Summary
Despite recent critical insights into the pluripotent state of embryonic stem (ES) cells, there is little agreement over the inaugural and subsequent steps leading to its generation in vitro [1–4]. Here we show that inner cell mass (ICM)-generated cells expressing Blimp1, a key transcriptional repressor of the somatic program during germ cell specification [5, 6], emerge on day 2 of blastocyst culture. Single-cell gene expression profiling indicated that many of these Blimp1-positive cells coexpress other genes generally associated with early germ cell specification. When genetically traced in vitro, these cells acquired properties normally associated with primordial germ cells, including the ability to mature in gonadal organ cultures and to colonize the genital ridges of E8.5 embryos in utero. Importantly, fate-mapping experiments revealed that ES cells commonly arise from Blimp1-positive precursors; indeed, prospective sorting of such cells from ICM outgrowths increased the rate of ES cell derivation more than 9-fold. Finally, using genetic ablation or distinct inhibitors of extracellular signal-regulated kinase (Erk) and glycogen synthase kinase-3 (Gsk3), or 2i culture conditions [7, 8], we show that epiblast cells can become ES cells without first acquiring Blimp1 positivity. Our findings suggest that the germ cell-like state is facultative for the stabilization of pluripotency in vitro. Thus, the association of Blimp1 expression with ES cell development furthers understanding of how the pluripotent state of these cells is established in vitro, and which enhance the generation of new stem cell lines from blastocysts.
Results
Mouse and human pluripotent embryonic stem (ES) cell lines can be readily generated in vitro from cultured preimplantation blastocyst stage embryos [1–4]. While mouse ES cells are capable of participating in normal development [9], the full developmental potential of human ES cells is less clear. The wide uses of mouse ES cells to study mammalian embryonic development and the growing importance of such cells in biomedical research have raised pivotal questions concerning their origin. Careful study of microdissected peri-implantation mouse embryos in the late 1990s conclusively showed that ES cells originate in the early epiblast, after its segregation from the hypoblast [10]. This observation led logically to the idea that a particular subpopulation of epiblast cells, selected during the derivation process, gives rise to ES cells. An attractive candidate for this role are epiblast cells predisposed to develop in the germ cell lineage [11]. Indeed, primordial germ cells (PGCs) can be induced to generate pluripotent cell lines that are virtually indistinguishable from ES cells [12, 13], and among all lineages that develop from epiblast, only germ cells regain expression of pluripotency-associated genes during the course of their specification [14, 15]. Nonetheless, a firm link between PGCs and ES cell generation has not been demonstrated. Here we use cell-fate mapping strategies and single-cell gene expression profiling to examine the developmental transitions of inner cell mass (ICM)-derived cells as they adapt to growth in ES cell derivation medium. Our results indicate the importance of Blimp1 gene expression, a key marker of germ cell specification, in this process and suggest that it may offer a critical window for understanding the acquisition and maintenance of pluripotentiality in ES cells.
Lineage Tracing and Single-Cell Analysis of Blimp1-Positive Cells
We first investigated in detail the presence of specific germ-line cell markers in ICM outgrowth cells. Blimp1 expression was of particular interest because it identifies the precursors of PGCs, which emerge from the proximal epiblast at the pre/no-streak stage (E6.25; initially 6 cells) and become lineage-restricted PGCs at E7.5 [5, 6, 16]. Since the autonomous growth program of cultured blastocysts seemed adequate to support embryonic development resembling the peri-implantational or egg-cylinder stage of murine development in vivo [17–21], we reasoned that PGC-like precursors would arise from the mature epiblast in vitro and perhaps contribute to the generation of ES cells.
