Abstract
Integrins participate in multiple cellular processes, including cell adhesion, migration, proliferation, survival, and the activation of growth factor receptors. Recent studies have shown that expression of αv integrins is elevated in the prostate cancer stem/progenitor cell subpopulation compared with more differentiated, committed precursors. Here, we examine the functional role of αv integrin receptor expression in the acquisition of a metastatic stem/progenitor phenotype in human prostate cancer. Stable knockdown of αv integrins expression in PC-3M-Pro4 prostate cancer cells coincided with a significant decrease of prostate cancer stem/progenitor cell characteristics (α2 integrin, CD44, and ALDHhi) and decreased expression of invasion-associated genes Snail, Snail2, and Twist. Consistent with these observations, αv-knockdown strongly inhibited the clonogenic and migratory potentials of human prostate cancer cells in vitro and significantly decreased tumorigenicity and metastatic ability in preclinical models of orthotopic growth and bone metastasis. Our data indicate that integrin αv expression is functionally involved in the maintenance of a highly migratory, mesenchymal cellular phenotype as well as the acquisition of a stem/progenitor phenotype in human prostate cancer cells with metastasis-initiating capacity.
Prostate cancer is the most commonly diagnosed cancer in men and the second leading cause of death. The 5-year survival rate for men with organ-confined disease is almost 100% because of the current treatment options.1 However, the prognosis becomes much worse when the cancer metastasizes to other organs. Greater than 80% of the patients with advanced prostate cancer will develop secondary lesions within the skeleton, indicating bone as a preferred site for the growth of disseminated disease.2, 3 Most carcinomas comprise a heterogeneous cell population with marked differences in their ability to proliferate and differentiate as well as their ability to reconstitute the tumor on transplantation. This led to the hypothesis that the entire population of tumor cells might arise from a small number of cells, the cancer stem/progenitor cells (CSCs) or tumor-initiating cells.4, 5, 6
CSCs have properties that resemble those of normal tissue stem cells, including the ability to self-renew, to reproducibly form tumors with the original cellular heterogeneity, and to undergo differentiation into more differentiated, nontumorigenic cells.7 Increasing evidence suggests that normal stem cells and their immediate progenitors are prime targets for oncologic transformation. Collins and co-workers4, 8, 9 have found that α2β1-high/CD44+/CD133+ prostate cancer cells displayed enhanced clonogenic ability in vitro and form prostate-like glands in vivo. Furthermore, CD44+ prostate cancer cells have higher proliferative, clonogenic, tumorigenic, invasive, and metastatic potential than CD44low cells.10, 11 Recently, our group demonstrated that αv expression is elevated in human prostate cancer cells with tumor- and metastasis-initiating properties identified by high aldehyde dehydrogenase (ALDH) activity.12
Evidence is mounting that “stemness” of normal and transformed epithelial cells is promoted by a process called epithelial-to-mesenchymal transition (EMT).13, 14 In cancer, EMT is fundamental for epithelial cells to acquire a mesenchymal, migratory phenotype. In support of this view, EMT has been linked to metastatic disease and poor prognosis of patients with carcinoma.14, 15, 16, 17 Before dissemination and growth at a metastatic site, prostate cancer cells must become motile and detach from the primary tumor and invade the surrounding stroma. The increase in cell motility is accompanied by changes in the adhesion receptor repertory of the cancer cells. Furthermore, the cellular and extracellular tumor microenvironments are not innocent bystanders but may actively contribute to promotion of tumorigenesis and metastasis.18, 19, 20, 21 Collagens, laminin, fibronectin, and vitronectin are main components of the extracellular matrices in neoplastic disease, where extracellular matrix proteins can integrate complex, multivalent signals to cancer cells.22, 23
Integrins are members of a family of transmembrane glycoprotein receptors that regulate cell-matrix and cell-cell interactions.24 Integrins transduce signals from the outside into the cell and vice versa to regulate cell adhesion and cell spreading, as well as cell survival, migration, proliferation, differentiation, angiogenesis, and remodeling of the extracellular matrix.25 In addition, there is considerable cross talk between integrins and several growth factors, including transforming growth factor (TGF)-β.25 TGF-β is a well-documented stroma-derived effector of EMT that may direct the acquisition of a migratory, mesenchymal phenotype in a number of primary carcinomas.13, 14, 26 Recent evidence suggests that EMT can generate cells with stem/progenitor-like properties, which are not only critically involved in prostate cancer initiation and progression but also in colonization and metastasis formation.12, 14, 27 During the process of carcinogenesis, which is often enabled by EMT, disseminated cancer cells seem to acquire self-renewal capability, similar to that displayed by stem cells. This raises the possibility that the EMT process may also impart a self-renewal capability to disseminated cancer cells.27, 28, 29, 30
The observed changes in integrin expression or function in malignant disease is implicated in tumor growth, angiogenesis, and metastasis, which make these receptors promising targets for novel anticancer therapies.31, 32 In this study, we generated human prostate cancer cell lines with a stable knockdown of αv integrins. Data are presented that indicate an essential role for these αv integrins in tumor growth and metastasis via the induction of prostate cancer cells with a stem/progenitor phenotype.
Materials and Methods
Cell Lines and Culture Conditions
The human osteotropic PC-3M-Pro4 prostate cancer cells were generated from PC-3 cells (ATCC, Manassas, VA; no. CRL-1435) by injecting PC-3 cells into athymic mouse prostates and selecting for clones with increasing metastatic potential by several rounds of re-injecting cells from xenograft tumors back into the mouse prostate. PC-3M-Pro4 cells were stably transfected with a cytomegalovirus promoter-driven mammalian expression vector containing firefly-luciferase (pcDNA3.1 CMV-ff-luc). One clone with high luciferase activity was selected with neomycin (800 μg/mL; Life Technologies, Basel, Switzerland) and successfully used for in vivo bioluminescent imaging (BLI).12, 33
The human prostate cancer cell lines PC-3M-Pro4lucA6 (Pro4luc) and C4-2B were maintained as described previously.12, 33 Puromycin in a concentration of 1 μg/mL was added for cells with stable short hairpin RNA interference (shRNAi) knockdown (see further below). HEK293T cells were maintained in Dulbecco's modified Eagle's medium containing 10% fetal calf serum. All cell lines were grown in a humidified incubator at 37°C and 5% CO2.
Suppressing Integrin αv Expression with a shRNA-Lentiviral Vector
shRNAi constructs (integrin αv clone nos. TRCN0000003239, TRCN0000003240, TRCN0000000768, and TRCN000000769) were derived from the MISSION library of Sigma-Aldrich (St. Louis, MO). HEK293T cells were lentivirally transfected with the short hairpin constructs together with the packaging plasmids REV, GAG, and VSV in a 1:1:1:1 ratio with the use of Fugene HD (Roche, Indianapolis, IN) as transfection reagent. The supernatant fluid of the culture medium containing the lentiviral vector was collected 48 hours after transfection.
Cells (Pro4lucA6 and C4-2B) were mixed with 1 mL of shRNA-lentiviral vector, and 8 μg of Polybrene (Sigma-Aldrich) was added. The mixture was incubated for 1 to 2 hours at room temperature. Scrambled shRNA (clone no. TRC1/1.5), which was used as control, lacks identity with any mammalian mRNA sequence. Cells stably expressing the shRNA were selected with puromycin (1 μg/mL; Sigma-Aldrich). The effects of integrin αv knockdown described in this study represent activities of the heterogeneous cell populations transduced with high efficiency by the lentivirus and not single-cell selected clones. The integrin αv knockdown cell line is further referred to as αv-kd-Pro4luc or αv-kd-C42B cells and the nontargeting control cell line as NT-Pro4luc or NT-C42B cells.
