Abstract
Background
Tobacco smoking is the leading preventable cause of death in the developed world. Identifying risk factors for smoking may lead to more effective treatments. Genome wide association studies revealed a relationship between development of nicotine dependence and a single-nucleotide polymorphism (SNP, rs16969968) of the nicotine acetylcholine receptor (nAChR) alpha-5 subunit gene (CHRNA5). The relationship between this SNP and other factors contributing to smoking behavior such as smoking cue reactivity is unclear.
Methods
We assessed the role of rs16969968 on brain functional MRI (fMRI) reactivity to smoking cues by studying nicotine dependent women with the nicotine dependence ‘risk’ allele (A allele, N=14) and without the ‘risk’ allele (G/G smokers, N=10). Nicotine dependence severity, as assessed with the Fagerstrom test for nicotine dependence, smoking pack-years, and expired carbon monoxide levels, were equivalent in these groups.
Results
We observed a group difference in fMRI reactivity; women without the A allele (G/G smokers) showed greater fMRI reactivity to smoking images in brain areas related to memory and habitual behavior such as the hippocampus and dorsal striatum.
Conclusions
Our finding suggests that nicotine-dependent smokers lacking the rs16969968 A allele are more likely to recall smoking-related memories and engage in habitual responding to smoking cues than A allele smokers. Although more studies are necessary to determine the mechanism underlying and significance of this cue reactivity difference, these data suggest that smokers may develop and remain nicotine dependent due to different factors including genetics and cue reactivity. This finding may have implications for personalizing smoking treatment.
Keywords: Tobacco smoking, fMRI, CHRNA5, nicotine dependence, dorsal striatum
1. Introduction
The public health impact of tobacco smoking is enormous, with one billion tobacco-related deaths projected for the twenty-first century (World Health Organization, 2008). Nicotine dependence develops due to a range of genetic, psychosocial, and environmental risk factors (Amos et al., 2010). A more comprehensive understanding of smoking risk factors and the interactions between them could improve the ability to prevent and treat smoking addiction. Nicotine dependence is highly heritable (Lessov-Schlaggar et al., 2008) and genetic risk factors for nicotine dependence have been revealed by genome wide association studies (Bierut, 2010; Bierut et al., 2007). However, not all nicotine-dependent smokers carry the same genetic risks, suggesting that factors other than genetics also may moderate nicotine dependence. Identifying differences between smokers with equivalent nicotine dependence severity but with dissimilar genetic profiles may help reveal other risk factors for nicotine dependence.
Nicotine derived from tobacco smoking acts largely via nicotinic acetylcholine receptors (nAChR) in the brain (Benowitz, 1996). Brain nAChRs are pentameric ligand-gated cation channels typically comprised of alpha and beta subunit combinations (Gotti et al., 2006). The alpha-5 subunit is an accessory subunit (Kuryatov et al., 2008) co-expressed only with other alpha and beta subunits; when present, alpha-5 subunits increase nAChR function (Gerzanich et al., 1998; Gotti and Clementi, 2004; Ramirez-Latorre et al., 1996). The genetic polymorphism most associated with development of smoking dependence is the non-synonymous coding single-nucleotide polymorphism (SNP) of the nAChR subunit alpha-5 (CHRNA5) gene rs16969968 (Bierut, 2010).
The rs16969968 SNP encodes an Asp398Asn polymorphism resulting in an aspartic acid (G allele) change to asparagine (A allele) at the 398th amino acid (Bierut et al., 2008; Saccone et al., 2007). Expression of the A ‘risk-allele’ reduces nAChR function (Bierut et al., 2008), which may enhance nicotine dependence susceptibility. It has been suggested that reduced nAChR function due to A allele expression may regulate dopamine-mediated reward signaling, thereby facilitating dependence (Bierut et al., 2008). However, others have suggested that smokers expressing the A allele may smoke to ameliorate cognitive impairments (Winterer et al., 2010). It is possible that genotypic differences, which impact nicotine dependence, reward, and cognition, may also influence other smoking-related phenotypes such as reactivity to smoking cues.
