Abstract
Objectives
Definitions of renal function in patients undergoing coronary artery bypass graft surgery (CABG) vary in the literature. We sought to investigate which method of estimating renal function is the best predictor of mortality after CABG.
Methods
We analysed the preoperative and postoperative renal function data from all patients undergoing isolated CABG from January 1998 through December 2007. Preoperative and postoperative renal function was estimated using serum creatinine (SeCr) levels, creatinine clearance (CrCl) determined by the Cockcroft-Gault formula and the glomerular filtration rate (e-GFR) estimated by the Modification of Diet in Renal Disease (MDRD) formula. Receiver operator characteristic (ROC) curves and area under the ROC curves were calculated.
Results
In 9987 patients, CrCl had the best discriminatory power to predict early as well as late mortality, followed by e-GFR and finally SeCr. The odds ratios for preoperative parameters for early mortality were closer to 1 than those of the postoperative parameters.
Conclusions
Renal function determined by the Cockcroft-Gault formula is the best predictor of early and late mortality after CABG. The relationship between renal function and mortality is non-linear. Renal function as a variable in risk scoring systems such as the EuroSCORE needs to be reconsidered.
Keywords: Coronary artery bypass grafts, CABG; Kidney, renal function; Statistics, regression analysis
Introduction
Renal insufficiency is associated with an increased risk of complications and mortality after coronary artery bypass grafting (CABG) [1–3]. Most studies assessed the relationship between renal insufficiency and early mortality [2, 3]; only a few studies described the association between renal insufficiency and late mortality after CABG [1, 4]. The gold standard to determine renal function is the measurement of the glomerular filtration rate, represented by the clearance of an exogenous inert substance such as inulin. This is a cumbersome and time-consuming method to determine GFR and hence some more practical formulas have been developed to estimate the GFR. The Cockcroft-Gault formula is used to estimate the creatinine clearance (CrCl) and more recently, the Modification of Diet in Renal Disease (MDRD) has been introduced to estimate the glomerular filtration rate (e-GFR).
Definitions of impairment of renal function after CABG vary in the literature. Serum creatinine levels (SeCr) [4, 5], CrCl [5–7] and e-GFR [5, 6, 8] have been used, describing renal function as a risk factor for mortality after CABG. We analysed the preoperative and postoperative renal function data of all patients undergoing isolated CABG in our hospital from 1998 until 2007. We used three different definitions of renal function (SeCr, CrCl and e-GFR) in order to investigate the relationship between these parameters and early and late mortality after CABG and to compare their predictive values.
Patients and methods
Patients
This study retrospectively analysed the data of all patients undergoing isolated CABG or off-pump coronary artery bypass (OPCAB) surgery in a single centre (Catharina Hospital in Eindhoven the Netherlands) between January 1998 and December 2007. Clinical data, including demographics and renal function parameters, were prospectively collected in our database. The Institutional Research Review Board approved this study and waived the need for patient consent.
Operative technique
In CABG surgery, all patients received short-acting anaesthetic drugs to facilitate early extubation. Normothermic extracorporeal circulation was performed using non-pulsatile flow. Cold crystalloid cardioplegia (St Thomas’ solution) or warm blood cardioplegia was used to induce and maintain cardioplegic cardiac arrest, according to the surgeon’s preference. All patients undergoing CABG received low-dose aprotinin (2 million kallikrein inactivating units) during extracorporeal circulation, administered in the prime solution according to the hospital protocol. The anaesthetic management in OPCAB surgery was the same as in CABG surgery, but aprotinin was omitted.
Estimation of renal function
Renal function was determined by SeCr, CrCl (using the Cockcroft-Gault formula) [9], which is different between men 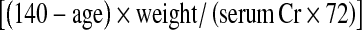 and women:
and women:  , and estimated glomerular filtration rate (using the MDRD formula [10]:
, and estimated glomerular filtration rate (using the MDRD formula [10]: 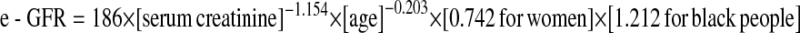 ). Calculations were based on the most recent preoperative SeCr and the largest value of SeCr within the first week postoperatively or if sooner before discharge.