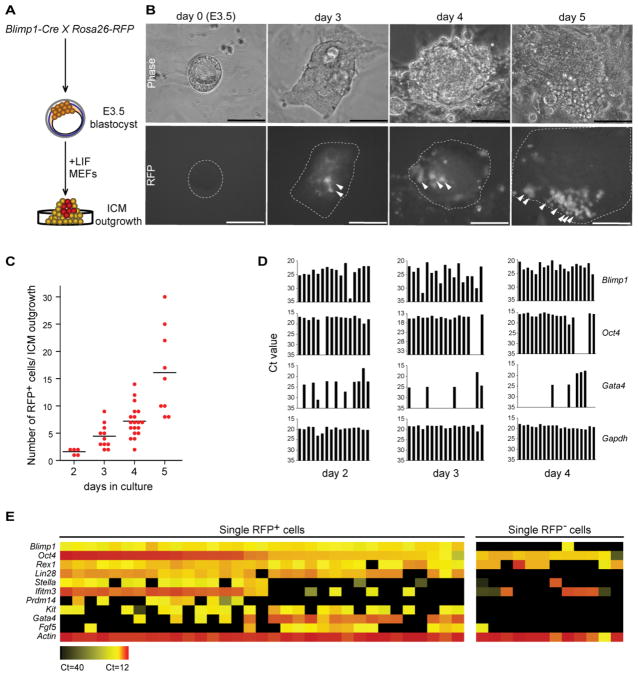
To confirm the presence of Blimp1-positive cells in the ICM outgrowth and track their developmental fate, we used a Cre/loxP system with stop-floxed Rosa26-RFP knockin reporter mice (Figure 1A) [22]. Genetic fate-mapping experiments during ICM outgrowth were performed with a Blimp1-Cre driver that faithfully recapitulates endogenous Blimp1 expression [5, 16]. A close examination of ICM outgrowth between 2 and 3 days of culture revealed small clusters of RFP+ cells (Figure 1B), indicating that these cells were expressing or had expressed Blimp1. The RFP+ cells typically had a large nucleus-to-cytoplasm ratio, formed long extensions, appeared to be highly migratory and increased progressively in number from day 3 to day 5 of culture (Figures 1B and 1C).
Figure 1.
Lineage tracing and single-cell analysis of Blimp1-positive cells from ICM outgrowth. (A) Schematic of experimental strategy. Blimp1-Cre mice were crossed with Rosa26-RFP reporter mice to obtain E3.5 blastocysts that were cultured for various times depending on the analysis. (B) Progressive increase in RFP+ cells over 5 days in cultured ICM outgrowths (Blimp1-Cre; Rosa26-RFP). Arrowheads indicate RFP+ cells within the outgrowth. Scale bars = 100 μm. (C) Quantification of RFP+ cells per ICM outgrowth from days 2 to 5 of culture. From 5 to 18 outgrowths were observed at each time point; bars represent mean values. (D) Single cells were analyzed by qPCR for Oct4+, Blimp1+ and Gata4+ expression. From 16 to 19 single cells expressing Blimp1 were selected for analysis at each time point. Gapdh served as the internal control. Y-axis shows Ct (cycle threshold) value. (E) Upregulation of PGC markers in relation to Blimp1 positivity. Expression levels of the 11 comparison genes were simultaneously determined with the BioMark system in single RFP+ (n=33) or RFP- (n=12) cells sorted from day-4 ICM outgrowths. The heat map is based on averaged Ct values of triplicate qPCR reactions with each single-cell cDNA. Ct, cycle threshold.
Blimp1 expression is restricted to PGCs and visceral endoderm (VE) cells during the early postimplantation stage [5, 6, 16], but is undetectable in preimplantation embryos (see Figure S1A available online), suggesting that the RFP+ cells seen in our blastocyst culture experiment likely represent such cell types in vitro. To test this prediction, we performed immunostaining of RFP+ cells for Gata4, a marker of primitive endoderm on day-4 ICM outgrowths, and found that 64.2 ± 9.9% (mean ± SEM) of the RFP+ cells expressed this protein (Figures S1B and S1C), suggesting a primitive VE-like fate in vitro. Importantly, a substantial subset of RFP+ cells remained negative for Gata4 expression (Figures S1B and S1C), consistent with their development in the germ cell lineage. Control experiments with cultured primary postimplantation epiblast fragments revealed a Blimp1 expression pattern (Figure S1D) that reiterated previous observations [23–25]. This finding, together with the reported robustness of the Blimp1-Cre driver transgene [5, 16], makes it unlikely that the Cre driver was aberrantly activated in vitro.
To study the RFP+ cells in greater detail, we performed single-cell gene expression profiling to test for the presence of PGC markers as well as Gata4. Quantitative PCR (qPCR) analysis of single RFP+ cells from day-2 to day-4 ICM outgrowths indicated a correlation between Blimp1 and Oct4 expression, while Gata4 tended to be expressed in the absence of these markers as ICM outgrowths developed (Figure 1D). Notably, not all RFP+ cells were Blimp1 positive at any given point in the analysis, probably because of the relatively narrow window of Blimp1 expression [25] and, in rare instances, because of improper activation of the reporter gene. Finally, by broadening our single-cell qPCR analyses, we could detect expression of Oct4 together with other common PGC markers: Ifitm3, Lin28, Prdm14, Stella and c-Kit (Figure 1E).
