Summary
Purpose
Cardiac arrhythmias and respiratory disturbances have been proposed as likely causes for sudden unexpected death in epilepsy. Oxygen desaturation occurs in one-third of patients with localization-related epilepsy (LRE) undergoing inpatient video-EEG telemetry (VET) as part of their pre-surgical workup. Ictal-related oxygen desaturation is accompanied by hypercapnia. Both abnormal lengthening and shortening of the corrected QT interval (QTc) on the electrocardiogram (EKG) have been reported with seizures. QTc abnormalities are associated with increased risk of sudden cardiac death. We hypothesized that there may be an association between ictal hypoxemia and cardiac repolarization abnormalities.
Methods
VET data from patients with refractory LRE were analyzed. Consecutive patients having at least one seizure with accompanying oxygen desaturation below 90% and artifact free EKG data were selected. EKG during the one minute prior to seizure onset (PRE) and during the ictal/postictal period with accompanying oxygen desaturation below 90%(DESAT) was analyzed. Consecutive QT and RR intervals were measured. In the same patients, DESAT seizures were compared with seizures without accompanying oxygen desaturation below 90% (NODESAT). For NODESAT seizures, QT and RR intervals for 2 minutes after seizure onset were measured.
Key findings
37 DESAT seizures were analyzed in 17 patients with localization-related epilepsy. A total of 2448 QT and RR intervals were analyzed during PRE. During DESAT, 1554QT and RR intervals were analyzed. 12 of the 17patients had at least one NODESAT seizure. A total of 19 NODESAT seizures were analyzed, including 1558 QT and RR intervals during PRE and 3408 QT and RR intervals during NODESAT. The odds ratio for an abnormally prolonged (>457 msec) QTcH (Hodges correction method) during DESAT relative to PRE was 10.64(p<0.0001). The odds ratio for an abnormally shortened (<372 msec) QTcH during DESAT relative to PRE was 1.65 (p<0.0001). Seizure-related shortening and prolongation of QTc during DESAT were also observed when Fridericia correction of the QT was applied. During DESAT seizures, the mean range of QT values (QTr)(61.14 msec) was significantly different from that during PRE (44.43 msec) (p=0.01). There was a significant association between DESAT QTr and oxygen saturation nadir (p=0.025) and between DESAT QTr and duration of oxygen desaturation (p < 0.0001). Both QTcH prolongation and shortening also occurred with NODESAT seizures. A seizure-associated prolonged QTcH was more likely during DESAT than NODESAT with an odds-ratio of 4.30 (p<0.0001). A seizure-associated shortened QTcH was more likely during DESAT than NODESAT with an odds-ratio of 2.13 (p<0.0001).
Significance
We have shown that the likelihood of abnormal QTcH prolongation is increased 4.3-fold with seizures that are associated with oxygen desaturation when compared with seizures that are not accompanied with oxygen desaturation. The likelihood of abnormally shortened QTcH increases with seizures that are accompanied by oxygen desaturation with an odds-ratio of 2.13 compared with that in seizures without desaturations. There is a significant association between the depth and duration of oxygen desaturation and QTr increase. These findings may be related to the pathophysiology of SUDEP.
Keywords: epilepsy, QT interval, electrocardiogram, SUDEP
INTRODUCTION
Sudden unexpected death in epilepsy (SUDEP) occurs with an incidence as high as 6.3 – 9.5/1000 patient years in patients with refractory epilepsy (Tomson, et al., 2008). Cardiac arrhythmias and respiratory disturbances have been proposed as likely causes (Surges, et al., 2009).
We have previously demonstrated that oxygen desaturation occurs in one-third of patients with localization-related epilepsy (LRE) undergoing inpatient video-EEG telemetry (VET) as part of their pre-surgical workup (Bateman, et al., 2008). Ictal-related oxygen desaturation is accompanied by hypercapnia (Seyal, et al., 2010) and in a subset of patients seizures may be accompanied by severe oxygen desaturation (< 60%) and marked rise in end-tidal CO2 (>70 mm Hg)(Seyal, et al., 2010).
