Abstract
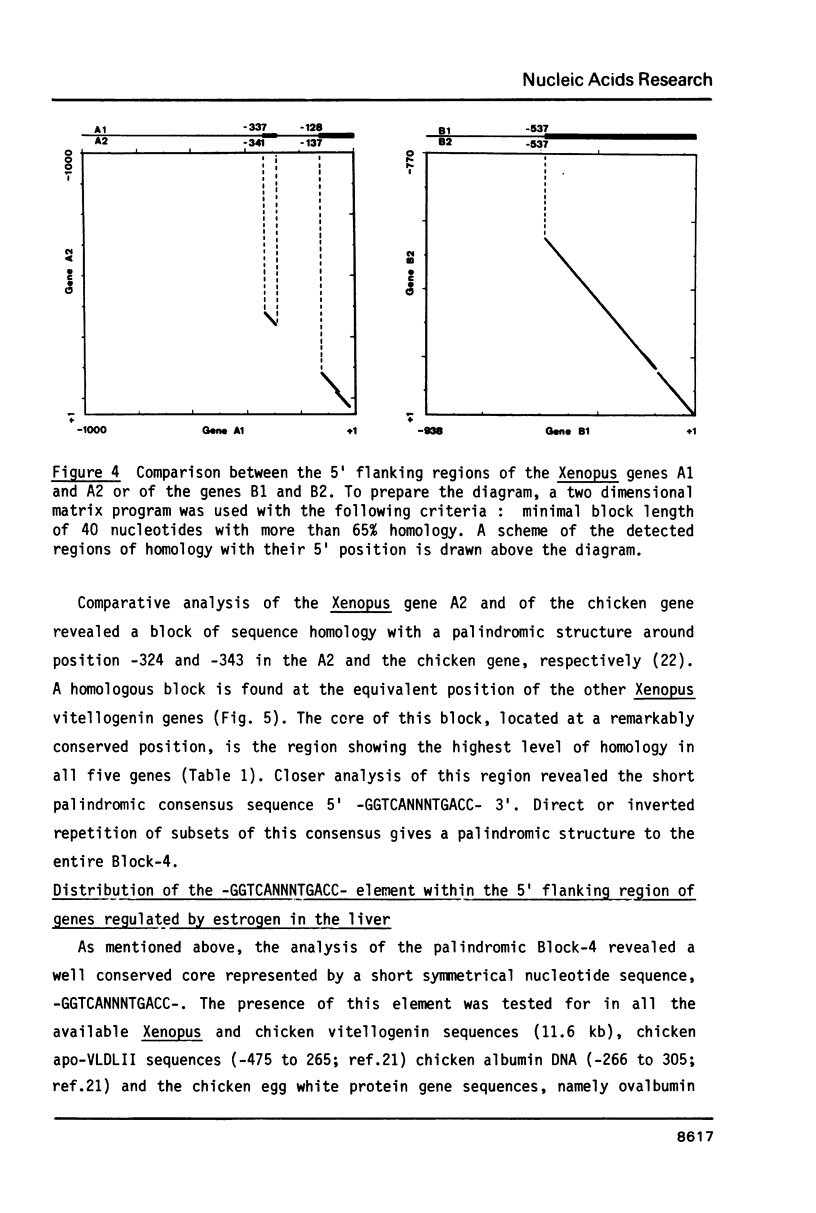
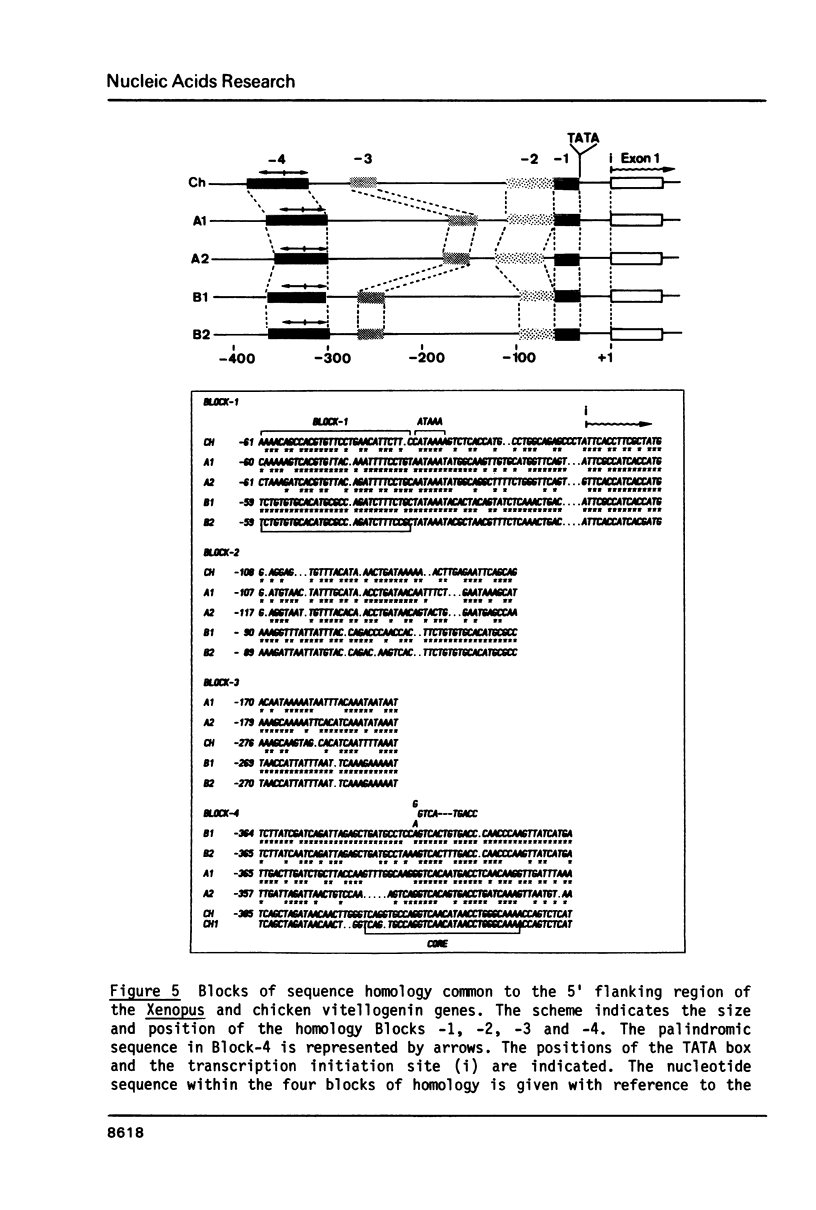
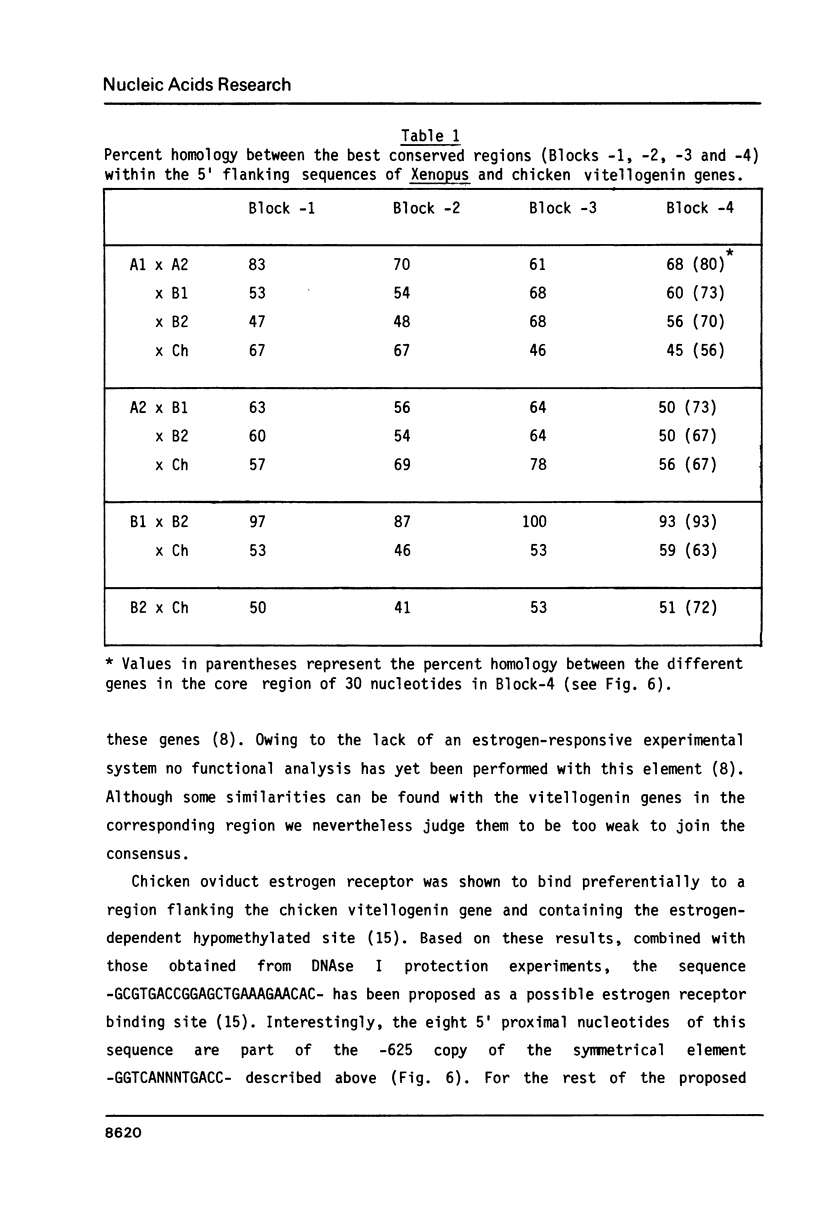
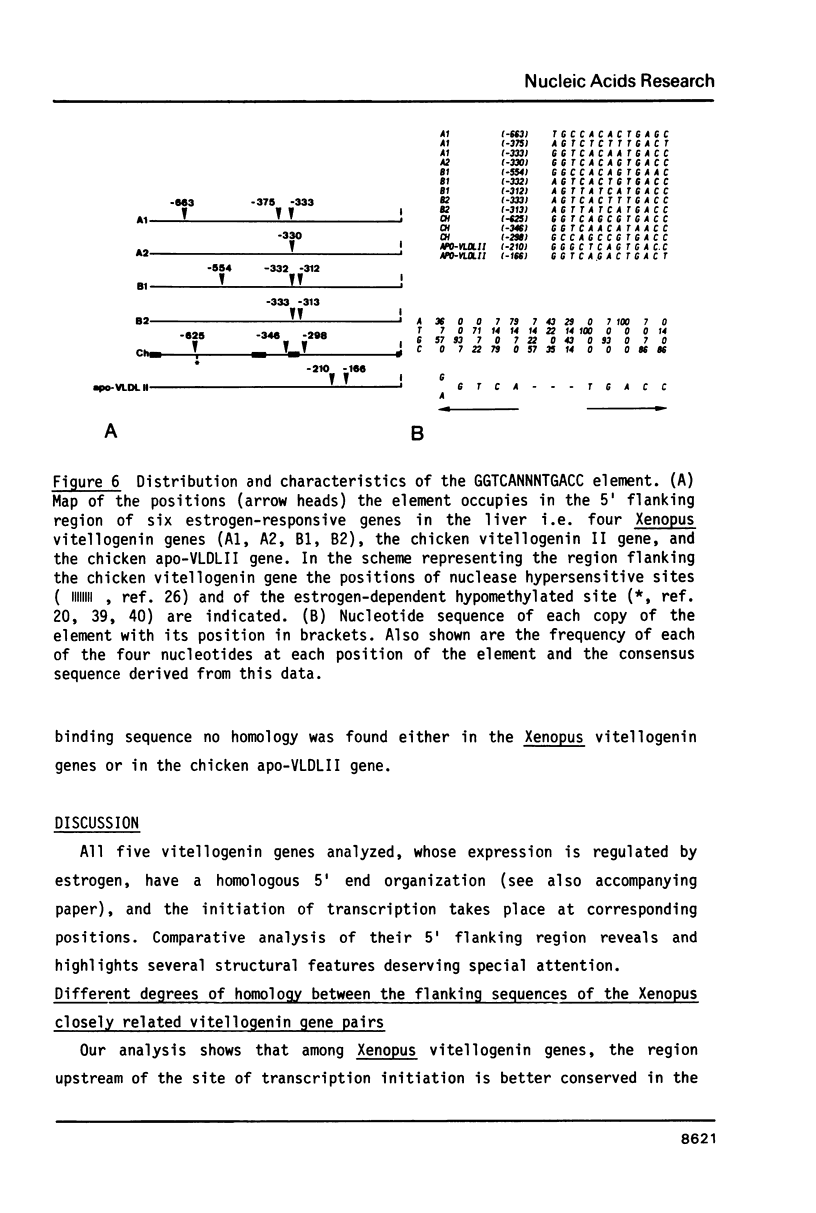
In the liver of oviparous vertebrates vitellogenin gene expression is controlled by estrogen. The nucleotide sequence of the 5' flanking region of the Xenopus laevis vitellogenin genes A1, A2, B1 and B2 has been determined. These sequences have been compared to each other and to the equivalent region of the chicken vitellogenin II and apo-VLDLII genes which are also expressed in the liver in response to estrogen. The homology between the 5' flanking region of the Xenopus genes B1 and B2 is higher than between the corresponding regions of the other closely related genes A1 and A2. Four short blocks of sequence homology which are present at equivalent positions in the vitellogenin genes of both Xenopus laevis and chicken are characterized. A short sequence with two-fold rotational symmetry (GGTCANNNTGACC) was found at similar positions upstream of the five vitellogenin genes and is also present in two copies close to the 5' end of the chicken apo-VLDLII gene. The possible functional significance of this sequence, common to liver estrogen-responsive genes, is discussed.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnberg A. C., Meijlink F. C., Mulder J., van Bruggen E. F., Gruber M., Geert A. B. Isolation and characterization of genomic clones covering the chicken vitellogenin gene. Nucleic Acids Res. 1981 Jul 24;9(14):3271–3286. doi: 10.1093/nar/9.14.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes W. M., Bevan M. Kilo-sequencing: an ordered strategy for rapid DNA sequence data acquisition. Nucleic Acids Res. 1983 Jan 25;11(2):349–368. doi: 10.1093/nar/11.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoist C., O'Hare K., Breathnach R., Chambon P. The ovalbumin gene-sequence of putative control regions. Nucleic Acids Res. 1980 Jan 11;8(1):127–142. doi: 10.1093/nar/8.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisbee C. A., Baker M. A., Wilson A. C., Haji-Azimi I., Fischberg M. Albumin phylogeny for clawed frogs (Xenopus). Science. 1977 Feb 25;195(4280):785–787. doi: 10.1126/science.65013. [DOI] [PubMed] [Google Scholar]
