Scholar]
- 23.Den Ruijter HM, Berecki G, Verkerk AO, Bakker D, Baartscheer A, Schumacher CA, et al. Acute administration of fish oil inhibits triggered activity in isolated myocytes from rabbits and patients with heart failure. Circulation. 2008;117:536–44. doi: 10.1161/CIRCULATIONAHA.107.733329. 10.1161/CIRCULATIONAHA.107.733329 [DOI] [PubMed] [Google Scholar]
- 24.Burr ML, Ashfield-Watt PA, Dunstan FD, Fehily AM, Breay P, Ashton T, et al. Lack of benefit of dietary advice to men with angina: Results of a controlled trial. Eur J Clin Nutr. 2003;57:193–200. doi: 10.1038/sj.ejcn.1601539. 10.1038/sj.ejcn.1601539 [DOI] [PubMed] [Google Scholar]
- 25.Coronel R, Wilms-Schopman FJ, Den Ruijter HM, Belterman CN, Schumacher CA, Opthof T, et al. Dietary n-3 fatty acids promote arrhythmias during acute regional myocardial ischemia in isolated pig hearts. Cardiovasc Res. 2007;73:386–94. doi: 10.1016/j.cardiores.2006.10.006. 10.1016/j.cardiores.2006.10.006 [DOI] [PubMed] [Google Scholar]
- 26.Calder PC. Polyunsaturated fatty acids, inflammation, and immunity. Lipids. 2001;36:1007–24. doi: 10.1007/s11745-001-0812-7. 10.1007/s11745-001-0812-7 [DOI] [PubMed] [Google Scholar]
- 27.Harbige LS. Fatty acids, the immune response, and autoimmunity: A question of n-6 essentiality and the balance between n-6 and n-3. Lipids. 2003;38:323–41. doi: 10.1007/s11745-003-1067-z. 10.1007/s11745-003-1067-z [DOI] [PubMed] [Google Scholar]
- 28.Mori TA, Beilin LJ. Omega-3 fatty acids and inflammation. Curr Atheroscler Rep. 2004;6:461–7. doi: 10.1007/s11883-004-0087-5. 10.1007/s11883-004-0087-5 [DOI] [PubMed] [Google Scholar]
- 29.Kalman JM, Munawar M, Howes LG, Louis WJ, Buxton BF, Gutteridge G, et al. Atrial fibrillation after coronary artery bypass grafting is associated with sympathetic activation. Ann Thorac Surg. 1995;60:1709–15. doi: 10.1016/0003-4975(95)00718-0. 10.1016/0003-4975(95)00718-0 [DOI] [PubMed] [Google Scholar]
- 30.Halonen J, Halonen P, Jarvinen O, Taskinen P, Auvinen T, Tarkka M, et al. Corticosteroids for the prevention of atrial fibrillation after cardiac surgery: A randomized controlled trial. Jama. 2007;297:1562–7. doi: 10.1001/jama.297.14.1562. 10.1001/jama.297.14.1562 [DOI] [PubMed] [Google Scholar]
- 31.Kowey PR, Reiffel JA, Ellenbogen KA, Naccarelli GV, Pratt CM. Efficacy and Safety of Prescription Omega-3 Fatty Acids for the Prevention of Recurrent Symptomatic Atrial Fibrillation: A Randomized Controlled Trial. JAMA. 2010;304:2363–72. doi: 10.1001/jama.2010.1735. [DOI] [PubMed] [Google Scholar]