Clinical and preclinical studies indicate that cue reactivity plays a role in smoking behavior and nicotine seeking (Cohen et al., 2005; Ferguson and Shiffman, 2009; Shiffman et al., 1996). Enhanced cue reactivity as measured by fMRI may confer relapse vulnerability in smokers (Janes et al., 2010a) and in other drug-dependent populations (Grüsser et al., 2004; Kosten et al., 2006). However, it has been argued that the role of cue-reactivity in dependence is unclear (Perkins, 2009). Inconsistencies between cue-reactivity studies may partly be explained by genetic differences in study cohorts suggesting that interactions between genetics and cue-reactivity should be studied. Currently, it is not known whether rs16969968 moderates smoking cue reactivity in nicotine dependent smokers. Expression of the A allele could both enhance risk for initial development of nicotine dependence and enhance brain-reactivity to smoking cues, which may influence relapse risk in smokers who try to quit. Alternatively, while the G allele is not an equivalent risk factor for developing nicotine dependence, G/G smokers who do develop dependence may have greater smoking cue-reactivity, possibly in brain regions involved in the maintenance of smoking-cue associations, including the dorsal striatum (Porrino et al., 2004), which could explain their continued smoking. To determine whether rs16969968 moderates fMRI-measured brain reactivity to smoking cues, we compared two groups of women whose nicotine dependence severity was equivalent, with one group expressing an A allele and the other expressing 2 copies of the G allele.
2. Methods
2.1 Subjects
Following methods previously described (Janes et al., 2009; Janes et al., 2010a; Janes et al., 2010b), women smokers (N=24) underwent neuroimaging at McLean Hospital as part of a smoking cessation clinical trial at Massachusetts General Hospital (MGH, NCT00218465). Subjects reported smoking 10 cigarettes/day in the last 6 months and had expired air carbon monoxide (CO)>10 ppm at screening. Subjects met DSM-IV criteria for current nicotine dependence and subjects were excluded if they had the following conditions: alcohol use disorder and/or major depressive episode in the past 6 months, lifetime DSM-IV diagnosis of organic mental disorder, bipolar disorder or any schizophrenia spectrum disorder. Smokers also were excluded for current unstable medical illness or pregnancy, current psychotropic drug use, recent drug use or excessive alcohol use (QuickTox 11 Panel Drug Test Card, Branan Medical Corporation, Irvine California; Alco-Sensor IV, Intoximeters Inc., St. Louis, MO). Ancestry was determined by self-report. Two subjects reported African-American ancestry, one reported Pacific Islander ancestry, and the remaining 21 were Caucasian. Only women were enrolled since the parent clinical trial involved an investigational medication not approved for use in men. Institutional Review Boards at MGH and McLean Hospital approved this study, which was conducted in adherence with the principles of the Declaration of Helsinki. Subjects provided written informed consent and were compensated for participation. Subjects were not overnight abstinent and were allowed to smoke until shortly before imaging.
Smoking behavior was characterized by recording pack-years of tobacco use, average number of cigarettes smoked per day, number of cigarettes smoked the morning prior to imaging, and by measuring end expiratory CO levels prior to scanning (Bedfont Micro IV Smokerlyzer, Bedfont Scientific, Kent, England). Degree of nicotine dependence was assessed with the Fagerstrom Test for Nicotine Dependence (FTND) (Fagerström, 1978). Group (homozygous expression of the dominant G allele vs. any expression of the minor A allele) differences in demographic characteristics were assessed with two-sided Student’s t-tests.
The rs16969968 SNP was genotyped by mass spectrometry using the iPlex assay (Sequenom) from an assay designed using SpectroDESIGNER software. The Hardy-Weinberg equilibrium p = 0.99, the call rate = 100%, and the minor allele frequency was 0.35.
2.2 Functional Neuroimaging
Subjects viewed images of smoking-related (people smoking, hands holding cigarettes, or cigarettes alone) or neutral (general content-matched but no smoking cues) images (Due et al., 2002; Gilbert and Rabinovich, 1999). Animal images (not used in data analyses) were shown to prompt subjects to press a button to ensure they were attending to the images. A total of 42 smoking-related, 40 neutral, and 8 animal images were presented in 6 equal length blocks. Each image was presented pseudo-randomly for 4 seconds with no more than two of the same stimulus type appearing consecutively. A fixation-cross appeared for 14 seconds between images.
Scans were acquired on a Siemens Trio 3 Tesla scanner (Erlangen, Germany) with a circularly polarized (CP) head coil. Multiplanar rapidly acquired gradient-echo structural images (TR=2.1 sec, TE=2.7 msec, slices=128, matrix=256×256, flip angle=12, resolution= 1.0×1.0×1.33 mm) and gradient echo echo-planar images (TR=2 sec, TE=30 msec, matrix=64×64, field of view=224, flip angle=75, slices=30, resolution=3.5mm isotropic with 0mm gap) were acquired.