There is lengthening of the corrected QT interval (QTc) of the electrocardiogram (EKG) during epileptic seizures (Brotherstone, et al., 2010, Kandler, et al., 2005). QTc prolongation is associated with torsade de pointes and sudden cardiac death whether the QTc prolongation is genetically determined or acquired (Elming, et al., 2002, Kannankeril & Roden, 2007, Morita, et al., 2008, Pater, 2005, Schouten, et al., 1991). Drug-induced torsade de pointes is associated with QTc prolongation of at least 60 msec (Fenichel, et al., 2004). Abnormal shortening of the QTc occurs postictally and is most commonly present with secondarily generalized convulsive seizures (Surges, et al., 2010b). Genetically determined QTc shortening may increase the risk of ventricular tachycardia and cardiac sudden death (Schimpf, et al., 2008). QTc shortening may be acquired and some antiepileptic drugs have been implicated in QTc shortening (Cheng-Hakimian, et al., 2006, DeSilvey & Moss, 1980). There is lengthening of the corrected QT interval (QTc) of the electrocardiogram (EKG) during epileptic seizures in children and adults who have died of SUDEP (Brotherstone, et al., 2010, Kandler, et al., 2005, Surges, et al. 2010a).
Both hypoxemia and hypercapnia have been associated with QT prolongation (Kiely, etal., 1996, Roche, et al., 2003). We hypothesized that there may be an association between ictal hypoxemia and cardiac repolarization abnormalities. This study was done to determine whether or not a linkage exists between ictal hypoxemia and alterations in cardiac repolarization.
METHODS
In the present study, details of the methodology for acquiring VET data in patients with medically refractory LRE, including EKG and oxygen saturation, were the same as published previously (Bateman, et al., 2008, Seyal, et al., 2010). Single channel EKG data was used for EKG analysis. Prior approval for the study was obtained from the local institutional review board. Consecutive patients with at least one seizure that had an accompanying oxygen desaturation below 90% and artifact free EKG data were selected. Patients were required to have segments of artifact free EKG during the one minute prior to seizure onset (PRE) and during the ictal/postictal period with accompanying oxygen desaturation below 90%. Seizures with accompanying oxygen desaturation below 90% (DESAT) were compared with control seizures without accompanying oxygen desaturation below 90% (NODESAT) in the same patients. For DESAT seizures, consecutive QT and RR intervals were measured during the ictal/post ictal period of oxygen desaturation below 90%. For NODESAT seizures, QT and RR intervals for 2 minutes after seizure onset were measured. A two minute period of analysis for NODESAT was chosen to include approximately equivalent periods after seizure onset for DESAT and NODESAT seizures as hypoxemia onset and nadir are delayed after seizure start (Bateman, et al., 2008). If NODESAT duration extended beyond 2 minutes, measurements were continued to the end of the seizure. For each seizure, consecutive QT and RR intervals during PRE were measured for one minute, starting one minute before seizure onset. QT and RR intervals obscured by electromyographic activity or movement artifact were excluded. The QT was measured from the start of the QRS complex to the intersection of the downward limb of the T-wave with the isoelectric line. The U-wave, if present, was excluded from the measurement. QT and RR intervals were measured using manually placed electronic cursors on the computer screen. Such measurements have a precision of ± 5 msec (Fenichel, et al., 2004). EKG measurements were not blinded or masked from the EEG data.
Primary QTc calculations were performed using the Hodges method (QTcH = QT + 105 (1/RR – 1)) (Luo, et al., 2004). Of the several QT correction formulae available, the Hodges correction is recommended as the default method and has the least heart rate dependence of QT correction formulae (Chiladakis, et al., 2010, Luo, et al., 2004). We used 457 msec as the upper normal limit for QTcH and 372 msec as the lower normal limit using published combined male and female values for all heart rates (Luo, et al., 2004).
Additional QTc calculations were done using the Fridericia correction method, a commonly used nonlinear formula for QT correction (QTcF=QT (RR)−1/3). QTcF upper and lower limits of normal of 460 and 365 msec, respectively, were used (Luo et al., 2004) to assess odds ratios for abnormally prolonged and shortened QTcF between DESAT, NODESAT and their corresponding PRE.
In order to assess the range of QT values (QTr) during PRE, DESAT and NODESAT, we computed the difference between the longest and shortest QT interval for the preictal period and similarly for the ictal/postictal period.
