Abstract
We present the case of a 75-year-old woman with a medical history of rheumatoid arthritis treated with hydroxychloroquine, who was admitted with acute left-sided heart failure due to a hydroxychloroquine-induced cardiomyopathy as supported by endomyocardial biopsy.
Keywords: Hydroxychloroquine cardiomyopathy, Heart failure, Endomyocardial biopsy
Case report
A 75-year-old woman with a medical history of rheumatoid arthritis treated with hydroxychloroquine (HCQ) for about 10 years was admitted to our cardiac care unit with acute dyspnoea. The patient had no cardiovascular risk factors or known cardiovascular medical history. Cardiac screening 5 years ago had shown a normal electrocardiogram (ECG) and transthoracic echocardiogram (TTE).
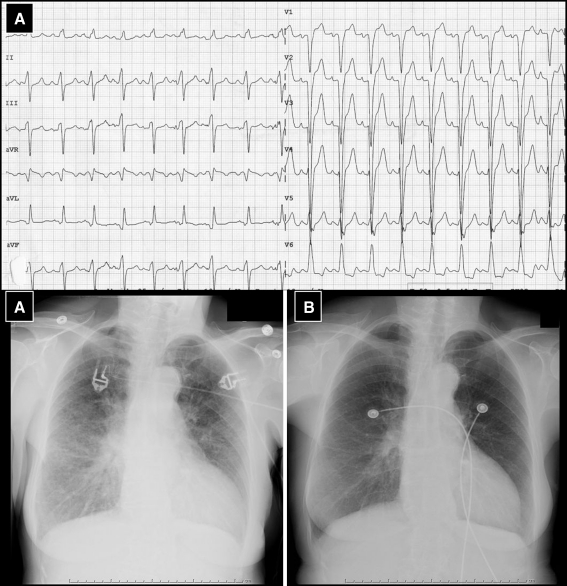
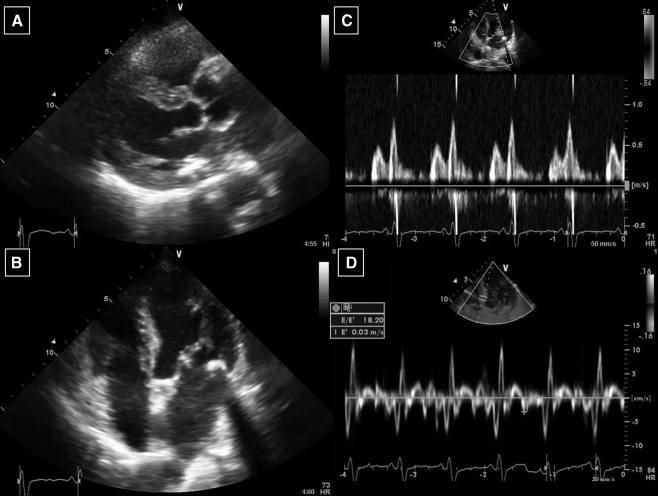
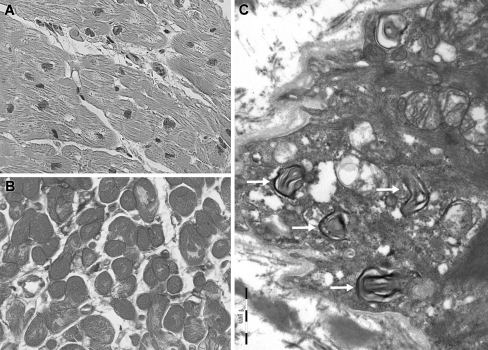
At admission the blood pressure was 150/60 mmHg with a regular heart rate of 100 beats/min and oxygen saturation measured 94% with 3 l O2. Further physical examination revealed bilateral pulmonary rales, a third heart sound and no peripheral oedema. The ECG showed sinus tachycardia with a left bundle branch block and chest X-ray confirmed bilateral lung oedema (Fig. 1). Standard laboratory measures were normal. The patient was treated with diuretics, nitroglycerine and morphine intravenously and the symptoms improved quickly. Further investigations revealed a normal coronary angiogram and globally depressed left ventricular systolic function (ejection fraction 25%) with moderate diastolic dysfunction, concentric left ventricular hypertrophy (14 mm) and slightly elevated right-sided filling pressures on TTE (Fig. 2). Family history for hypertrophic cardiomyopathy was negative, the patient had no signs of the x-linked Fabry disease, and alpha-galactosidase levels were in normal range [4]. To evaluate the presence of HCQ-induced cardiomyopathy endomyocardial biopsy was performed [1–3]. On light microscopy the myocardium was mildly hypertrophic without myocardial disarray. Many cardiomyocytes showed clear perinuclear vacuoles filled with lipofuscin-like material (Fig. 3a and b). Electron microscopy (Fig. 3c) revealed intracellular membrane-bound concentric lamellar structures, aggregates of curvilinear bodies, and mitochondria of different sizes. These findings could fit with the diagnosis of HCQ-induced cardiomyopathy [1–5]. HCQ was withdrawn and the patient had a stable clinical course and was discharged with an angiotensin-converting-enzyme inhibitor and β-blocker. At 6-month follow-up systolic function showed improvement (ejection fraction 38%).