FACS Analysis
Expression of integrin αv, and a number of previously described stem and EMT markers, was measured by fluorescence-activated cell sorting (FACS) analysis with the use of the Calibur2 flow cytometer (BD Biosciences, San Jose, CA) and FCS Express 3 software (De Novo Software, Los Angeles, CA). The cells (1 × 105) were incubated for 45 minutes at 4°C in a solution of 90 μL of FACS wash buffer containing PBS + 1% fetal calf serum + 0.1% natriumazide NaN3 and 10 μL of antibody (αv-phosphatidylethanolamine, α2-fluorescein isothiocyanate, CD44-allophycocyanin, CD44v6-allophycocyanin; Miltenyi Biotec Inc., Auburn, CA). To determine E-cadherin/vimentin ratios, cells were harvested and labeled with E-cadherin-fluorescein isothiocyanate (BD Biosciences; 1:10) in FACS buffer for 30 minutes at 4°C in the dark. Then cells were washed with 1 mL of FACS buffer and fixed with freshly prepared 2% formaldehyde for 15 minutes. Cells were washed with ice-cold PBS and subsequently incubated for 30 minutes at 4°C in the dark with vimentin rabbit polyclonal antibody (1:200 in FACS buffer; Abcam Inc., Cambridge, MA). Cells were washed twice with 1 mL of FACS buffer and incubated for 30 minutes at 4°C with goat anti-rabbit IgG-allophycocyanin antibody (Invitrogen, Carlsbad, CA). After the last incubation step, the cells were washed and centrifuged for 5 minutes, followed by adding 250 μL of FACS wash buffer. ALDH activity was measured as described earlier.12
RNA Isolation and Real-Time qPCR
RNA was extracted with the use of Trizol (Invitrogen), according to the manufacturer's instructions. Real-time quantitative PCR (qPCR) was run and analyzed with a Bio-Rad IQ5 cycler (Bio-Rad, Hercules, CA). For primer sequences, see Table 1. Gene expression was measured relative to GAPDH expression with the use of the following formula: relative transcript abundance = 10,000/2(Ctgene−CtGAPDH).
Table 1.
q-PCR Primer Sequences
| Primer | Sequence |
|---|---|
| Alpha-v forward | 5′-GCTGGACTGTGGAGAAGAC-3′ |
| Alpha-v reverse | 5′-AAGTGAGGTTCAGGGCATTC-3′ |
| Alpha-2 forward | 5′-TTTGGTAGTGTGCTGTGTTC-3′ |
| Alpha-2 reverse | 5′-GACTCTTCCTTCCTCTTTCTTTAG-3′ |
| N-Cadherin forward | 5′-CAGACCGACCCAAACAGCAAC-3′ |
| N-Cadherin reverse | 5′-GCAGCAACAGTAAGGACAAACATC-3′ |
| CD44 forward | 5′-TGGCACCCGCTATGTCCAG-3′ |
| CD44 reverse | 5′-GTGACAGGGATTCTGTCTG-3′ |
| Osteopontin forward | 5′-CAAAGTCAGCCGTGAATTCCA-3′ |
| Osteopontin reverse | 5′-AACCCAATAAACTGAGAAAGAAGC-3′ |
| Snail forward | 5′-TGCAGGACTCTAATCCAAGTTTACCC-3′ |
| Snail reverse | 5′-GTGGGATGGCTGCCAGC-3′ |
| Snail2 forward | 5′-TGTGTGGACTACCGCTGC-3′ |
| Snail2 reverse | 5′-TCCGGAAAGAGGAGAGAGG-3′ |
| Twist forward | 5′-TGTCCGCGTCCCACTAGC-3′ |
| Twist reverse | 5′-TGTCCATTTTCTCCTTCTCTGGA-3′ |
| ALDH7A1 shRNAi clone TRCN0000003239 | 5′-CCGGGACTGAGCTAATCTTGAGAATCTCGAGATTCTCAAGATTAGCTCAGTCTTTTT-3′ |
| ALDH7A1 shRNAi clone TRCN0000003240 | 5′-CCGGCTCTGTTGTATATCCTTCATTCTCGAGAATGAAGGATATACAACAGAGTTTTT-3′ |
| ALDH7A1 shRNAi clone TRCN0000000768 | 5′-CCGGGTGAGGTCGAAACAGGATAAACTCGAGTTTATCCTGTTTCGACCTCACTTTTT-3′ |
| ALDH7A1 shRNAi clone TRCN0000000769 | 5′-CCGGCGACAGGCTCACATTCTACTTCTCGAGAAGTAGAATGTGAGCCTGTCGTTTTT-3′ |
| Nontarget control TRC1/1.5 | 5′-CCGGCAACAAGATGAAGAGCACCAACTCGAGTTGGTGCTCTTCATCTTGTTGTTTTT-3′ |
Soft Agar Colony Assay
Cell suspensions were generated12 and overlaid onto a 60-mm dish containing a solidified bottom layer of 0.6% Noble agarose (Becton Dickinson, Franklin Lakes, NJ) in medium. Medium (1 mL) was placed on top of the solidified cell layer. Plates were incubated for 1 to 3 weeks until colonies were visible. The colonies on the soft agar plates were counted with light microscopy (Zeiss Axiovert 200M, Sliedrecht, The Netherlands). Three individual and representative fields of each well were counted. The mean number of colonies/field was calculated.
Colony-Forming Assay
Cells were seeded into a 96-well plate containing an average of 1 cell per well. Plates were monitored twice a week and maintained in Dulbecco's modified Eagle's medium/10% FCII medium. After 1 to 3 weeks, colonies were clearly visible, and the mean number of positive wells/plate was counted by microscopy (Zeiss Axiovert 200M).
Migration Assay
Tumor cell migration was performed in Transwell migration chambers (Costar, Cambridge, MA).12 Three random fields were counted for each well, and mean numbers of migrated cells/field were calculated.
Annexin V/Propidium Iodide Apoptosis Assay
For apoptotic analysis, harvested cells were stained with Annexin V/propidium iodide (Alexa Fluor 488 Annexin V/Dead Cell Apoptosis Kit; Invitrogen), incubated for 15 minutes according to the manufacturer's protocol. Samples were analyzed with FACSCalibur2 (BD Biosciences) and FCS Express 3 software (DeNovo Software).
Proliferation Assay
Cells were seeded at a density of 2500/cm2 and allowed to grow for 24, 48, and 72 hours, respectively After the cell incubation, 20 μL of MTS was added to the medium, and mitochondrial activity was measured at 490 nm after 2 hours of incubation at 37°C (CellTiter96 Aqueous Non-radioactive Cell proliferation assay; Promega, Madison, WI).
In Vivo Animal Experiments
Mouse Strains
Male nude (BALB/c nu/nu) mice were housed in individual ventilated cages under sterile condition according to the local guidelines for the care and use of laboratory animals (DEC07026 and 09052). Mice were anesthetized before surgical and analytical procedures were performed.