Blimp1+ Cells Display PGC-Like Activity
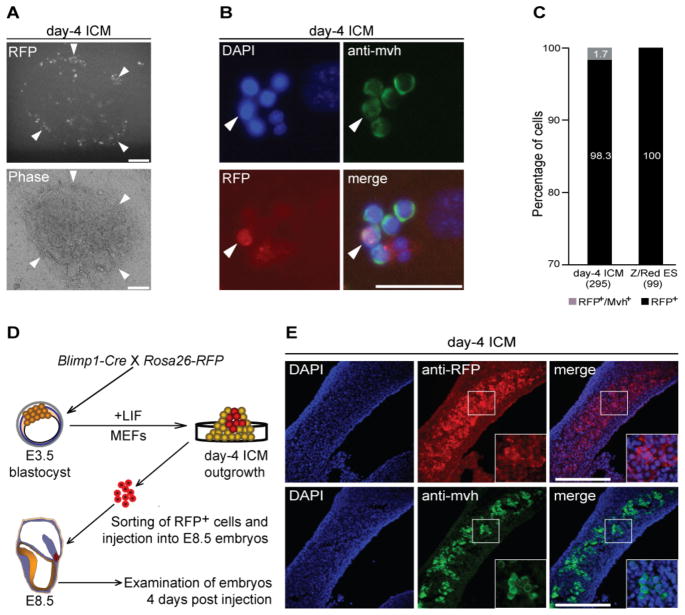
To functionally characterize the RFP+ cells within ICM outgrowths, we undertook ex vivo organ culture experiments similar to those used to propagate germ cells in vitro [25]. When purified RFP+ cells were cocultured with fragments of E13.5 female genital ridges for 3–5 days, they proliferated slowly, forming small clumps and migrating actively (Figure 2A). Staining of organ cocultures with antibody against the mouse vasa homolog (Mvh) protein showed that the RFP+ cell population colocalized with this marker (Figures 2B and 2C). To obtain additional evidence that these RFP+ cells have the potential to differentiate in the germ cell pathway, we tested their ability to colonize genital ridges by sorting and injecting them into individual E8.5 embryos obtained from heterozygous W/Wv matings [26]. Previous studies had shown that donor cells injected into such embryos can migrate to the amniotic cavity [26, 27], where they have ready access to the gut of the developing embryo. We therefore predicted that if the RFP+ donor cells indeed possessed PGC traits, they would migrate to the genital ridges as development continued. In experiments to test this hypothesis (Figure 2D), we detected RFP+ cells along genital ridges that colocalized with Mvh antibody staining within the embryo (Figures 2E, S2 and Table S1). This indicates that the donor cells were able to respond to chemoattractive signals released from the genital ridges within the host embryo and eventually colonize them, properties typically associated with functional germ cells.
Figure 2.
Blimp1-expressing cells display PGC-like activity. (A) Representative image indicates that RFP+ cells sorted from day-4 ICM outgrowths tend to aggregate close to fragments of genital ridges after 3 days of coculture. Arrowheads indicate clusters of RFP+ cells. Scale bars = 100 μm. (B) Representative images of cocultured RFP+ cells stained with anti-Mvh antibody after 3 days of growth. Arrowheads indicate RFP+ /Mvh+ cells. Scale bar = 50 μm. (C) Percentage of RFP+ /Mvh+ cells (gray) among total RFP+ cells from day-4 ICM outgrowth (n=295) compared with controls (n= 99). (D) Schematic of experimental strategy. Blimp1-Cre was crossed with Rosa26-RFP reporter mice to obtain blastocysts. RFP+ cells were then sorted from day-4 ICM outgrowths and injected into E8.5 embryos in utero. Examination of host embryos was performed on day-4 postinjection. (E) Cross-sections (10 μm) of host genital ridges dissected from in utero-injected embryos stained for RFP or Mvh. Inserts show higher magnification views of the boxed areas. Scale bars = 100 μm.
Together, these data demonstrate the emergence of cells from the Blimp1-positive fraction of ICM outgrowth that possess PGC-like properties. This acquisition of a PGC-specific transcriptional program by a small subset of early epiblast cells would be expected to provide a signaling milieu conducive to stabilization of the pluripotent state in cells that otherwise are poised to adopt a somatic fate [5,28].