2.3 fMRI Analyses
Using methods previously described (Janes et al., 2009; Janes et al., 2010a; Janes et al., 2010b), images were analyzed using Brain Voyager QX 1.10.4 (Brain Innovation, Maastricht, Netherlands). Images were slice-time corrected, motion corrected, spatially smoothed (6mm Gaussian kernel), resampled to 3×3×3mm isotropic voxels, and spatially normalized into Talairach space. To reduce motion-related variability, a program based on (Lemieux et al., 2007) was used to model out time points exhibiting motion >1.75mm (½ voxel size).
To compare brain reactivity across all smokers in response to smoking > neutral images a whole-brain random-effects general linear model (GLM) approach was used. The model included image regressors (smoking, neutral, and animal images) and motion confound regressors. The 2-gamma hemodynamic response function was convolved with square waves defined by the onset/offset of each image presentation. Brain activation during smoking and neutral image presentation then was compared. An individual voxel statistical threshold of p< 0.01 was enforced. To account for multiple comparisons, data was cluster corrected to p < 0.05 using a minimum cluster extent of 540 mm3 (Slotnick et al., 2003).
To assess the influence of genotype on brain reactivity to smoking images, a whole-brain mixed-effects approach was used. First a whole-brain fixed effects general linear model was used, which included the same regressors and hemodynamic response function convolution as stated above. Beta maps comparing smoking to neutral images were created for each subject. These maps were used in a random-effects analysis of covariance (ANCOVA) to compare fMRI activity between subjects carrying at least one copy of the risk allele (A/A or A/G) vs. smokers homozygous for the major ‘non-risk allele’ (G/G). Due to the relationship between rs16969968 and FTND score, we covaried for FTND in this analysis. As in the first analysis, an individual voxel statistical threshold of p< 0.01 was enforced and was corrected for to p < 0.05 using the same method as described above.
3. Results
3.1 Demographics
Smokers were stratified into 2 groups, those carrying at least one allele previously associated with risk for developing nicotine dependence (N=14, 3/14 = A/A) and those homozygous for the major allele (G/G, N=10). There were no SNP group differences in age or on any smoking behavior measure (Table 1).
Table 1.
Group Demographics
No statistical difference was found between A-allele and G/G-allele smokers.
| Group | A-allele (n = 14) | G/G allele (n =10) | p-value |
|---|---|---|---|
| Age (years) | 46.1 ± 11.4 | 43.1 ±8.1 | p > 0.4 |
| Carbon Monoxide (ppmv) | 21.2 ± 9.8 | 18.7 ± 7.3 | p > 0.5 |
| FTND | 5.6 ± 2.4 | 5.8 ± 1.9 | p > 0.8 |
| Pack-Years | 26.3 ± 16.0 | 27.8 ±19.9 | p > 0.8 |
| Cig. Smoked before Scan | 4.5 ± 2.3 | 4.0 ± 1.9 | p > 0.5 |
| Avg. Smoked/Day | 18.5 ± 7.6 | 19.3 ± 8.5 | p > 0.8 |
3.2 Functional MRI Results
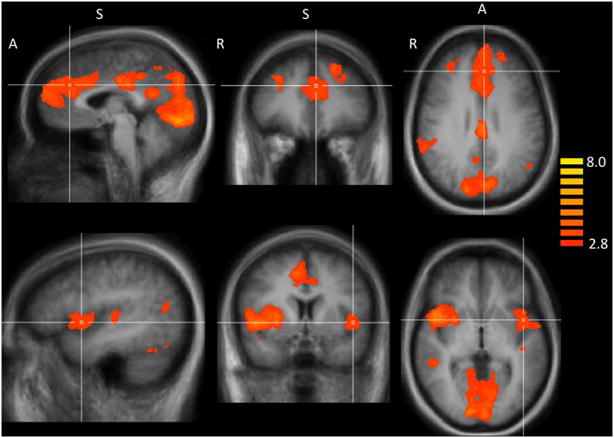
The whole-brain random effects analysis showed that smokers have greater brain reactivity to smoking relative to neutral images in several cortical regions (including midline structures and insula) and subcortically in the putamen. These activation patterns overlap with the results reported by our group and others (Due et al., 2002; Franklin et al., 2007; Janes et al., 2009; McClernon et al., 2008) and are listed in Table 2 and Figure 1.