Subcutaneous Inoculation of Pro4luc Cells
A 10-μL single-cell suspension of 1 × 105 αv-kd-Pro4luc cells or NT-Pro4luc cells in PBS was combined with 10 μL of growth factor-reduced Matrigel (BD Biosciences) and injected subcutaneously in anesthetized 6-week-old male nude mice. The progression of cancer cell growth was monitored weekly by BLI.12
Orthotopic Inoculation of Pro4luc into the Mouse Prostate
A single-cell suspension of 1 × 105 αv-kd-Pro4luc cells or NT-Pro4luc cells/10 μL of PBS was combined with 10 μL of growth factor-reduced Matrigel (BD Biosciences) and surgically inoculated into the prostate of anesthetized 6-week-old male nude mice.12, 34 The cutaneous wound was sutured. The progression of cancer cell growth was monitored weekly by BLI.
Intracardiac Inoculation Pro4luc Cells to Induce Systemic Metastases
A single-cell suspension of 1 × 105 αv-kd-Pro4luc cells or NT-Pro4luc cells per 100 μL of PBS was injected into the left cardiac ventricle of anesthetized 5-week-old male nude mice, and cancer cell growth was monitored weekly by BLI.14
Whole-Body BLI and Quantification of the Bioluminescent Signal
Luciferin (Perbio Science Nederland B.V., Etten-Leur, The Netherlands) was injected intraperitoneally. BLI of tumors induced by the luciferase-expressing human prostate cancer cell lines was performed with the Xenogen IVIS100. Analyses for each metastatic site were performed after definition of the region of interest and quantified with Living Image 4.2 (Caliper Life Sciences, Teralfene, Belgium). Values are expressed as photons per second.
Statistical Analysis
Statistical analysis was performed with GraphPad Prism 4.0 software (GraphPad Software Inc., San Diego, CA) with the use of either t test (for comparison between two groups) or analysis of variance (for comparison between more than two groups). Unless otherwise stated, data are presented as the mean ± SEM. P values ≤ 0.05 were regarded as being statistically significant (*P < 0.05, **P < 0.01, and ***P < 0.001).
Results
Expression and Stable Knockdown of Integrin αv in PC-3M-Pro4luc Prostate Cancer Cells
Strong αv integrin expression is associated with the basal layer of the human prostate (Figure 1A, left panel), and its expression was previously found to be increased in prostate cancer cells possessing stem/progenitor characteristics.9, 12 Integrins αv mRNA expression is observed in prostate cancer tissue, prostate cancer cell lines, and primary cultures (Figure 1A, middle and right panels). In line with these transcriptional data, flow cytometric analysis of prostate cancer cell lines and primary prostate cancer cultures (derived from radical prostatectomy specimens) show detectable integrin αv expression levels (Table 2). The malignant origin of the isolated primary epithelial cell cultures was confirmed by qPCR, showing the expression of the TMPRSS:ERG fusion gene as described previously12 (see Supplemental Figure S1 at http://ajp.amjpathol.org).
Figure 1.
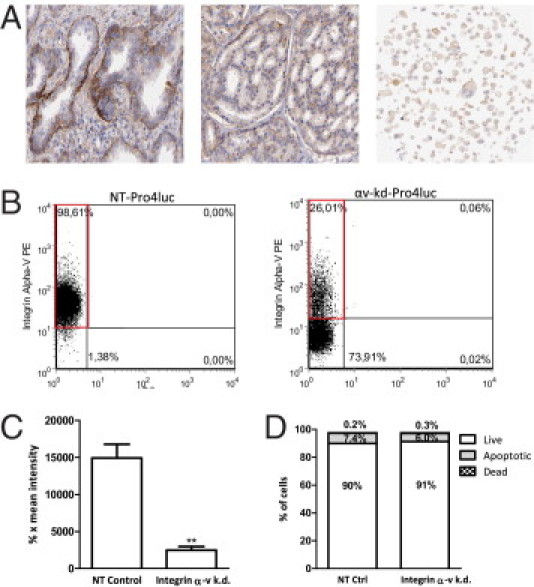
Established integrin αv expression knockdown in PC-3M-Pro4luc prostate cancer cells. A: Expression of integrin αv in normal prostate tissue (left panel), prostate cancer tissue (middle panel), and prostate cancer cell lines (right panel). Adapted from www.proteinatlas.org. B: Flow cytometric analysis of αv expression in Pro4luc cells stably infected with a short hairpin for integrin αv. Expression levels were compared with control cells infected with a nontargeting short hairpin. C: Mean intensities of the αv fluorescence in αv-kd-Pro4luc or NT-Pro4luc cells. D: Percentage of live, apoptotic, or dead cells in total knockdown or control cell populations.
Table 2.
Flow Cytometric Analysis of Cell Lines and Primary Prostate Cancer Cultures According to Integrin αv Expression
| αv Expression (%) | |
|---|---|
| PCa cell lines | |
| C4 | 70.5 |
| C4-2 | 71 |
| C4-2B | 76.5 |
| PC-3 M-Pro4luc | 98.6 |
| PCa primary cultures | |
| 633 | 0.3 |
| 169 | 1.2 |
| 713 | 0.1 |
| 805 | 1.3 |
| 567_1 | 23.2 |
| 567_2 | 51.9 |
Human prostate cancer cell lines and primary cultures were stained with an antibody for integrin αv and analyzed with flow cytometry. Data are presented as percentage of cells with αv expression after analysis by flow cytometry.
Next, we studied the functional involvement of integrin αv in tumorigenicity and metastasis formation. For this, integrin αv expression was blocked with lentiviral-mediated shRNAi. FACS analysis of nontargeted Pro4 cells (NT-Pro4luc) and cell clones with a lentivirally induced knockdown of αv integrins (αv-kd-Pro4luc) showed a strong and significant down-regulation of αv expression in αv-kd-Pro4luc (Figure 1, B and C). On knockdown of integrin αv expression, the prostate cancer cells also displayed structural changes. The cells no longer adhered to plastic and grew in suspension where they clustered together and formed small clumps of viable cells (see Supplemental Figure S2 at http://ajp.amjpathol.org). An Annexin-V/propidium iodide apoptosis assay showed no significant differences in the proportion of cultured apoptotic or necrotic cells in the NT-Pro4luc and αv-kd-Pro4luc cell lines (Figure 1D). In addition, no changes in cell viability were observed (data not shown).
Integrin αv Knockdown Leads to Decreased Clonogenicity and Migratory Capability in Vitro
Functional differences in stem/progenitor phenotype between integrin αv-kd-Pro4luc cells and NT-Pro4luc control prostate cancer cells were assessed by different in vitro clonogenic assays (Figure 2, A–D). Blocking the expression of integrin αv in Pro4luc cells resulted in a significantly decreased proliferation rate after 24, 48, and 72 hours (Figure 2A). The ability of the cells to grow anchorage independently (soft agar assay) was strongly affected by αv knockdown. The αv-kd-Pro4luc cells formed significantly less colonies than the control cell population (Figure 2B). When plated in vitro at low density (1 cell/well), the αv-kd-Pro4luc cells displayed significantly less single-cell growth than the control prostate cancer cells (Figure 2C).
Figure 2.