Blimp1-Positive Cells Contribute to ES Cell Formation
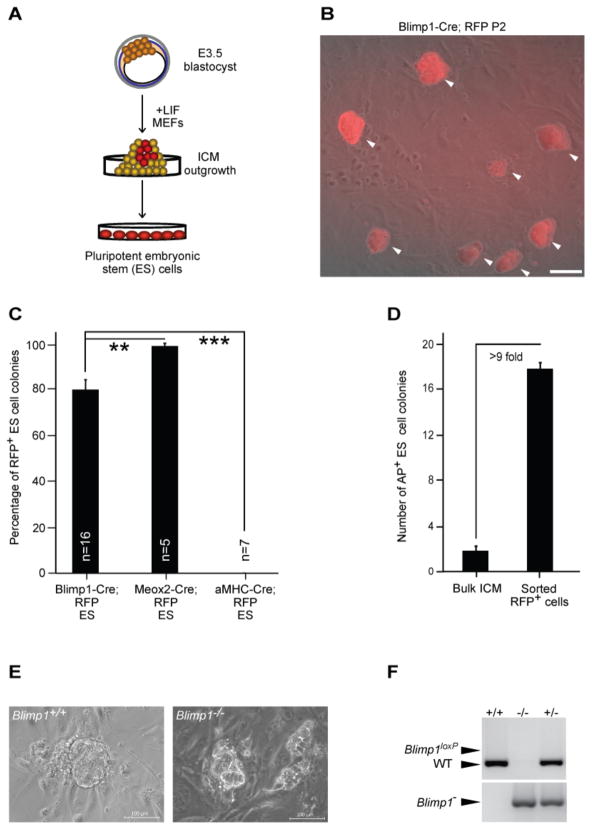
We next asked if RFP+ cells, which express or previously expressed Blimp1, can give rise to ES cells. When we isolated and replated individual cells emerging from the ICM of blastocysts cultured for 5 days (Figure 3A), most of the resultant primary colonies were RFP+ (Figure 3B). In three independent experiments, we established 21 ES cell lines from a total of 55 ICM outgrowths (41.8% efficiency). Importantly, a majority of the primary ES cell colonies (mean 79.7 ± 4.5% SEM) generated from Blimp1-Cre; Rosa26-RFP embryos were RFP+ (Figure 3C). Control experiments with α MHC-Cre [29] or Meox2-Cre [30] mice crossed with Rosa26-RFP reporter mice uniformly yielded either RFP− or RFP+ ES cell lines. Each of the ES cell lines that we tested formed embryoid bodies in vitro and contributed to teratoma formation after injection into immunodeficient mice (Figure S3). Interestingly, attempts to derive ES cells from PGCs isolated from E7.5 embryos were unsuccessful, indicating that the tissue culture environment may accelerate the development and maturation of the nascent PGC precursors, as previously reported [31–34]. Critically, prospective sorting of RFP+ cells from day-4 ICM outgrowths enhanced the generation of ES cell lines by more than 9-fold (mean 1.7 ± 0.4% SEM vs. 17.0 ± 0.5% ) (Figure 3D).
Figure 3.
Blimp1-expressing cells contribute to ES cell formation. (A) Schematic of experimental strategy. Different Cre drivers were crossed with Rosa26-RFP reporter mice to obtain E3.5 blastocysts. ICM outgrowths were dissociated and replated on day 5 under conditions that support ES cell derivation. (B) Representative image of an ES cell line (passage 2) derived from Blimp1-Cre; Rosa26-RFP embryos. Arrowheads indicate RFP+ ES-like colonies. (C) Quantitative summary of results from fate-mapping experiments in which ES cell lines were derived from individual embryos (Cre; RFP+). Mean ± SEM percentages are shown. *** P< 0.001, ** P< 0.01 (two-tailed t-test). (D) Number of AP+ ES colonies per 100 cells plated from bulk ICM outgrowths (n=22) or RFP+ cells sorted from day-4 ICM outgrowths (n=737). (E) Representative images for Blimp1+/+ and Blimp1−/− day 5 ICM outgrowths. (F) Representative genotyping results for ES cell lines derived from Blimp1 heterozygous intercross. All scale bars = 100 μm.