). Calculations were based on the most recent preoperative SeCr and the largest value of SeCr within the first week postoperatively or if sooner before discharge.
Follow-up
Follow-up data concerning mortality were obtained from the databases of health insurance companies. The data of 9% of the total patient group could initially not be retrieved from these databases. We therefore contacted the general practitioners to get information about mortality data of these patients or if necessary, the city authorities of the cities where the patients lived at the time of the operation. Early mortality was defined as death within 30 days postoperatively or death at anytime if the patient did not leave the hospital or a transfer tertiary hospital alive, whereas late mortality was defined as any-cause mortality that occurred at anytime after hospital discharge.
Statistical analyses
Univariate logistic regression analyses were performed to investigate the impact of biomedical variables on early mortality. If significant at p < 0.05, the variables were included into the multivariable logistic regression analyses together with the renal function parameters. For each renal function parameter a separate multivariate regression analysis model was made. For late mortality univariate and multivariate Cox regression analyses were performed. Hazard ratios (HR) with 95% confidence interval (CI) with p-values are reported. A p-value <0.05 was used for all tests to indicate statistical significance. To compare the discriminatory power of all renal function parameters, receiver operating characteristic (ROC) curves and the area under the curve (AUC) were calculated. Logistic regression analyses were used to calculate the predicted probabilities (hazard ratio) of SeCr, eGFR and CrCl levels. All statistical analyses were performed using SPPS version 15.0 (SPSS Inc, Chicago, IL).
Results
Between January 1998 and December 2007, 10,626 patients underwent isolated CABG or OPCAB surgery in our institution. Preoperative SeCr was missing in 267 patients and postoperative SeCr was missing in 102 patients due to mortality before renal function could be determined. In 433 patients, we were not able to calculate the CrCl or e-GFR due to missing data. We excluded 122 patients who were lost to follow-up and 24 patients who were on haemodialysis leaving 9987 patients with complete data included in this study. Patients’ preoperative and perioperative demographic and clinical characteristics are presented in Table 1. Renal function parameters are shown in Table 2.
Table 1.
Preoperative and perioperative demographic and clinical characteristics
| Variables | Incidence |
|---|---|
| Male gender | 7698 (77.1) |
| Age | 64.6 ± 9.6 |
| Angina class | 2.7 ± 0.9 |
| Hypertension | 4161 (41.7) |
| Diabetes | 2098 (21.0) |
| BMI > 35 kg.m−2 | 233 (2.3) |
| COPD | 1242 (12.4) |
| PVD | 1148 (11.5) |
| EF < 35% | 326 (3.3) |
| Emergency | 363 (3.6) |
| Preop Hb, g/dL | 13.9 ± 1.3 |
| No of grafts | 3.4 ± 1.1 |
| Use of IMA | 8858 (88.7) |
| Number of RBC transfusions | 1.0 ± 2.2 |
| Re-exploration | 531 (5.3) |
| Periop MI | 282 (2.8) |
| Early mortality | 198 (2.0) |
| Late mortality | 1111 (11.1) |
Results are expressed as numbers (percentage) or mean ± standard deviation
BMI body mass index; COPD chronic obstructive pulmonary disease; EF estimated left ventricular ejection fraction; IABP need for perioperative intra aortic balloon pump support; IMA internal mammary artery; periop MI perioperative myocardial infarction; PVD peripheral vascular disease; RBC red blood cell; Hb hemoglobin
Table 2.