The data are presented as mean ± standard deviation (median, range). The two-sided Wilcoxon rank-sum test was used to compare QTcH and QTcF between DESAT and the corresponding PRE and between NODESAT and the corresponding PRE. The two-sided Wilcoxon signed-rank test was used to compare heartrate and QTr between DESAT and the corresponding PRE and between NODESAT and the corresponding PRE. The chi-squared exact test was used to compare the proportion of abnormally prolonged QTcH and QTcF values (QTcH > 457 msec, QTcF >460) and the proportion of abnormally shortened QTcH and QTcF values (QTcH < 372 msec, QTcF < 365) between DESAT and the corresponding PRE, between NODESAT and the corresponding PRE, and between the DESAT and NODESAT. Outliers in data were detected, so robust linear regression was used to study the association between the DESAT QTr and oxygen saturation nadir, between the DESAT QTr and duration of desaturation between the DESAT QTr and duration of the seizure and between DESAT and NODESAT QTcH values and duration of the seizure. All analyses were performed with SAS (Version 9.2) software (SAS Institute, Cary NC). A two-sided p-value < 0.05 was considered statistically significant.
RESULTS
37 DESAT seizures were analyzed in 17 patients with localization-related epilepsy. A total of 2448 QT and RR intervals were analyzed during PRE. In the ictal/post-ictal period, 1554QT and RR intervals were analyzed during the time when oxygen desaturation was below 90%.
12 of the 17 (70.6%) patients had at least one NODESAT seizure. In these 12 patients, 19 seizures with adequate EKG data were available for analysis. For these 19 seizures, 1558 QT and RR intervals were analyzed during PRE and 3408 QT and RR intervals during the 2 minutes or longer following seizure onset.
The mean age of all patients was 35.47 ± 12.93 years (35, 16–59). For the 37 DESAT seizures, 25 (67.6%) seizures were of right temporal lobe onset, 5 (13.5%) were left temporal seizures, 1 (2.7%) was of near simultaneous bitemporal onset, 4 (10.8%) were of frontal onset, and the onset could not be determined in the remaining 2 (5.4%) seizures. Of these seizures, 6 (16.2%) evolved to generalized convulsions, 22 (59.5%) were complex partial seizures, 1 (2.7%) was a simple partial seizure, and 5 (13.5%) were electrographic seizures without clear clinical correlates. Adequate testing to determine seizure type was not performed in the remaining three (8.1%). For the 19 NODESAT seizures, 9 (47.3%) seizures were of right temporal onset, 5 (26.3%) seizures were of left temporal onset, 4(21.1%) seizures were of frontal onset, and in one (5.3%) the onset could not be determined. Of these seizures, 2 (10.5%) progressed to generalized convulsions, 4 (21.1%) were complex partial seizures, one (5.3%) was a simple partial seizure, and adequate testing was not performed in the remaining seizures.
The mean oxygen saturation nadir during DESAT was 78.78 ± 9.56% (82, 53–89). The mean duration of oxygen desaturation below 90% was 78.76 ± 74.65 sec (43, 6–254). The mean PRE heart rate was 82.14 ± 14.17 beats per minute (78, 60–114). The peak DESAT heart rate was 119.33 ±20.07 beats per minute (117, 84–168). The DESAT peak heart rate was significantly different from the mean PRE heart rate (p<0.0001)(Table 1).
Table 1.
| Seizure-related QTcH change (Hodges correction method) and QTr | ||||
|---|---|---|---|---|
| DESATSEIZURES | ||||
| PRE | DESAT | Odds-ratio for seizure QTcH versus PRE | p-value | |
| Mean heart rate (beats per minute) | 82.14 | 119.33 | <0.0001* | |
| Prolonged QTcH intervals (%) | 0.3 | 3.0 | 10.64 | <0.0001** |
| Shortened QTcH intervals (%) | 20.1 | 29.3 | 1.65 | <0.0001** |
| Mean QTcH (msec) | 389.48 | 393.08 | 0.003*** | |
| QTr (msec) | 44.43 | 61.14 | 0.01* | |
| NODESAT SEIZURES | ||||
| PRE | NODESAT | Odds-ratio for seizure QTcH versus PRE | p-value | |
| Mean heart rate (beats per minute) | 91.67 | 113.17 | <0.001* | |
| Prolonged QTcH intervals (%) | 0.06 | 0.7 | 11.04 | 0.004** |
| Shortened QTcH (%) | 21.3 | 16.3 | 0.72 | <0.0001** |
| Mean QTcH (msec) | 388.32 | 397.06 | <0.0001*** | |
| QTr (msec) | 59.05 | 91.1 | 0.002* | |
Two-sided Wilcoxon signed-rank test;
chi-squared exact test;
two-sided Wilcoxon rank-sum test
For NODESAT, the mean PRE heart rate was91.67 ±26.66 beats per minute (84, 54–144). The peak ictal heart rate during NODESAT was 113.17 ±26.43 beats per minute (111, 60–162). The peak ictal heart rate was significantly different from the PRE heart rate (p<0.001) (Table 1).
