Abstract
Pulmonary infections and pneumonitis occur frequently after hematopoietic stem cell transplantation. Using a syngeneic mouse model of bone marrow transplantation (BMT), we have previously demonstrated that BMT mice are more susceptible to acute gammaherpesvirus 68 (MHV-68) replication at day 7 after infection. By day 21, the virus is latent in lungs of BMT and control mice, and there is no difference in viral load. Despite similar latent viral load, BMT mice develop severe pneumonitis associated with reduced oxygen saturation, fibrosis, peripheral inflammation, hyaline membranes, and foamy alveolar macrophages, a phenotype that persists for 7 weeks after infection. BMT mice demonstrate increased bronchoalveolar lavage (BAL) cells, and this population is enriched in neutrophils and T cells. Alternatively, activated macrophages appear earlier than do classically activated macrophages. BAL fluid from BMT mice at day 21 after infection contains increased levels of hydrogen peroxide, nitrite, and transforming growth factor–β (TGF-β). Mice expressing the dominant-negative transgene dn-TGFβRII in multiple cell types were used as BMT donors. BMT mice with T-cell dnTGFβRII are largely protected from the pneumonitis phenotype, whereas mice with CD11c-dnTGFβRII BMT mice are only modestly protected from pneumonitis. Protection in BMT mice with T-cell dnTGFβRII is associated with decreased TGF-β derived from parenchymal cells in the BAL fluid, lower nitrite levels, and reduced apoptosis, whereas alternatively activated macrophage markers are unchanged.
Hematopoietic stem cell transplantation (HSCT) is an important therapy in the treatment of many malignant diseases and some inherited and autoimmune disorders.1 In preparation for transplantation, patients are frequently treated using a myeloablative conditioning regimen including chemotherapy and/or total body irradiation. After conditioning, patients receive an infusion of hematopoietic stem cells from a donor (allogeneic transplant) or their own cells that had been harvested before conditioning (autologous transplant). These cells are derived from bone marrow, cord blood, or mobilized peripheral cells.1, 2 Several serious transplant-related complications can occur after HSCT, and pulmonary complications are a substantial cause of morbidity and mortality in transplant recipients.3, 4, 5
Pulmonary complications can be infectious or noninfectious. Infectious pneumonia can be caused by fungi (eg, Aspergillus), bacteria, or viruses including cytomegalovirus, respiratory viruses, or adenovirus.6 Noninfectious pulmonary complications that occur after transplantation include idiopathic pneumonia syndrome, which involves pneumonia and alveolar injury in the absence of infection in both allogeneic7 and autologous7, 8, 9 transplantation procedures, and bronchiolitis obliterans as a manifestation of graft-versus-host disease (GvHD) in allogeneic transplantations.7 Both total body irradiation and chemotherapeutic agents used in conditioning, including cyclophosphamide and busulfan, can cause pulmonary toxicity.10 In addition, HSCT recipients demonstrate abnormal pulmonary function test results over the long term after transplantation.11, 12
Mouse models of bone marrow transplantation (BMT) enable controlled study of how conditioning and transfer of hematopoietic stem cells alters subsequent pulmonary immune responses in the transplant host. The syngeneic mouse model enables study of conditioning and transplantation in the absence of the confounding effects of GvHD or immunosuppressive drug therapy. In a syngeneic BMT mouse model, we have previously demonstrated that mice undergoing BMT are more susceptible to lytic pulmonary infection with the murine gammaherpesvirus 68 (MHV-68), even after reconstitution of immune cell numbers. Our data suggested that this increased susceptibility was related to decreased interferon-γ production by CD4 cells and increased transforming growth factor (TGF)–β1 in the lung.13 In the present study, we characterized virus-induced pneumonitis that occurs during MHV-68 latency14 in BMT mice and persists even 49 days after infection, long after the lytic infection has resolved. In our model, lungs from latently infected BMT mice were characterized by fibrosis, foamy alveolar macrophages, peripheral inflammation, and diffuse alveolar damage. Our data suggested that TGF-β has an important role in promoting pneumonitis, which supports a previous report of herpes simplex type 1–induced pneumonitis in allogeneic mice with GvHD that could be alleviated with use of anti–TGF-β therapy.15 We observed that blocking of TGF-β signaling in the BMT setting can alleviate development of pneumonitis during latent MHV-68 infection.
Materials and Methods
Mice
C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME). Mice expressing dominant-negative TGF-β receptor II (dnTGFβRII) under the permissive CD4 promoter16 backcrossed onto the C57BL/6 background were obtained from Jackson Laboratory and bred at the University of Michigan. Because of the nature of the promoter construct, these mice lack functional TGF-β receptor II in both CD4 and CD8 T cells.16 Mice expressing the same construct under the CD11c promoter17 were bred at the University of Michigan. Mice were housed in specific pathogen-free conditions and were monitored daily by veterinary staff. Experiments were approved by the University of Michigan Committee on the Use and Care of Animals.
Bone Marrow Transplantation
BMT was performed as described previously.13, 18, 19 In brief, recipient mice were treated with 13 Gy total body irradiation using a cesium 137 irradiator, delivered in two doses separated by 3 hours. Donor whole bone marrow cells, 5 × 106, from a genetically identical donor in 0.2 mL Dulbecco's modified Eagle's medium (DMEM; Invitrogen Corp., Carlsbad, CA) without serum were injected into irradiated mice via the tail vein. Mice were given acidified water (pH 3.3) for the first 3 weeks after BMT. Total numbers of hematopoietic cells were fully reconstituted in the lungs and spleen at 5 weeks after BMT, with lung lymphocytes 93% donor-derived at that time.13 All infections were initiated at 5 to 6 weeks after BMT.
MHV-68 Infection
MHV-68, 5 × 104 plaque-forming units (pfu) (American Type Culture Collection, Manassas, VA), was diluted in 20 μL PBS and delivered intranasally in mice that had been anesthetized using ketamine and xylazine. In some experiments, mice were infected with different dosages of virus, as noted in the text.
Real-Time RT-PCR
Real-time RT-PCR was performed using a thermocycler (ABI Prism 7000; Applied Biosystems, Inc., Foster City, CA) using a previously described protocol.19 Gene-specific primers and probes (Table 1) were designed using Primer Express software (Applied Biosystems, Inc.).
Table 1.
Primers and Probes for Semiquantitative Real-Time RT-PCR
| Gene | Oligo | Primer sequence |
|---|---|---|
| DNA polymerase (ORF9) | Forward | 5′-ACAGCAGCTGGCCATAAAGG-3′ |
| Reverse | 5′-TCCTGCCCTGGAAAGTGATG-3′ | |
| Probe | 5′-CCTCTGGAATGTTGCCTTGCCTCCA-3′ | |
| Envelope gene gB (ORF8) | Forward | 5′-CGCTCATTACGGCCCAAA-3′ |
| Reverse | 5′-ACCACGCCCTGGACAACTC-3′ | |
| Probe | 5′-TTGCCTATGACAAGCTGACCACCA-3′ | |
| β-Actin | Forward | 5′-CCGTGAAAAGATGACCCAGATC-3′ |
| Reverse | 5′-CACAGCCTGGATGGCTACGT-3′ | |
| Probe | 5′-TTTGAGACCTTCAACACCCCCAGCCA-3′ | |
| M3 | Forward | 5′-AGTGGGCTCACGCTGTACTTGT-3′ |
| Reverse | 5′-TGTCTCTGCTCACTCCATTTGG-3′ | |
| Probe | 5′-CATGGGCAAGTGTTCATCTTAGCC-3′ | |
| Collagen 1 | Forward | 5′-TGACTGGAAGAGCGGAGAGTACT-3′ |
| Reverse | 5′-GGTCTGACCTGTCTCCATGTTG-3′ | |
| Probe | 5′-CTGCAACCTGGACGCCATCAAGG-3′ | |
| Collagen 3 | Forward | 5′-GGATCTGTCCTTTGCGATGAC-3′ |
| Reverse | 5-GCTGTGGGCATATTGCACAA-3′ | |
| Probe | 5′-TGCCCCAACCCAGAGATCCCATTT-3′ | |
| iNOS | Forward | 5′-ACATCAGGTCGGCCATCACT-3′ |
| Reverse | 5′-CGTACCGGATGAGCTGTGAAT-3′ | |
| Probe | 5′-CCCCAGCGGAGTGACGGCA-3′ | |
| Arginase 1 | Forward | 5′-ACCACAGTCTGGCAGTTGGAA-3′ |
| Reverse | 5′-GCATCCACCCAAATGACACA-3′ | |
| Probe | 5′-CTGGCCACGCCAGGGTCCAC-3′ |
Histologic Analysis
Lungs were harvested for histologic analysis as previously described.20 In brief, lungs from euthanized animals were perfused with PBS, inflated with 1 mL 10% buffered formalin, removed, and fixed for 6 to 24 hours in formalin. Lungs were placed in ethanol for at least 24 hours, embedded in paraffin, and cut into 3-μm sections. Sections were placed on slides and stained using H&E or Masson's trichrome blue for collagen deposition.
Apoptosis Staining
Lung sections were stained with fluorescein isothiocyanate–labeled E-cadherin as a marker for epithelial cells and an in situ cell death detection kit (TUNEL-TMR red; Roche Applied Science, Indianapolis, IN) according to the manufacturer's instructions. Apoptotic cells were counted in four high-power fields in two tissue sections per group.
Pathology Scoring
H&E- and trichrome-stained slides were analyzed by a pathologist (S.F.) in a blinded fashion. Lungs were scored for severity of fibrosis, perivascular inflammation, and peripheral inflammation on a scale of 0 (absent) to 3 (severe). The presence of foamy alveolar macrophages and hyaline membranes was graded as 0 (absent) or 1 (present). The scores for each factor were totaled to give a pathology score, with 11 indicating the most severe phenotype.
Oxygen Saturation Measurements
Oxygen saturation was measured using a pulse oximeter (Mouse Ox; Starr Life Sciences Corp., Oakmont, PA) according to the manufacturer's instructions. In brief, mice were anesthetized, and their neck hair was removed using a depilatory cream. After 24 hours, collar clip sensors were placed on mice that were awake, and oxygen saturation was measured as an average over 15 seconds per mouse.