Renal function parameters
| Parameter | Value |
|---|---|
| Preoperative SeCr | 101 ± 27 |
| Postoperative SeCr | 99.9 ± 44.6 |
| Preoperative CrCl | 74.5 ± 24.4 |
| Postoperative CrCl | 79.4 ± 28.3 |
| Preoperative e-GFR | 67.4 ± 16.4 |
| Postoperative e-GFR | 72.6 ± 21.2 |
Values are expressed as mean ± standard deviation
CrCl creatinine clearance; e-GFR estimated glomerular filtration rate; SeCr serum creatinine level
Risk factors for early mortality, which were identified using univariate analyses and were entered into the multivariate analysis, were: age, diabetes, chronic obstructive pulmonary disease (COPD), left ventricular ejection fraction less than 35% (EF < 35%), the number of transfused units of red blood cells (RBCs) and perioperative myocardial infarction (Table 3). These independent risk factors were entered into the multivariate analyses together with the renal function parameters (Table 4). The odds ratios of the preoperative parameters for early mortality were closer to 1 than those of the postoperative parameters.
Table 3.
Multivariate logistic regression analyses for early mortality and Cox regression analyses for late mortality
| OR early mortality | p-value | HR late mortality | p-value | |
|---|---|---|---|---|
| Age | 1.087 (1.062–1.113) | <.0001 | 1.082 (1.073–1.091) | <.0001 |
| Female | 0.85 (0.58–1.26) | .430 | 0.62 (0.53–0.73) | <.0001 |
| Diabetes | 1.66 (1.16–2.37) | .005 | 1.44 (1.25–1.65) | <.0001 |
| Hypertension | 1.11 (0.98–1.26) | .098 | ||
| COPD | 1.88 (1.27–2.78) | .002 | 1.69 (1.45–1.96) | <.0001 |
| PVD | 0.98 (0.62–1.57) | .959 | 1.68 (1.44–1.97) | <.0001 |
| EF < 35% | 4.77 (2.88–7.88) | <.0001 | 2.29 (1.80–2.92) | <.0001 |
| Preop Hb | 0.912 (0.811–1.026) | .125 | 0.829 (0.789–0.871) | <.0001 |
| Emergency | 1.76 (0.82–3.73) | .141 | ||
| No of grafts | 1.00 (0.94–1.06) | .934 | ||
| Redo surgery | 1.34 (0.78–2.29) | .281 | 1.262 (1.015–1.570) | .036 |
| RBC units transfusion | 1.220 (1.160–1.284) | <.0001 | 1.01 (0.98–1.04) | .369 |
| Re-exploration | 1.70 (0.99–2.90) | .051 | 1.36 (1.03–1.79) | .027 |
| Periop MI | 4.62 (2.81–7.61) | <.0001 | 1.73 (1.26–2.38) | .001 |
Only significant factors in the univariate analysis were entered into the multivariate analysis
COPD chronic obstructive pulmonary disease; EF estimated left ventricular ejection fraction; HR hazard Ratio; Hb haemoglobin level; PVD peripheral vascular disease; periop MI perioperative myocardial infarction
Table 4.
Multivariate logistic regression analyses for early mortality and Cox regression analyses for late mortality
| HR early mortality | p-value | HR late mortality | p-value | |
|---|---|---|---|---|
| Preop SeCr | 1.003 (1.000–1.007) | .026 | 1.005 (1.004–1.006) | <.0001 |
| Postop SeCr | 1.007 (1.006–1.009) | <.0001 | 1.004 (1.003–1.005) | <.0001 |
| Preop CrCl | 0.988 (0.978–0.998) | .018 | 0.988 (0.983–0.992) | <.0001 |
| Postop CrCl | 0.958 (0.950–0.966) | <.0001 | 0.987 (0.983–0.990) | <.0001 |
| Preop e-GFR | 0.989 (0.979–0.999) | .026 | 0.984 (0.979–0.988) | <.0001 |
| Postop e-GFR | 0.960 (0.952–0.968) | <.0001 | 0.985 (0.982–0.989) | <.0001 |
SeCr serum creatinine level; CrCl creatinine clearance; e-GFR estimated glomerular filtration rate
Age, male gender, diabetes, COPD, peripheral vascular disease (PVD), EF < 35%, preoperative haemoglobin level, previous cardiac surgery, re-exploration and perioperative myocardial infarction were significant risk factors for late mortality as identified by Cox regression analysis. These factors were entered into the multivariate analysis. The results are shown in Table 3. Subsequently, the renal function parameters were added to the multivariate analyses together with the independent risk factors. The results of these analyses are shown in Table 4. The hazard ratios for late mortality of the preoperative parameters are close to the hazard ratios of the postoperative parameters (range 0.984–1.005).