QTcH and QTr changes with DESAT seizures
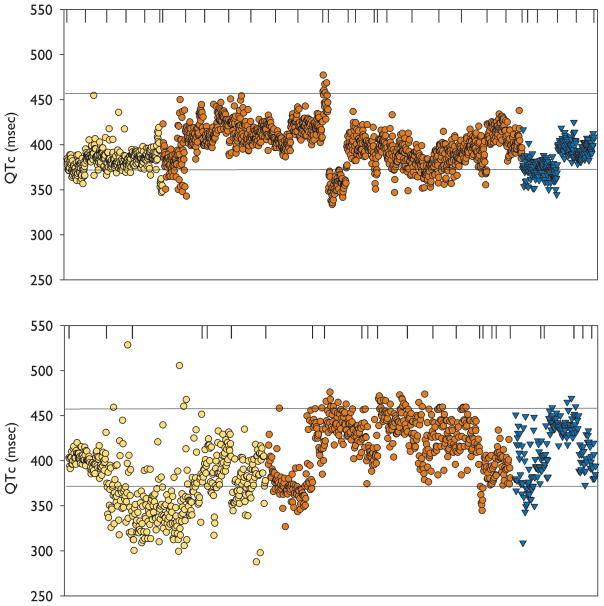
The results are summarized in Table 1. Forty six of 1554 (3%) QTcH values in the DESAT period were abnormally prolonged (QTcH > 457 msec). Seven of 2448 (0.3%) QTcH values in the corresponding PRE periods were abnormally prolonged (Figure 1). The odds ratio for an abnormally prolonged QTcH during DESAT relative to PRE was 10.64(95% confidence interval 4.75–27.98, p<0.0001). 456 of 1554 (29.3%) QTcH values during DESAT were abnormally shortened (QTcH < 372 msec). 492 of 2448 (20.1%) QTcH values during PRE were abnormally shortened (Figure 1). The odds ratio for an abnormally shortened QTcH during DESAT relative to PRE was 1.65(95% confidence interval 1.42–1.92, p<0.0001).
Figure 1.
Individual QTcH intervals for all DESAT seizures during PRE (Top) and during DESAT (Bottom). The upper and lower horizontal lines represent the limits of normal QTcH values computed by Hodges method. The vertical lines at the top of the figures separate QTcH values from individual seizures. The ordinate shows QTcH values (msec). Individual QTcH values from secondary generalized seizures (yellow circles), right temporal partial seizures (dark red circles) and left temporal partial seizures(triangles)are shown.
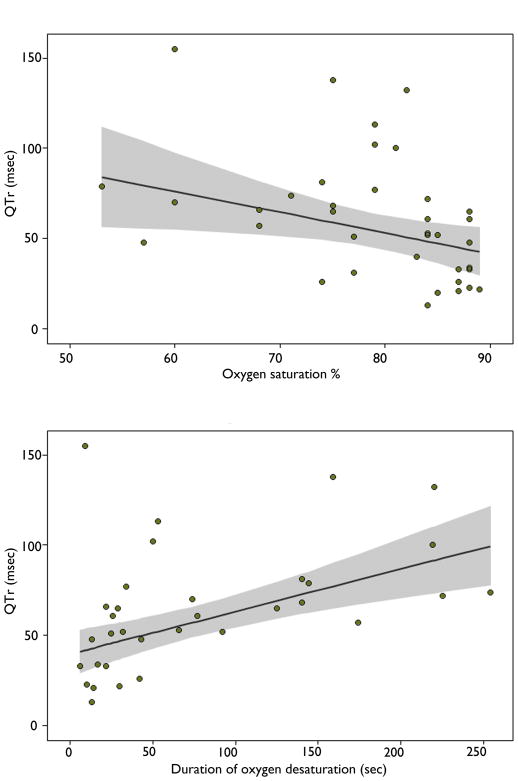
The mean QTcH duration during DESAT was 393.08 ± 36.94msec (392.77, 287.72–528.43). The mean QTcH during PRE was 389.48 ± 25.3 msec (390.32, 272.2–490.5). The difference was significant (p=0.003). The mean QTr during PRE was 44.43 ± 18msec (40, 15–95). The mean QTr during DESAT was 61.14 ±34.35 msec (57, 13–155). The difference in DESAT and PRE QTr was significant (p=0.01). There was a significant association between DESAT QTr and oxygen saturation nadir (p=0.025) and between DESAT QTr and duration of desaturation (p< 0.0001)(Figure 2). There was no significant association between DESAT QTr and duration of the seizure (p=0.079).