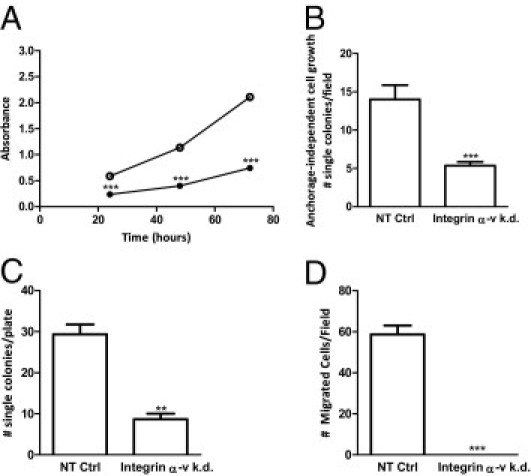
Integrin αv knockdown prostate cancer cells show decreased clonogenicity and migratory ability in vitro. A: Absorbance measured at 490 nm after 24, 48, and 72 hours of incubation in αv-kd-Pro4luc (closed circle) and NT-Pro4luc cells (open circle). B: The number of colonies growing anchorage independently in the αv-kd-Pro4luc and NT-Pro4luc cells. C: The number of colonies per 96-well plate in the single-cell diluted cultures after 2 weeks in the αv-kd-Pro4luc and NT-Pro4luc cells. D: Mean number of migrated αv-kd-Pro4luc and NT-Pro4luc cells per field. Data are representative for 3 independent experiments.
In addition to the clonogenic ability, Transwell/Boyden chambers showed that αv-kd-Pro4luc cells were significantly less migratory than the control NT-Pro4luc cells (Figure 2D).
Differential Expression of Stem/Progenitor Markers and Invasiveness-Associated Genes on Integrin αv Expression Knockdown
The expression of αv integrins was previously found to be up-regulated in prostate cancer cells with a stem/progenitor cell phenotype.9, 12 Next, we investigated and compared the expression of previously identified prostate cancer stem cell markers (integrin α2, CD44, and ALDHhi) in established control and αv-knockdown cell lines.4, 5, 7, 9, 11, 12, 35 Real-time qPCR analysis showed decreased expression levels of integrin α2 and CD44 in the αv-kd-Pro4luc cells (Figure 3A). In line with these observations, flow cytometric analysis showed decreased protein levels of α2 integrin and CD44v6 in the αv-kd-Pro4luc cells. Furthermore, the subpopulation of cells with high ALDH activity (measured by ALDEFLUOR), indicative of stem/progenitor phenotypes, was significantly decreased in the αv-kd-Pro4luc cell line compared with NT-Pro4luc cells (Figure 3B). Note that no CD133 staining was observed in both types of PC-3M-Pro4 clones, as expected.5, 7, 12, 35
Figure 3.
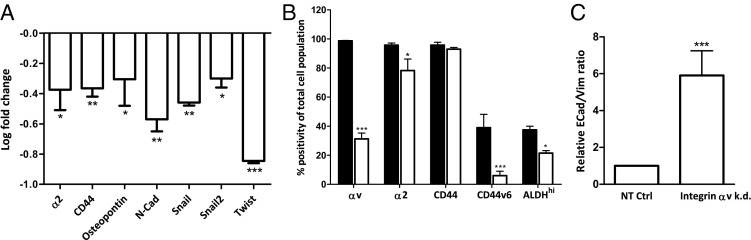
Differential expression of stem/progenitor markers and invasiveness-associated genes in prostate cancer cells on integrin αv expression knockdown. A: qPCR analysis of stem/progenitor markers and invasiveness-associated genes (α2, CD44, Osteopontin, N-cadherin, Snail, Snail2, and Twist). Relative expression levels in αv knockdown cells were shown compared with the NT-Pro4luc control cells. All values were normalized for GAPDH and presented as mean ± SEM. B: Flow cytometric analysis of stem cell markers (integrin αv, α2, CD44, CD44v6, and ALDHhi) in αv-kd-Pro4luc (white bars) and NT-Pro4luc cells (black bars). C: Relative E-cadherin/vimentin ratios of αv-kd-Pro4luc and NT-Pro4luc cells as measured by flow cytometry. Data are representative for 3 independent experiments.
Acquisition of an invasive phenotype is a requirement for metastasis whereby transformed epithelial cells can switch from a sessile, epithelial, to a motile, mesenchymal phenotype by EMT. Whether integrin αv is functionally involved in the EMT-like switch in human prostate cancer has remained unclear. Therefore, we examined the effect of αv knockdown on the expression of EMT transcription factors such as Snail, Snail2, and Twist, and the expression of E-cadherin and vimentin (E-cadherin/vimentin ratio) as indicators of epithelial and mesenchymal phenotypes, respectively. In αv-kd-Pro4luc cells, reduced levels of Snail, Snail2, and Twist transcripts were observed, coinciding with strongly diminished expression levels of the invasion-associated factors osteopontin (OPN) and N-cadherin (Figure 3A).36, 37 Furthermore, FACS analysis showed a significantly increased E-cadherin/vimentin ratio in the prostate cancer cells with stable knockdown of αv integrin, indicating a reversal to a more sessile epithelial phenotype (Figure 3C).
Knockdown of αv integrin of the human C4-2B prostate cancer cells showed similar effects (ie, loss of adhesion to tissue culture plastic; see Supplemental Figure S3B at http://ajp.amjpathol.org) and an increased E-cadherin/vimentin ratio (see Supplemental Figure S3C at http://ajp.amjpathol.org). We compared the expression of previously identified prostate cancer stem cell markers (integrin α2 and ALDHhi) in the control and αv-knockdown cell lines. Flow cytometric analysis showed that protein levels of α2 integrin as well as the subpopulation of cells with high ALDH activity (measured by ALDEFLUOR), indicative of stem/progenitor phenotypes, was significantly decreased in the αv-kd-C4-2B cell line compared with NT-C4-2B cells (see Supplemental Figure S3D at http://ajp.amjpathol.org).
Integrin αv knockdown also resulted in a significantly decreased proliferation rate after 24, 48, and 72 hours (see Supplemental Figure S3E at http://ajp.amjpathol.org), as well as migration of C4-2B cells (see Supplemental Figure S3F at http://ajp.amjpathol.org). When plated in vitro at low density (1 cell/well), the αv-kd-C4-2B cells displayed significantly less single-cell growth than the NT-C4-2B cells (see Supplemental Figure S3G at http://ajp.amjpathol.org).
Integrin αv Knockdown, Tumorigenicity, and Metastasis in Vivo
Our in vitro data showed that prostate cancer cells with a strongly diminished integrin αv expression are poorly clonogenic and migratory compared with control cells. Subsequently, we analyzed and compared the tumor-initiating and metastasis-initiating abilities of both cell lines in preclinical models. To monitor and compare tumorigenicity in vivo, 100,000 luciferase-expressing PC-3M-Pro4luc cells were implanted subcutaneously on the back of immunocompromised mice and measured weekly by BLI for 42 days (Figure 4).12 The tumor take after inoculation of viable αv-kd-Pro4luc cells was strikingly lower compared with the Pro4luc control cells, and tumor burden was significantly decreased (P < 0.05; Figure 4A).
Figure 4.