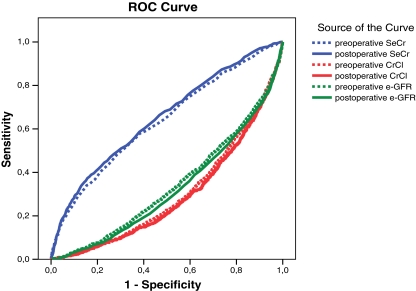
The ROC curves for the different renal function parameters as risk factors for early mortality are shown in Fig. 1. The areas under the curve (AUC) are presented in Table 5. The postoperative level of CrCl and e-GFR has the highest discriminatory power. For both preoperative and postoperative levels, CrCl showed the highest discriminatory power, followed by e-GFR and at least SeCr.
Fig. 1.
ROC curves of renal function parameters as risk factors for early mortality
Table 5.
Area under the ROC curve for renal function parameters
| Area early mortality | SE | Area late mortality | SE | |
|---|---|---|---|---|
| Preop SeCr | .632 | .023 | .634 | .009 |
| Postop SeCr | .778 | .020 | .651 | .009 |
| Preop CrCl | .710 | .019 | .303 | .008 |
| Postop CrCl | .721 | .017 | .291 | .008 |
| Preop e-GFR | .664 | .021 | .342 | .009 |
| Postop e-GFR | .803 | .018 | .326 | .009 |
SE standard error; SeCr serum creatinine level; CrCl creatinine clearance; e-GFR estimated glomerular filtration rate
Figure 2 shows the ROC curves for the renal function parameters as risk factors for late mortality. The corresponding areas under the curves are shown in Table 5. For the three renal function parameters, the postoperative levels had a significantly better discriminatory power than the preoperative levels. Both preoperative and postoperative CrCl had a higher discriminatory power compared with e-GFR, which in turn was better than SeCr.
Fig. 2.
ROC curves for renal function parameters as risk factors for late mortality
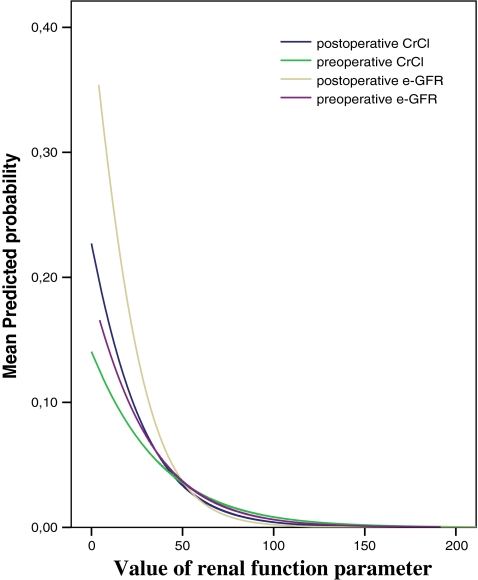
Figure 3 shows the non-linear relationship between the preoperative and postoperative CrCl and e-GFR and predicted probability of mortality (hazard ratio).
Fig. 3.
Mean predicted probability of mortality for the pre- and postoperative e-GFR and CrCl
Discussion
In this study of 9987 patients undergoing CABG in a single institution, renal dysfunction measured by CrCl showed the highest discriminatory power to predict early and late mortality. The predictive power of CrCl was greater than that of e-GFR and SeCr. The postoperative values of all the three parameters had a higher discriminatory power than the preoperative values. All the three parameters were revealed as independent risk factors for early as well as late mortality confirming the findings of others [4, 6, 7, 11].
