Abstract
Background
Previous studies have suggested that women do not accrue equal therapeutic benefit from antiplatelet medication as compared with men. The physiological mechanism and clinical implications behind this gender disparity have yet to be established.
Methods
On-treatment platelet reactivity was determined in 717 men and 234 women on dual antiplatelet therapy, undergoing elective coronary stent implantation. Platelet function testing was performed using arachidonic acid and adenosine diphosphate-induced light transmittance aggregometry (LTA) and the VerifyNow P2Y12 and Aspirin assays. Also the incidence of all-cause death, non-fatal acute myocardial infarction, stent thrombosis and ischaemic stroke was evaluated.
Results
Women had higher baseline platelet counts than men. Women exhibited a higher magnitude of on-aspirin platelet reactivity using LTA, but not using the VerifyNow Aspirin assay. The magnitude of on-clopidogrel platelet reactivity was significantly higher in women as compared with men with both tests used. The cut-off value to identify patients at risk as well as the incidence of clinical endpoints was similar between women and men (16/234[6.8%] vs. 62/717[8.6%], p = 0.38).
Conclusion
Although the magnitude of platelet reactivity was higher in women, the absolute difference between genders was small and both the cut-off value to identify patients at risk and the incidence of the composite endpoint were similar between genders. Thus, it is unlikely that the difference in platelet reactivity accounts for a worse prognosis in women.
Keywords: Gender, Platelet function testing, Clopidogrel, Aspirin, Antiplatelet therapy
Introduction
Coronary artery disease is the main cause of mortality and morbidity worldwide [1, 2]. Throughout the last decade improvements in the diagnosis and treatment of atherosclerosis have caused a marked reduction in the morbidity and mortality in men, whereas the rate of recurrent atherothrombotic events, including cardiovascular death, in women has increased [3, 4]. Since platelet reactivity plays a pivotal role in thrombus formation and atherosclerosis, dual antiplatelet therapy with both aspirin and clopidogrel has become the cornerstone in the treatment of patients undergoing coronary stent implantation and those presenting with acute coronary syndrome (ACS) [5, 6]. However, previous studies have suggested that women do not accrue equal therapeutic benefit of antithrombotic therapy [7, 8]. Although multiple contributing factors have been described, the physiological mechanism behind this gender disparity remains unclear [9]. Therefore, the aim of the present study is to compare the magnitude of on-treatment platelet reactivity between genders in patients on dual antiplatelet therapy undergoing elective coronary stenting.
Methods
Population and study design
The POPular study (The Do Platelet Function Assays Predict Clinical Outcomes in Clopidogrel Pretreated Patients Undergoing Elective PCI study) was a prospective, observational study including consecutive patients with established coronary artery disease scheduled for elective coronary stent implantation. The entry and exclusion criteria were described in the original publication. The POPular study has established that patients exhibiting a high on-treatment platelet reactivity status were at higher risk for adverse events post-PCI [10].
In the present sub-analysis all patients were on dual antiplatelet therapy with adequate clopidogrel treatment (defined as a maintenance dose of 75 mg daily for >5 days, a loading dose of 300 mg at least 24 h before PCI or 600 mg at least 4 h prior to PCI) and low-dose aspirin of 80–100 mg daily for at least 10 days. Patients were excluded when they had a whole blood platelet count <150,000/μL or had used medication (other than aspirin or clopidogrel) known to have any effect on platelet reactivity (i.e. NSAIDs, dipyramidole, glycoprotein (GP) IIb/IIIa-inhibitors) within 1 week prior to inclusion. The study was conducted according to the principles of the Declaration of Helsinki and the laws and regulations applicable in the Netherlands. All patients gave written informed consent.
Clinical endpoint
The clinical endpoint was a combination of all-cause death, non-fatal myocardial infarction (defined as the occurrence of ischaemic symptoms as well as a spontaneous troponin T value or creatine kinase MB greater than the upper limit of normal), definite stent thrombosis (according to the Academic Research Consortium criteria) [11] and ischaemic stroke. An independent committee, blinded for platelet function data, adjudicated all endpoints through review of source documents of medical records.