Bronchoalveolar Lavage
Bronchoalveolar lavage (BAL) was performed as previously described, with modifications.19 In brief, cells were isolated from the alveolar space via lavage using 20 mL PBS containing 5 mmol/L EDTA in successive 1-mL aliquots. BAL fluid was harvested by instilling 1 mL PBS containing 5 mmol/L EDTA into lung and removing fluid via suction. The BAL fluid was centrifuged to remove cells. In some experiments, alveolar macrophages were isolated via plastic adherence in serum-free medium for 2 hours. BAL fluid or cell supernatants were assayed for total TGF-β1 levels after acidification and neutralization using the DuoSet ELISA Development System kit (R&D Systems, Inc., Minneapolis, MN), for hydrogen peroxide using the Amplex Red Hydrogen Peroxide/Peroxidase Assay Kit (Molecular Probes, Inc., Eugene, OR), and for nitrite using the Griess Reagent System (Promega Corp., Madison, WI) following the manufacturers' instructions.
Lung Collagenase Digestion
Lung leukocytes were isolated from collagenase and DNase digested lungs as previously described.13 B and T cells were collected via magnetic sorting using anti-CD3 beads or biotinylated CD19 antibody followed by incubation with streptavidin beads (Miltenyi Biotec, Inc., Auburn, CA). Cell supernatants were assayed for TGF-β using and enzyme-linked immunosorbent assay (ELISA).
Anti-CD25 Treatment
On day 10 after infection, BMT mice were subjected to 100 μL anti-CD25 ascites or 100 μg isotype control.
Flow Cytometry
Cells, 1 × 106, were stained for flow cytometry using fluorochrome-conjugated antibodies against the cell surface markers CD4, CD8, and CD19 (BD Biosciences, San Jose, CA) after incubation with anti-CD16/CD32 (FcBlock; BD Biosciences). Data were analyzed using flow cytometry analysis software (FlowJo, version 7.5; Tree Star, Inc., Ashland, OR).
Hydroxyproline Assays
Collagen content was measured in lungs using a hydroxyproline assay. In brief, lungs were homogenized in 1 mL PBS and hydrolyzed via addition of 1 mL 12 N hydrochloric acid. Samples were then baked at 110°C for 12 hours. Five-microliter aliquots were then assayed by adding cloramine T solution for 20 minutes followed by development with Erlich's reagent at 65°C for 15 minutes, as previously described.21 Absorbance was measured at 550 nm, and the amount of hydroxyproline was determined against a standard curve generated using known concentrations of a cis-hydroxyproline standard (Sigma-Aldrich, St. Louis, MO).
Alveolar Epithelial Cell Purification
Type II alveolar epithelial cells (AECs) were isolated after dispase digestion of the lower airways and removal of CD32- and CD45-expressing cells via magnetic sorting. Fibroblasts were removed via overnight adherence to plastic, before AECs were enumerated and plated on fibronectin-coated plates, as described previously.22
Viral Genome Quantification
DNA was prepared from MHV-68–infected lungs using the DNeasy Blood and Tissue Kit (Qiagen, Inc., Valencia, CA), and PCR was performed to detect the glycoprotein B (gB) viral coding sequence as previously described.23, 24 Values were compared with a standard curve consisting of gB plasmid DNA diluted at known copy numbers. Reported values were normalized to 100 ng input DNA for each reaction. For gB DNA analysis, the forward primer was 5′-GGCCCAAATTCAATTTGCCT-3′, the reverse primer was 5′-CCCTGGACAACTCCTCAAGC-3′, and the probe was 5′-6-(FAM)-ACAAGCTGACCACCAGCGTCAACAAC-3′(TAMRA).
Reagents
The complete medium used was DMEM (Lonza Walkersville, Inc., Walkersville, MD) with 10% fetal bovine serum (Fisher Scientific, Pittsburgh, PA), 1% penicillin-streptomycin (Gibco-BRL, Invitrogen Corp.), 1% l-glutamine (Fisher Scientific), and 0.1% amphotericin B (Lonza Walkersville, Inc.). The serum-free medium used was DMEM with 1% bovine serum albumin (Sigma-Aldrich), 1% penicillin-streptomycin, 1% l-glutamine, and 0.1% amphotericin B.
Statistical Analysis
Statistical significance between the two groups was measured using Student's t-test with Prism 5 software (GraphPad Software, Inc., La Jolla, CA). Data are given as mean ± SEM. P < 0.05 was considered significant.
Results
BMT and Control Mice Demonstrate an Equivalent Viral Load at Day 21 after Infection
We have previously demonstrated that syngeneic BMT mice are more susceptible to lytic MHV-68 infection measured at day 7 after challenge despite complete immune cell reconstitution.13 To determine whether BMT mice exhibited an increased viral load during MHV-68 latency, syngeneic BMT mice at 5 weeks after transplantation and control mice that had not undergone transplantation were infected with 5 × 104 pfu MHV-68 intranasally, and lungs were harvested at day 21 after infection, a time when MHV-68 is latent in the lung.25 Using real-time RT-PCR on whole-lung RNA, we observed that expression of the predominantly latent viral gene M3 was expressed at much higher levels than the lytic viral gene gB, which suggests that MHV-68 was latent at this time in both groups of mice. In addition, no significant difference was observed between levels of either gene in lungs of BMT or control mice (Figure 1A). To confirm that there was no difference in latent viral load, DNA was harvested from lungs at day 21 after infection, and copy numbers of the viral genome were quantified using real-time PCR and a plasmid standard containing known copy numbers of gB DNA. No significant difference was observed in quantities of viral genomes between BMT and control mice (Figure 1B).
Figure 1.
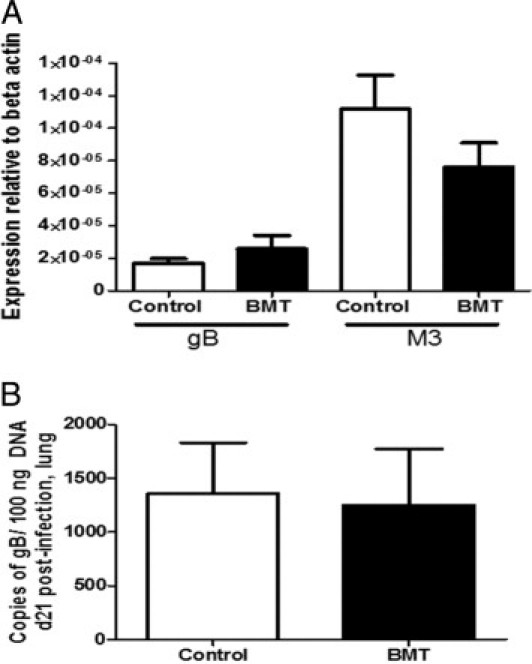
BMT and control mice have similar latent viral load at day 21 after infection with MHV-68. Control and syngeneic BMT mice at 5 weeks after transplantation were infected with 5 × 104 pfu MHV-68 intranasally. A: At day 21 after infection, lungs were harvested for RNA, and expression of the lytic viral envelope glycoprotein gene gB and the predominantly latent viral gene M3 were measured using real-time RT-PCR. There was no significant difference in expression of either of these genes between BMT and control mice (n = 11 mice per group; data representative of two independent experiments). B: At day 21 after infection, lungs were harvested for DNA, and copy number of viral gB gene was quantified using real-time PCR using a standard with known quantities of gB DNA. There was no significant difference in latent viral genome load between BMT and control groups (n = 3 mice per group; data representative of two independent experiments).
BMT Mice Exhibit Histologic Changes in Lung during Latent MHV-68 Infection
Despite observing no difference in viral load at day 21 after infection with MHV-68, lungs from BMT mice demonstrated significant histologic changes compared with lungs from control mice that did not undergo transplantation. Lung sections from BMT and control mice were stained using H&E at day 21 after infection (Figure 2A). H&E- and trichrome-stained slides were analyzed in a blinded fashion by a pulmonary pathologist (S.F.), and lungs from BMT mice exhibited mild to moderate fibrosis, foamy alveolar macrophages, peripheral inflammation, and diffuse alveolar damage. Both control and BMT mice exhibited perivascular inflammation (Figure 2B). Lungs were scored on a scale of 0 (normal lung) to 11 (most severe disease) (see Materials and Methods). Compared with control mice, BMT mice demonstrated a significant increase in histology score (Figure 2C). To determine whether disease observed in BMT mice was virus-induced or due only to the conditioning regimen, lung sections from uninfected BMT mice at 8 weeks after transplantation were analyzed. H&E staining demonstrated that uninfected BMT lungs did not exhibit the pathologic features observed in latently infected BMT lungs (Figure 2A). In addition, compared with latently infected BMT mice, uninfected BMT mice demonstrated a significantly lower histology score (Figure 2C).
Figure 2.
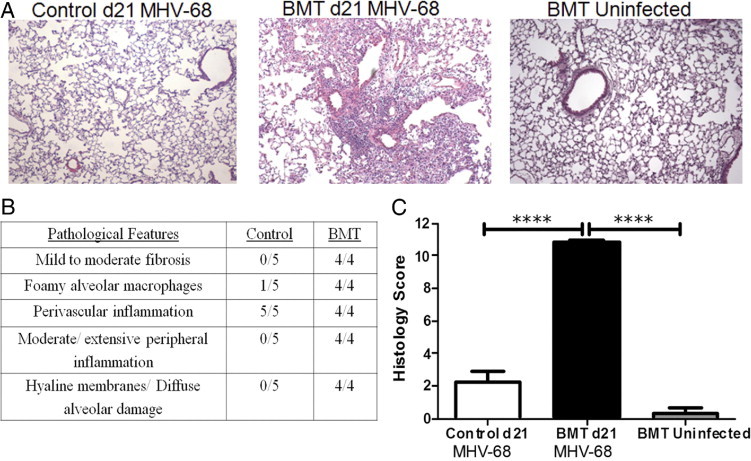
BMT mice, but not control mice, demonstrated virus-induced lung disease during latent MHV-68 infection. A: Lungs from syngeneic BMT and control mice were harvested for histologic analysis, either at day 21 after infection with MHV-68 or uninfected at 8 weeks after BMT, and sections were stained using H&E (×100 magnification). B: H&E-stained lung sections from BMT and control mice at day 21 after infection with MHV-68 were analyzed for the presence of pathologic features in a blinded fashion (n = 5 control mice and 4 BMT mice; data representative of two separate experiments). C: Lung sections were scored on the basis of presence and severity of pathologic features, and a composite score was generated for each mouse. A mean histology score was generated for each group (n = 7 infected BMT mice, 5 infected control mice, and 3 uninfected BMT; data representative of three separate experiments; ****P ≤ 0.0001).