- Borgmeyer U., Nowock J., Sippel A. E. The TGGCA-binding protein: a eukaryotic nuclear protein recognizing a symmetrical sequence on double-stranded linear DNA. Nucleic Acids Res. 1984 May 25;12(10):4295–4311. doi: 10.1093/nar/12.10.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock M. L., Shapiro D. J. Estrogen regulates the absolute rate of transcription of the Xenopus laevis vitellogenin genes. J Biol Chem. 1983 May 10;258(9):5449–5455. [PubMed] [Google Scholar]
- Buetti E., Diggelmann H. Glucocorticoid regulation of mouse mammary tumor virus: identification of a short essential DNA region. EMBO J. 1983;2(8):1423–1429. doi: 10.1002/j.1460-2075.1983.tb01601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch J. B. Identification and sequence analysis of the 5' end of the major chicken vitellogenin gene. Nucleic Acids Res. 1984 Jan 25;12(2):1117–1135. doi: 10.1093/nar/12.2.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch J. B., Weintraub H. Temporal order of chromatin structural changes associated with activation of the major chicken vitellogenin gene. Cell. 1983 May;33(1):65–76. doi: 10.1016/0092-8674(83)90335-5. [DOI] [PubMed] [Google Scholar]
- Cochet M., Gannon F., Hen R., Maroteaux L., Perrin F., Chambon P. Organization and sequence studies of the 17-piece chicken conalbumin gene. Nature. 1979 Dec 6;282(5739):567–574. doi: 10.1038/282567a0. [DOI] [PubMed] [Google Scholar]
- Compton J. G., Schrader W. T., O'Malley B. W. DNA sequence preference of the progesterone receptor. Proc Natl Acad Sci U S A. 1983 Jan;80(1):16–20. doi: 10.1073/pnas.80.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. C., Knoll B. J., Riser M. E., O'Malley B. W. A 5'-flanking sequence essential for progesterone regulation of an ovalbumin fusion gene. Nature. 1983 Oct 6;305(5934):551–554. doi: 10.1038/305551a0. [DOI] [PubMed] [Google Scholar]