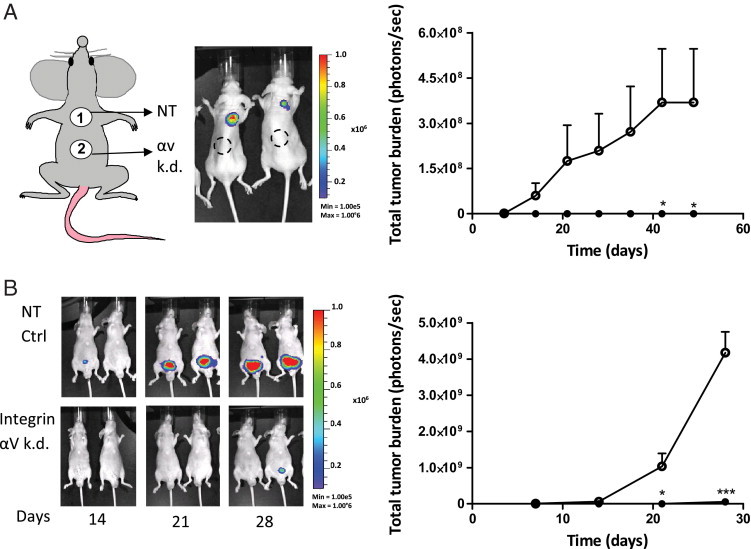
Integrin αv knockdown leads to decreased tumorigenicity in vivo. A, left panel: Shown are representative images of 2 mice 14 days after subcutaneous injection with either 100,000 NT-Pro4 (number 1, upper back) or αv-kd-Pro4luc (number 2, lower back) prostate cancer cells. A, right panel: Shown are total tumor burden of mice injected subcutaneously with 100,000 αv-kd-Pro4 cells (closed circle) compared with mice injected with 100,000 NT-Pro4 control cells (open circle) (n = 5/group). B, left panel: Shown are representative images of 2 mice 14, 21, and 28 days after orthotopic injection with either 100,000 αv-kd-Pro4luc or NT-Pro4 prostate cancer cells. B, right panel: Shown are total tumor burden for the mice injected with the αv-kd-Pro4luc knockdown population (closed circle) or the NT-Pro4luc control population (open circle) (n = 8/group).
In addition to the PC-3M-Pro4 cells, we compared the tumorigenicity of C4-2B cells sorted for integrin αv expression (ie, integrin αvhi and C4-2B integrin αv− cells) by implanting the cells subcutaneously on the back of immunocompromised mice. The C4-2B-integrin αvhi cells grew readily subcutaneously, whereas the tumor take after inoculation of viable C4-2B integrin αv− cells was strikingly lower, and tumor burden was significantly decreased (see Supplemental Figure S4 at http://ajp.amjpathol.org).
To compare tumorigenicity and metastatic ability of both PC-3M-Pro4 cell populations in a more clinically relevant model system, both prostate cancer cell lines were surgically and orthotopically implanted into the mouse prostate.12 Total tumor burden was significantly lower for the mice inoculated with αv-kd-Pro4luc knockdown cells than with NT-Pro4luc control cells at various time points (Figure 4B) (*P < 0.05 at day 21, ***P < 0.01 at day 28). Furthermore, both prostate cancer cell lines were evaluated in a preclinical model of bone metastasis.12, 33 In line with our subcutaneous and orthotopic models, tumor take, the total number of bone metastases, and the metastatic tumor burden in the αv-kd-Pro4luc group were significantly diminished compared with control conditions (Figure 5, A–D).
Figure 5.
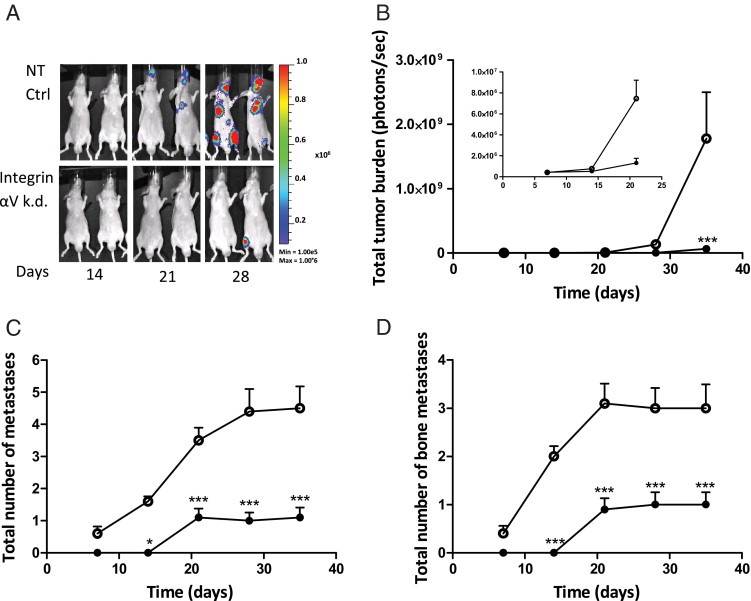
Integrin αv knockdown leads to decreased metastatic growth in vivo. A: Representative images of mice inoculated by intracardiac injection with either 100,000 αv-kd-Pro4luc knockdown or NT-Pro4luc control cells at day 14, 21, and 28 after inoculation (n = 10/group). B: Total tumor burden of αv-kd-Pro4luc cells (closed circle) compared with NT-Pro4luc cells (open circle). The first 20 days after inoculation are shown as inset in the presented graph. C: Total number of metastases per mouse in the mice injected with either αv knockdown PC-3M-Pro4 (closed circle) or NT control (open circle) cells. D: Total number of bone metastases per mouse in the mice injected with either αv knockdown PC-3M-Pro4 (closed circle) or control (open circle) cells.
Discussion
Greater than 80% of the patients with advanced prostate cancer will develop secondary lesions within the skeletal compartment, indicating bone as a preferred site for the growth of disseminated disease.32 Once cancers have spread to the skeleton, treatment options are predominantly focused on palliation and the prevention of pathologic bone fractures. To date, there is no cure for metastatic bone disease, and novel therapy for advanced prostate cancer is urgently needed. Changes in integrin expression or function are directly involved in the regulation of tumor growth, angiogenesis, and metastasis, making these receptors promising targets for novel anticancer therapies.31, 32
We show here that αv integrin expression in human prostate cancer cells is functionally required for subcutaneous growth, orthotopic growth, and the formation of distant metastases. In addition, we show for the first time that the level of integrin αv expression is involved in the maintenance of a mesenchymal, migratory phenotype in human prostate cancer via an EMT-like process and that αv expression contributes to the acquisition of a migratory stem/progenitor phenotype. In addition, on αv integrin expression knockdown, a concomitant decrease of prostate CSC phenotypes was observed as exemplified by diminished α2 integrin, CD44v6, and ALDH expression.8, 11, 12 Furthermore, a number of genes/factors involved in migration, invasion (EMT), and metastasis were affected. In addition, the clonogenic and migratory abilities of the αv-kd-Pro4luc cells was significantly decreased in vitro. In line with decreased clonogenic abilities in vitro, αv-kd-Pro4luc cells displayed decreased tumorigenicity and metastatic ability in vivo in several preclinical models of orthotopic growth and experimental metastasis. Importantly, significant lower numbers of (bone) metastases were formed on integrin αv knockdown.