Latently Infected BMT Lungs Are Fibrotic
To determine whether MHV-68 induced a fibrotic response in the lungs of BMT mice, lung sections from BMT and control mice at day 21 after infection were harvested and stained with trichrome. Blue coloration indicating collagen deposition was observed throughout the lungs in the BMT mice but not in the control mice. Lungs from uninfected BMT mice at 8 weeks after transplantation did not show abnormal collagen deposition (Figure 3A). To quantify collagen expression in lungs of BMT and control mice in response to latent MHV-68 infection, we performed hydroxyproline assays to quantify collagen deposition. Figure 3B demonstrates that, compared with control mice, BMT mice exhibited significantly increased collagen content in their lungs at day 21 after infection. In addition, RNA was harvested from lungs at day 21 after infection, and expression of collagen 1 and collagen 3 transcripts was determined using real time RT-PCR. BMT lungs expressed an approximate twofold increase in both genes (Figure 3C). Thus, lungs from latently infected BMT mice are fibrotic. To determine whether the pneumonitis and fibrotic phenotype persisted, we infected BMT and control mice and harvested their lungs for histologic analysis at day 49 after infection. At that time, we observed persistence of pneumonitis and fibrosis, as demonstrated by H&E and trichrome staining, and the histology score was significantly increased in BMT mice compared with control mice (see Supplemental Figure S1 at http://ajp.amjpathol.org).
Figure 3.
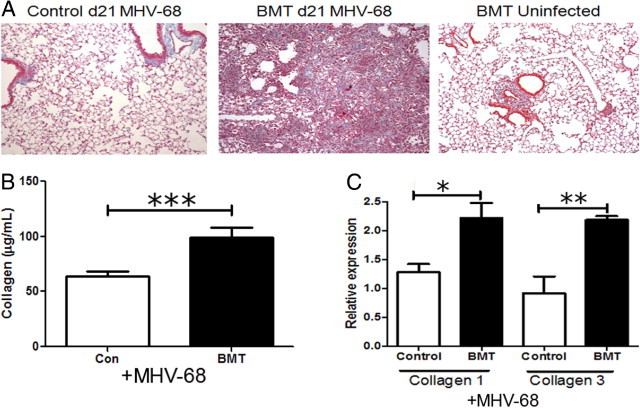
BMT lungs, but not control lungs, are fibrotic during latent MHV-68 infection. Syngeneic BMT and control mice were infected with 5 × 104 pfu MHV-68, and lungs were harvested at day 21 after infection. A: Lung sections from infected or not infected BMT and control mice were stained using Masson's trichrome (magnification ×100). B: Collagen content in lungs of control or BMT mice at day 21 after infection were measured using a hydroxyproline assay (n = 5). ***P = 0.0057. C: Lungs were harvested for RNA, and expression of collagen 1 and collagen 3 was analyzed using real-time RT-PCR. Expression was normalized to the control group for each gene. Collagen 1 and collagen 3 were significantly increased in BMT lungs (n = 3 mice per group; *P = 0.0348 and **P = 0.0129, respectively; data representative of two independent experiments).
BMT Mice Have Increased Inflammatory Cells in the Alveolar Space during Latent MHV-68 Infection
To characterize the cells present in the alveolar space at day 21 after infection, BAL was performed in BMT and control mice, and total cells were counted. There was a significant increase in total cell numbers harvested from BMT lungs compared with control lungs (Figure 4A). BAL cells from control mice were largely monocytes and macrophages, as determined via differential counting. However, BMT BAL cells included an influx of lymphocytes and neutrophils (Figure 4B). To determine lymphocyte populations in the alveolar space, cells were analyzed using flow cytometry. Compared with control mice, BMT mice demonstrated significant increases in numbers of both CD4 and CD8 cells, and few B cells were present in either group (Figure 4C).
Figure 4.
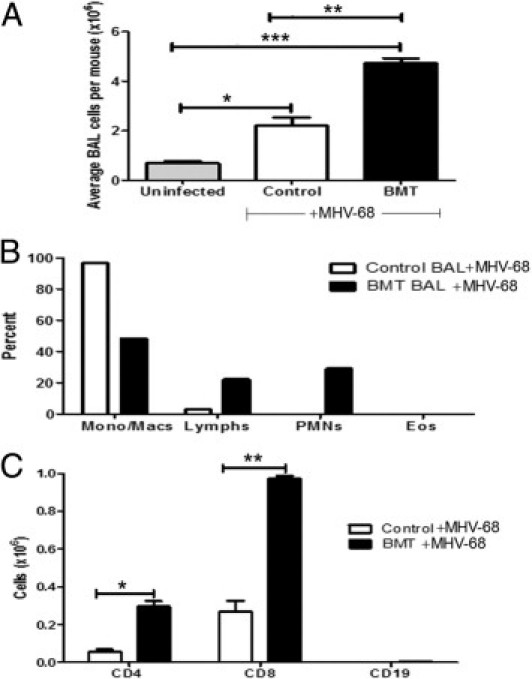
BMT mice demonstrate increased infiltration of inflammatory cells in the alveolar space during latent MHV-68 infection. At day 21 after infection with 5 × 104 pfu MHV-68, cells were harvested from the alveolar space of BMT and control mice using BAL. A: Compared with control mice, BMT mice exhibited a significant increase in total number of cells harvested by BAL (**P ≤ 0.01; n = 5 mice per group; data representative of three independent experiments); *P ≤ 0.05; ***P ≤ 0.001. B: BMT mice have increased percentages of lymphocytes and neutrophils in the alveolar space, as determined by differential counting (n = 5 mice per group; data representative of two independent experiments). C: BAL cells were harvested and stained for flow cytometry for CD4, CD8, and CD19. Numbers of CD4 and CD8 cells were significantly increased in BMT mice (*P = 0.0166 and **P = 0.0074, respectively; n = 5 mice per group; data representative of two independent experiments).
Reduced Oxygen Saturation in Latently Infected BMT Mice
To understand whether pneumonitis in BMT mice led to changes in lung physiologic features, we used the Mouse Ox pulse oximeter (Starr Life Sciences Corp.) to measure oxygen saturation in mice at day 21 after infection. A significant decrease in oxygen saturation was observed in BMT mice compared with control mice (see Supplemental Figure S2 at http://ajp.amjpathol.org). However, there was no difference in oxygen saturation between these groups before infection (data not shown). These data indicate that gammaherpesvirus infection in BMT mice leads to reduced oxygen saturation.
Alveolar Macrophages Show a Mixed Classic and Alternative Activation Phenotype
Lungs from BMT mice latently infected with MHV-68 contained foamy alveolar macrophages (Figure 2). H&E staining at ×400 magnification demonstrated these macrophages more clearly (Figure 5A). Because alternative activation of macrophages (reviewed by Gordon and Martinez26) has been implicated in contributing to pulmonary fibrosis in mouse models,27 we wondered whether macrophages in our model expressed markers of alternative or classic activation. Alveolar macrophages were harvested from control or BMT mice via plastic adherence, and expression of the classic marker inducible nitric oxide synthesis (iNOS) and the alternative activation marker arginase 1 was determined using real time RT-PCR. A significant increase was observed in expression of both genes in BMT cells compared with control cells at day 21 after infection (Figure 5B). To determine the kinetics for macrophage activation, we also collected alveolar macrophages on days 9, 13, and 19 after infection. Expression of arginase 1 was significantly elevated by day 13 after infection, whereas expression of iNOS was not elevated until day 21. In addition, the fold increase in arginase 1 was much greater than that noted for iNOS (see Supplemental Figure S3 at http://ajp.amjpathol.org).
Figure 5.
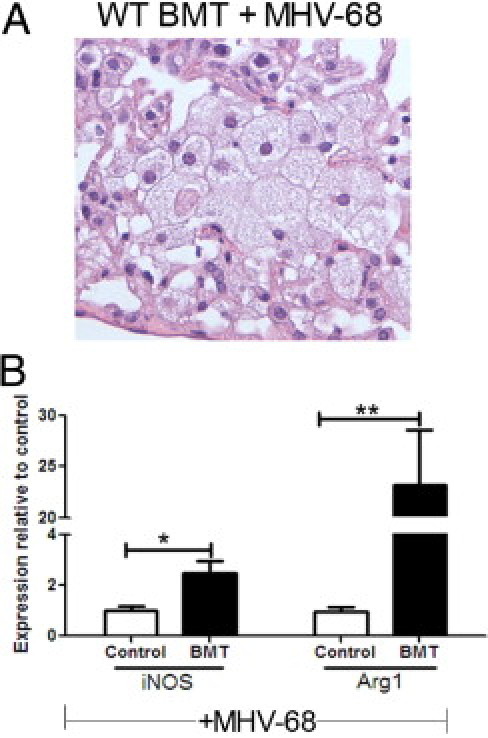
BMT alveolar macrophages are foamy and express increased iNOS and arginase 1. BMT and control mice were infected with 5 × 104 pfu MHV-68 for 21 days. A: H&E-stained BMT lung section demonstrates foamy alveolar macrophages (magnification ×400). B: BAL cells from infected BMT and control mice were plated for 2 hours in serum-free DMEM. Nonadherent cells were removed, and adherent cells were harvested for RNA. Expression of the classic activation marker iNOS and an alternative activation marker arginase 1 (Arg1) were both significantly increased in BMT cells (*P = 0.0270 and **P = 0.0065, respectively) as determined using real-time RT-PCR. Expression of each gene in control mice was set to 1 (n = 4 mice per group; data representative of two independent experiments).
Despite the steady increase in arginase 1 expression, alveolar macrophages from BMT mice did not express IL-4 or IL-13 mRNA, cytokines thought to be critical for alternative activation,26 at day 21 after infection (data not shown). In addition, IL-4 and IL-13 protein could not be detected using an ELISA in collagenase-digested lungs, and little IL-13 and no IL-4 mRNA could be detected in cultured BAL cells including lymphocytes in BMT mice on day 21 after infection (data not shown).