Blood sampling
Prior to heparinisation, whole blood was drawn from the femoral or radial artery sheath. Blood samples were collected into Vacuette® tubes (Greiner Bio-one, Frickenhausen, Germany) containing 3.2% sodium citrate for all platelet function tests. Blood samples for whole blood count were drawn into tubes containing K3-EDTA. Platelet function testing was performed within 2 h after blood withdrawal.
Platelet function testing
Light transmittance aggregometry
Light transmittance aggregometry (LTA) was performed using an APACT 4004 aggregometer (LABiTec, Arensburg, Germany) at 37°C. Platelet poor plasma (PPP) was used as a reference for 100% aggregation and maximal platelet aggregation (%) was measured in non-adjusted platelet rich plasma after stimulation with arachidonic acid (AA) in a final concentration of 0.5 mg/ml to determine on-aspirin platelet reactivity and adenosine diphosphate (ADP) in a final concentration of 20 μmol/L to determine on-clopidogrel platelet reactivity.
VerifyNow® system
The VerifyNow® (Accumetrics, San Diego, USA) is a whole blood assay designed to measure agonist-induced platelet aggregation. Aspirin-induced platelet reactivity was measured with the aspirin assay, which contains arachidonic acid (AA) (1 mmol/L) and clopidogrel response was measured with the P2Y12 assay. This assay contains 20 μmol/L ADP to induce P2Y12-dependent platelet aggregation, and 22 nmol/L prostaglandin E1 (PGE1) to minimise the contribution of the ADP-activated P2Y1 receptor to platelet aggregation. Results are described as Aspirin Reaction Units (ARU) and P2Y12 Reaction Units (PRU) respectively.
Statistical analysis
Continuous variables were expressed as mean ± SD, unless otherwise specified, and categorical variables as frequencies (%). All distributions were checked for normality. Differences in continuous variables were compared by independent t-test or Mann–Whitney U test, as appropriate. Dichotomous variables were compared by chi-square test or Fisher’s exact test.
Covariate adjustment using a propensity score was performed to reduce confounding factors in the comparison of the magnitude of platelet reactivity between genders. The propensity score was defined as the probability of being a man depending on the baseline characteristics of each patient and used to account for imbalances in the distribution of these characteristics between genders. Prior to calculation of the propensity score, missing data were imputed using the program R. The propensity score for each patient was determined using the following characteristics as covariates in a logistic regression model: clinical characteristics (i.e. classic cardiovascular risk factors, previous myocardial infarction (MI), previous percutaneous coronary intervention (PCI) or previous coronary artery bypass graft surgery (CABG), renal failure, left ventricular ejection fraction <45%), co-medication (i.e. use of clopidogrel loading dose and concomitant use of statins, β-blockers, angiotensin converting enzyme inhibitors, coumarin derivates, calcium channel blocker, proton pump inhibitor, upstream GP IIb/IIIa inhibitor-therapy), laboratory parameters (platelet count, mean platelet volume, white blood cell count, red blood cell count, haemoglobin and haematocrit) and procedural risk factors (i.e. total stent length, number of lesions treated, number of stents implanted, bifurcation stenting, graft stenting, left anterior descending coronary artery (LAD), type of stent implanted (bare-metal stent (BMS), drug-eluting stent (DES) or both) and minimal stent diameter). Subsequently, linear regression analysis was performed to compare the magnitude of platelet reactivity between genders, using the propensity scores as a covariate.
To evaluate whether the cut-off value to identify patients at higher risk of atherothrombotic events was similar between genders, a receiver-operator characteristic (ROC) curve analysis was calculated for each test in both genders. The optimal cut-off level was calculated by determining the smallest distance between the ROC curve and the upper left corner of the graph. To determine whether the cut-off for both genders was similar, a heterogeneity index was calculated. All data were analysed with SPSS version 17.0 (SPSS, Chicago, IL) and R (version 2.9 http://r-project.org) and a two-sided p-value <0.05 was considered significant.
Results
Study population
A total of 1069 consecutive patients undergoing elective PCI with stent implantation were enrolled, of whom 951 were on aspirin >10 days. The latter comprised the present study population. Due to irregularities in platelet assay supply, as well as technical failure in a minority of platelet function tests, not all platelet function assays were performed in every patient. ADP-induced LTA was performed in 936 patients; AA-induced LTA was performed in 925 patients and the VerifyNow P2Y12 Assay in 940 patients. Since use of the VerifyNow® Aspirin cartridge did not start until halfway through the POPular study, this assay was performed in less than half of the population (n = 422). Of the patients, 234 were female (24.6%) and 717 were male (75.4%). Baseline characteristics are depicted in Table 1. Women were significantly older than men and were more likely to have a familial history of coronary artery disease (CAD). Furthermore, women had a higher platelet count and a lower haemoglobin value.