Our data indicate that αv integrins play an important role in the formation and maintenance of the prostate CSC pool. As we have recently shown, the expression of integrin αv is increased in human prostate cancer cells with high ALDH activity, a population that is enriched for tumor- and metastasis-initiating cells.12, 38 In addition, integrin αv is increased in a prostate cancer stem cell population (α2β1hi, CD44+, and CD133+) compared with the committed population.9 Migration of mammary and prostate carcinoma cells is stimulated by OPN via interactions with integrins and CD44 cell surface receptors,39 which is further supported by our findings described here. The presence of OPN can mediate preferential adhesion, migration, and growth of prostate cancer cells expressing integrin αv.40 OPN functions through the interaction with two cell adhesion molecules: integrins and CD44v6,39, 41, 42 and the latter has been implicated in the progression of a variety of carcinomas.39, 41 The observed diminished CD44v6 and OPN expression levels in the αv-kd-Pro4luc cells are, therefore, in line with these previous observations. Moreover, we found that αv knockdown lead to a more epithelial, less invasive cell phenotype (increased E-cadherin/vimentin ratio; decrease in Snail, Snail2, Twist, and N-cadherin levels).43 The prometastatic transcription factor Twist induces EMT and promotes the tumor-initiating capability in other epithelial cancers,44 thus linking Twist expression and EMT to the acquisition of stem cell properties in cancer cells. This is in line with our data in human prostate cancer because decreased αv expression leads to diminished Twist expression and a reduction in stem/progenitor cell properties in human prostate cancer cells. Our data support the notion that the gain of N-cadherin expression in prostate cancer is important in the regulation of cell migration, invasion, and survival.38, 45 Strikingly, the loss of N-cadherin mRNA expression in PC-3 prostate carcinoma depends, at least in part, on the basic helix-loop-helix transcription factor Twist,45 which fits our findings.
Other studies have provided further evidence that EMT plays a critical role not only in invasion and metastasis but also in tumor recurrence, which is believed to be tightly linked with the biology of CSCs.13, 14, 46, 47, 48 Factors, such as TGF-β that induce E-cadherin repressors Snail and Twist, have now been implicated in the generation of cancer cells with stem/progenitor cell properties that are capable of tumor-initiation and maintenance.13, 30 Intriguingly, extensive cross talk between integrins and TGF-β exists.25, 49 Integrins can modulate the signaling cascade elicited by several growth factors, including TGF-β, and TGF-β in turn controls the transcription of genes that encode numerous integrins. 25 The exact mechanisms by which EMT can support the generation of the stem-like cells has remained largely elusive.
In cancer, EMT is fundamental for epithelial cells to become more invasive. We show here that αv integrin expression is required for prostate cancer cells to acquire a metastatic phenotype and to form bone metastases. Factors such as TGF-β and OPN are prominent in the bone microenvironment where they seemed to play important roles in skeletal metastasis.2, 3, 39, 50, 51, 52, 53 This finding is further supported by our findings on the role of αv integrins and the fact that bone morphogenetic protein 7 inhibits the TGF-β-driven EMT process and bone metastasis.33, 34 Our new data suggest a potential role of integrin αv in the therapeutic response of bone morphogenetic protein 7.
In conclusion, we show for the first time that integrin αv expression is required for the acquisition of a α2hi/CD44+/ALDHhi prostate cancer stem/progenitor phenotype, invasiveness via EMT-like processes, and metastasis formation by this prostate cancer stem/progenitor subpopulation. The data presented here strengthen the role of αv integrins in prostate cancer and provide the therapeutic rationale for αv integrin blockade.
Footnotes
Supported by a European grant FP6-LSH-5-2004-018858PROMET.
Supplemental material for this article can be found at http://ajp.amjpathol.org or at doi: 10.1016/j.ajpath.2011.07.011.
Supplementary Data
RT-PCR expression of the TMPRSS:ERG fusion gene in primary prostate cancer cell lines.
Representative images of NT-Pro4luc (A) and αv-kd-Pro4luc (B) cells.
A: Mean intensities of the αv fluorescence in αv-kd-C4-2B or NT-C4-2B cells. B: Representative images of C4-2B-NT and αv-kd-C4-2B cells. C: Relative E-cadherin/vimentin ratios of αv-kd-C4-2B and NT-C4-2B cells as measured by flow cytometry. D: Flow cytometric analysis of stem cell markers (integrin αv, α2, and ALDHhi) in αv-kd-C4-2B (white bars) and NT-C4-2B cells (black bars). E: Absorbance measured at 490 nm after 24, 48, and 72 hours of incubation in αv-kd-C4-2B (closed circle) and NT-C4-2B cells (open circle). F: Mean number of migrated αv-kd-C4-2B and NT-C4-2B cells per field. G: The number of colonies per 96-well plate in the single-cell diluted cultures after 2 weeks in the αv-kd-C4-2B and NT-C4-2B cells. Data are representative for 2 independent experiments.
The bulk population of C4-2Bluc2 cells was sorted for expression of integrin αv. We compared the cells with no expression of integrin αv (C4-2B-luc integrin αvneg) with cells with high expression of integrin αv (C4-2B-luc integrin αvhi). A: When plated in vitro at low density (1 cell/well), the C4-2B-luc integrin αvneg displayed significantly less single-cell growth than the C4-2B-luc integrin αvhi cells. B: To monitor and compare tumorigenicity in vivo, 0.5 or 1 × 106 luciferase-expressing C4-2B-integrin αvhi cells or C4-2B integrin αvneg cells were implanted subcutaneously on the back of immunocompromised mice and measured by BLI.