This study confirms the superiority of the Cockcroft-Gault formula (as expressed by CrCl) over the MDRD equation (as expressed by e-GFR) in terms of predictive power for in-hospital mortality, confirming the finding of Lin et al. [6]. This may be explained by the fact the Cockcroft-Gault formula includes weight as a variable which indirectly reflects the body mass index (BMI). BMI is known to be an independent risk factor for early mortality after CABG [12]. Other studies [13, 14] revealed that the MDRD equation tends to underestimate the actual GFR in patients with chronic kidney disease but normal serum creatinine. This may explain why renal function estimated by the MDRD equation is a less accurate predictor for early mortality when compared with renal function estimated by the Cockcroft-Gault formula. In an earlier study [15] we showed the value of preoperative creatinine clearance in predicting early and late mortality after CABG.
We found a small non-significant decrease in the mean value of postoperative serum creatinine level compared with the preoperative value. This is in disagreement with some earlier reports [16]. This small change in serum creatinine in our patient group could be explained by the perioperative intravenous crystalloid administration protocol in our patients, which is different from that in other centres.
For SeCr, CrCl and e-GFR, the postoperative renal function has a higher discriminatory power for predicting early mortality compared with the preoperative renal function. This seems logical because the postoperative renal function is the result of the preoperative renal function and the subsequent trauma of the operation [10, 17]. This operative trauma may lead to further deterioration of the renal function with subsequent higher early and late mortality. Furthermore, the hazard ratios for the postoperative levels were further away from the value ‘1’ compared with the hazard ratios of the preoperative levels. This means that when the preoperative and postoperative renal function measurements are equally deteriorated, it is the postoperative value which strongly predicts the poor outcome. In other words, the preoperative renal function is mostly a stable situation whereas the postoperative renal function is, as stated above, the result of the surgical trauma on top of the preoperative renal function. On the other hand, postoperative renal function measurement is strongly dependent on the preoperative value. This association is also stronger with the estimated CrCl than with serum creatinine levels [18].
In patients who survive the operation, the postoperative renal function reflects a stable situation. The hazard ratios for late mortality do not differ between preoperative and postoperative renal function parameters. In addition, the areas under the ROC curves of preoperative and postoperative parameters are almost the same. We also found that the Cockcroft-Gault formula is a better predictor for late mortality after CABG followed by the MDRD equation and then the SeCr.
We found a non-linear relationship between CrCl and e-GFR and the predicted probability of early mortality (=odds ratio), calculated by logistic regression analysis. Any arbitrary cut-off point in a risk scoring system will be inevitably inaccurate. Considering the non-linear predicted probability curves, commonly described cut-off points for CrCl and e-GFR of 30–59 mL/min as moderate renal failure and <30 mL/min as severe renal failure do not seem to be accurate when used as a dichotomous variable in risk scoring systems. The cut-off point of serum creatinine of >200 μmol/L used in the European System for Cardiac Operative Risk Evaluation (EuroSCORE) [19, 20] will inevitably be inaccurate and needs to be reconsidered.
Conclusions
In patients undergoing isolated coronary artery bypass grafting, renal function estimated by the Cockcroft-Gault formula has the highest discriminatory power to predict early and late mortality followed by the Modification of Diet in Renal Disease (MDRD) formula and finally the serum creatinine level. The postoperative parameters were better predictors for early and late mortality compared with the preoperative parameters. The relationship between renal function and mortality is non-linear. Any cut-off point used in a risk scoring system will be inaccurate. Renal function as a variable in the EuroSCORE risk scoring system needs to be reconsidered.