Figure 2.
Figure 2 (Top). Plot of QTr values during DESAT (ordinate) versus oxygen desaturation nadir (abscissa). The regression line is plotted and 95% confidence intervals are shaded.
Figure 2 (Bottom). Plot of QTr values during DESAT (ordinate) versus duration of oxygen desaturation (abscissa).
QTcH and QTr changes with NODESAT seizures
The results are summarized in Table 1. The mean PRE QTcH was 388.32± 20.27msec (386.86, 309.17–462.57). The mean QTcH during NODESAT was 397.06 ±24.5 msec (396.43, 315.16–477.84). The difference in QTcH between PRE and during NODESAT was significant (p<0.0001). 24 of 3408 QTcH (0.7%) intervals during NODESAT were prolonged beyond 457 msec. One of 1558 QTcH (0.06%) intervals was prolonged in PRE (Table 1). The odds ratio for an abnormally prolonged QTcH during NODESAT relative to PRE was 11.04(95% confidence interval 1.80–454.39, p=0.004). The mean PRE QTr was 59.05 ± 20.91msec (57, 32–110). The mean QTr during NODESAT was 91.11±29.87msec (89, 40–149). The difference in NODESAT and PRE QTr was significant (p=0.002). 557 of 3408 (16.3%) QTcH intervals were abnormally shortened during NODESAT and 331 of 1558 (21.3%) QTcH intervals were abnormally shortened in the corresponding PRE. The odds ratio for an abnormally shortened QTcH during NODESAT relative to PRE was 0.72(95% confidence interval 0.62–0.85, p<0.0001).
QTcH comparisons of DESAT and NODESAT seizures
A seizure-associated prolonged QTcH (QTcH>457 msec) was more likely during DESAT than NODESAT with an odds-ratio of 4.30 (95% confidence interval 2.56–7.39, p<0.0001). A seizure-associated shortened QTcH (QTcH <372 msec) was more likely during DESAT than NODESAT with an odds-ratio of 2.13(95% confidence interval 1.84–2.46, p<0.0001).
QTcH changes with generalized and non-generalized seizures
During DESAT the mean ictal QTcH values were 378.91 ± 29.03 msec (373.88, 287.72 – 528.43) and 396.33 ± 37.79 msec (397.90, 295.98–505.59), respectively, in the secondarily generalized seizure and non-generalized seizure groups. The difference in ictal QTcH value between the generalized seizure and non-generalized seizure groups was significant (p < 0.0001).
QTcH changes and seizure duration
The mean seizure duration during DESAT was 75.33 ± 34.32 sec (71, 12–204). For NODESAT, the mean seizure duration was 91.72 ± 101.24 sec (49, 10–360). There was no difference in seizure duration for DESAT and NODESAT seizures (p=0.340). For DESAT seizures alone, there was no statistically significant association between QTcH and seizure duration (p=0.208). For NODESAT seizures alone, there was no statistically significant association between QTcH and seizure duration (p=0.335). For DESAT and NODESAT seizures combined, there was no statistically significant association between QTcH and seizure duration (p=0.748).
Seizure-related QT changes applying Fridericia correction
The results are summarized in Table 2. For DESAT seizures, the results using QTcF were similar to those found using QTcH. There was a significantly increased odds ratio for abnormally prolonged as well as abnormally shortened QTcF during DESAT relative to PRE. For NODESAT seizures, the odds ratio for prolonged QTcF was not significantly different from PRE (p=0.0757), and the odds ratio for abnormally shortened QTcF was significantly higher during the seizure relative to PRE.