References
- 1.Frydenberg M., Stricker P.D., Kaye K.W. Prostate cancer diagnosis and management. Lancet. 1997;349:1681–1687. doi: 10.1016/S0140-6736(96)07393-X. [DOI] [PubMed] [Google Scholar]
- 2.Mundy G.R. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 3.Buijs J.T., van der Pluijm G. Osteotropic cancers: from primary tumor to bone. Cancer Lett. 2009;273:177–193. doi: 10.1016/j.canlet.2008.05.044. [DOI] [PubMed] [Google Scholar]
- 4.Collins A.T., Berry P.A., Hyde C., Stower M.J., Maitland N.J. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 5.Guzman-Ramirez N., Voller M., Wetterwald A., Germann M., Cross N.A., Rentsch C.A., Schalken J., Thalmann G.N., Cecchini M.G. In vitro propagation and characterization of neoplastic stem/progenitor-like cells from human prostate cancer tissue. Prostate. 2009;69:1683–1693. doi: 10.1002/pros.21018. [DOI] [PubMed] [Google Scholar]
- 6.Al-Hajj M., Wicha M.S., Benito-Hernandez A., Morrison S.J., Clarke M.F. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfeiffer M.J., Schalken J.A. Stem cell characteristics in prostate cancer cell lines. Eur Urol. 2009;57:246–254. doi: 10.1016/j.eururo.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Collins A.T., Habib F.K., Maitland N.J., Neal D.E. Identification and isolation of human prostate epithelial stem cells based on alpha(2)beta(1)-integrin expression. J Cell Sci. 2001;114:3865–3872. doi: 10.1242/jcs.114.21.3865. [DOI] [PubMed] [Google Scholar]
- 9.Birnie R., Bryce S.D., Roome C., Dussupt V., Droop A., Lang S.H., Berry P.A., Hyde C.F., Lewis J.L., Stower M.J., Maitland N.J., Collins A.T. Gene expression profiling of human prostate cancer stem cells reveals a pro-inflammatory phenotype and the importance of extracellular matrix interactions. Genome Biol. 2008;9:R83. doi: 10.1186/gb-2008-9-5-r83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klarmann G.J., Hurt E.M., Mathews L.A., Zhang X., Duhagon M.A., Mistree T., Thomas S.B., Farrar W.L. Invasive prostate cancer cells are tumor initiating cells that have a stem cell-like genomic signature. Clin Exp Metastasis. 2009;26:433–446. doi: 10.1007/s10585-009-9242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patrawala L., Calhoun T., Schneider-Broussard R., Li H., Bhatia B., Tang S., Reilly J.G., Chandra D., Zhou J., Claypool K., Coghlan L., Tang D.G. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25:1696–1708. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- 12.van den Hoogen C., van der Horst G., Cheung H., Buijs J.T., Lippitt J.M., Guzman-Ramirez N., Hamdy F.C., Eaton C.L., Thalmann G.N., Cecchini M.G., Pelger R.C., van der Pluijm G. High aldehyde dehydrogenase activity identifies tumor-initiating and metastasis-initiating cells in human prostate cancer. Cancer Res. 2010;70:5163–5173. doi: 10.1158/0008-5472.CAN-09-3806. [DOI] [PubMed] [Google Scholar]
- 13.Mani S.A., Guo W., Liao M.J., Eaton E.N., Ayyanan A., Zhou A.Y., Brooks M., Reinhard F., Zhang C.C., Shipitsin M., Campbell L.L., Polyak K., Brisken C., Yang J., Weinberg R.A. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kong D., Banerjee S., Ahmad A., Li Y., Wang Z., Sethi S., Sarkar F.H. Epithelial to mesenchymal transition is mechanistically linked with stem cell signatures in prostate cancer cells. PLoS One. 2010;5:e12445. doi: 10.1371/journal.pone.0012445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurt E.M., Farrar W.L. Cancer stem cells: the seeds of metastasis? Mol Interv. 2008;8:140–142. doi: 10.1124/mi.8.3.7. [DOI] [PubMed] [Google Scholar]
- 16.Meng H.M., Zheng P., Wang X.Y., Liu C., Sui H.M., Wu S.J., Zhou J., Ding Y.Q., Li J.M. Overexpression of nanog predicts tumor progression and poor prognosis in colorectal cancer. Cancer Biol Ther. 2010;9:295–302. doi: 10.4161/cbt.9.4.10666. [DOI] [PubMed] [Google Scholar]
- 17.Kim M.A., Lee H.S., Lee H.E., Kim J.H., Yang H.K., Kim W.H. Prognostic importance of epithelial-mesenchymal transition-related protein expression in gastric carcinoma. Histopathology. 2009;54:442–451. doi: 10.1111/j.1365-2559.2009.03247.x. [DOI] [PubMed] [Google Scholar]
- 18.Mueller M.M., Fusenig N.E. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 19.Karnoub A.E., Dash A.B., Vo A.P., Sullivan A., Brooks M.W., Bell G.W., Richardson A.L., Polyak K., Tubo R., Weinberg R.A. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 20.Albini A., Sporn M.B. The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer. 2007;7:139–147. doi: 10.1038/nrc2067. [DOI] [PubMed] [Google Scholar]
- 21.Fidler I.J., Poste G. The “seed and soil” hypothesis revisited. Lancet Oncol. 2008;9:808. doi: 10.1016/S1470-2045(08)70201-8. [DOI] [PubMed] [Google Scholar]
- 22.Hurt E.M., Chan K., Serrat M.A., Thomas S.B., Veenstra T.D., Farrar W.L. Identification of vitronectin as an extrinsic inducer of cancer stem cell differentiation and tumor formation. Stem Cells. 2010;28:390–398. doi: 10.1002/stem.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hynes R.O. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pontes-Junior J., Reis S.T., Dall'oglio M., Neves de Oliveira L.C., Cury J., Carvalho P.A., Ribeiro-Filho L.A., Moreira Leite K.R., Srougi M. Evaluation of the expression of integrins and cell adhesion molecules through tissue microarray in lymph node metastases of prostate cancer. J Carcinog. 2009;8:3. doi: 10.4103/1477-3163.48453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Margadant C., Sonnenberg A. Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 2010;11:97–105. doi: 10.1038/embor.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalluri R., Weinberg R.A. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Pluijm G. Epithelial plasticity, cancer stem cells and bone metastasis formation. Bone. 2011;48:37–43. doi: 10.1016/j.bone.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 28.Brabletz T., Jung A., Spaderna S., Hlubek F., Kirchner T. Opinion: migrating cancer stem cells—an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5:744–749. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- 29.Kelly K., Yin J.J. Prostate cancer and metastasis initiating stem cells. Cell Res. 2008;18:528–537. doi: 10.1038/cr.2008.50. [DOI] [PubMed] [Google Scholar]
- 30.Morel A.P., Lievre M., Thomas C., Hinkal G., Ansieau S., Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nemeth J.A., Nakada M.T., Trikha M., Lang Z., Gordon M.S., Jayson G.C., Corringham R., Prabhakar U., Davis H.M., Beckman R.A. Alpha-v integrins as therapeutic targets in oncology. Cancer Invest. 2007;25:632–646. doi: 10.1080/07357900701522638. [DOI] [PubMed] [Google Scholar]
- 32.McCabe N.P., De S., Vasanji A., Brainard J., Byzova T.V. Prostate cancer specific integrin alphavbeta3 modulates bone metastatic growth and tissue remodeling. Oncogene. 2007;26:6238–6243. doi: 10.1038/sj.onc.1210429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buijs J.T., Rentsch C.A., van der Horst G., van Overveld P.G., Wetterwald A., Schwaninger R., Henriquez N.V., Ten Dijke P., Borovecki F., Markwalder R., Thalmann G.N., Papapoulos S.E., Pelger R.C., Vukicevic S., Cecchini M.G., Lowik C.W., van der Pluijm G. BMP7, a putative regulator of epithelial homeostasis in the human prostate, is a potent inhibitor of prostate cancer bone metastasis in vivo. Am J Pathol. 2007;171:1047–1057. doi: 10.2353/ajpath.2007.070168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buijs J.T., Henriquez N.V., van Overveld P.G., van der Horst G., Que I., Schwaninger R., Rentsch C., Ten D.P., Cleton-Jansen A.M., Driouch K., Lidereau R., Bachelier R., Vukicevic S., Clezardin P., Papapoulos S.E., Cecchini M.G., Lowik C.W., van der Pluijm G. Bone morphogenetic protein 7 in the development and treatment of bone metastases from breast cancer. Cancer Res. 2007;67:8742–8751. doi: 10.1158/0008-5472.CAN-06-2490. [DOI] [PubMed] [Google Scholar]