Table 1.
Baseline characteristics of study population
| Total population | Women | Men | p-value | |
|---|---|---|---|---|
| (n = 951) | (n = 234) | (n = 717) | ||
| Clinical parameters | ||||
| Age (years) | 64 ± 10.6 | 67 ± 9.9 | 63 ± 10.6 | <0.001 |
| BMI (kg/m2) | 27.3 ± 3.9 | 27.2 ± 4.7 | 27.3 ± 3.6 | 0.607 |
| Current smoker | 102 (10.7%) | 24 (10.3%) | 78 (10.9%) | 0.785 |
| Diabetes mellitus | 175 (18.4%) | 53 (22.6%) | 122 (17.0%) | 0.053 |
| Hypertension | 737 (77.5%) | 186 (79.5%) | 551 (76.8%) | 0.401 |
| Hypercholesterolaemia | 769 (80.9%) | 189 (80.8%) | 580 (81.0%) | 0.936 |
| Familial history | 580 (61.4%) | 171 (74.0%) | 409 (57.4%) | <0.001 |
| Prior myocardial infarction | 432 (45.4%) | 94 (40.2%) | 338 (47.1%) | 0.063 |
| Impaired ejection fraction | 133 (14.0%) | 35 (15.0%) | 98 (13.7%) | 0.622 |
| Renal failure | 93 (9.8%) | 22 (9.4%) | 71 (9.9%) | 0.809 |
| Medication | ||||
| Loading dose clopidogrel | 489/951 (51.4%) | 116 (51.1%) | 373 (53.5%) | 0.527 |
| Proton pump inhibitor | 270 (29.4%) | 70 (31.1%) | 200 (28.9%) | 0.528 |
| Coumarin derivates | 24 (2.5%) | 2 (0.9%) | 21 (3.0%) | 0.073 |
| Calcium channel blocker | 365 (39.8%) | 91 (40.4%) | 274 (39.6%) | 0.821 |
| Laboratory parameters | ||||
| Platelet count (× 10^9/L) | 273 ± 79 | 290 ± 78 | 268 ± 78 | <0.001 |
| White blood cell count (× 10^9/L) | 7.7 ± 2.6 | 7.9 ± 3.3 | 7.7 ± 2.3 | 0.359 |
| Haemoglobin (mmol/L) | 8.5 ± 1.0 | 7.9 ± 0.8 | 8.7 ± 0.9 | <0.001 |
| Procedural parameters | ||||
| Mean no. of stents implanted | 1.57 | 1.55 ± 0.9 | 1.57 ± 0.8 | 0.15 |
| Minimal stent diameter (mm) | 3.1 ± 0.8 | 3.1 ± 1.4 | 3.1 ± 0.6 | 0.67 |
| Total stent length (mm) | 28.3 ± 17.1 | 27.4 ± 17.2 | 28.6 ± 17.1 | 0.33 |
| Left anterior descending artery | 450/951 (47.3%) | 118/234 (50.4%) | 448/713 (46.3%) | 0.27 |
| Bifurcation lesion | 32/951 (3.4%) | 9/234 (3.8%) | 23/717 (3.2%) | 0.64 |
| Drug-eluting stent | 604/946 (63.8%) | 156/233 (67.0%) | 332/717 (62.8%) | 0.26 |
Definitions
Hypertension: Systolic blood pressure >140 mmHg or diastolic blood pressure >90 mmHg
Hypercholesterolaemia: A fasting LDL cholesterol ≥3.4 mmol/L or being on statin therapy at the time of inclusion