ES Cell Derivation from Blimp1-Negative Precursors Recruited directly from Epiblast Cells
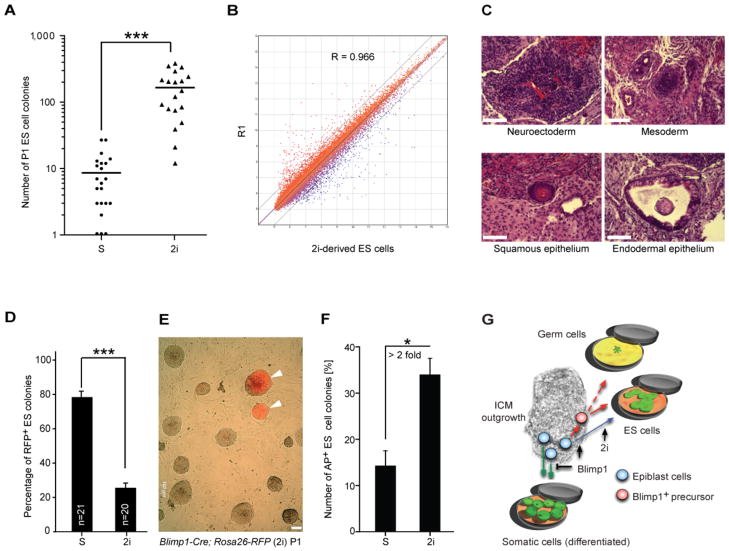
To assess the functional role of Blimp1 during ES cell derivation, we performed ES cell derivation experiments from embryos obtained from Blimp1 heterozygous intercrosses. Both Blimp1−/ − and Blimp1+/+ embryos gave rise to ICM outgrowths (Figure 3E). We found that the loss of Blimp1 expression was not associated with a decrease in the ability of ICM outgrowth to produce ES cells (Figures 3F and S4A). Nor is there any obvious qualitative phenotype of established Blimp1−/− ES cell lines (Figures S4B and S4C). Even so, since a hallmark of Blimp1 molecular activity is transcriptional repression we speculated that further repression of epiblast differentiation via inhibition of fibroblast growth factor / extracellular signal-regulated kinase (Fgf/Erk) signaling and glycogen synthase kinase-3 (Gsk3) activity, using the so-called 2i regimen [7, 8], might allow more Blimp1-negative epiblast cells to be recruited to an ES cell fate. In experiments to test this prediction, 2i treatment of ICM outgrowths from Blimp1-Cre; Rosa26-RFP embryos for 4 days resulted in a 19.2-fold increase of ES cell-like colonies compared to those generated in conventional derivation medium alone (Figure 4A). Microarray analysis of ES cell lines derived under conventional or 2i derivation conditions showed no major changes in gene expression (Figures 4B and S3B), nor were there any substantial differences in the ability of these cells to differentiate properly (Figure 4C), in agreement with previous results [7]. Finally, we noted that approximately 74.4 ± 2.6% (mean ± SEM) of the ES cell colonies emerging under 2i conditions were RFP− (Figures 4D and 4E), suggesting that a substantial fraction of nascent epiblast cells not induced toward a germ cell fate can directly acquire ES cell-like characteristics [8]. Interestingly, prospective sorting of RFP+ cells from day-4 ICM outgrowths treated with 2i enhanced the generation of ES cell colonies by more than 2-fold (Figure 4F), indicating a positive proliferative effect of 2i on the Blimp1-positive precursor cells in the ICM outgrowth.
Figure 4.
ES cell derivation from Blimp1-negative precursors through direct recruitment from epiblast cells. (A) Number of P1 ES cell colonies obtained per embryo. Each symbol represents a single embryo. Bars denote mean values (***P< 0.001, two-tailed t-test). Y-axis is shown in log scale. S, standard conditions; 2i, 2i conditions. (B) Pairwise comparison of global transcriptional profiles between 2i-derived ES cells and R1 ES cells. R, linear coefficient. (C) Histological analysis of teratoma from 2i-derived ES cell lines after injection into SCID mice. (D) Summary of fate-mapping experiments with Blimp1-Cre; Rosa26-RFP embryos. The data are means ± SEM (***P< 0.001, two-tailed t-test). S, standard conditions; 2i, 2i conditions. (E) Representative merged RFP/phase image of an ES cell line (passage 1) derived from Blimp1-Cre; Rosa26-RFP embryo under 2i conditions. Arrowheads indicate RFP+ ES-like colonies. All scale bar = 100 μm. (F) Number of AP+ ES colonies per 100 cells sorted from day-4 ICM outgrowths treated with or without 2i. The data are means ± SEM (*P< 0.05, two-tailed t-test). S, standard conditions; 2i, 2i conditions. (G) Proposed model for generation of ES cells from ICM outgrowth in vitro. Blimp1+ precursors (red) arise from epiblast cells (blue) and differentiate to ES cells in ES cell-derivation medium. These precursors also have the potential to display PGC-like features in the appropriate growth environment. A proportion of epiblast cells not induced toward a germ cell fate can directly acquire ES cell-like characteristics in tissue culture medium supplemented with inhibitors of Erk signaling and Gsk activity (“2i cocktail”; blue line).