- 35.Li T., Su Y., Mei Y., Leng Q., Leng B., Liu Z., Stass S.A., Jiang F. ALDH1A1 is a marker for malignant prostate stem cells and predictor of prostate cancer patients' outcome. Lab Invest. 2010;90:234–244. doi: 10.1038/labinvest.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eaton C.L., Colombel M., van der Pluijm G., Cecchini M., Wetterwald A., Lippitt J., Rehman I., Hamdy F., Thalman G. Evaluation of the frequency of putative prostate cancer stem cells in primary and metastatic prostate cancer. Prostate. 2010;70:875–882. doi: 10.1002/pros.21121. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka H., Kono E., Tran C.P., Miyazaki H., Yamashiro J., Shimomura T., Fazli L., Wada R., Huang J., Vessella R.L., An J., Horvath S., Gleave M., Rettig M.B., Wainberg Z.A., Reiter R.E. Monoclonal antibody targeting of N-cadherin inhibits prostate cancer growth, metastasis and castration resistance. Nat Med. 2010;16:1414–1420. doi: 10.1038/nm.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomita K., van B.A., van Leenders G.J., Ruijter E.T., Jansen C.F., Bussemakers M.J., Schalken J.A. Cadherin switching in human prostate cancer progression. Cancer Res. 2000;60:3650–3654. [PubMed] [Google Scholar]
- 39.Khan S.A., Cook A.C., Kappil M., Gunthert U., Chambers A.F., Tuck A.B., Denhardt D.T. Enhanced cell surface CD44 variant (v6, v9) expression by osteopontin in breast cancer epithelial cells facilitates tumor cell migration: novel post-transcriptional, post-translational regulation. Clin Exp Metastasis. 2005;22:663–673. doi: 10.1007/s10585-006-9007-0. [DOI] [PubMed] [Google Scholar]
- 40.Cooper C.R., Chay C.H., Pienta K.J. The role of alpha(v)beta(3) in prostate cancer progression. Neoplasia. 2002;4:191–194. doi: 10.1038/sj.neo.7900224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee J.L., Wang M.J., Sudhir P.R., Chen G.D., Chi C.W., Chen J.Y. Osteopontin promotes integrin activation through outside-in and inside-out mechanisms: oPN-CD44V interaction enhances survival in gastrointestinal cancer cells. Cancer Res. 2007;67:2089–2097. doi: 10.1158/0008-5472.CAN-06-3625. [DOI] [PubMed] [Google Scholar]
- 42.van der Pluijm G., Vloedgraven H., Papapoulos S., Lowick C., Grzesik W., Kerr J., Robey P.G. Attachment characteristics and involvement of integrins in adhesion of breast cancer cell lines to extracellular bone matrix components. Lab Invest. 1997;77:665–675. [PubMed] [Google Scholar]
- 43.Emadi Baygi M., Soheili Z.S., Schmitz I., Sameie S., Schulz W.A. Snail regulates cell survival and inhibits cellular senescence in human metastatic prostate cancer cell lines. Cell Biol Toxicol. 2010;26:553–567. doi: 10.1007/s10565-010-9163-5. [DOI] [PubMed] [Google Scholar]
- 44.Yang M.H., Hsu D.S., Wang H.W., Wang H.J., Lan H.Y., Yang W.H., Huang C.H., Kao S.Y., Tzeng C.H., Tai S.K., Chang S.Y., Lee O.K., Wu K.J. Bmi1 is essential in Twist1-induced epithelial-mesenchymal transition. Nat Cell Biol. 2010;12:982–992. doi: 10.1038/ncb2099. [DOI] [PubMed] [Google Scholar]
- 45.Alexander N.R., Tran N.L., Rekapally H., Summers C.E., Glackin C., Heimark R.L. N-cadherin gene expression in prostate carcinoma is modulated by integrin-dependent nuclear translocation of Twist1. Cancer Res. 2006;66:3365–3369. doi: 10.1158/0008-5472.CAN-05-3401. [DOI] [PubMed] [Google Scholar]
- 46.Kasper S. Identification, characterization, and biological relevance of prostate cancer stem cells from clinical specimens. Urol Oncol. 2009;27:301–303. doi: 10.1016/j.urolonc.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santisteban M., Reiman J.M., Asiedu M.K., Behrens M.D., Nassar A., Kalli K.R., Haluska P., Ingle J.N., Hartmann L.C., Manjili M.H., Radisky D.C., Ferrone S., Knutson K.L. Immune-induced epithelial to mesenchymal transition in vivo generates breast cancer stem cells. Cancer Res. 2009;69:2887–2895. doi: 10.1158/0008-5472.CAN-08-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van der Horst G., van den Hoogen C., Buijs J.T., Cheung H., Bloys H., Pelger R.C., Lorenzon G., Heckmann B., Feyen J., Pujuguet P., Blanque R., Clement-Lacroix P., van der Pluijm G. Targeting of alpha(v)-Integrins in Stem/Progenitor Cells and Supportive Microenvironment Impairs Bone Metastasis in Human Prostate Cancer. Neoplasia. 2011;13:516–525. doi: 10.1593/neo.11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Worthington J.J., Klementowicz J.E., Travis M.A. TGFbeta: a sleeping giant awoken by integrins. Trends Biochem Sci. 2011;36:47–54. doi: 10.1016/j.tibs.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 50.Desai B., Rogers M.J., Chellaiah M.A. Mechanisms of osteopontin and CD44 as metastatic principles in prostate cancer cells. Mol Cancer. 2007;6:18. doi: 10.1186/1476-4598-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van der Pluijm G., Sijmons B., Vloedgraven H., Deckers M., Papapoulos S., Lowik C. Monitoring metastatic behavior of human tumor cells in mice with species-specific polymerase chain reaction: elevated expression of angiogenesis and bone resorption stimulators by breast cancer in bone metastases. J Bone Miner Res. 2001;16:1077–1091. doi: 10.1359/jbmr.2001.16.6.1077. [DOI] [PubMed] [Google Scholar]
- 52.Yoneda T., Hiraga T. Crosstalk between cancer cells and bone microenvironment in bone metastasis. Biochem Biophys Res Commun. 2005;328:679–687. doi: 10.1016/j.bbrc.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 53.Yin J.J., Selander K., Chirgwin J.M., Dallas M., Grubbs B.G., Wieser R., Massague J., Mundy G.R., Guise T.A. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest. 1999;103:197–206. doi: 10.1172/JCI3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RT-PCR expression of the TMPRSS:ERG fusion gene in primary prostate cancer cell lines.
Representative images of NT-Pro4luc (A) and αv-kd-Pro4luc (B) cells.
A: Mean intensities of the αv fluorescence in αv-kd-C4-2B or NT-C4-2B cells. B: Representative images of C4-2B-NT and αv-kd-C4-2B cells. C: Relative E-cadherin/vimentin ratios of αv-kd-C4-2B and NT-C4-2B cells as measured by flow cytometry. D: Flow cytometric analysis of stem cell markers (integrin αv, α2, and ALDHhi) in αv-kd-C4-2B (white bars) and NT-C4-2B cells (black bars). E: Absorbance measured at 490 nm after 24, 48, and 72 hours of incubation in αv-kd-C4-2B (closed circle) and NT-C4-2B cells (open circle). F: Mean number of migrated αv-kd-C4-2B and NT-C4-2B cells per field. G: The number of colonies per 96-well plate in the single-cell diluted cultures after 2 weeks in the αv-kd-C4-2B and NT-C4-2B cells. Data are representative for 2 independent experiments.
The bulk population of C4-2Bluc2 cells was sorted for expression of integrin αv. We compared the cells with no expression of integrin αv (C4-2B-luc integrin αvneg) with cells with high expression of integrin αv (C4-2B-luc integrin αvhi). A: When plated in vitro at low density (1 cell/well), the C4-2B-luc integrin αvneg displayed significantly less single-cell growth than the C4-2B-luc integrin αvhi cells. B: To monitor and compare tumorigenicity in vivo, 0.5 or 1 × 106 luciferase-expressing C4-2B-integrin αvhi cells or C4-2B integrin αvneg cells were implanted subcutaneously on the back of immunocompromised mice and measured by BLI.