BAL Fluid from BMT Mice Contains Pro-Fibrotic Mediators
Because oxidative stress has been implicated in contributing to pulmonary fibrosis in studies in both humans28 and mice,29 we tested levels of ROS in the BAL fluid of BMT and control mice at day 21 after infection. Levels of H2O2 and NO2− were significantly increased in BAL fluid from BMT mice compared with control mice (Figure 6, A and B) at day 21 after infection. We next determined whether the level of TGF-β, a potent pro-fibrotic mediator,30, 31 was increased in BAL fluid from BMT mice. Our previous studies had demonstrated that whole lung homogenates from uninfected BMT mice at 5 weeks after transplantation had significantly increased total TGF-β1 protein.13 We now report that at day 21 after infection, compared with control mice, BMT mice demonstrated a significant increase in total TGF-β1 protein in BAL fluid (Figure 6C).
Figure 6.
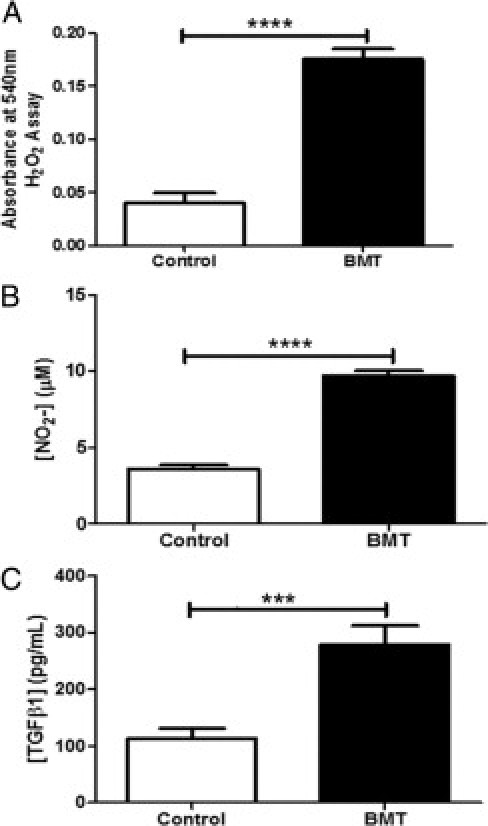
BMT mice exhibited increased H202, NO2−, and TGF-β1 in BAL fluid. BAL fluid was harvested from BMT and control mice at day 21 after infection with 5 × 104 pfu MHV-68. The relative levels of H202 (A), NO2 (B), and total TGF-β1 (C) were significantly increased in BAL fluid from BMT mice compared with infected control mice (***P = 0.0027, and ****P < 0.0001 respectively; n = 5 mice per group; data representative of two independent experiments).
To determine the kinetics of TGF-β secretion, we analyzed BAL fluid for TGF-β levels on days 9, 13, 19, and 21 after infection. Levels were increasing by day 19 after infection, and reached statistical significance by day 21 (Supplemental Figure S4 at http://ajp.amjpathol.org). Generation of ROS, as assessed by nitrite production in BAL fluid, mirrored the increases in TGF-β levels (see Supplemental Figure S4 at http://ajp.amjpathol.org).
Blocking TGF-β Signaling in T Cells Significantly Improves Pneumonitis and Fibrosis in BMT Mice
To understand whether TGF-β was contributing to gammaherpesvirus-induced pneumonitis and fibrosis in this model, we used mice expressing the dnTGFβRII transgene in T cells as BMT donors. Using the same conditioning regimen as in previous experiments, recipient BMT mice underwent transplantation using bone marrow from a T-cell dnTGFβRII donor16 (expressing the dnTGFβRII transgene under the permissive CD4 promoter and blocking TGF-β signaling in both CD4 and CD8 T cells). This approach enabled us to determine whether TGF-β was promoting pneumonitis via effects on donor T cells at day 21 after infection. We have previously published that whereas wild-type (WT) BMT mice demonstrate reduced ability to control lytic MHV-68 replication in the lung at day 7 after infection, T-cell dnTGFβRII BMT mice do not.13 At day 21 after infection, we observed a drastic reduction in pneumonitis, fibrosis, and overall histology score in infected BMT recipients of T-cell dnTGFβRII bone marrow (Figure 7, A and B). Considering only the fibrosis component of the composite histology score, infected BMT mice scored 2.86 ± 0.1 units, whereas infected T-cell dnTGFβRII BMT mice scored 1.0 ± 0.3 units (P < .001). We next measured levels of TGF-β in BAL fluid at day 21 in infected mice. BAL fluid levels of TGF-β were diminished on day 21 after infection when donor T cells were unresponsive to TGF-β signaling (Figure 7C). Levels of nitrite were intermediate between control and BMT values (Figure 7D). In addition, expression of arginase 1 and iNOS, markers of alternatively or classically activated macrophages, was also unchanged. When levels of these transcripts were measured using real-time RT-PCR in isolated alveolar macrophages on day 21 after infection and compared with those in control mice normalized to 1, iNOS levels were 2.7 ± 0.6-fold higher and arginase 1 levels were 30.5 ± 3.5-fold higher (n = 6). These levels are similar to those measured in WT BMT mice (Figure 5). When we considered transcription of collagen 1 and collagen 3, the T-cell dnTGFβRII BMT mice demonstrated reductions when compared with the WT BMT mice; however, in these experiments, the changes did not quite reach statistical significance (Figure 7, E and F).
Figure 7.
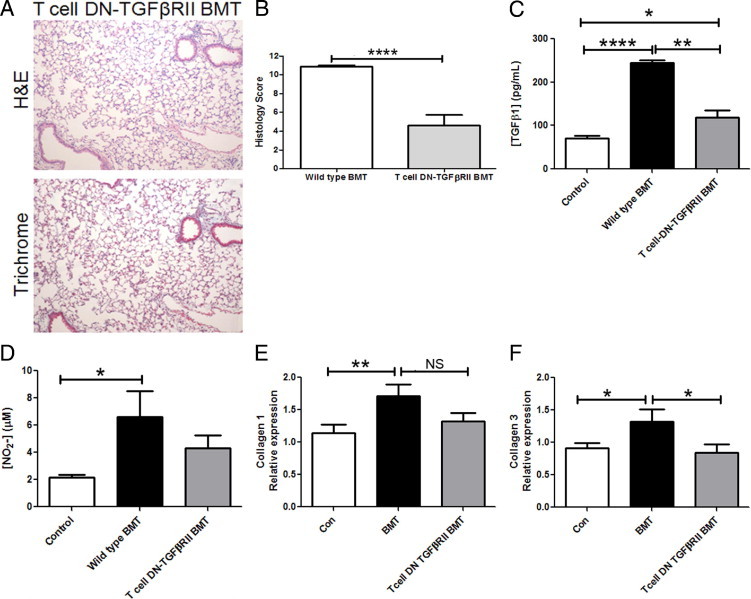
In mice, transplantation of T-cell dnTGFβRII bone marrow significantly reduces pneumonitis, fibrosis, and TGF-β levels during latent MHV-68 infection. BMT mice who received T-cell dnTGFβRII bone marrow were infected with 5 × 104 pfu MHV-68. A: Lungs were harvested at day 21 after infection for histologic analysis and stained using H&E and Masson's trichrome (magnification ×100; n = 6 mice; data representative of two independent experiments. B: Lung sections were scored in a blinded fashion by a pathologist (S.F.) on the basis of presence and severity of pathologic features. C: TGF-β levels were measured in BAL fluid at day 21 after infection (n = 4 or 5 mice per group; data representative of two independent experiments). D: Levels of nitrite were measured in BAL fluid at day 21 after infection (n = 3 to 5 mice per group). E and F: Expression of collagen 1 or collagen 3 mRNA was measured in lungs collected at day 21 after infection (n = at least 10 mice per group; data representative of three experiments; *P ≤ 0.05, **P ≤ 0.01, ****P ≤ 0.0001).
Pneumonitis and Fibrosis in WT BMT Mice Are Not Related to Regulatory T Cells
Given the importance of TGF-β in the development of pneumonitis and fibrosis in BMT mice and the importance of TGF-β in the biology of regulatory T cells (Tregs), we investigated the possible role of Tregs. We have previously demonstrated that Tregs were not impairing clearance of lytic virus at day 7 after infection.13 To determine whether Tregs were important for generation of TGF-β during latent infection, we injected mice with anti-CD25 or isotype control antibody on day 10 after infection. On day 21 after infection, mice treated with anti-CD25 demonstrated equivalent levels of fibrosis as did mice treated with isotype control (see Supplemental Figure S5A at http://ajp.amjpathol.org). In addition, there were similar increases in TGF-β (see Supplemental Figure S5B at http://ajp.amjpathol.org). Nitrite levels in the BAL fluid were reduced, but not significantly (see Supplemental Figure S5C at http://ajp.amjpathol.org). These data indicate that Tregs are not the primary source of TGF-β during MHV-68 latency in BMT mice.
Increases in TGF-β after Infection in BMT Mice Are from Parenchymal Cells in the Lung
We next sought to identify the potential cellular source of TGF-β in our BMT mice at day 21 after infection. Alveolar macrophages were isolated via BAL, and T cells and B cells from lung digests from control and BMT mice on day 21 after infection. These cell populations were cultured for 24 hours, and TGF-β was measured in cell supernatants. None of these cell types demonstrated increased production of TGF-β when isolated from BMT mice at day 21 after infection (see Supplemental Figure S6, A–C, at http://ajp.amjpathol.org). In addition, culture of total BAL cells (containing additional leukocytes such as neutrophils) at day 21 after infection did not demonstrate increased TGF-β (data not shown). These results suggest that parenchymal cells may be the source of increased TGF-β production. To determine whether AECs were the source, we attempted to purify AECs from control and BMT mice on day 21 after infection. However, in three separate experiments, only half the number of AECs could be recovered from infected BMT mice compared with control mice (see Supplemental Figure S6D at http://ajp.amjpathol.org). Of the AECs that were recovered, there were no differences in TGF-β production (see Supplemental Figure S6E at http://ajp.amjpathol.org), although we think it likely that the injured AECs which could not be isolated may be a source of TGF-β in vivo.