Table 2.
Brain reactivity is greater for smoking vs. neutral images across all subjects.
| Brain Area | Brodmann Area | X | Y | Z | Cluster Size (mm3) | t |
|---|---|---|---|---|---|---|
| Medial Temporal Gyrus, Angular Gyrus, Supramarginal Gyrus | 21,22,40 | 56 | −44 | 33 | 4213 | 4.32 |
| Inferior Parietal Lobule | 40 | 60 | −23 | 24 | 571 | 3.75 |
| Insula, Putamen, Claustrum, Superior Temporal Gyrus | 13, 22 | 47 | 10 | 0 | 9880 | 5.16 |
| Lateral Occiptial Gyrus, Fusiform Gyrus | 19, 37 | 35 | −59 | −18 | 1622 | 4.66 |
| Middle Frontal Gryus | 9 | 32 | 34 | 36 | 1145 | 3.84 |
| Posterior Cingulate Cortex, Precuneus, Cuneus, Lingual Gyrus, Parahippocampal Gyrus, Retrosplenial Cortex | 7, 17, 18, 23, 30, 31 | 8 | −89 | 12 | 51456 | 6.48 |
| Superior Frontal Gyrus, Medial Frontal Gyrus, Anterior Cingulate Cortex, | 8, 9, 10, 24, 32 | −4 | 58 | 21 | 24512 | 6.05 |
| Insula, Superior Temporal Gyrus | 13, 21 | −40 | 4 | −3 | 3362 | 4.51 |
| Fusiform Gyrus | 37 | −37 | −68 | −21 | 762 | 4.48 |
| Superior Temporal Gyrus | 39 | −40 | −65 | 12 | 1092 | 3.65 |
| Superior Temporal Gyrus | 41 | −46 | −29 | 9 | 702 | 3.72 |
Brain area and Brodmann area refer to the location of each cluster of contiguous voxels. Talairach and Tournoux coordinates (Tal X, Y, and Z; Talairach and Tournoux, 1988) refer to the region of maximum cluster activation for each cluster. The volume refers to the total volume of each cluster in cubic millimeters. The t value refers to the maximum t-statistic in each cluster (p cluster corrected < 0.05).
Figure 1.
Smokers have greater fMRI reactivity to smoking vs. neutral images. Top panel crosshairs located in the dorsal anterior cingulate cortex at Talairach (Talairach and Tournoux, 1988) coordinates: x = −3, 34, 28. A, anterior; S, superior; R, right. Bottom panel crosshairs located in the Insula cortex at Talairach corrdinates: x = −45, y = 5, z = 0.
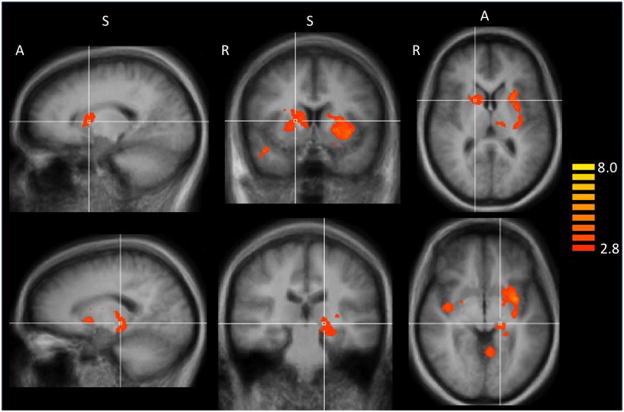
A whole-brain mixed effects analysis reveled that homozygous G/G smokers had greater brain reactivity to smoking vs. neutral images than smokers with an A allele. Differential brain activation was noted cortically in the superior temporal gyrus (BA 38), in the posterior cingulate cortex (PCC, BA 31) including the retrosplenial cortex (BA 30), and in the right posterior insula (BA 13). Differential subcortical activation was identified in the caudate nucleus, putamen, hippocampus, and thalamus (pcluster corrected < 0.05; Table 3; Figure 2). Relative to the G/G smokers, smokers with an A allele showed no increased brain activation to smoking vs. neutral images.
Table 3.