- Geiser M., Mattaj I. W., Wilks A. F., Seldran M., Jost J. P. Structure and sequence of the promoter area and of a 5' upstream demethylation site of the estrogen-regulated chicken vitellogenin ii gene. J Biol Chem. 1983 Jul 25;258(14):9024–9030. [PubMed] [Google Scholar]
- Geisse S., Scheidereit C., Westphal H. M., Hynes N. E., Groner B., Beato M. Glucocorticoid receptors recognize DNA sequences in and around murine mammary tumour virus DNA. EMBO J. 1982;1(12):1613–1619. doi: 10.1002/j.1460-2075.1982.tb01363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber-Huber S., May F. E., Westley B. R., Felber B. K., Hosbach H. A., Andres A. C., Ryffel G. U. In contrast to other Xenopus genes the estrogen-inducible vitellogenin genes are expressed when totally methylated. Cell. 1983 May;33(1):43–51. doi: 10.1016/0092-8674(83)90333-1. [DOI] [PubMed] [Google Scholar]
- Gerlinger P., Krust A., LeMeur M., Perrin F., Cochet M., Gannon F., Dupret D., Chambon P. Multiple initiation and polyadenylation sites for the chicken ovomucoid transcription unit. J Mol Biol. 1982 Dec 5;162(2):345–364. doi: 10.1016/0022-2836(82)90531-9. [DOI] [PubMed] [Google Scholar]
- Germond J. E., ten Heggeler B., Schubiger J. L., Walker P., Westley B., Wahli W. Vitellogenin B2 gene in Xenopus laevis: isolation, in vitro transcription and relation to other vitellogenin genes. Nucleic Acids Res. 1983 May 25;11(10):2979–2997. doi: 10.1093/nar/11.10.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan M. V., Spiess E., Majors J. Purified glucocorticoid receptor-hormone complex from rat liver cytosol binds specifically to cloned mouse mammary tumor virus long terminal repeats in vitro. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5157–5161. doi: 10.1073/pnas.79.17.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grez M., Land H., Giesecke K., Schütz G., Jung A., Sippel A. E. Multiple mRNAs are generated from the chicken lysozyme gene. Cell. 1981 Sep;25(3):743–752. doi: 10.1016/0092-8674(81)90182-3. [DOI] [PubMed] [Google Scholar]