T-Cell dnTGFβRII BMT Mice Demonstrate Reduced Levels of Apoptosis Compared with BMT Mice after Infection
Our findings of reduced numbers of AECs in BMT mice on day 21 after infection (see Supplemental Figure S6D at http://ajp.amjpathol.org) suggested that epithelial cell injury may contribute to development of pneumonitis and fibrosis. To explore this further, we stained lung sections from control, BMT, and T-cell dnTGFβRII BMT mice for apoptosis using a TUNEL assay and E-cadherin to highlight epithelial cells. Increased levels of apoptosis were noted in BMT mice compared with control mice (Figure 8). Counts of apoptotic cells in lung sections demonstrated that infected control mice exhibited 26 ± 5.4 cells per high-power field, whereas BMT mice exhibited 55 ± 5.6 apoptotic cells (P = 0.004). In addition, there was a trend toward lower levels of apoptosis in the infected T-cell dnTGFβRII BMT mice, ie, 38 ± 2 cells per high-power field, than in infected WT BMT mice (P = 0.06).
Figure 8.
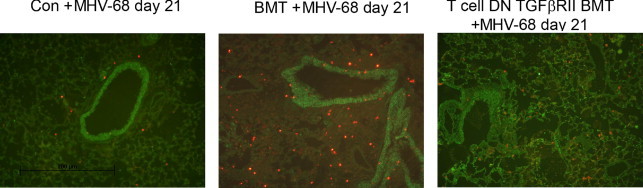
BMT mice demonstrated increased apoptosis at day 21 after infection with MHV-68. Lung sections from control, BMT, or T-cell dnTGFβRII BMT mice were stained using FITC-labeled E-cadherin (green) to mark epithelial cells and TUNEL (TMR red) to indicate apoptosis. Overlays are representative of data from two mice in each group.
CD11c-dnTGFβRII Mice Are Only Modestly Protected from Virus-Induced Pneumonitis and Fibrosis
To determine whether TGF-β signaling in CD11c-expressing donor cells including alveolar macrophages, dendritic cells, and natural killer cells would affect the development of pneumonitis and fibrosis, we transplanted WT or CD11c-dnTGFβRII bone marrow into mice and examined the mice at days 7 and 21 after infection with MHV-68. CD11c-dnTGFβRII BMT mice demonstrated increased lytic viral replication at day 7 after infection, which is similar to levels in WT BMT mice (see Supplemental Figure S7A at http://ajp.amjpathol.org). Accordingly, these mice demonstrated far less protection from disease (see Supplemental Figure S7B at http://ajp.amjpathol.org) and lower histology score (see Supplemental Figure S7C at http://ajp.amjpathol.org) than did the T-cell dnTGFβRII BMT mice (Figure 7).
Within BMT Mice, Lytic Viral Load Corresponds with Subsequent Degree of Pneumonitis and Fibrosis
Data from the present study demonstrated that T-cell dnTGFβRII BMT mice demonstrated restored ability to control lytic MHV-68 replication13 and that these mice are largely protected from development of pneumonitis at day 21 after infection (Figure 7). Correspondingly, CD11c-dnTGFβRII BMT mice were not able to restore control of lytic replication and proceed to development of severe pneumonitis and fibrosis (see Supplemental Figure S7 at http://ajp.amjpathol.org). Considered together, these results suggest that initial control of lytic virus can prevent subsequent pneumonitis and fibrosis. To determine whether degree of lytic replication corresponds with subsequent disease in BMT mice, we infected BMT mice with increasing dosages of virus. We have previously reported that BMT mice challenged with 1 × 103 pfu MHV-68 demonstrate an approximately twofold increase in viral replication at day 7 after infection, BMT mice infected with 5 × 104 pfu demonstrate an approximately sixfold increase over control mice, and BMT mice infected with 1 × 106 pfu demonstrate a ninefold increase in lytic virus gene expression.13 We now demonstrate that development of lung disease in BMT mice correlates with the ability to control lytic replication. The lower lytic viral dosage is associated with less disease (Figure 9A) and a lower histology score (Figure 9B) at day 21 than noted with the higher viral titer. In addition, lower initial lytic virus results in lower levels of nitrite (Figure 9C) and ultimately in lower TGF-β levels in the BAL fluid (Figure 9D). It should be noted that control mice infected with the lower viral dosage exhibit essentially no signs of disease at day 21 after infection (data not shown). Infection of BMT mice with a higher dose of virus, 3 × 105 pfu, resulted in death of all mice by day 14. Together, these findings suggest that impaired ability to control lytic viral replication in BMT mice leads to increased apoptosis and lung damage, increased TGF-β levels in the BAL fluid, and ultimately pneumonitis and fibrosis during viral latency.
Figure 9.
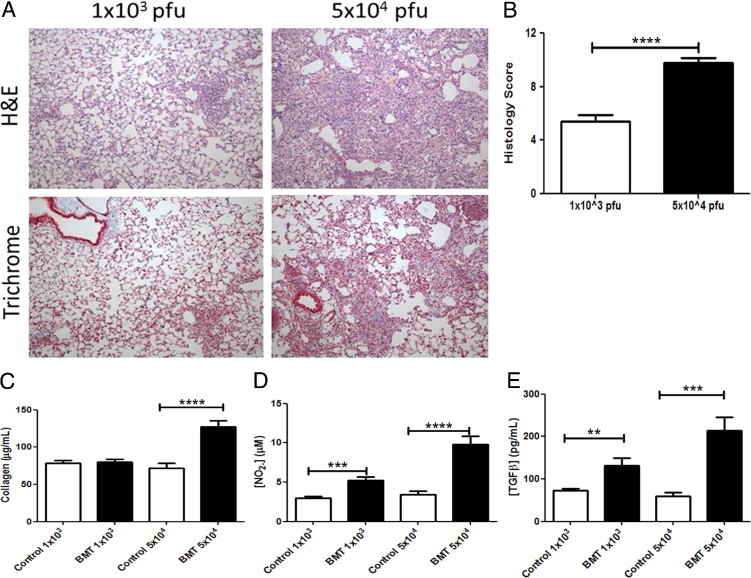
Increasing lytic viral dose worsens subsequent lung disease. BMT mice were infected with 1 × 103 or 5 × 104 pfu MHV-68, and measurements were made at day 21 after infection. A: Representative H&E- and trichrome-stained sections. B: Histology score based on data from five mice demonstrate the higher dosage results in worsened disease. C: Control or BMT mice were infected with both dosages of virus, and collagen content was measured in lungs via hydroxyproline content. Only the 5 × 104 pfu dose of virus caused significant increases in lung fibrosis (n = 7 or 8 per group). D: Levels of nitrite were increased in BAL fluid of BMT mice compared with control mice infected with both dosages at day 21 after infection (n = 7 or 8 per group). E: Levels of TGF-β from BAL fluid were analyzed using an ELISA. TGF-β was significantly increased in BAL fluid from BMT mice compared with control mice with both dosages of virus (n = 7 or 8 per group; **P ≤ 0.01, ***P ≤ 0.001, ***P ≤ 0.001).
Discussion
We have previously demonstrated in our myeloablative syngeneic BMT model that mice have fully restored hematopoietic cell numbers in the lung at 5 weeks after transplantation, yet have increased susceptibility to pulmonary lytic infection with MHV-68 measured at day 7 after challenge.13 In the present study, we observed that BMT mice develop severe persistent MHV-68–induced pneumonitis during virus latency (days 21 to 49) despite equivalent latent viral loads. By day 21 after infection, BMT mice demonstrate peripheral inflammation in the lung, as well as presence of foamy alveolar macrophages, fibrosis, and diffuse alveolar damage. This phenotype persists even 7 weeks after infection. Compared with infected control mice who have not undergone transplantation, BMT mice exhibit increased infiltration of neutrophils and of CD4, and CD8 cells into the alveolar space during MHV-68 latency. Accordingly, BMT mice demonstrate reduced oxygen saturation during virus latency. Alveolar macrophages from BMT mice are histologically large and foamy, and, as a population, expression of the alternative activation marker arginase 1 precedes expression of the classic activation marker iNOS. BAL fluid from BMT mice during MHV-68 latency contains increased levels of H2O2 and NO2−, indicating oxidative stress, as well as an increase in the pro-fibrotic cytokine TGF-β1. BMT mice also exhibit increased evidence of apoptosis within the lung. BMT mice that are transplant recipients of bone marrow from T-cell dnTGFβRII mice demonstrate a drastic reduction in severity of pneumonitis, whereas recipients of bone marrow from CD11c-dnTGFβRII mice exhibit only a moderate reduction in pneumonitis severity. The protection in T-cell dnTGFβRII BMT mice is associated with less apoptosis and reduced levels of TGF-β in the BAL fluid, and a trend toward reduced nitrite production. Considered together, these data suggest that severe gammaherpesvirus-induced pneumonitis can occur in BMT mice long after the lytic phase of virus infection has resolved and that this may be alleviated by blocking TGF-β signaling, especially in T cells. We hypothesized that the prevention of disease in the T-cell dnTGFβRII BMT mice was associated with the ability of these mice to control lytic MHV-68 replication because severity of disease increased with increasing viral challenge in WT BMT mice.
We have previously reported that BMT mice demonstrated increased susceptibility to lytic MHV-68 infection, as noted by higher expression of lytic viral genes at day 7 after infection, viral immunohistochemistry, and plaque assay.13 We now report that these mice have equivalent latent viral loads (Figure 1) at day 21 after infection as control mice given the same dosage of virus. It was surprising to note that BMT mice have equivalent latent viral loads after having increased lytic virus; however, our results are consistent with previous studies that demonstrated that lytic virus titers do not necessarily correlate with latent levels of MHV-68.32, 33 We hypothesize that much of the lytic replication at day 7 may occur in cell types that do not remain resident in the lung by day 21. It is important to note, however, that while levels of lytic infection may not influence the levels of latent viral load at day 21 with MHV-68, the degree of lung damage induced by lytic virus in BMT mice may still be important. In BMT mice, infection with 5 × 104 pfu MHV-68 led to more severe pneumonitis and fibrosis than did infection with 1 × 103 pfu (Figure 9), whereas in control mice, no disease was observed after infection with 1 × 103 pfu virus (data not shown), and only mild disease was noted at the higher dosage (Figure 2). These results highlight that lungs of BMT recipients are uniquely susceptible to induction of viral-induced disease.