Brain reactivity to smoking vs. neutral images is greater in homozygous G/G versus A-allele smokers, with FTND as a covariate.
| Brain Area | Brodmann Area | X | Y | Z | Cluster Size (mm3) | t |
|---|---|---|---|---|---|---|
| Superior Temporal Gyrus | 38 | 41 | 4 | −18 | 649 | 5.49 |
| Insula | 13 | 38 | −8 | 0 | 546 | 4.28 |
| Caudate, Putamen | 20 | 7 | 3 | 2160 | 4.50 | |
| Hippocampus, Thalamus | −19 | −26 | −3 | 1648 | 5.17 | |
| Posterior Cingulate Cortex | 31 | −7 | −35 | 36 | 675 | 3.84 |
| Retrosplenial Cortex | 30 | −7 | −56 | 0 | 742 | 4.18 |
| Putamen, Insula | 13 | −28 | −20 | 3 | 6647 | 6.13 |
Brain area and Brodmann area refer to the location of each cluster of contiguous voxels. Talairach and Tournoux coordinates (Tal X, Y, and Z; Talairach and Tournoux, 1988) refer to the region of maximum cluster activation for each cluster. The volume refers to the total volume of each cluster in cubic millimeters. The t value refers to the maximum t-statistic in each cluster (p cluster corrected < 0.05).
Figure 2.
Homozygous G/G smokers have greater fMRI reactivity to smoking vs. neutral images than A-allele smokers in several brain regions (Table 2). Top panel crosshairs located in the putamen at Talairach (Talairach and Tournoux, 1988) coordinates: x=16, y=7, z=8. A, anterior; S, superior; R, right. Bottom panel crosshairs located in the hippocampus at Talairach corrdinates: x = −16, y = −24, z = −3.
4. Discussion
As a group, smokers exhibited greater brain reactivity to smoking vs. neutral images in many brain regions including the anterior and posterior cingulate, the insula, and putamen (Table 2, Figure 1). Greater brain reactivity to smoking vs. neutral images has been reported in these regions by our group and by others (For example: Due et al., 2002; Franklin et al., 2007; Janes et al., 2009; McClernon et al., 2008). Since this finding largely replicates previously published work, we focus our discussion on the main finding of a CHRNA5 genotype influence on brain reactivity to smoking images.
In comparison to smokers with an A allele, G/G smokers had greater fMRI reactivity to smoking-related versus neutral images. This result may seem counterintuitive, as one might have expected that smokers expressing the “risk” allele (A) for developing nicotine dependence would have greater cue reactivity than G/G smokers. Thus we observed an apparent dissociation among nicotine dependent smokers between genetic risk for development of nicotine dependence and enhanced cue reactivity. This dissociation may, in part, explain the equivalent level of nicotine dependence severity reported by the two groups in this sample.
The relative enhancement of cue-reactivity we observed in G/G smokers may be explained in part by genotype moderation of nAChR function. In this regard, A allele expression reduces nAChR function leading to reduced intracellular calcium influx following application of a nicotinic agonist (Bierut et al., 2008). This is key as nicotine facilitates long-term potentiation (LTP), the molecular process underlying learning and memory, in a calcium-dependent manner in the hippocampus (Jia et al., 2010), a brain area critically involved in learning and memory (Eichenbaum et al., 2007; Fortin et al., 2004; Poldrack and Packard, 2003). Notably, the hippocampus expresses alpha-5 subunit-containing nAChRs (Wada et al., 1990). Relative to G/G smokers, smokers with reduced nAChR function due to A allele expression may have decreased nicotine-induced calcium influx during smoking which may lead to diminished formation of drug-cue associations. It has been reported that smokers with an A allele have disrupted working memory as assessed with the n-back task, supporting the idea that expression of the A allele may impair memory function (Winterer et al., 2010). This suggests that rs16969968 may have the capacity to moderate memory processes. Based on these findings, we speculate that smokers with an A allele may form relatively weaker smoking-cue associations that could result in diminished fMRI cue reactivity. Our findings support the idea that G/G smokers may have more cue-associated memory as G/G smokers had more smoking cue-induced reactivity in memory-related brain regions including portions of the temporal lobe, hippocampus, and posterior cingulate cortex (PCC, including the retrosplenial cortex; Eichenbaum et al., 2007; Fortin et al., 2004; Piefke et al., 2005; Poldrack et al., 2003). Collectively, this suggests that nicotine dependent G/G smokers may experience more smoking-related memories when exposed to cues than smokers with an A allele, which may contribute to their continued smoking. However, this hypothesis is speculative and the role of the A allele in memory and the mechanism by which G/G smokers become relatively more reactive to smoking cues requires further study.