Discussion
This study of the developmental fate and gene expression profiles of ICM cells in different culture conditions yielded three key findings. (i) Close examination of ICM outgrowths in conventional ES cell derivation medium revealed cells harboring molecular markers that are associated with germ cell development, consistent with previous studies [5, 15]. (ii) These cells possessed properties typically associated with authentic PGCs and showed a strong propensity to transition into a stable pluripotent state in vitro (and hence become ES cells). This outcome may reflect the acquisition of a PGC-like transcriptional program early in blastocyst culture, which would be expected to provide a milieu conducive to stabilization of the pluripotent state in cells that otherwise are poised to adopt a somatic fate [5, 28, 35]. (iii) Blimp1 expression was facultative for ES cell production. That is, specific alterations in Erk signaling and Gsk3 activity (“2i” regimen) enable effective direct recruitment of ES cell precursors from newly formed epiblast (Figure 4G) [7, 8].
Whether Blimp1-positive cells directly give rise to ES cells or transition through one or more intermediate steps cannot be resolved from our data, which focus on a single window of the derivation process. Nonetheless, the striking difference in gene expression profiles of these cell types strongly argues for the presence of additional developmental steps beyond the PGC-like stage. Our findings also suggest that ES cell generation from cultured blastocysts is a far more complex process than initially thought, a view supported by descriptions of other pluripotent stem cell states, both in vitro and in vivo, that differ from those reported here [21, 23, 24]. Thus, it appears that the mechanisms responsible for the generation of stable pluripotency in vitro may be biologically overengineered [36]. On the other hand, our results may not unequivocally resolve the question of whether an early germ cell precursor state is essential for the derivation of pluripotent cell lines under standard serum conditions, as the loss of Blimp1 function does not entirely abrogate the formation of founder PGC precursor population, but instead affects their proliferation in vivo [5, 6].
Indeed, the present gap in knowledge concerning the specific intermediate states leading to stable pluripotency in vitro may well account for the repeated failures to derive human pluripotent cells with the same phenotypic traits as their murine counterparts. It will be difficult to realize the full biomedical potential of either human ES or induced pluripotent stem (iPS) cells without first defining the exact sequence of events leading to their acquisition of pluripotency. Our demonstration that ES cell derivation efficiency can be improved by prescreening for Blimp1 positivity suggests that PGC-permissive or inductive cues could be exploited to improve the generation of new stem cell lines from cultured blastocysts, especially in humans [37, 38]. Meanwhile, efforts to understand pluripotent cellular states might profit from closer consideration of the genetic programs that promote and maintain fidelity to the germ cell lineage [39]. We also anticipate that such strategies could be exploited to enhance the production of ES-like cells through reprogramming of somatic cells and might open new opportunities to study the early and intermediate steps of epigenetic reprogramming [40, 41].
Supplementary Material
Highlights.
Blimp1+ cells emerging from ICM outgrowths display PGC-like activity
Blimp1+ cells contribute to ES cell formation
ES cells are derived from Blimp1− precursors recruited directly from epiblast cells
Acknowledgments
We thank A.J. Cooney, R.R. Behringer and M. Dejosez for critical discussions and reading of the manuscript; H.J. Fehling for providing Rosa26-RFP reporter mouse line; M. Nussenzweig and M. Schneider for providing Blimp1-Cre and α MHC-Cre mouse lines; K.K. Hirschi and M.E. Dickinson for providing microscopy expertise; Baylor Cytometry and Cell Sorting Core for technical assistance; and J. Gilbert for editorial advice. This work was supported by the Huffington Foundation and National Institutes of Health grants R01 EB005173-01, 1R01 GM077442-01, P20 EB007076 and P01 GM81627.
Footnotes
Supplemental Information includes four figures, two tables, and Supplemental Experimental Procedures and can be found with this article online.