- Haché R. J., Wiskocil R., Vasa M., Roy R. N., Lau P. C., Deeley R. G. The 5' noncoding and flanking regions of the avian very low density apolipoprotein II and serum albumin genes. Homologies with the egg white protein genes. J Biol Chem. 1983 Apr 10;258(7):4556–4564. [PubMed] [Google Scholar]
- Heilig R., Muraskowsky R., Kloepfer C., Mandel J. L. The ovalbumin gene family: complete sequence and structure of the Y gene. Nucleic Acids Res. 1982 Jul 24;10(14):4363–4382. doi: 10.1093/nar/10.14.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig R., Muraskowsky R., Mandel J. L. The ovalbumin gene family. The 5' end region of the X and Y genes. J Mol Biol. 1982 Mar 25;156(1):1–19. doi: 10.1016/0022-2836(82)90455-7. [DOI] [PubMed] [Google Scholar]
- Hong G. F. A systemic DNA sequencing strategy. J Mol Biol. 1982 Jul 5;158(3):539–549. doi: 10.1016/0022-2836(82)90213-3. [DOI] [PubMed] [Google Scholar]
- Hynes N., van Ooyen A. J., Kennedy N., Herrlich P., Ponta H., Groner B. Subfragments of the large terminal repeat cause glucocorticoid-responsive expression of mouse mammary tumor virus and of an adjacent gene. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3637–3641. doi: 10.1073/pnas.80.12.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost J. P., Seldran M., Geiser M. Preferential binding of estrogen-receptor complex to a region containing the estrogen-dependent hypomethylation site preceding the chicken vitellogenin II gene. Proc Natl Acad Sci U S A. 1984 Jan;81(2):429–433. doi: 10.1073/pnas.81.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Haslinger A., Holtgreve H., Richards R. I., Krauter P., Westphal H. M., Beato M. Characterization of DNA sequences through which cadmium and glucocorticoid hormones induce human metallothionein-IIA gene. Nature. 1984 Apr 5;308(5959):513–519. doi: 10.1038/308513a0. [DOI] [PubMed] [Google Scholar]
- Ladiges W. C., Raff R. F., Brown S., Deeg H. J., Storb R. The canine major histocompatibility complex. Supertypic specificities defined by the primed lymphocyte test (PLT). Immunogenetics. 1984;19(4):359–365. doi: 10.1007/BF00345410. [DOI] [PubMed] [Google Scholar]