Nicotine dependent smokers expressing the G/G genotype also had relatively greater smoking cue-induced dorsal striatal activation. The dorsal striatum is important for developing stimulus-response associations with experience (van der Meer et al., 2010) and for maintaining cue-induced behavioral reactivity, once drug seeking has become habitual (Porrino et al., 2004). The apparently greater dorsal striatal fMRI cue reactivity we found in G/G smokers suggests that they may be more likely to habitually respond to smoking-related cues, possibly contributing to continued nicotine dependence.
The insula has become a region of great interest since it may play a critical role in maintaining smoking behavior (Naqvi et al., 2007), it activates in response to smoking cues (Franklin et al., 2007), and increased anterior insula reactivity to smoking cues is related to smoking relapse vulnerability and cigarette craving (Brody et al., 2002; Janes et al., 2010a). While we did not detect a moderating effect of the rs16969968 SNP on anterior insula reactivity, we did detect an effect in posterior insula. The posterior insula receives information about the homeostatic physical condition of the body and sends direct projections to the anterior insula (Craig, 2010). Thus, our findings could indicate that cue exposure and smoking memory recall may trigger greater physiologic reactions and interoceptive effects in G/G smokers versus smokers with an A allele.
The pattern of increased brain reactivity we report in G/G smokers suggests that upon exposure to smoking cues, G/G smokers are more likely to recall smoking-related memories and engage in habitual responding to the cues, possibly leading to the continuation of smoking. Our findings do not shed further light on exactly how the A allele contributes to smoking behavior. However, prior studies suggest that A allele smokers may experience fewer negative effects of smoking. Preclinical research suggests that mice lacking the alpha5 receptor subunit experience decreased aversive reactions to nicotine, which may allow them to find otherwise aversive doses of nicotine rewarding (Jackson et al., 2010). Additionally, deletion of the alpha5 receptor subunit in the habenula removes the inhibitory motivational signal that limits nicotine consumption (Fowler et al., 2011). These studies are supported by clinical research which shows that smokers with an A allele reported increased subjective ‘pleasurable buzz’ during early smoking (Sherva et al., 2008). Such heightened pleasure could increase initial risk for developing dependence. Additionally, it has been reported that smokers with an A allele have disrupted functional connectivity in reward-related brain circuits (Hong et al., 2010). These studies suggest that the risk conferred by the A allele may be enhanced reward responsiveness to nicotine, possibly due to reduced reward inhibition, which may contribute to the risk for initial development and possibly maintenance of nicotine dependence. By contrast, smokers in this study with the G/G genotype were equally nicotine dependent but exhibited greater fMRI smoking-cue reactivity. Thus, G/G carriers who develop nicotine dependence may continue smoking due to heightened smoking cue-reactivity.
5. Limitations
This study included a relatively small sample size and was limited to women so it remains unknown whether the genotype effect we observed occurs in men. Further, the G/G group included 3 non-Caucasian smokers, which could have confounded the data via an ancestry effect. However, removing non-Caucasian subjects from the analysis had a limited effect on the data, leading us to believe that ancestry had a small if any effect on our observations.
In addition, women’s hormonal status was not taken into consideration and we were unable to determine whether menstrual cycle phase impacted our results. Focusing on menstrual cycle phase will be important as sex steroid hormone changes may influence smoking behavior (Mazure et al., 2010). However, it seems unlikely that all G/G smokers would have randomly been scanned during one menstrual phase while smokers with the A allele were scanned at another phase. The impact of hormonal status, genetics, and brain reactivity to smoking cues are possible next avenues of research. Determining links between genetics, neuroimaging, and a range of behavioral measures will create a clearer picture of interactions and dissociations between various smoking dependence risk factors. Finally, we do not have smoking cessation outcome measures for these smokers. Further research is needed to determine whether rs16969968 moderates relapse vulnerability and whether treatments targeting nicotine reward or smoking cue-reactivity may be more effective for a particular genotype.