References
- 1.Brown JR, Cochran RP, MacKenzie TD, et al. Long-term survival after cardiac surgery is predicted by estimated glomerular filtration rate. Ann Thorac Surg. 2008;86:4–12. doi: 10.1016/j.athoracsur.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Cooper WA, O’Brien SM, Thourani VH, et al. Impact of renal dysfunction on outcomes of coronary artery bypass surgery. Circulation. 2006;113:1063–1070. doi: 10.1161/CIRCULATIONAHA.105.580084. [DOI] [PubMed] [Google Scholar]
- 3.Chertow GM, Levy EM, Hammermeister KE, et al. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. 1998;104:343–348. doi: 10.1016/S0002-9343(98)00058-8. [DOI] [PubMed] [Google Scholar]
- 4.Holtzmann MJ, Hammar N, Ahnve S, et al. Renal insufficiency and long-term mortality and incidence of myocardial infarction in patients undergoing coronary artery bypass grafting. Eur Hear J. 2007;28:865–871. doi: 10.1093/eurheartj/ehl508. [DOI] [PubMed] [Google Scholar]
- 5.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 6.Lin Y, Zheng Z, Li Y, et al. Impact of renal dysfunction on long-term survival after isolated coronary artery bypass surgery. Ann Thorac Surg. 2009;87:1079–1084. doi: 10.1016/j.athoracsur.2009.01.065. [DOI] [PubMed] [Google Scholar]
- 7.Zakeri R, Freemantle N, Barnett V, et al. Relation between mild renal dysfunction and outcomes after coronary artery bypass grafting. Circulation. 2005;112(9 Suppl):I270–I275. doi: 10.1161/CIRCULATIONAHA.104.522623. [DOI] [PubMed] [Google Scholar]
- 8.Jin R, Grunkemeier GL, Brown JR, et al. Estimated glomerular filtration rate and renal function. Ann Thorac Surg. 2008;86:1–3. doi: 10.1016/j.athoracsur.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 10.Provenchere S, Plantefeve G, Hufnagel G, et al. Renal dysfunction after cardiac surgery with normothermic cardiopulmonary bypass: incidence, risk factors and effect on clinical outcome. Anesth Analg. 2003;96:1258–1264. doi: 10.1213/01.ANE.0000055803.92191.69. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Bosch JP, Lewis JB, et al. More accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 12.Reeves BC, Ascione R, Chamberlain MH, et al. Effect of body mass index on early outcomes in patients undergoing coronary artery bypass surgery. J Am Coll Cardiol. 2003;20:668–676. doi: 10.1016/S0735-1097(03)00777-0. [DOI] [PubMed] [Google Scholar]
- 13.Bostom AG, Kronenberg R, Ritz E. Predictive performance of renal function equations for patients with chronic kidney disease and normal serum creatinine levels. J Am Soc Nephrol. 2002;13:2140–2144. doi: 10.1097/01.ASN.0000022011.35035.F3. [DOI] [PubMed] [Google Scholar]
- 14.Rule AD, Larson TS, Bergstrahl EJ, et al. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141:929–937. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]
- 15.Straten AH, Soliman Hamad MA, Zundert AA, et al. Preoperative renal function as a predictor of survival after coronary artery bypass grafting: comparison with a matched general population. J Thorac Cardiovasc Surg. 2009;138(4):971–976. doi: 10.1016/j.jtcvs.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 16.Brown JR, Cochran RP, Dacey LJ, et al. Perioperative increases in serum creatinine are predictive of increased 90-day mortality after coronary artery bypass graft surgery. Circulation. 2006;114:I-409–I-413. doi: 10.1161/CIRCULATIONAHA.105.000596. [DOI] [PubMed] [Google Scholar]
- 17.Brown JR, Cochran RP, Leavitt BJ, et al. Multivariable prediction of renal insufficiency developing after cardiac surgery. Circulation. 2007;116(11 Suppl):139–143. doi: 10.1161/CIRCULATIONAHA.106.677070. [DOI] [PubMed] [Google Scholar]
- 18.Noyez L, Plesiewicz I, Verheugt FW. Estimated creatinine clearance instead of plasma creatinine level as prognostic test for postoperative renal function in patients undergoing coronary artery bypass surgery. Eur J Cardiothorac Surg. 2006;29(4):461–465. doi: 10.1016/j.ejcts.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 19.Dourado R, Queiroz e Melo J, et al. Serum creatinine values underestimate surgical risk. Rev Port Cardiol. 2009;28(3):269–278. [PubMed] [Google Scholar]
- 20.Roques F, Nashef SA, Michel P, et al. Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19030 patients. Eur J Cardiothorac Surg. 1999;15(6):816–822. doi: 10.1016/S1010-7940(99)00106-2. [DOI] [PubMed] [Google Scholar]