Author contributions
L.C. performed the experiments and with T.P.Z. analyzed the data. M.A.S. and R.J. designed experiments and provided critical feedback. T.P.Z. designed the project and with L.C. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 2.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proceedings of the National Academy of Sciences of the United States of America. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 4.Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nature biotechnology. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 5.Ohinata Y, Payer B, O'Carroll D, Ancelin K, Ono Y, Sano M, Barton SC, Obukhanych T, Nussenzweig M, Tarakhovsky A, et al. Blimp1 is a critical determinant of the germ cell lineage in mice. Nature. 2005;436:207–213. doi: 10.1038/nature03813. [DOI] [PubMed] [Google Scholar]
- 6.Vincent SD, Dunn NR, Sciammas R, Shapiro-Shalef M, Davis MM, Calame K, Bikoff EK, Robertson EJ. The zinc finger transcriptional repressor Blimp1/Prdm1 is dispensable for early axis formation but is required for specification of primordial germ cells in the mouse. Development. 2005;132:1315–1325. doi: 10.1242/dev.01711. [DOI] [PubMed] [Google Scholar]
- 7.Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P, Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nichols J, Silva J, Roode M, Smith A. Suppression of Erk signalling promotes ground state pluripotency in the mouse embryo. Development. 2009;136:3215–3222. doi: 10.1242/dev.038893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradley A, Evans M, Kaufman MH, Robertson E. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature. 1984;309:255–256. doi: 10.1038/309255a0. [DOI] [PubMed] [Google Scholar]
- 10.Brook FA, Gardner RL. The origin and efficient derivation of embryonic stem cells in the mouse. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:5709–5712. doi: 10.1073/pnas.94.11.5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zwaka TP, Thomson JA. A germ cell origin of embryonic stem cells? Development. 2005;132:227–233. doi: 10.1242/dev.01586. [DOI] [PubMed] [Google Scholar]
- 12.Matsui Y, Zsebo K, Hogan BL. Derivation of pluripotential embryonic stem cells from murine primordial germ cells in culture. Cell. 1992;70:841–847. doi: 10.1016/0092-8674(92)90317-6. [DOI] [PubMed] [Google Scholar]
- 13.Resnick JL, Bixler LS, Cheng L, Donovan PJ. Long-term proliferation of mouse primordial germ cells in culture. Nature. 1992;359:550–551. doi: 10.1038/359550a0. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi K, de Sousa Lopes SM, Surani MA. Germ cell specification in mice. Science. 2007;316:394–396. doi: 10.1126/science.1137545. [DOI] [PubMed] [Google Scholar]
- 15.Yamaji M, Seki Y, Kurimoto K, Yabuta Y, Yuasa M, Shigeta M, Yamanaka K, Ohinata Y, Saitou M. Critical function of Prdm14 for the establishment of the germ cell lineage in mice. Nat Genet. 2008;40:1016–1022. doi: 10.1038/ng.186. [DOI] [PubMed] [Google Scholar]
- 16.Robertson EJ, Charatsi I, Joyner CJ, Koonce CH, Morgan M, Islam A, Paterson C, Lejsek E, Arnold SJ, Kallies A, et al. Blimp1 regulates development of the posterior forelimb, caudal pharyngeal arches, heart and sensory vibrissae in mice. Development. 2007;134:4335–4345. doi: 10.1242/dev.012047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pienkowski M, Solter D, Koprowski H. Early mouse embryos: growth and differentiation in vitro. Exp Cell Res. 1974;85:424–428. doi: 10.1016/0014-4827(74)90145-1. [DOI] [PubMed] [Google Scholar]
- 18.Wiley LM, Pedersen RA. Morphology of mouse egg cylinder development in vitro: a light and electron microscopic study. J Exp Zool. 1977;200:389–402. doi: 10.1002/jez.1402000309. [DOI] [PubMed] [Google Scholar]
- 19.Gonda MA, Hsu YC. Correlative scanning electron, transmission electron, and light microscopic studies of the in vitro development of mouse embryos on a plastic substrate at the implantation stage. Journal of embryology and experimental morphology. 1980;56:23–39. [PubMed] [Google Scholar]
- 20.Wu TC, Wan YJ, Damjanov I. Positioning of inner cell mass determines the development of mouse blastocysts in vitro. Journal of embryology and experimental morphology. 1981;65:105–117. [PubMed] [Google Scholar]