Diabetes mellitus: According to the World Health Organization criteria
Family history: One or more first-degree relatives have developed CAD before the age of 55 years (men) or 65 years (women)
Renal insufficiency: Serum creatinine >120 μmol/L
Gender-specific differences in platelet reactivity
On-aspirin platelet reactivity
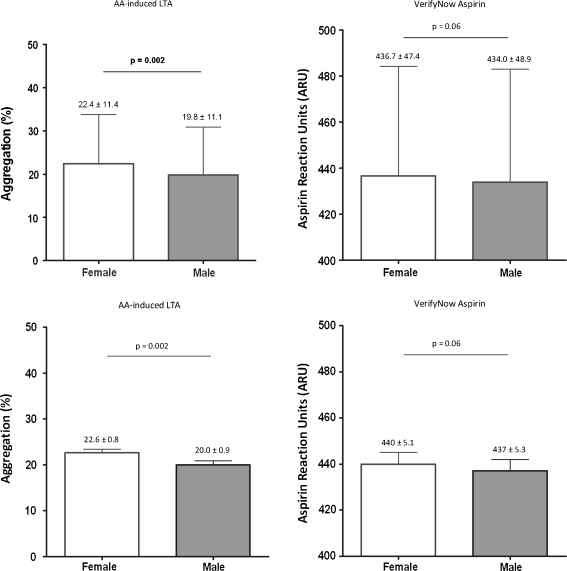
Women exhibited a higher magnitude of on-aspirin platelet reactivity as compared with men when measured with LTA (22.4 ± 11.4% vs. 19.8 ± 11.1%, p = 0.002). After adjustment for potential confounders, the difference remained significant (22.6 ± 0.8% vs. 20.0 ± 0.9%, p = 0.002 [mean ± standard error of the mean (SEM)]). In contrast, women had a similar magnitude of on-aspirin platelet reactivity using the VerifyNow® Aspirin Assay (437 ± 4.6 vs. 434 ± 5.6, p = 0.06 after adjustment [mean ± SEM]). (Fig. 1)
Fig. 1.
On-aspirin platelet reactivity. The magnitude of on-aspirin platelet reactivity as assessed by AA-induced LTA and the VerifyNow Aspirin assay prior to (upper part: mean ± SD) and after adjustment for potential confounders (lower part; mean ± SEM)
On-clopidogrel platelet reactivity
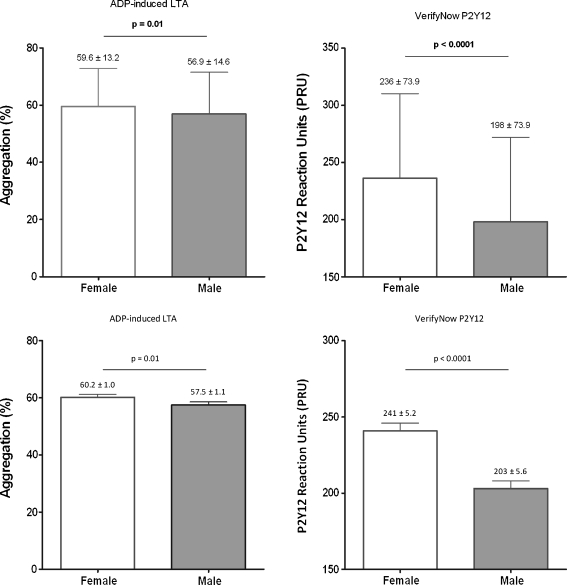
The magnitude of on-clopidogrel platelet reactivity was significantly higher in women as compared with men when measured with either LTA (59.6 ± 13.2% vs. 56.9 ± 14.6%, p = 0.01) or the VerifyNow® P2Y12 assay (236 ± 73.9 vs. 198 ± 73.9, p < 0.0001). All differences remained significant after adjustment for potential confounders in multivariate analysis (Fig. 2).
Fig. 2.
On-clopidogrel platelet reactivity. The magnitude of on-aspirin platelet reactivity as assessed by ADP-induced LTA and the VerifyNow P2Y12 assay prior to (upper part: mean ± SD) and after adjustment for potential confounders (lower part; mean ± SEM)
Gender-specific differences in clinical outcome
Table 2 summarises 1-year clinical outcome. The occurrence of the composite endpoint (62/717 [8.6%] in males vs. 16/234 [6.8%] in females, p = 0.58), as well as its single components, was similar between men and women (13/717 [1.8%] men died vs. 3/234 [1.3%] women).
Table 2.