Notwithstanding these limitations, our findings suggest that in nicotine-dependent smokers, G/G smokers may be more reactive to smoking-related cues than smokers with an A allele. Accordingly, it seems plausible to suggest that G/G smokers may benefit to a greater extent than smokers with an A allele from treatment targeted at reducing smoking cue-reactivity. Future research into this and other nAChR genetic polymorphisms may help clarify the role CHRNA5 genetic variation plays in initiation and maintenance of nicotine dependence, which could lead to more personalized treatment options.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amos CI, Spitz MR, Cinciripini P. Chipping away at the genetics of smoking behavior. Nat Genet. 2010;42:366–368. doi: 10.1038/ng0510-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL. Pharmacology of nicotine: addiction and therapeutics. Annu Rev Pharmacol Toxicol. 1996;36:597–613. doi: 10.1146/annurev.pa.36.040196.003121. [DOI] [PubMed] [Google Scholar]
- Bierut LJ. Convergence of genetic findings for nicotine dependence and smoking related diseases with chromosome 15q24–25. Trends Pharmacol Sci. 2010;31:46–51. doi: 10.1016/j.tips.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ, Madden PAF, Breslau N, Johnson EO, Hatsukami D, Pomerleau OF, Swan GE, Rutter J, Bertelsen S, Fox L, Fugman D, Goate AM, Hinrichs AL, Konvicka K, Martin NG, Montgomery GW, Saccone NL, Saccone SF, Wang JC, Chase GA, Rice JP, Ballinger DG. Novel genes identified in a high-density genome wide association study for nicotine dependence. Hum Mol Genet. 2007;16:24–35. doi: 10.1093/hmg/ddl441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, Saccone NL, Saccone SF, Bertelsen S, Fox L, Horton WJ, Breslau N, Budde J, Cloninger CR, Dick DM, Foroud T, Hatsukami D, Hesselbrock V, Johnson EO, Kramer J, Kuperman S, Madden PAF, Mayo K, Nurnberger J, Pomerleau O, Porjesz B, Reyes O, Schuckit M, Swan G, Tischfield JA, Edenberg HJ, Rice JP, Goate AM. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry. 2008;165:1163–1171. doi: 10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, Ho ML, Saxena S, Baxter LR, Madsen D, Jarvik ME. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Craig AD. Once an island, now the focus of attention. Brain Struct Funct. 2010;214:395–396. doi: 10.1007/s00429-010-0270-0. [DOI] [PubMed] [Google Scholar]
- Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. Am J Psychiatry. 2002;159:954–960. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerström KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Fortin NJ, Wright SP, Eichenbaum H. Recollection-like memory retrieval in rats is dependent on the hippocampus. Nature. 2004;431:188–191. doi: 10.1038/nature02853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular α5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471:597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, Ehrman R, Kampman K, O’Brien CP, Detre JA, Childress AR. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology. 2007;32:2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- Gerzanich V, Wang F, Kuryatov A, Lindstrom J. alpha 5 Subunit alters desensitization, pharmacology, Ca++ permeability and Ca++ modulation of human neuronal alpha 3 nicotinic receptors. J Pharmacol Exp Ther. 1998;286:311–320. [PubMed] [Google Scholar]
- Gotti C, Clementi F. Neuronal nicotinic receptors: from structure to pathology. Prog Neurobiol. 2004;74:363–396. doi: 10.1016/j.pneurobio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci. 2006;27:482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Hong LE, Hodgkinson CA, Yang Y, Sampath H, Ross TJ, Buchholz B, Salmeron BJ, Srivastava V, Thaker GK, Goldman D, Stein EA. A genetically modulated, intrinsic cingulate circuit supports human nicotine addiction. Proc Natl Acad Sci USA. 2010;107:13509–13514. doi: 10.1073/pnas.1004745107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Marks MJ, Vann RE, Chen X, Gamage TF, Warner JA, Damaj MI. Role of alpha5 nicotinic acetylcholine receptors in pharmacological and behavioral effects of nicotine in mice. J Pharmacol Exp Ther. 2010;334:137–146. doi: 10.1124/jpet.110.165738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Frederick BD, Richardt S, Burbridge C, Merlo-Pich E, Renshaw PF, Evins AE, Fava M, Kaufman MJ. Brain fMRI reactivity to smoking-related images before and during extended smoking abstinence. Exp Clin Psychopharmacol. 2009;17:365–373. doi: 10.1037/a0017797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, Frederick B, Chuzi S, Pachas G, Culhane MA, Holmes AJ, Fava M, Evins AE, Kaufman MJ. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry. 2010a;67:722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, Frederick B, Holmes AJ, Sousa J, Fava M, Evins AE, Kaufman MJ. Neural substrates of attentional bias for smoking-related cues: an fMRI study. Neuropsychopharmacology. 2010b;35:2339–45. doi: 10.1038/npp.2010.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Yamazaki Y, Nakauchi S, Ito K-I, Sumikawa K. Nicotine facilitates long-term potentiation induction in oriens-lacunosum moleculare cells via Ca2+ entry through non-alpha7 nicotinic acetylcholine receptors. Eur J Neurosci. 2010;31:463–476. doi: 10.1111/j.1460-9568.2009.07058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov A, Onksen J, Lindstrom J. Roles of accessory subunits in α4β2* nicotinic receptors. Mol Pharmacol. 2008;74:132–43. doi: 10.1124/mol.108.046789. [DOI] [PubMed] [Google Scholar]
- Lessov-Schlaggar CN, Pergadia ML, Khroyan TV, Swan GE. Genetics of nicotine dependence and pharmacotherapy. Biochem Pharmacol. 2008;75:178–195. doi: 10.1016/j.bcp.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazure CM, Toll B, McKee SA, Wu R, O’Malley SS. Menstrual cycle phase at quit date and smoking abstinence at 6 weeks in an open label trial of bupropion. Drug Alcohol Depend. 2011;114:68–72. doi: 10.1016/j.drugalcdep.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Rose JE. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI BOLD responses to smoking cues. Neuropsychopharmacology. 2008;33:2148–2157. doi: 10.1038/sj.npp.1301618. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. The MPOWER package. Geneva: 2008. WHO report on the global tobacco epidemic, 2008. [Google Scholar]
- Perkins K. Does smoking cue-induced craving tell us anything important about nicotine dependence? Addiction. 2009;104:1610–6. doi: 10.1111/j.1360-0443.2009.02550.x. [DOI] [PubMed] [Google Scholar]
- Piefke M, Weiss PH, Markowitsch HJ, Fink GR. Gender differences in the functional neuroanatomy of emotional episodic autobiographical memory. Hum Brain Mapp. 2005;24:313–324. doi: 10.1002/hbm.20092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Packard MG. Competition among multiple memory systems: converging evidence from animal and human brain studies. Neuropsychologia. 2003;41:245–251. doi: 10.1016/s0028-3932(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J Neurosci. 2004;24:3554–3562. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Latorre J, Yu CR, Qu X, Perin F, Karlin A, Role L. Functional contributions of alpha5 subunit to neuronal acetylcholine receptor channels. Nature. 1996;380:347–351. doi: 10.1038/380347a0. [DOI] [PubMed] [Google Scholar]
- Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PAF, Breslau N, Johnson EO, Hatsukami D, Pomerleau O, Swan GE, Goate AM, Rutter J, Bertelsen S, Fox L, Fugman D, Martin NG, Montgomery GW, Wang JC, Ballinger DG, Rice JP, Bierut LJ. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet. 2007;16:36–49. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherva R, Wilhelmsen K, Pomerleau CS, Chasse SA, Rice JP, Snedecor SM, Bierut LJ, Neuman RJ, Pomerleau OF. Association of a single nucleotide polymorphism in neuronal acetylcholine receptor subunit alpha 5 (CHRNA5) with smoking status and with ‘pleasurable buzz’ during early experimentation with smoking. Addiction. 2008;103:1544–1552. doi: 10.1111/j.1360-0443.2008.02279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer MAA, Johnson A, Schmitzer-Torbert NC, Redish AD. Triple dissociation of information processing in dorsal striatum, ventral striatum, and hippocampus on a learned spatial decision task. Neuron. 2010;67:25–32. doi: 10.1016/j.neuron.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada E, McKinnon D, Heinemann S, Patrick J, Swanson LW. The distribution of mRNA encoded by a new member of the neuronal nicotinic acetylcholine receptor gene family (alpha 5) in the rat central nervous system. Brain Res. 1990;526:45–53. doi: 10.1016/0006-8993(90)90248-a. [DOI] [PubMed] [Google Scholar]
- Winterer G, Mittelstrass K, Giegling I, Lamina C, Fehr C, Brenner H, Breitling LP, Nitz B, Raum E, Müller H, Gallinat J, Gal A, Heim K, Prokisch H, Meitinger T, Hartmann AM, Möller H-J, Gieger C, Wichmann H-E, Illig T, Dahmen N, Rujescu D. Risk gene variants for nicotine dependence in the CHRNA5-CHRNA3-CHRNB4 cluster are associated with cognitive performance. Am J Med Genet B Neuropsychiatr Genet. 2010;5:1448–58. doi: 10.1002/ajmg.b.31126. [DOI] [PubMed] [Google Scholar]