Table 2.
| Seizure-related QTcF changes (Fridericia correction formula) | ||||
|---|---|---|---|---|
| DESAT SEIZURES | ||||
| PRE | DESAT | Odds-ratio for DESAT QTcF versus PRE | p-value | |
| Prolonged QTcF intervals (%) | 0.12 | 0.71 | 5.81 | 0.003** |
| Shortened QTcF intervals (%) | 20.63 | 50.26 | 3.89 | <0.0001** |
| Mean QTcF (msec) | 385.5 | 369.4 | <0.0001*** | |
| NODESAT SEIZURES | ||||
| PRE | NODESAT | Odds ratio for seizure QTcF versus PRE | p-value | |
| Prolonged QTcF intervals (%) | 0.06 | 0.35 | 5.5 | 0.0757** |
| Shortened QTcF intervals (%) | 43.13 | 51.35 | 1.39 | <0.0001** |
| Mean QTcF (msec) | 370.85 | 367.27 | <0.001*** | |
Two-sided Wilcoxon signed-rank test;
chi-squared exact test;
two-sided Wilcoxon rank-sum test
DISCUSSION
We have shown that individual QTc intervals are both abnormally prolonged and abnormally shortened with seizures of partial onset. Our data indicate that seizure-related respiratory dysfunction is associated with prolongation of QTc. Other factors contributing to seizure-related increase in QTc cannot be excluded as QTc increase also occurred with seizures that were not accompanied by a drop in oxygen saturation below90%but with a lower likelihood than in seizures with accompanying hypoxemia. We did not have sufficient data for sub analysis of possible differential effects of age and gender on the QTc.
Seizures are associated with enhanced shortening of the QTc (Surges, et al.,2010b). We have now shown that the likelihood of abnormally shortened QTc intervals increases with seizures that had associated oxygen desaturation. Other seizure-associated factors may also contribute to QTc changes. Increases in circulating catecholamines result in QT shortening (Arrowood, et al., 1993). Prolonged rather than brief adrenergic stimulation appears necessary for QT shortening (Arrowood, et al., 1993). Other metabolic derangements including hyperkalemia, hypercalcemia, hyperthermia and acidosis also contribute to short QT intervals (Lu, et al., 2008). With more than 20% of seizures, QT shortening was present pre-ictally suggesting the possibility that this preictal shortening reflects a persistent effect on the myocardium of recurrent seizures in the epilepsy monitoring unit. Nevertheless, the likelihood of QT shortening increased during DESAT relative to the preictal period. Susceptibility to malignant ventricular tachyarrhythmias in short QT syndromeis probably related to the large dispersion of ventricular refractoriness caused by inhomogeneous abbreviation of the action potential within the ventricle and within different layers of the ventricular wall (Borggrefe, et al., 2005, Gaita, et al., 2003).
Increased QT variability is associated with increased mortality in patients with heart failure and with increased risk of ventricular tachycardia and ventricular fibrillation (Dobson, et al., 2011, Haigney, et al., 2004). These studies analyzed temporal variability of repolarization from single channel EKG data. Although we did not directly assess QT beat to beat variability, we have shown that the range of QT intervals increases with seizures that have accompanying oxygen desaturation. The seizure-associated increased range of QT intervals may be related to increased QT variability.
Seizure-related abnormal shortening and prolongation of QTc are more likely with seizures that result in oxygen desaturation. Increases in QTr are associated with the depth and duration of hypoxemia. These findings suggest that seizure-associated respiratory dysfunction may be a factor in cardiac repolarization abnormalities. Our study does not exclude other possible ictal-related mechanisms causing cardiac dysfunction and, in fact, the preictal QTc shortening suggests a persistent effect of seizures on the myocardium. Seizure-related prolongation and shortening of QT interval as well as persistent interictal QT abnormalities have also been shown in people with epilepsy who have died suddenly (Surges, et al., 2010a). According to the aforementioned study, abnormalities of cardiac repolarization do not seem to reliably distinguish those who have a higher risk of SUDEP from those who have not. However, our findings may be related to the pathophysiology of SUDEP in a subset of patients and contribute to the understanding of underlying cardio-respiratory interactions.
Acknowledgments
All co-authors have been substantively involved in the study and/or the preparation of the manuscript; b) no undisclosed groups or persons have had a primary role in the study and/or in manuscript preparation (i.e., there are no “ghost-writers”); and c) all co-authors have seen and approved the submitted version of the paper and accept responsibility for its content.