- Majors J., Varmus H. E. A small region of the mouse mammary tumor virus long terminal repeat confers glucocorticoid hormone regulation on a linked heterologous gene. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5866–5870. doi: 10.1073/pnas.80.19.5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijlink F. C., Philipsen J. N., Gruber M., Ab G. Methylation of the chicken vitellogenin gene: influence of estradiol administration. Nucleic Acids Res. 1983 Mar 11;11(5):1361–1373. doi: 10.1093/nar/11.5.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvihill E. R., LePennec J. P., Chambon P. Chicken oviduct progesterone receptor: location of specific regions of high-affinity binding in cloned DNA fragments of hormone-responsive genes. Cell. 1982 Mar;28(3):621–632. doi: 10.1016/0092-8674(82)90217-3. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Mulvihill E. R., Shepherd J. H., McKnight G. S. Steroid hormone regulation of ovalbumin and conalbumin gene transcription. A model based upon multiple regulatory sites and intermediary proteins. J Biol Chem. 1981 Aug 10;256(15):7910–7916. [PubMed] [Google Scholar]
- Payvar F., Wrange O., Carlstedt-Duke J., Okret S., Gustafsson J. A., Yamamoto K. R. Purified glucocorticoid receptors bind selectively in vitro to a cloned DNA fragment whose transcription is regulated by glucocorticoids in vivo. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6628–6632. doi: 10.1073/pnas.78.11.6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfahl M. Specific binding of the glucocorticoid-receptor complex to the mouse mammary tumor proviral promoter region. Cell. 1982 Dec;31(2 Pt 1):475–482. doi: 10.1016/0092-8674(82)90140-4. [DOI] [PubMed] [Google Scholar]
- Renkawitz R., Schütz G., von der Ahe D., Beato M. Sequences in the promoter region of the chicken lysozyme gene required for steroid regulation and receptor binding. Cell. 1984 Jun;37(2):503–510. doi: 10.1016/0092-8674(84)90380-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y., Ohlendorf D. H., Anderson W. F., Matthews B. W. DNA-binding proteins. Science. 1983 Sep 9;221(4615):1020–1026. doi: 10.1126/science.6308768. [DOI] [PubMed] [Google Scholar]