Clinical outcome
| Female (n = 234) | Male (n = 717) | OR (95 CI) | p-value | |
|---|---|---|---|---|
| Death, MI, ST, stroke | 16 (6.8%) | 62 (8.6%) | 0.78 (0.43–1.35) | 0.58 |
| Death | 3 (1.3%) | 13 (1.8%) | 0.70 (0.13–2.59) | 0.47 |
| MI | 11 (4.7%) | 43 (6.0%) | 0.77 (0.35–1.53) | 0.35 |
| ST | 1 (0.4%) | 8 (1.1%) | 0.38 (0.01–2.86) | 0.36 |
| Stroke | 4 (1.7%) | 7 (0.8%) | 1.76 (0.37–7.01) | 0.38 |
MI myocardial infarction, ST stent thrombosis
Gender-specific differences in ROC curve derived cut-off values
Receiver operator characteristic curve (ROC) analysis demonstrated that the cut-off value to identify patients at higher risk of atherothrombotic events was not significantly different between genders (all p-values for heterogeneity > 0.10).
Discussion
Evidence that gender differences play a role in platelet reactivity was first reported over 30 years ago [12] and this observation has been confirmed in more recent studies [13–16]. Differences in vessel wall biology between men and women, as well as the direct influence of sex hormones (oestrogens, progesterone or androgens) on platelets or their indirect effect on the vasculature, might be underlying conditions from a biological point of view [4, 17].
Since platelet reactivity plays a pivotal role in thrombus formation and atherosclerosis, dual antiplatelet therapy with both aspirin and clopidogrel has become the mainstay in the treatment of patients undergoing coronary stent implantation and those presenting with ACS [5, 6, 18, 19]. However, both drugs result in a wide interindividual range in platelet inhibition [20, 21] and the association between high on-treatment platelet reactivity and the occurrence of adverse events is well established [22–25]. As a consequence, identification of particular subgroups of patients with high on-treatment platelet reactivity has gained much attention [26]. Women have been reported to exhibit a higher magnitude of both on-aspirin and on-clopidogrel platelet reactivity more often [13, 27]. However, the cause and clinical implications of these findings are uncertain.
The results from the present study support previous findings that women have higher platelet counts [28] and a higher magnitude of on-treatment platelet reactivity than men [12, 13, 29]. Women exhibited a higher magnitude of on-aspirin platelet reactivity as compared with men using light transmittance aggregometry, but not using the VerifyNow Aspirin assay. This observation is in line with previous studies reporting a poor correlation between platelet function tests [30, 31] and might also be due to a decreased statistical power, since the VerifyNow Aspirin sample was performed in only half of the patient population [32]. The magnitude of on-clopidogrel platelet reactivity was significantly higher in women as compared with men regardless of the test used. In addition, the cut-offs to identify patients at higher risk of atherothrombotic events as well as the prevalence of the primary endpoint were similar between genders. Thus, the present study does not support the hypothesis that higher on-treatment platelet reactivity could account for the gender differences in clinical outcome and it remains highly questionable whether this gender-related difference in platelet reactivity has clinical relevance.
Previously reported in vitro data suggest that although women have a higher magnitude of platelet reactivity, the response to aspirin is similar or even larger as compared with men [13, 33]. This is in line with the observation of a gender-specific meta-analysis on the role of aspirin in primary prevention of cardiovascular disease, demonstrating that aspirin is effective in reducing cardiovascular events in both women and men [7, 34]. To date, there are few data on the effects of clopidogrel in women versus men. Whereas conflicting results have been reported on the association between on-clopidogrel platelet reactivity and gender [9], a recent meta-analysis has established that clopidogrel reduces cardiovascular risk in both men and women [35].
Some issues merit mention. First, the magnitude of platelet reactivity was determined with a single assessment while patients were already on antiplatelet therapy. In this setting, it is impossible to establish whether the difference in platelet reactivity should be attributed to a higher intrinsic (baseline) platelet reactivity in women or to less response. Second, the role of hormonal influences remains unclear, as menopausal status and menstrual cycle were not assessed in our study, but the numbers of premenopausal women in our study are presumably low.