Statistical support for this publication was made possible by Grant Number UL1 RR024146from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
Footnotes
Statement on ethical publication
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosure of Conflicts of Interest
The authors report no conflicts of interest.
References
- Arrowood JA, Kline J, Simpson PM, Quigg RJ, Pippin JJ, Nixon JV, Mohanty PK. Modulation of the QT interval: effects of graded exercise and reflex cardiovascular stimulation. J Appl Physiol. 1993;75:2217–2223. doi: 10.1152/jappl.1993.75.5.2217. [DOI] [PubMed] [Google Scholar]
- Bateman LM, Li CS, Seyal M. Ictal hypoxemia in localization-related epilepsy: analysis of incidence, severity and risk factors. Brain. 2008;131:3239–3245. doi: 10.1093/brain/awn277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borggrefe M, Wolpert C, Antzelevitch C, Veltmann C, Giustetto C, Gaita F, Schimpf R. Short QT syndrome. Genotype-phenotype correlations. J Electrocardiol. 2005;38:75–80. doi: 10.1016/j.jelectrocard.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotherstone R, Blackhall B, McLellan A. Lengthening of corrected QT during epileptic seizures. Epilepsia. 2010;51:221–232. doi: 10.1111/j.1528-1167.2009.02281.x. [DOI] [PubMed] [Google Scholar]
- Cheng-Hakimian A, Anderson GD, Miller JW. Rufinamide: Pharmacology, clinical trials, and role in clinical practice. Int J Clin Pract. 2006;60:1497–1501. doi: 10.1111/j.1742-1241.2006.01173.x. [DOI] [PubMed] [Google Scholar]
- Chiladakis J, Kalogeropoulos A, Arvanitis P, Koutsogiannis N, Zagli F, Alexopoulos D. Heart rate-dependence of QTc intervals assessed by different correction methods in patients with normal or prolonged repolarization. Pacing Clin Electrophysiol. 2010;33:553–560. doi: 10.1111/j.1540-8159.2009.02657.x. [DOI] [PubMed] [Google Scholar]
- DeSilvey DL, Moss AJ. Primidone in the treatment of the long QT syndrome: QT shortening and ventricular arrhythmia suppression. Ann Intern Med. 1980;93:53–54. doi: 10.7326/0003-4819-93-1-53. [DOI] [PubMed] [Google Scholar]
- Dobson CP, La Rovere MT, Pinna GD, Goldstein R, Olsen C, Bernardinangeli M, Veniani M, Midi P, Tavazzi L, Haigney M. QT Variability Index on 24-Hour Holter Independently Predicts Mortality in Patients with Heart Failure: Analysis of GISSI-HF Trial Data. Heart Rhythm. 2011 doi: 10.1016/j.hrthm.2011.03.055. [DOI] [PubMed] [Google Scholar]
- Elming H, Brendorp B, Kober L, Sahebzadah N, Torp-Petersen C. QTc interval in the assessment of cardiac risk. Card Electrophysiol Rev. 2002;6:289–294. doi: 10.1023/a:1016345412555. [DOI] [PubMed] [Google Scholar]
- Fenichel RR, Malik M, Antzelevitch C, Sanguinetti M, Roden DM, Priori SG, Ruskin JN, Lipicky RJ, Cantilena LR. Drug-induced torsades de pointes and implications for drug development. J Cardiovasc Electrophysiol. 2004;15:475–495. doi: 10.1046/j.1540-8167.2004.03534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaita F, Giustetto C, Bianchi F, Wolpert C, Schimpf R, Riccardi R, Grossi S, Richiardi E, Borggrefe M. Short QT Syndrome: a familial cause of sudden death. Circulation. 2003;108:965–970. doi: 10.1161/01.CIR.0000085071.28695.C4. [DOI] [PubMed] [Google Scholar]
- Haigney MC, Zareba W, Gentlesk PJ, Goldstein RE, Illovsky M, McNitt S, Andrews ML, Moss AJ. QT interval variability and spontaneous ventricular tachycardia or fibrillation in the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II patients. J Am Coll Cardiol. 2004;44:1481–1487. doi: 10.1016/j.jacc.2004.06.063. [DOI] [PubMed] [Google Scholar]