- 21.Najm FJ, Chenoweth JG, Anderson PD, Nadeau JH, Redline RW, McKay RD, Tesar PJ. Isolation of epiblast stem cells from preimplantation mouse embryos. Cell stem cell. 2011;8:318–325. doi: 10.1016/j.stem.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luche H, Weber O, Nageswara Rao T, Blum C, Fehling HJ. Faithful activation of an extra-bright red fluorescent protein in "knock-in" Cre-reporter mice ideally suited for lineage tracing studies. Eur J Immunol. 2007;37:43–53. doi: 10.1002/eji.200636745. [DOI] [PubMed] [Google Scholar]
- 23.Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RD. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- 24.Brons IG, Smithers LE, Trotter MW, Rugg-Gunn P, Sun B, Chuva de Sousa Lopes SM, Howlett SK, Clarkson A, Ahrlund-Richter L, Pedersen RA, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi K, Surani MA. Self-renewing epiblast stem cells exhibit continual delineation of germ cells with epigenetic reprogramming in vitro. Development. 2009;136:3549–3556. doi: 10.1242/dev.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huszar D, Sharpe A, Jaenisch R. Migration and proliferation of cultured neural crest cells in W mutant neural crest chimeras. Development. 1991;112:131–141. doi: 10.1242/dev.112.1.131. [DOI] [PubMed] [Google Scholar]
- 27.Jaenisch R. Mammalian neural crest cells participate in normal embryonic development on microinjection into post-implantation mouse embryos. Nature. 1985;318:181–183. doi: 10.1038/318181a0. [DOI] [PubMed] [Google Scholar]
- 28.Saitou M, Barton SC, Surani MA. A molecular programme for the specification of germ cell fate in mice. Nature. 2002;418:293–300. doi: 10.1038/nature00927. [DOI] [PubMed] [Google Scholar]
- 29.Agah R, Frenkel PA, French BA, Michael LH, Overbeek PA, Schneider MD. Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J Clin Invest. 1997;100:169–179. doi: 10.1172/JCI119509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tallquist MD, Soriano P. Epiblast-restricted Cre expression in MORE mice: a tool to distinguish embryonic vs. extra-embryonic gene function. Genesis. 2000;26:113–115. doi: 10.1002/(sici)1526-968x(200002)26:2<113::aid-gene3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 31.Hubner K, Fuhrmann G, Christenson LK, Kehler J, Reinbold R, De La Fuente R, Wood J, Strauss JF, 3rd, Boiani M, Scholer HR. Derivation of oocytes from mouse embryonic stem cells. Science. 2003;300:1251–1256. doi: 10.1126/science.1083452. [DOI] [PubMed] [Google Scholar]
- 32.Toyooka Y, Tsunekawa N, Akasu R, Noce T. Embryonic stem cells can form germ cells in vitro. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:11457–11462. doi: 10.1073/pnas.1932826100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geijsen N, Horoschak M, Kim K, Gribnau J, Eggan K, Daley GQ. Derivation of embryonic germ cells and male gametes from embryonic stem cells. Nature. 2004;427:148–154. doi: 10.1038/nature02247. [DOI] [PubMed] [Google Scholar]
- 34.Surani MA. Stem cells: how to make eggs and sperm. Nature. 2004;427:106–107. doi: 10.1038/427106a. [DOI] [PubMed] [Google Scholar]
- 35.Xu X, Pantakani DV, Luhrig S, Tan X, Khromov T, Nolte J, Dressel R, Zechner U, Engel W. Stage-Specific Germ-Cell Marker Genes Are Expressed in All Mouse Pluripotent Cell Types and Emerge Early during Induced Pluripotency. PloS one. 2011;6:e22413. doi: 10.1371/journal.pone.0022413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gray MW, Lukes J, Archibald JM, Keeling PJ, Doolittle WF. Cell biology. Irremediable complexity? Science. 2010;330:920–921. doi: 10.1126/science.1198594. [DOI] [PubMed] [Google Scholar]
- 37.Buecker C, Chen HH, Polo JM, Daheron L, Bu L, Barakat TS, Okwieka P, Porter A, Gribnau J, Hochedlinger K, et al. A murine ESC-like state facilitates transgenesis and homologous recombination in human pluripotent stem cells. Cell stem cell. 2010;6:535–546. doi: 10.1016/j.stem.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanna J, Cheng AW, Saha K, Kim J, Lengner CJ, Soldner F, Cassady JP, Muffat J, Carey BW, Jaenisch R. Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9222–9227. doi: 10.1073/pnas.1004584107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohinata Y, Ohta H, Shigeta M, Yamanaka K, Wakayama T, Saitou M. A signaling principle for the specification of the germ cell lineage in mice. Cell. 2009;137:571–584. doi: 10.1016/j.cell.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 40.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 41.Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.