Higher platelet reactivity at baseline among women has been described previously. Although we support the finding that the magnitude of platelet reactivity is higher in women, the absolute difference between genders is small and both the cut-off value to identify patients at risk and the incidence of the composite endpoint was similar between genders. Thus, it is unlikely that the difference in platelet reactivity accounts for a worse prognosis in women.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Lopez AD, Mathers CD, Ezzati M, et al. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 3.Chandra NC, Ziegelstein RC, Rogers WJ, et al. Observations of the treatment of women in the United States with myocardial infarction: a report from the National Registry of Myocardial Infarction-I. Arch Intern Med. 1998;158:981–988. doi: 10.1001/archinte.158.9.981. [DOI] [PubMed] [Google Scholar]
- 4.Capodanno D, Angiolillo DJ. Impact of race and gender on antithrombotic therapy. Thromb Haemost. 2010;104:471–484. doi: 10.1160/TH10-04-0232. [DOI] [PubMed] [Google Scholar]
- 5.Steinhubl SR, Berger PB, Mann JT, III, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288:2411–2420. doi: 10.1001/jama.288.19.2411. [DOI] [PubMed] [Google Scholar]
- 6.Mehta SR, Yusuf S, Peters RJ, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001;358:527–533. doi: 10.1016/S0140-6736(01)05701-4. [DOI] [PubMed] [Google Scholar]
- 7.Berger JS, Roncaglioni MC, Avanzini F, et al. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. JAMA. 2006;295:306–313. doi: 10.1001/jama.295.3.306. [DOI] [PubMed] [Google Scholar]
- 8.Boersma E, Harrington RA, Moliterno DJ, et al. Platelet glycoprotein IIb/IIIa inhibitors in acute coronary syndromes: a meta-analysis of all major randomised clinical trials. Lancet. 2002;359:189–198. doi: 10.1016/S0140-6736(02)07442-1. [DOI] [PubMed] [Google Scholar]
- 9.Jochmann N, Stangl K, Garbe E, et al. Female-specific aspects in the pharmacotherapy of chronic cardiovascular diseases. Eur Heart J. 2005;26:1585–1595. doi: 10.1093/eurheartj/ehi397. [DOI] [PubMed] [Google Scholar]
- 10.Breet NJ, van Werkum JW, Bouman HJ, et al. Comparison between platelet function tests in predicting clinical outcome in patients undergoing coronary stent placement. JAMA. 2010;303:754–762. doi: 10.1001/jama.2010.181. [DOI] [PubMed] [Google Scholar]
- 11.Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 12.Johnson M, Ramey E, Ramwell PW. Sex and age differences in human platelet aggregation. Nature. 1975;253:355–357. doi: 10.1038/253355a0. [DOI] [PubMed] [Google Scholar]
- 13.Becker DM, Segal J, Vaidya D, et al. Sex differences in platelet reactivity and response to low-dose aspirin therapy. JAMA. 2006;295:1420–1427. doi: 10.1001/jama.295.12.1420. [DOI] [PubMed] [Google Scholar]
- 14.Gum PA, Kottke-Marchant K, Poggio ED, et al. Profile and prevalence of aspirin resistance in patients with cardiovascular disease. Am J Cardiol. 2001;88:230–235. doi: 10.1016/S0002-9149(01)01631-9. [DOI] [PubMed] [Google Scholar]
- 15.Blais N, Pharand C, Lordkipanidze M, et al. Response to aspirin in healthy individuals. Cross-comparison of light transmission aggregometry, VerifyNow system, platelet count drop, thromboelastography (TEG) and urinary 11-dehydrothromboxane B(2) Thromb Haemost. 2009;102:404–411. doi: 10.1160/TH09-02-0126. [DOI] [PubMed] [Google Scholar]
- 16.Yee DL, Sun CW, Bergeron AL, et al. Aggregometry detects platelet hyperreactivity in healthy individuals. Blood. 2005;106:2723–2729. doi: 10.1182/blood-2005-03-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bailey AL, Scantlebury DC, Smyth SS. Thrombosis and antithrombotic therapy in women. Arterioscler Thromb Vasc Biol. 2009;29:284–288. doi: 10.1161/ATVBAHA.108.179788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruggeri ZM. Platelets in atherothrombosis. Nat Med. 2002;8:1227–1234. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- 19.Goldschmidt PJ, Lopes N, Crwaford LE, et al. Atherothrombosis and coronary artery disease. Platelets. Elsevier/Academic Press; 2007, p. 629–96