- Kandler L, Fiedler A, Scheer K, Wild F, Frick U, Schneider P. Early post-convulsive prolongation of QT time in children. Acta Paediatr. 2005;94:1243–1247. doi: 10.1111/j.1651-2227.2005.tb02083.x. [DOI] [PubMed] [Google Scholar]
- Kannankeril PJ, Roden DM. Drug-induced long QT and torsade de pointes: recent advances. Curr Opin Cardiol. 2007;22:39–43. doi: 10.1097/HCO.0b013e32801129eb. [DOI] [PubMed] [Google Scholar]
- Kiely DG, Cargill RI, Lipworth BJ. Effects of hypercapnia on hemodynamic, inotropic, lusitropic, and electrophysiologic indices in humans. Chest. 1996;109:1215–1221. doi: 10.1378/chest.109.5.1215. [DOI] [PubMed] [Google Scholar]
- Lu HR, Vlaminckx E, Hermans AN, Rohrbacher J, Van Ammel K, Towart R, Pugsley M, Gallacher DJ. Predicting drug-induced changes in QT interval and arrhythmias: QT-shortening drugs point to gaps in the ICHS7B Guidelines. Br J Pharmacol. 2008;154:1427–1438. doi: 10.1038/bjp.2008.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Michler K, Johnston P, Macfarlane PW. A comparison of commonly used QT correction formulae: the effect of heart rate on the QTc of normal ECGs. J Electrocardiol. 2004;37(Suppl):81–90. doi: 10.1016/j.jelectrocard.2004.08.030. [DOI] [PubMed] [Google Scholar]
- Morita H, Wu J, Zipes DP. The QT syndromes: long and short. Lancet. 2008;372:750–763. doi: 10.1016/S0140-6736(08)61307-0. [DOI] [PubMed] [Google Scholar]
- Pater C. Methodological considerations in the design of trials for safety assessment of new drugs and chemical entities. Curr Control Trials Cardiovasc Med. 2005;6:1. doi: 10.1186/1468-6708-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche F, Reynaud C, Pichot V, Duverney D, Costes F, Garet M, Gaspoz JM, Barthelemy JC. Effect of acute hypoxia on QT rate dependence and corrected QT interval in healthy subjects. Am J Cardiol. 2003;91:916–919. doi: 10.1016/s0002-9149(03)00040-7. [DOI] [PubMed] [Google Scholar]
- Schimpf R, Borggrefe M, Wolpert C. Clinical and molecular genetics of the short QT syndrome. Curr Opin Cardiol. 2008;23:192–198. doi: 10.1097/HCO.0b013e3282fbf756. [DOI] [PubMed] [Google Scholar]
- Schouten EG, Dekker JM, Meppelink P, Kok FJ, Vandenbroucke JP, Pool J. QT interval prolongation predicts cardiovascular mortality in an apparently healthy population. Circulation. 1991;84:1516–1523. doi: 10.1161/01.cir.84.4.1516. [DOI] [PubMed] [Google Scholar]
- Seyal M, Bateman LM, Albertson TE, Lin TC, Li CS. Respiratory changes with seizures in localization-related epilepsy: analysis of periictal hypercapnia and airflow patterns. Epilepsia. 2010;51:1359–1364. doi: 10.1111/j.1528-1167.2009.02518.x. [DOI] [PubMed] [Google Scholar]
- Surges R, Adjei P, Kallis C, Erhuero J, Scott CA, Bell GS, Sander JW, Walker MC. Pathologic cardiac repolarization in pharmacoresistant epilepsy and its potential role in sudden unexpected death in epilepsy: a case-control study. Epilepsia. 2010a;51:233–242. doi: 10.1111/j.1528-1167.2009.02330.x. [DOI] [PubMed] [Google Scholar]
- Surges R, Scott CA, Walker MC. Enhanced QT shortening and persistent tachycardia after generalized seizures. Neurology. 2010b;74:421–426. doi: 10.1212/WNL.0b013e3181ccc706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surges R, Thijs RD, Tan HL, Sander JW. Sudden unexpected death in epilepsy: risk factors and potential pathomechanisms. Nat Rev Neurol. 2009 doi: 10.1038/nrneurol.2009.118. [DOI] [PubMed] [Google Scholar]
- Tomson T, Nashef L, Ryvlin P. Sudden unexpected death in epilepsy: current knowledge and future directions. Lancet Neurol. 2008;7:1021–1031. doi: 10.1016/S1474-4422(08)70202-3. [DOI] [PubMed] [Google Scholar]