Despite observing no difference in latent viral load between control and BMT mice, we noted that BMT mice developed severe persistent pneumonitis and fibrosis during latent MHV-68 infection (Figure 2). It is important to note that in our model, the pneumonitis phenotype was virus-induced. Uninfected BMT mice exhibited no signs of pneumonitis or fibrosis at 2 months after transplantation (Figure 2, Figure 3). Although irradiated C57BL/6 mice develop irradiation-induced fibrosis, this does not occur until 20 to 30 weeks after irradiation, long after the 8-week point used in our studies.34 A recent prospective study of pediatric allogeneic HSCT recipients closely monitored respiratory viral infections after transplantation and observed that early respiratory virus infection after transplantation correlated significantly with development of idiopathic pneumonia syndrome and bronchiolitis obliterans in their cohort. Patients had recovered from initial respiratory symptoms before development of later respiratory complications.35 It is interesting to speculate that an inflammatory stimulus such as a virus can lead to pneumonitis and lung injury at later time points, even after resolution of the initial lytic insult.
In accordance with the severe inflammatory and fibrotic phenotype in BMT mice at day 21 after infection with MHV-68, our data demonstrate that these mice also exhibit reduced oxygen saturation (see Supplemental Figure S2 at http://ajp.amjpathol.org). It is important to highlight that this reduced oxygen saturation measures values at rest and might be even more dramatic if mice are exercised. The reduced oxygen saturation in our BMT mice correlated with reports of abnormal pulmonary function test results that persist over the long term in HSCT recipients.11
In our model, we observed infiltration of inflammatory cells including neutrophils and lymphocytes in the alveolar space during latent MHV-68 infection in BMT mice (Figure 4). The largest lymphocyte population present in the alveolar space in both BMT and control mice was CD8 cells, which are an expanded population during MHV-68 latency.36 We speculate that infiltration of these cells to the alveolar space may be indicative of unresolved inflammation due to the initial virus infection because a percentage of CD8 cells were specific for a viral epitope (data not shown). We also observed the presence of foamy alveolar macrophages in lungs of BMT mice (Figure 5) during latent MHV-68 infection. When the alveolar macrophage population was enriched from BMT mice at day 21 after infection, these cells expressed the classic activation marker iNOS, an enzyme that catalyzes production of nitric oxide, as well as the alternative activation marker arginase 1, an enzyme that promotes fibroblast proliferation (Figure 5B). Classically activated macrophages are thought to promote inflammation, whereas alternatively activated macrophages are associated with wound repair and fibrosis.26, 37 The kinetics of arginase 1 and iNOS expression suggest that alternatively activated macrophages develop more quickly after infection and that the magnitude of arginase 1 expression is greater than that of iNOS (see Supplemental Figure S3 at http://ajp.amjpathol.org). The dichotomy of function between these macrophage subsets correlates well with the inflammatory and fibrotic phenotypes in the lungs in BMT mice during MHV-68 latency.
Alternatively activated macrophages have been implicated in promoting pulmonary fibrosis during chronic MHV-68 infection in IFNγR−/− mice; the appearance of these macrophages was attributed to the type 2 helper T-cell (Th2) environment and expression of IL-1327 in these Th2-biased mice. In our model, we observed no evidence of up-regulated IL-4 or IL-13 production by BAL cells and alveolar macrophages or in whole-lung homogenates (data not shown). However, we have previously reported a significant decrease in numbers of IFN-γ–producing CD4 cells in the lungs of BMT mice during lytic MHV-68 infection (day 7 after infection),13 which suggests that the development of arginase 1–expressing alveolar macrophages may occur in the absence of an overwhelming Th1 response and not simply in the presence of a Th2 response. It is also possible that the protracted expression of IL-10 in the lungs of BMT mice (data not shown) may drive alternative activation of the alveolar macrophages.38 Alveolar macrophages are known to express CD11c; thus, the fact that CD11c-dnTGFβRII BMT mice still exhibit evidence of foamy macrophages at histologic analysis of the lungs as part of the composite score (see Supplemental Figure S7 at http://ajp.amjpathol.org) may suggest that TGF-β signaling in alveolar macrophages is not required for alternative activation. However, we cannot rule out the possibility that the alternatively activated macrophages may have arisen from CD11c-negative monocytes that were still responsive to TGF-β signaling in those transplants.
We also report increased oxidative stress in BMT mice at day 21 after infection with MHV-68, as evidenced by increased levels of H2O2 and NO2− in BAL fluid (Figure 6). These reactive intermediates promote tissue damage and have been implicated in promoting pulmonary fibrosis (reviewed by Kliment and Oury39). For example, in animal studies, reactive nitrogen intermediates stimulated production of TGF-β.40 Accordingly, we observed a significant increase in levels of TGF-β in BAL fluid from BMT mice at day 21 after infection (Figure 6), and kinetic increases in nitrite and TGF-β are similar after infection (see Supplemental Figure S4 at http://ajp.amjpathol.org). It is also possible that the increased presence of neutrophils in the BMT mice contributed to the secretion of oxidants into the alveolar space. While ROS were noted in the BMT mice, it is unknown how much they contributed to the disease we observed. Compared with infected BMT mice, infected T-cell dnTGFβRII BMT mice demonstrate significantly reduced pneumonitis and fibrosis scores. When measuring levels of nitrite in the BAL fluid, the T-cell dnTGFβRII BMT mice demonstrated an intermediate phenotype between that of control and BMT mice (Figure 7). Conversely, the anti-CD25–treated mice exhibited reduced nitrite levels but not improved fibrosis (see Supplemental Figure S5 at http://ajp.amjpathol.org). Thus, it is not clear whether nitrite alterations have a biological effect on fibrosis. It is also interesting that the T-cell dnTGFβRII BMT mice did not demonstrate decreases in the alternative activation marker arginase 1 despite improved pathologic findings. In the final analysis, the factor that most closely tracked with fibrosis and pneumonitis was the level of TGF-β observed in the BAL fluid at day 21 in all cases.
Our data demonstrate that blocking of TGF-β signaling in T cells in the BMT setting leads to a dramatic reduction in inflammation, fibrosis, and lung histology score (Figure 7). These data suggest direct or indirect involvement of T cells in development of pneumonitis in this model. Previous studies from our laboratory have demonstrated that BMT mice expressed increased levels of TGF-β1 in the lung before infection.13 Our data suggested that TGF-β limited effector Th1 responses to lytic MHV-68 infection, thus causing an increase in lytic viral load.13 Subsequent pneumonitis and fibrosis during latent infection may result from the enhanced lytic virus insult, possibly causing increased epithelial cell destruction, the unresolved inflammatory response that accompanies the increased lytic viral load, or altered wound repair mechanisms to control tissue damage. Support for these hypotheses come from several of our observations: i) increasing lytic viral pfu correlates with increased disease during latency in BMT mice (Figure 9); ii) infected BMT mice exhibit evidence of apoptosis that is lessened in T-cell dnTGFβRII BMT mice and control mice (Figure 8); iii) fewer AECs can be isolated from infected BMT mice than from infected control mice at day 21 (see Supplemental Figure S6 at http://ajp.amjpathol.org); and iv) the inflammatory response in BMT mice is much greater than that in infected control mice (Figure 4). The T-cell dnTGFβRII BMT mice express decreased levels of TGF-β1 before infection and exhibit restored numbers of Th1 cells in response to lytic MHV-68 infection.13 These mice also have reduced levels of TGF-β in BAL fluid at day 21 after infection. While we have not definitively proved the source of increased TGF-β, we have ruled out the contribution of Tregs (see Supplemental Figure S5 at http://ajp.amjpathol.org), alveolar macrophages, and B and T cells (see Supplemental Figure S6 at http://ajp.amjpathol.org), which suggests that the source is a parenchymal cell type in the lung. We speculate that injured alveolar epithelial cells that are unable to be isolated at 21 days after infection may contribute; however, it is also possible that endothelial or mesenchymal cell types may participate as well.
We also observed a modest but significant reduction in lung histology score in BMT mice that received donor cells expressing a dnTGFβRII transgene under the CD11c promoter (see Supplemental Figure S7 at http://ajp.amjpathol.org). When analyzing the response to lytic infection, we observed that CD11c-dnTGFβRII BMT mice demonstrated high lytic viral loads at day 7 after infection, similar to that in WT BMT mice (see Supplemental Figure S7A at http://ajp.amjpathol.org). These data suggest that TGF-β signaling has a small role in CD11c-expressing cells to control lytic infection. However, the fact that the CD11c-dnTGFβRII BMT mice demonstrated a modestly improved pneumonitis phenotype suggests that some CD11c-expressing cell type may influence lung disease at a later time. We do not yet know whether the improved phenotype of the CD11c-dnTGFβRII BMT mice is due to blockade of TGF-β signaling in alveolar macrophages, dendritic cells, or natural killer cells; however, the pathologist (S.F.) was still able to detect foamy alveolar macrophages at histologic evaluation of the CD11c-dnTGFβRII BMT mice. However, whether these mice show reduced ROS generation or alternatively activated macrophages remains a formal possibility and will require further study.
Previous work in an allogeneic GvHD model demonstrated that induction of herpes simplex virus type 1 (HSV-1)–induced pneumonitis late after transplantation was also mediated by the effects of TGF-β.15 Although our model also implicates TGF-β in promoting pneumonitis, there are several important differences between the models. Pneumonitis in the HSV-1 model developed at day 5 to 7 after infection and involved periluminal but not parenchymal inflammation and exhibited no indication of fibrosis. In addition, those authors concluded that pneumonitis in the HSV-1 model resulted from GvHD because allogeneic BMT mice without GvHD did not develop lung disease.15 However, because of the difference in timing, it is interesting to speculate that fibrosis and lung disease in the allogeneic mice without GvHD would be manifested later after-HSV-1 as well.
Studies have indicated a correlation between GvHD and noninfectious lung complications such as idiopathic pneumonia syndrome and bronchiolitis obliterans.7 In addition, studies have also reported occurrence of idiopathic pneumonia syndrome in transplant recipients after autologous transplantation,7, 8, 9 which suggests that pneumonitis development in HSCT recipients is multifactorial. In our model, we hypothesized that up-regulation of TGF-β pre-infection in the lungs of BMT mice resulted in poor control of lytic viral replication. In turn, this leads to lung injury, inflammatory cell accumulation, alternative activation of macrophages, induction of ROS, and further increases in TGF-β production during viral latency. The end result is persistent pneumonitis and fibrosis that diminish lung function. Blocking TGF-β signaling, especially in T cells, in the BMT setting is sufficient to significantly reduce virus-induced pathologic changes in this model. These results suggest that anti–TGF-β therapy may be effective in limiting the complications of pneumonitis and fibrosis in the HSCT setting. The focus of future studies will be to determine whether TGF-β blockade at late times (during latency) is efficacious in limiting lung disease.