- 20.Gurbel PA, Bliden KP, Hiatt BL, et al. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation. 2003;107:2908–2913. doi: 10.1161/01.CIR.0000072771.11429.83. [DOI] [PubMed] [Google Scholar]
- 21.Lau WC, Gurbel PA, Watkins PB, et al. Contribution of hepatic cytochrome P450 3A4 metabolic activity to the phenomenon of clopidogrel resistance. Circulation. 2004;109:166–171. doi: 10.1161/01.CIR.0000112378.09325.F9. [DOI] [PubMed] [Google Scholar]
- 22.Gurbel PA, Bliden KP, Guyer K, et al. Platelet reactivity in patients and recurrent events post-stenting: results of the PREPARE POST-STENTING Study. J Am Coll Card. 2005;46:1820–1826. doi: 10.1016/j.jacc.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 23.Sibbing D, Braun S, Morath T, et al. Platelet reactivity after clopidogrel treatment assessed with point-of-care analysis and early drug-eluting stent thrombosis. J Am Coll Cardiol. 2009;53:849–856. doi: 10.1016/j.jacc.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 24.Marcucci R, Gori AM, Paniccia R, et al. Cardiovascular death and nonfatal myocardial infarction in acute coronary syndrome patients receiving coronary stenting are predicted by residual platelet reactivity to ADP detected by a point-of-care assay: a 12-month follow-up. Circulation. 2009;119:237–242. doi: 10.1161/CIRCULATIONAHA.108.812636. [DOI] [PubMed] [Google Scholar]
- 25.Bonello L, Tantry US, Marcucci R, et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol. 2010;56:919–933. doi: 10.1016/j.jacc.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 26.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, et al. Platelet function profiles in patients with type 2 diabetes and coronary artery disease on combined aspirin and clopidogrel treatment. Diabetes. 2005;54:2430–2435. doi: 10.2337/diabetes.54.8.2430. [DOI] [PubMed] [Google Scholar]
- 27.Serebruany VL, Steinhubl SR, Berger PB, et al. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol. 2005;45:246–251. doi: 10.1016/j.jacc.2004.09.067. [DOI] [PubMed] [Google Scholar]
- 28.Stevens RF, Alexander MK. A sex difference in the platelet count. Br J Haematol. 1977;37:295–300. doi: 10.1111/j.1365-2141.1977.tb06847.x. [DOI] [PubMed] [Google Scholar]
- 29.Zuern CS, Lindemann S, Gawaz M. Platelet function and response to aspirin: gender-specific features and implications for female thrombotic risk and management. Semin Thromb Hemost. 2009;35:295–306. doi: 10.1055/s-0029-1222608. [DOI] [PubMed] [Google Scholar]
- 30.Lordkipanidze M, Pharand C, Schampaert E, et al. A comparison of six major platelet function tests to determine the prevalence of aspirin resistance in patients with stable coronary artery disease. Eur Heart J. 2007;28:1702–1708. doi: 10.1093/eurheartj/ehm226. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen HL, Kristensen SD, Thygesen SS, et al. Aspirin response evaluated by the VerifyNow Aspirin System and light transmission aggregometry. Thromb Res. 2008;123:267–273. doi: 10.1016/j.thromres.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 32.Breet NJ, van Werkum JW, Bouman HJ, et al. High on-aspirin platelet reactivity as measured with aggregation based, COX-1 inhibition sensitive platelet function tests is associated with the occurrence of atherothrombotic events. J Thromb Haemost. 2010;8:2140–2148. doi: 10.1111/j.1538-7836.2010.04017.x. [DOI] [PubMed] [Google Scholar]
- 33.Harrison MJ, Weisblatt E. A sex difference in the effect of aspirin on “spontaneous” platelet aggregation in whole blood. Thromb Haemost. 1983;50:773–774. [PubMed] [Google Scholar]
- 34.Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berger JS, Bhatt DL, Cannon CP, et al. The relative efficacy and safety of clopidogrel in women and men a sex-specific collaborative meta-analysis. J Am Coll Cardiol. 2009;54:1935–1945. doi: 10.1016/j.jacc.2009.05.074. [DOI] [PubMed] [Google Scholar]