Fig. 1.
Electrocardiogram showed sinus tachycardia with left-bundle branch block (a). Chest X-ray during acute decompensated left heart failure (a) and after initial therapy (b)
Fig. 2.
Echocardiogram showed left ventricular hypertrophy with reduced left ventricular systolic function (a,b) and signs of diastolic dysfunction (c,d)
Fig. 3.
Endomyocardial biopsy shows hypertrophic myocytes with mild interstitial fibrosis, and perinuclear vacuoles filled with lipofuscin-like granulated material (Light microcopic magnification 400×, a and b). Electron microscopy revealed numerous intracellular membrane-bound concentric lamellar structures (c, arrows), aggregates of curvilinear bodies, and large amounts of mitochondria of different size suggestive of HCQ-induced cardiomyopathy [1–5]
Discussion
HCQ and chloroquine (CQ) are established (antimalarial) drugs used for the treatment of rheumatic diseases. In general, long-term treatment is safe although rarely both drugs can induce toxicities including retinopathy, neuropathy, skeletal myopathy, and cardiomyopathy [1–3]. Cardiomyopathy may develop without any clinically evident toxic effects in other organs and manifest as myocardial hypertrophy, systolic dysfunction or restrictive cardiomyopathy, eventually with conduction disorders (i.e., bundle branch block) [1–5]. The duration of previous HCQ treatment in patients with cardiotoxicities varies from 1 to 30 years with cumulative dosages from 290 to 4380 g [1–5]. The exact pathophysiological mechanism of HCQ-induced cardiomyopathy remains unclear. There may be interference with lysosomal digestion with intracellular accumulation of glycogen and phospholipids resulting in the vacuolar type of myopathy [1–3]. Support for HCQ cardiotoxicity can be derived from electron microscopy, which shows dense residual bodies with folded membranous aggregates, curvilinear bodies and megamitochondria [1–5].
The prognosis ranges from death, heart transplantation to partial recovery of cardiac function [1–5]. The potential of improvement after withdrawal of the drug and the potential fatal clinical course in undiagnosed cases emphasise the importance of recognising the early signs of toxicity—for example by regular screening with ECG and TTE [1–3]. The current case should remind us of the rare association of HCQ and cardiac toxicity and underlines the importance of performing cardiac biopsies in suspected cases.
Acknowledgments
Conflict of interest & funding: nothing to be declared
References
- 1.Soong TR, Barouch LA, Champion HC, et al. New clinical and ultrastructural findings in hydroxychloroquine-induced cardiomyopathy—a report of 2 cases. Hum Pathol. 2007;38:1858–1863. doi: 10.1016/j.humpath.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Costedoat-Chalumeau N, Hulot JS, Amoura Z, et al. Cardiomyopathy related to antimalarial therapy with illustrative case report. Cardiology. 2007;107:73–80. doi: 10.1159/000094079. [DOI] [PubMed] [Google Scholar]
- 3.Nord JE, Shah PK, Rinaldi RZ, et al. Hydroxychloroquine cardiotoxicity in systemic lupus erythematosus: a report of 2 cases and review of the literature. Semin Arthritis Rheum. 2004;33:336–351. doi: 10.1016/j.semarthrit.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Roos JM, Aubry C, Edwards WE. Chloroquine cardiotoxicity: clinicopathologic features in three patients and comparison with three patients with Fabry disease. Cardiovasc Pathol. 2002;11:277–283. doi: 10.1016/S1054-8807(02)00118-7. [DOI] [PubMed] [Google Scholar]
- 5.August C, Holzhausen HJ, Schmoldt A, et al. Histological and ultrastructural findings in chloroquine-induced cardiomyopathy. J Mol Med. 1995;73:73–77. doi: 10.1007/BF00270580. [DOI] [PubMed] [Google Scholar]