Acknowledgments
We thank Drs. Keith Bishop and Susan Faust for providing the T-cell dnTGFβRII mice, and Dr. Jason Weinberg for providing the protocol and plasmid for quantification of viral genomes.
Footnotes
Supported by grants AI065543 and HL087846 from the NIH and by the Herman and Dorothy Miller Fund for Innovative Immunology Research.
Supplemental material for this article can be found on http://ajp.amjpathol.org or at doi: 10.1016/j.ajpath.2011.08.002.
Supplementary Data
Pneumonitis in BMT mice persists even 7 weeks after infection. Lungs from syngeneic BMT and control mice infected with 5 × 104 pfu MHV-68 were harvested for histologic analysis at day 49 after infection. A: Representative H&E- and trichrome-stained lung sections from BMT and control mice at 49 days after infection (magnification ×100). B: Lung sections from BMT and control mice at day 49 after infection were analyzed in a blinded fashion by a pathologist (S.F.) and scored on the basis of the presence and severity of pathologic features (n = 4 BMT mice and 5 control mice; ****P ≤ 0.0001).
Oxygen saturation is reduced in BMT mice during latent MHV-68 infection. Oxygen saturation was measured using a Mouse Ox pulse oximeter at day 21 after infection with 5 × 104 pfu MHV-68 in syngeneic BMT and control mice. Compared with control mice, BMT mice demonstrated significantly decreased oxygen saturation (*P ≤ 0.05; n = 11 mice per group; data combined from three independent experiments).
Expression of alternative macrophage activation markers precede classic activation markers after infection. Alveolar macrophages were collected via BAL from control or BMT mice on day 9 (A and B), day 13 (C and D), or day 19 (E and F) after infection. Total RNA was prepared and analyzed for expression of iNOS as a marker of classic activation (A, C, and E) or arginase 1 (B, D, and F) as a marker of alternative activation (n = 5 mice per group per time point; **P ≤ 0.01, ***P ≤ 0.001).
TGF-β and nitrite levels increase in BAL fluid with time after infection. BAL fluid was collected from control or BMT mice on days 9 (A and E), 13 (B and F), 19 (C and G), or 21 (D and H) after infection. Expression of both mediators was significantly elevated by day 19 after infection and remained elevated at day 21 (n = 5 mice per group per time point; **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001).
Treg depletion does not diminish fibrosis or TGF-β elevation in infected mice after BMT. Control or BMT mice were infected with 5 × 104 PFU γ-HV-68 at week 5 after transplantation. On day 10 after infection, BMT mice were given 100 μL anti-CD25 ascites or 100 μg isotype control. A: On day 21 after infection, lungs were harvested for collagen determination via hydroxyproline assay (n = 5 per group). BAL fluid was collected on day 21 and analyzed for TGF-β levels (B) or nitrite (C) (n = 5 mice per group; *P ≤ 0.05, **P ≤ 0.01).
Sources of TGF-β after infection. Control or BMT mice were infected with 5 × 104 pfu MHV-68, and on day 21 after infection, BAL was performed to collect cell pellets. CD3+ cells (A) or CD19 cells (B) were isolated via magnetic sorting. C: Alveolar macrophages (AMs) were collected via adherence purification. Isolated cell types were cultured for 24 hours, and TGF-β was measured in cell supernatants using an ELISA (n = 4 mice per group). D: Alveolar epithelial cells (AECs) were isolated from control or BMT mice at day 21 after infection, and numbers were determined by counting using a hemocytometer (n = 3). E: AECs isolated from control or BMT mice at day 21 after infection were cultured on fibronectin for 48 hours, medium was changed, and conditioned medium was collected 24 hours later for measurement of TGF-β using an ELISA (n = 4; *P ≤ 0.05, ****P ≤ 0.0001).
Poor control of lytic MHV-68 infection correlates with lung disease during virus latency in CD11c dnTGFβRII BMT mice. Control, BMT, or CD11c dnTGFβRII BMT mice were infected with 5 × 104 pfu MHV-68. A: At day 7 after infection, lungs were harvested for RNA, and expression of the viral envelope glycoprotein gene gB was determined using real time RT-PCR with control levels set to 1. A: CD11c dnTGFβRII BMT mice exhibited a significant increase in viral gB expression when compared with control mice that did not undergo transplantation. There was no significant difference between WT BMT and CD11c dnTGFβRII BMT mice (n = 4 control, 5 WT BMT, and 5 CD11c dnTGFβRII BMT mice). B: H&E and trichrome staining of representative lung sections from CD11c dnTGFβRII BMT mice. C: Histology score representing seven BMT and nine CD11c dnTGFβRII BMT mice (data combined from two experiments; **P ≤ 0.01).
References
- 1.Copelan E.A. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 2.Barfield R.C., Kasow K.A., Hale G.A. Advances in pediatric hematopoietic stem cell transplantation. Cancer Biol Ther. 2008;7:1533–1539. doi: 10.4161/cbt.7.10.7046. [DOI] [PubMed] [Google Scholar]
- 3.Soubani A.O., Miller K.B., Hassoun P.M. Pulmonary complications of bone marrow transplantation. Chest. 1996;109:1066–1077. doi: 10.1378/chest.109.4.1066. [DOI] [PubMed] [Google Scholar]
- 4.Sharma S., Nadrous H.F., Peters S.G., Tefferi A., Litzow M.R., Aubry M.C., Afessa B. Pulmonary complications in adult blood and marrow transplant recipients: autopsy findings. Chest. 2005;128:1385–1392. doi: 10.1378/chest.128.3.1385. [DOI] [PubMed] [Google Scholar]
- 5.Roychowdhury M., Pambuccian S.E., Aslan D.L., Jessurun J., Rose A.G., Manivel J.C., Gulbahce H.E. Pulmonary complications after bone marrow transplantation: an autopsy study from a large transplantation center. Arch Pathol Lab Med. 2005;129:366–371. doi: 10.5858/2005-129-366-PCABMT. [DOI] [PubMed] [Google Scholar]
- 6.Wingard J.R., Hsu J., Hiemenz J.W. Hematopoietic stem cell transplantation: an overview of infection risks and epidemiology. Infect Dis Clin North Am. 2010;24:257–272. doi: 10.1016/j.idc.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Afessa B., Litzow M.R., Tefferi A. Bronchiolitis obliterans and other late onset non-infectious pulmonary complications in hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;28:425–434. doi: 10.1038/sj.bmt.1703142. [DOI] [PubMed] [Google Scholar]
- 8.Wong R., Rondon G., Saliba R.M., Shannon V.R., Giralt S.A., Champlin R.E., Ueno N.T. Idiopathic pneumonia syndrome after high-dose chemotherapy and autologous hematopoietic stem cell transplantation for high-risk breast cancer. Bone Marrow Transplant. 2003;31:1157–1163. doi: 10.1038/sj.bmt.1704141. [DOI] [PubMed] [Google Scholar]
- 9.Bilgrami S.F., Metersky M.L., McNally D., Naqvi B.H., Kapur D., Raible D., Bona R.D., Edwards R.L., Feingold J.M., Clive J.M., Tutschka P.J. Idiopathic pneumonia syndrome following myeloablative chemotherapy and autologous transplantation. Ann Pharmacother. 2001;35:196–201. doi: 10.1345/aph.10071. [DOI] [PubMed] [Google Scholar]
- 10.Limper A.H. Chemotherapy-induced lung disease. Clin Chest Med. 2004;25:53–64. doi: 10.1016/S0272-5231(03)00123-0. [DOI] [PubMed] [Google Scholar]
- 11.Gower W.A., Collaco J.M., Mogayzel P.J., Jr Lung function and late pulmonary complications among survivors of hematopoietic stem cell transplantation during childhood. Paediatr Respir Rev. 2010;11:115–122. doi: 10.1016/j.prrv.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Marras T.K., Szalai J.P., Chan C.K., Lipton J.H., Messner H.A., Laupacis A. Pulmonary function abnormalities after allogeneic marrow transplantation: a systematic review and assessment of an existing predictive instrument. Bone Marrow Transplant. 2002;30:599–607. doi: 10.1038/sj.bmt.1703700. [DOI] [PubMed] [Google Scholar]
- 13.Coomes S.M., Wilke C.A., Moore T.A., Moore B.B. Induction of TGF-beta 1, not regulatory T cells, impairs antiviral immunity in the lung following bone marrow transplant. J Immunol. 2010;184:5130–5140. doi: 10.4049/jimmunol.0901871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coomes S.M., Moore B.B. Pleiotropic effects of transforming growth factor-beta in hematopoietic stem-cell transplantation. Transplantation. 2010;90:1139–1144. doi: 10.1097/TP.0b013e3181efd018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adler H., Beland J.L., Kozlow W., Del-Pan N.C., Kobzik L., Rimm I.J. A role for transforming growth factor-beta1 in the increased pneumonitis in murine allogeneic bone marrow transplant recipients with graft-versus-host disease after pulmonary herpes simplex virus type 1 infection. Blood. 1998;92:2581–2589. [PubMed] [Google Scholar]
- 16.Gorelik L., Flavell R.A. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 17.Laouar Y., Sutterwala F.S., Gorelik L., Flavell R.A. Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nat Immunol. 2005;6:600–607. doi: 10.1038/ni1197. [DOI] [PubMed] [Google Scholar]
- 18.Ojielo C.I., Cooke K., Mancuso P., Standiford T.J., Olkiewicz K.M., Clouthier S., Corrion L., Ballinger M.N., Toews G.B., Paine R., III, Moore B.B. Defective phagocytosis and clearance of Pseudomonas aeruginosa in the lung following bone marrow transplantation. J Immunol. 2003;171:4416–4424. doi: 10.4049/jimmunol.171.8.4416. [DOI] [PubMed] [Google Scholar]
- 19.Ballinger M.N., Aronoff D.M., McMillan T.R., Cooke K.R., Olkiewicz K., Toews G.B., Peters-Golden M., Moore B.B. Critical role of prostaglandin E2 overproduction in impaired pulmonary host response following bone marrow transplantation. J Immunol. 2006;177:5499–5508. doi: 10.4049/jimmunol.177.8.5499. [DOI] [PubMed] [Google Scholar]