- Wahli W., Dawid I. B., Ryffel G. U., Weber R. Vitellogenesis and the vitellogenin gene family. Science. 1981 Apr 17;212(4492):298–304. doi: 10.1126/science.7209528. [DOI] [PubMed] [Google Scholar]
- Wahli W., Dawid I. B., Wyler T., Weber R., Ryffel G. U. Comparative analysis of the structural organization of two closely related vitellogenin genes in X. laevis. Cell. 1980 May;20(1):107–117. doi: 10.1016/0092-8674(80)90239-1. [DOI] [PubMed] [Google Scholar]
- Wahli W., Germond J. E., ten Heggeler B., May F. E. Vitellogenin genes A1 and B1 are linked in the Xenopus laevis genome. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6832–6836. doi: 10.1073/pnas.79.22.6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker P., Brown-Luedi M., Germond J. E., Wahli W., Meijlink F. C., van het Schip A. D., Roelink H., Gruber M., Ab G. Sequence homologies within the 5' end region of the estrogen-controlled vitellogenin gene in Xenopus and chicken. EMBO J. 1983;2(12):2271–2279. doi: 10.1002/j.1460-2075.1983.tb01734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilks A. F., Cozens P. J., Mattaj I. W., Jost J. P. Estrogen induces a demethylation at the 5' end region of the chicken vitellogenin gene. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4252–4255. doi: 10.1073/pnas.79.14.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilks A., Cato A. C., Cozens P. J., Mattaj I. W., Jost J. P. Isolation and fine structure organisation of an avian vitellogenin gene coding for the major estrogen-inducible mRNA. Gene. 1981 Dec;16(1-3):249–259. doi: 10.1016/0378-1119(81)90081-0. [DOI] [PubMed] [Google Scholar]
- Woo S. L., Beattie W. G., Catterall J. F., Dugaiczyk A., Staden R., Brownlee G. G., O'Malley B. W. Complete nucleotide sequence of the chicken chromosomal ovalbumin gene and its biological significance. Biochemistry. 1981 Oct 27;20(22):6437–6446. doi: 10.1021/bi00525a024. [DOI] [PubMed] [Google Scholar]