- 20.Kolodsick J.E., Toews G.B., Jakubzick C., Hogaboam C., Moore T.A., McKenzie A., Wilke C.A., Chrisman C.J., Moore B.B. Protection from fluorescein isothiocyanate–induced fibrosis in IL-13–deficient, but not IL-4–deficient, mice results from impaired collagen synthesis by fibroblasts. J Immunol. 2004;172:4068–4076. doi: 10.4049/jimmunol.172.7.4068. [DOI] [PubMed] [Google Scholar]
- 21.Thrall R.S., McCormick J.R., Jack R.M., McReynolds R.A., Ward P.A. Bleomycin-induced pulmonary fibrosis in the rat: inhibition by indomethacin. Am J Pathol. 1979;95:117–130. [PMC free article] [PubMed] [Google Scholar]
- 22.Moore B.B., Peters-Golden M., Christensen P.J., Lama V., Kuziel W.A., Paine R., III, Toews G.B. Alveolar epithelial cell inhibition of fibroblast proliferation is regulated by MCP-1/CCR2 and mediated by PGE2. Am J Physiol Lung Cell Mol Physiol. 2003;284:L342–L349. doi: 10.1152/ajplung.00168.2002. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen Y., McGuffie B.A., Anderson V.E., Weinberg J.B. Gammaherpesvirus modulation of mouse adenovirus type 1 pathogenesis. Virology. 2008;380:182–190. doi: 10.1016/j.virol.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoolman J.S., Vannella K.M., Coomes S.M., Wilke C.A., Sisson T.H., Toews G.B., Moore B.B. Latent infection by γherpesvirus stimulates profibrotic mediator release from multiple cell types [published online ahead of print October 2010] Am J Physiol Lung Cell Mol Physiol. 2011;300:L274–L285. doi: 10.1152/ajplung.00028.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vannella K.M., Luckhardt T.R., Wilke C.A., van Dyk L.F., Toews G.B., Moore B.B. Latent herpesvirus infection augments experimental pulmonary fibrosis. Am J Respir Crit Care Med. 2010;181:465–477. doi: 10.1164/rccm.200905-0798OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gordon S., Martinez F.O. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Mora A.L., Torres-Gonzalez E., Rojas M., Corredor C., Ritzenthaler J., Xu J., Roman J., Brigham K., Stecenko A. Activation of alveolar macrophages via the alternative pathway in herpesvirus-induced lung fibrosis. Am J Respir Cell Mol Biol. 2006;35:466–473. doi: 10.1165/rcmb.2006-0121OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Psathakis K., Mermigkis D., Papatheodorou G., Loukides S., Panagou P., Polychronopoulos V., Siafakas N.M., Bouros D. Exhaled markers of oxidative stress in idiopathic pulmonary fibrosis. Eur J Clin Invest. 2006;36:362–367. doi: 10.1111/j.1365-2362.2006.01636.x. [DOI] [PubMed] [Google Scholar]
- 29.Carpenter M., Epperly M.W., Agarwal A., Nie S., Hricisak L., Niu Y., Greenberger J.S. Inhalation delivery of manganese superoxide dismutase-plasmid/liposomes protects the murine lung from irradiation damage. Gene Ther. 2005;12:685–693. doi: 10.1038/sj.gt.3302468. [DOI] [PubMed] [Google Scholar]
- 30.Border W.A., Noble N.A. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 31.Martin M., Lefaix J., Delanian S. TGF-beta1 and radiation fibrosis: a master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys. 2000;47:277–290. doi: 10.1016/s0360-3016(00)00435-1. [DOI] [PubMed] [Google Scholar]
- 32.Stevenson P.G., Belz G.T., Castrucci M.R., Altman J.D., Doherty P.C. A gamma-herpesvirus sneaks through a CD8(+) T cell response primed to a lytic-phase epitope. Proc Natl Acad Sci USA. 1999;96:9281–9286. doi: 10.1073/pnas.96.16.9281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clambey E.T., Virgin H.W.T., Speck S.H. Disruption of the murine gammaherpesvirus 68 M1 open reading frame leads to enhanced reactivation from latency. J Virol. 2000;74:1973–1984. doi: 10.1128/jvi.74.4.1973-1984.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore B.B., Hogaboam C.M. Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;294:L152–L160. doi: 10.1152/ajplung.00313.2007. [DOI] [PubMed] [Google Scholar]
- 35.Versluys A.B., Rossen J.W., van Ewijk B., Schuurman R., Bierings M.B., Boelens J.J. Strong association between respiratory viral infection early after hematopoietic stem cell transplantation and the development of life-threatening acute and chronic alloimmune lung syndromes. Biol Blood Marrow Transplant. 2010;16:782–791. doi: 10.1016/j.bbmt.2009.12.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doherty P.C., Christensen J.P., Belz G.T., Stevenson P.G., Sangster M.Y. Dissecting the host response to a gamma-herpesvirus. Philos Trans R Soc Lond B Biol Sci. 2001;356:581–593. doi: 10.1098/rstb.2000.0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez F.O., Helming L., Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 38.Schreiber T., Ehlers S., Heitmann L., Rausch A., Mages J., Murray P.J., Lang R., Holscher C. Autocrine IL-10 induces hallmarks of alternative activation in macrophages and suppresses antituberculosis effector mechanisms without compromising T cell immunity. J Immunol. 2009;183:1301–1312. doi: 10.4049/jimmunol.0803567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kliment C.R., Oury T.D. Oxidative stress, extracellular matrix targets, and idiopathic pulmonary fibrosis. Free Radic Biol Med. 2010;49:707–717. doi: 10.1016/j.freeradbiomed.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 40.Hsu Y.C., Wang L.F., Chien Y.W. Nitric oxide in the pathogenesis of diffuse pulmonary fibrosis. Free Radic Biol Med. 2007;42:599–607. doi: 10.1016/j.freeradbiomed.2006.11.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pneumonitis in BMT mice persists even 7 weeks after infection. Lungs from syngeneic BMT and control mice infected with 5 × 104 pfu MHV-68 were harvested for histologic analysis at day 49 after infection. A: Representative H&E- and trichrome-stained lung sections from BMT and control mice at 49 days after infection (magnification ×100). B: Lung sections from BMT and control mice at day 49 after infection were analyzed in a blinded fashion by a pathologist (S.F.) and scored on the basis of the presence and severity of pathologic features (n = 4 BMT mice and 5 control mice; ****P ≤ 0.0001).
Oxygen saturation is reduced in BMT mice during latent MHV-68 infection. Oxygen saturation was measured using a Mouse Ox pulse oximeter at day 21 after infection with 5 × 104 pfu MHV-68 in syngeneic BMT and control mice. Compared with control mice, BMT mice demonstrated significantly decreased oxygen saturation (*P ≤ 0.05; n = 11 mice per group; data combined from three independent experiments).
Expression of alternative macrophage activation markers precede classic activation markers after infection. Alveolar macrophages were collected via BAL from control or BMT mice on day 9 (A and B), day 13 (C and D), or day 19 (E and F) after infection. Total RNA was prepared and analyzed for expression of iNOS as a marker of classic activation (A, C, and E) or arginase 1 (B, D, and F) as a marker of alternative activation (n = 5 mice per group per time point; **P ≤ 0.01, ***P ≤ 0.001).
TGF-β and nitrite levels increase in BAL fluid with time after infection. BAL fluid was collected from control or BMT mice on days 9 (A and E), 13 (B and F), 19 (C and G), or 21 (D and H) after infection. Expression of both mediators was significantly elevated by day 19 after infection and remained elevated at day 21 (n = 5 mice per group per time point; **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001).
Treg depletion does not diminish fibrosis or TGF-β elevation in infected mice after BMT. Control or BMT mice were infected with 5 × 104 PFU γ-HV-68 at week 5 after transplantation. On day 10 after infection, BMT mice were given 100 μL anti-CD25 ascites or 100 μg isotype control. A: On day 21 after infection, lungs were harvested for collagen determination via hydroxyproline assay (n = 5 per group). BAL fluid was collected on day 21 and analyzed for TGF-β levels (B) or nitrite (C) (n = 5 mice per group; *P ≤ 0.05, **P ≤ 0.01).
Sources of TGF-β after infection. Control or BMT mice were infected with 5 × 104 pfu MHV-68, and on day 21 after infection, BAL was performed to collect cell pellets. CD3+ cells (A) or CD19 cells (B) were isolated via magnetic sorting. C: Alveolar macrophages (AMs) were collected via adherence purification. Isolated cell types were cultured for 24 hours, and TGF-β was measured in cell supernatants using an ELISA (n = 4 mice per group). D: Alveolar epithelial cells (AECs) were isolated from control or BMT mice at day 21 after infection, and numbers were determined by counting using a hemocytometer (n = 3). E: AECs isolated from control or BMT mice at day 21 after infection were cultured on fibronectin for 48 hours, medium was changed, and conditioned medium was collected 24 hours later for measurement of TGF-β using an ELISA (n = 4; *P ≤ 0.05, ****P ≤ 0.0001).
Poor control of lytic MHV-68 infection correlates with lung disease during virus latency in CD11c dnTGFβRII BMT mice. Control, BMT, or CD11c dnTGFβRII BMT mice were infected with 5 × 104 pfu MHV-68. A: At day 7 after infection, lungs were harvested for RNA, and expression of the viral envelope glycoprotein gene gB was determined using real time RT-PCR with control levels set to 1. A: CD11c dnTGFβRII BMT mice exhibited a significant increase in viral gB expression when compared with control mice that did not undergo transplantation. There was no significant difference between WT BMT and CD11c dnTGFβRII BMT mice (n = 4 control, 5 WT BMT, and 5 CD11c dnTGFβRII BMT mice). B: H&E and trichrome staining of representative lung sections from CD11c dnTGFβRII BMT mice. C: Histology score representing seven BMT and nine CD11c dnTGFβRII BMT mice (data combined from two experiments; **P ≤ 0.01).


